Summary
Background
Azathioprine is used as a first-line treatment to prevent relapses of neuromyelitis optica spectrum disorder (NMOSD). Tocilizumab has been reported to reduce NMOSD disease activity in retrospective case reports. We aimed to compare the safety and efficacy of tocilizumab and azathioprine in patients with highly relapsing NMOSD.
Methods
We did an open-label, multicentre, randomised, phase 2 trial at six hospitals in China. We recruited adult patients (aged ≥18 years) with highly relapsing NMOSD diagnosed according to 2015 International Panel for Neuromyelitis Optica Diagnosis criteria, who had an Expanded Disability Status Scale (EDSS) score of 7·5 or lower, and had a history of at least two clinical relapses during the previous 12 months or three relapses during the previous 24 months with at least one relapse within the previous 12 months. Patients were randomly assigned (1:1) to intravenous tocilizumab (8 mg/kg every 4 weeks) or oral azathioprine (2–3 mg/kg per day) by an independent statistician using computer-generated randomisation software with permuted blocks of four. The central review committee, EDSS raters, laboratory personnel, and radiologists were masked to the treatment assignment, but investigators and patients were aware of treatment allocation. The minimum planned duration of treatment was 60 weeks following randomisation. The primary outcome was time to first relapse in the full analysis set, which included all randomly assigned patients who received at least one dose of study drug, and the per-protocol population, which included all patients who used azathioprine or tocilizumab as monotherapy. For the analyses of the primary outcome, the patients were prespecified into two subgroups according to concomitant auto immune disease status. Safety was assessed in the full analysis set. This study is registered with ClinicalTrials.gov, NCT03350633.
Findings
Between Nov 1, 2017, and Aug 3, 2018, we enrolled 118 patients, of whom 59 were randomly assigned to tocilizumab and 59 were randomly assigned to azathioprine. All 118 patients received one dose of study drug and were included in the full analysis set. 108 participants were included in the per-protocol analysis (56 in the tocilizumab group and 52 in the azathioprine group). In the full analysis set, median time to the first relapse was longer in the tocilizumab group than the azathioprine group (78·9 weeks [IQR 58·3–90·6] vs 56·7 [32·9–81·7] weeks; p=0·0026). Eight (14%) of 59 patients in the tocilizumab group and 28 (47%) of 59 patients in the azathioprine group had a relapse at the end of the study (hazard ratio [HR] 0·236 [95% CI 0·107–0·518]; p<0·0001). In the per-protocol analysis, 50 (89%) of 56 patients in the tocilizumab group were relapse-free compared with 29 (56%) of 52 patients in the azathioprine group at the end of the study (HR 0·188 [95% CI 0·076–0·463]; p<0·0001); the median time to first relapse was also longer in the tocilizumab group than the azathioprine group (67·2 weeks [IQR 47·9–77·9] vs 38·0 [23·6–64·9]; p<0·0001). In the prespecified subgroup analysis of the full analysis set stratified by concomitant autoimmune diseases, among patients without concomitant autoimmune diseases, three (9%) of 34 patients in the tocilizumab group and 13 (35%) of 37 patients in the azathioprine group had relapsed by the end of the study. Among patients with concomitant autoimmune diseases, a lower proportion of patients in the tocilizumab group had a relapse than in the azathioprine group (five [20%] of 25 patients vs 15 [68%] of 22 patients; HR 0·192 [95% CI 0·070–0·531]; p=0·0004). 57 (97%) of 59 patients in the tocilizumab group and 56 (95%) of 59 patients in the azathioprine group had adverse events. Treatment-associated adverse events occurred in 36 (61%) of 59 tocilizumab-treated patients and 49 (83%) of 59 azathioprine-treated patients. One death (2%) occurred in the tocilizumab group and one (2%) in the azathioprine group, but neither of the deaths were treatment-related.
Interpretation
Tocilizumab significantly reduced the risk of a subsequent NMOSD relapse compared with azathioprine. Tocilizumab might therefore be another safe and effective treatment to prevent relapses in patients with NMOSD.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a severe disabling inflammatory autoimmune disease of the CNS frequently associated with a pathological humoral immune response against the aquaporin-4 (AQP-4) water channel. The disorder is most commonly characterised by recurrent relapses of optic neuritis and longitudinally extensive transverse myelitis.1,2 Frequent relapses result in stepwise accumulation of neurological disability. Therefore, prevention of relapse is of paramount importance to reduce the risk of systemic disability over time.3,4
Azathioprine, mycophenolate mofetil, and rituximab are the most commonly used therapies for patients with NMOSD. On the basis of retrospective, open-label studies, azathioprine (a purine analogue that blocks DNA synthesis) has been recommended as a first-line treatment to reduce relapse rate and ameliorate neurological disability in patients with NMOSD.5,6 However, a substantial number of patients relapse and have side-effects with prolonged use of azathioprine.7 Additionally, because azathioprine is often used in conjunction with cortico steroids, its efficacy as a monotherapy remains unclear.8
Tocilizumab, the first humanised anti-interleukin (IL)-6 receptor monoclonal antibody, has been extensively used in patients with rheumatoid arthritis and juvenile idiopathic arthritis and is now approved for the treatment of several autoimmune diseases including giant cell arteritis.9 In previous case series, tocilizumab has been found to reduce the frequency of relapses and disability in patients with NMOSD10–12 including patients who have not responded to treatment with several immunosuppressants or the B-cell-depleting antibody rituximab. The IL-6 receptor mono clonal antibody satralizumab has been shown to significantly reduce the risk of NMOSD relapse compared with placebo.13 Disruption of IL-6 signalling might affect NMOSD disease activity via multiple pathways: reduction in AQP-4 autoantibody (AQP4-IgG) production, inhibition of pro-inflammatory T-cell differentiation, and lowering of blood–brain barrier permeability.14
Head-to-head comparison of therapeutics that act on distinct pathways presumed to be involved in NMOSD pathogenesis is imperative. Furthermore, evidence is needed to assess the benefit-to-risk ratio of newer medications (eg, IL-6 receptor, CD19, and C5 monoclonal antibodies) compared with more commonly used drugs such as azathioprine. We hypothesised that tocilizumab is superior to azathioprine in reducing the risk of relapse in patients with NMOSD. Therefore, we aimed to compare the safety and efficacy of tocilizumab with azathioprine in reducing the risk of relapse and disability in patients with highly relapsing NMOSD.
Methods
Study design and participants
TANGO was an open-label, multicentre, randomised phase 2 trial that recruited patients from six hospitals in China (Tianjin Medical University General Hospital [Tianjin], First Hospital of Shanxi Medical University [Taiyuan], The Third Hospital of Sun Yat-sen University [Guangzhou], Beijing Tiantan Hospital [Beijing], The Third People’s Hospital of Datong [Datong], and Tianjin Huanhu Hospital [Tianjin]). Eligible patients were adults (≥18 years) with highly relapsing NMOSD diagnosed according to 2015 International Panel for Neuromyelitis Optica Diagnosis criteria,15 who had an Expanded Disability Status Scale (EDSS) score of 7·5 or lower, and a history of at least two clinical relapses during the previous 12 months or three relapses during the previous 24 months, with at least one relapse in the previous 12 months. Patients were excluded if they had evidence of clinically significant infection, were pregnant or planning to conceive during the trial period, had previously relapsed on azathioprine therapy, had a heterozygous or homozygous mutation in the thiopurine methyltransferase (TPMT) gene, had received rituximab or any experimental B-cell-depleting drug in the 6 months before screening, or presented with a percentage of CD19-positive B cells in peripheral blood mononuclear cells that was higher than 1%. The study was done in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol (appendix p 24) was approved by the institutional review board at each participating institution. All patients provided written informed consent before study inclusion.
Randomisation and masking
Eligible patients were randomly assigned (1:1) to receive either tocilizumab or azathioprine. Randomisation was done centrally by an independent biostatistician at Tianjin Medical University (Tianjin, China) using computer-generated randomisation with permuted block sizes of four and was overseen by the chair of the Statistician Panel (appendix p 3). The central review committee (appendix p 3) who adjudicated relapses, EDSS raters, laboratory personnel, and radiologists were all masked to the treatment assignment. Investigators and patients were aware of treatment allocation. The success of masking was assessed and monitored by members of the local ethics committees.
Procedures
Patients in the azathioprine group were given an initial dose of oral azathioprine 25 mg, which was increased stepwise in 25 mg per day increments until the daily target dose (2–3 mg/kg) was reached. During this loading period, patients who had medication-related side-effects were allowed symptomatic treatments, with the exception of any new immunosuppressants. Once the daily target dose had been reached, patients received their final, stable dosage of azathioprine daily until relapse, discontinuation, or the end of the trial. Patients in the azathioprine group were permitted to receive concomitant immunosuppressants during the first 24 weeks of treatment as follows: patients without previous azathioprine treatment received 24 weeks of concomitant immunosuppressants; patients who had received azathioprine for less than 24 weeks before randomisation received supplementary immunosuppressants until they had had 24 weeks of azathioprine treatment; and patients who had received azathioprine for 24 weeks or longer before randomisation received no concomitant immunosuppressants. All patients in the azathioprine group continued medication as monotherapy after 24 weeks of combined treatment.
Patients in the tocilizumab group were given intravenous tocilizumab 8 mg/kg every 4 weeks. Adjustment of the infusion rate and symptomatic treatment with prednisone or diphenhydramine were permitted to manage infusion-related reactions. Patients in the tocilizumab group were permitted to receive concomitant immunosuppressants for the first 12 weeks; thereafter, tocilizumab was used as monotherapy. The planned total duration of treatment in both groups was 60 weeks after randomisation.
At each study site, the investigator was responsible for assessing patient eligibility, supervising the administration of study medication, recording and managing adverse events, and assessing relapses. The investigators identified relapses, which were subsequently confirmed by the central review committee. Patients were assessed within 24 h after a possible relapse and at intervals of 1, 4, 6, and 12 weeks by the investigators and EDSS raters. Relapses could be treated with intravenous glucocorticoids, intravenous immunoglobulin, or plasma exchange as per the investigators’ discretion.
Brain and spinal cord MRI scans were done at baseline and week 60 using 3T scanners with identical scanning protocols used across the hospitals. MRI scans were also done at the time of suspected relapse. MRI scans were analysed centrally and independently by radiologists at the Department of Radiology at Tianjin Medical University General Hospital (Tianjin, China) and used to confirm relapse on the basis of protocol definitions (appendix p 7). AQP4-IgG seropositivity and titres were determined centrally using a live cell-binding assay (appendix p 8).
Laboratory tests including routine blood chemistry were done at baseline, every 2 weeks for the first 12 weeks, and every 6 weeks thereafter until study completion. Physical and neurological examinations were done every 4 weeks. Patients who withdrew or discontinued the study drug were monitored for a 24-week follow-up period, during which disability was assessed.
For vision assessment, low-contrast letter scores were measured using a retro-illuminated 2·5% Sloan chart (Precision Vision, LaSalle, IL, USA), and the logarithm of the minimum angle of resolution (logMAR) and high-contrast letter scores were measured using the retro-illuminated Early Treatment Diabetic Retinopathy Study chart (Precision Vision). High-resolution spectral domain optical coherence tomography images using RTVUE100–2 (Optovue, Fremont, CA, USA) including the peripapillary retinal nerve fibre layer (pRNFL) and macular volume were obtained at baseline and the last follow-up visit. Full-field visual evoked potentials were recorded at baseline, relapse, and the last follow-up using the Synergy system (EMG and Evoked Potential Response Unit, Nicolet, NE, USA).
Outcomes
The primary outcome was time to first relapse. Relapse was defined as new onset of neurological symptoms or worsening of existing neurological symptoms with an objective change on neurological examination that persisted for more than 24 h, with signs and symptoms attributable solely to NMOSD, and preceded by at least 30 days of clinical stability (appendix p 5). MRI was used to confirm cases of relapse for which clinical changes on examination did not meet relapse criteria. A relapse required a change in the EDSS score regardless of MRI (appendix pp 5–6). We did a prespecified subgroup analysis of patients with and without concomitant autoimmune diseases. We also did prespecified subgroup analyses of the primary outcome stratified by AQP4-IgG status, EDSS score at baseline, total number of previous relapses, disease duration, age at randomisation, and number of relapse 1 or 2 years before randomisation.
The secondary outcomes were analysis of the proportion of patients with confirmed disability progression for at least 12 weeks and change in serum AQP4-IgG titres between baseline and the end of the trial (60 weeks).Disability progression was defined as an increase in EDSS score of at least 1·0 point from baseline that was sustained on subsequent visits for at least 12 weeks if the baseline EDSS score was 5·5 or less, or an increase in the EDSS of at least 0·5 points that was sustained for at least 12 weeks if the baseline score was greater than 5·5. Patients with initial disability progression during the treatment period who discontinued tocilizumab or azathioprine early and did not have a subsequent visit with confirmatory measurement of the EDSS score were considered to have confirmed disability progression.
Exploratory outcomes were the proportion of patients with confirmed disability progression for at least 24 weeks using the same EDSS criteria as used for secondary outcomes; change in high-contrast visual acuity between baseline and 60 weeks; change in low-contrast letter acuity between baseline and 60 weeks; change in mean pRNFL thickness between baseline and 60 weeks; change in mean ganglion cell complex volume between baseline and 60 weeks; and change in P100 latency and amplitude between baseline and 60 weeks; number of new or enlarging T2 hyperintense lesions on brain and spinal cord MRI; and change in peripheral blood B-cell subset count. The change in mean ganglion cell complex volume between baseline and 60 weeks, number of new or enlarging T2 hyperintense lesions on brain and spinal cord MRI, and change in peripheral blood B-cell subset count exploratory outcomes will be published elsewhere.
Safety was assessed based on adverse events reported by study participants or investigators. All adverse events were recorded according to Common Terminology Criteria for Adverse Events (version 5.0) and were coded using the preferred terms in the Medical Dictionary for Regulatory Activities (version 22.0). Relapses were considered a severe adverse event when the relapse resulted in hospital admission for any reason other than routine treatment of NMOSD relapse (eg, for a treatment course beyond the standard treatment or when hospital stay is prolonged). Investigators assessed the association between adverse events and study treatment using predefined categories (not related and unlikely, possibly, probably, or definitely related).
Statistical analysis
The sample size for this trial was calculated on the basis of the estimated proportion of relapse-free patients at 12 months (85% for tocilizumab; 60% for azathioprine; HR 0·32). Assuming 1:1 randomisation, we calculated that 118 patients with 30 relapse events would provide 80% power to determine the prespecified between-group difference on the basis of a two-sided log-rank test with a probability of type I error of 5%, assuming a 10% dropout rate. Enrolment was planned to stop when 118 participants had been randomly allocated and at least 30 relapse events had occurred.
All analyses were prespecified in a detailed statistical analysis plan. The primary outcome was analysed in the full analysis set, which included all randomly assigned patients who received at least one dose of tocilizumab or azathioprine, and the per-protocol population, which included all patients who used azathioprine or tocilizumab as monotherapy. Analyses of the secondary and safety outcomes were done in the full analysis set. For the secondary outcome of confirmed disability progression that was sustained for 12 weeks or longer, patients with missing data for the EDSS score at baseline were excluded from the analysis. For the baseline variables, summary statistics were presented as frequencies and proportions for categorical data and means (SDs) or median (IQR) for continuous variables. Patient characteristics were compared using Fisher’s exact test for categorical outcomes and Student’s t test for continuous variables, as appropriate.
We used a two-sided log-rank test and Cox proportional hazards model to analyse between-group differences for the primary outcome. Data for patients who did not have a relapse were censored at the end of the trial period, including those who discontinued the trial early. Cox regression was used for estimation of HRs. We generated Kaplan-Meier plots of relapse-free survival for prespecified subgroups of patients with or without concomitant autoimmune diseases. We additionally did prespecified subgroup analyses to determine the effect size of treatment in the primary outcome variable stratified by AQP4-IgG status, EDSS score at baseline, total number of previous relapses, disease duration, age at randomisation, and number of relapses 1 or 2 years before randomisation. HRs and associated p values of each interaction term were estimated from separate logistic regression models across the prespecified subgroups.
Log-rank analyses were also applicable to the outcome of confirmed disability progression that was sustained for 12 weeks or longer.
Since serum AQP4-IgG titres are not normally distributed and contain extreme outlier values, the change and percentage change in AQP4-IgG titres were analysed using Wilcoxon rank sum test and Hodges-Lehmann estimation. Missing values were imputed by means of the last-observation-carried-forward method.
For the visual acuity, thickness of pRNFL, and P100 latency and amplitude exploratory outcomes, two independent-sample t tests were used to analyse the difference between treatment groups. The Satterthwaite method16 was used to correct results if homogeneity of variance was not satisfied.
The Bonferron-based chain procedure was used to control the overall type 1 error rate to α=0·05.17 The primary outcome was tested at α=0·05 in the full analysis set. If the primary endpoint was met in the full analysis set, then all secondary and exploratory outcomes were tested in the same manner, with each secondary outcome initially tested based on the chain procedure at α=0·025. If the null hypothesis for a secondary outcome was rejected across overall populations, the type 1 error saved was propagated equally to other nonrejected sets of secondary null hypotheses.
All analyses were done using SAS software (version 9.4). This study is registered with ClinicalTrials.gov, NCT03350633.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Results
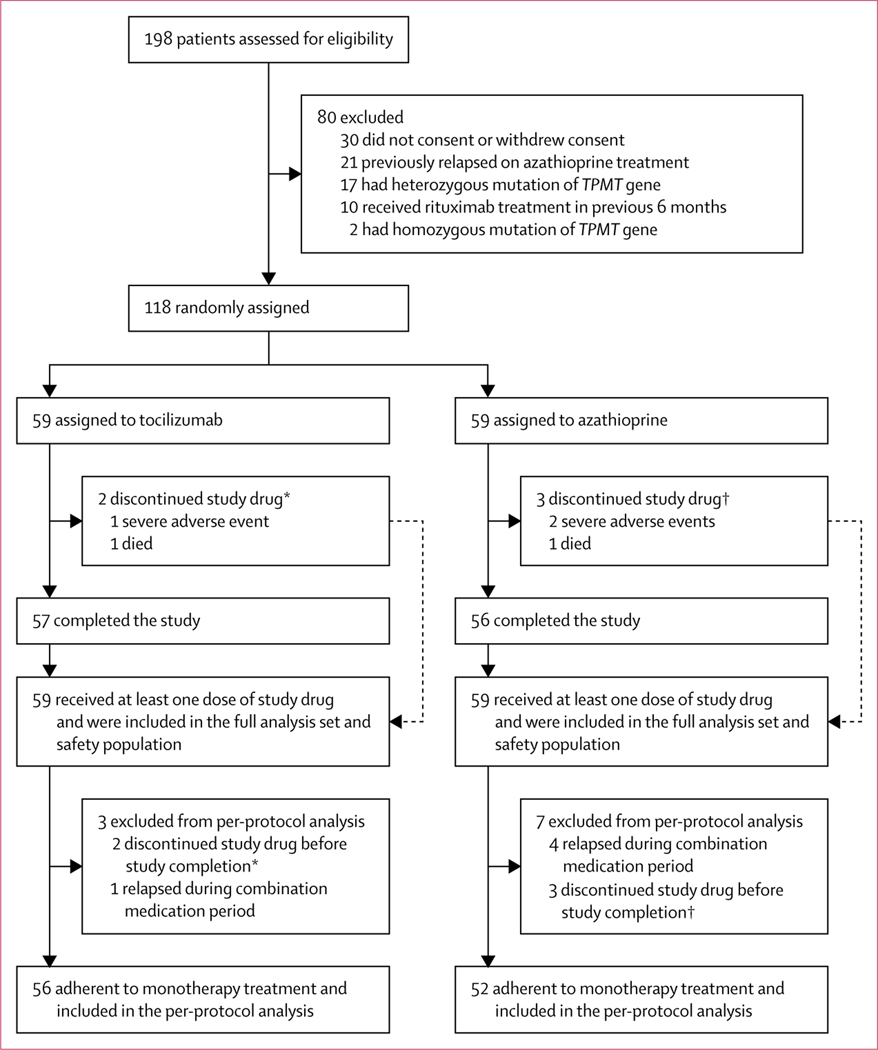
Between Nov 1, 2017, and Aug 3, 2018, 118 patients were enrolled and randomly assigned to tocilizumab (n=59) or azathioprine (n=59), all of whom received at least one dose of the study drug and were included in the full analysis set for the primary outcome. Three participants (one [2%] of 59 patients in the tocilizumab group and two [3%] of 59 patients in the azathioprine group) discontinued the trial due to severe adverse events (appendix p 22) and two patients died (one [2%] in the tocilizumab group and one [2%] in the azathioprine group). Five patients (one [2%] patient in the tocilizumab group and four [7%] patients in the azathioprine group) had a new relapse during the periods of concomitant immunosuppressant treatment. Thus, the remaining 56 patients assigned to tocilizumab and 52 to azathioprine were adherent to monotherapy treatment and were included in the per-protocol analysis for the primary outcome (figure 1).
Figure 1: Trial profile.
TPMT=thiopurine methyltransferase. Patients in both treatment groups had a washout period to taper baseline concomitant corticosteroids or immunosuppressants. *Represents the same two patients. †Represents the same three patients.
Baseline characteristics were generally balanced between the two groups (table 1). The mean (SD) annualised relapse rate during the previous 24 months was 1·69 (0·64), and the median score on the EDSS was 4·5 (IQR 4·0–5·5), indicating moderate-to-severe disability. 47 (40%) of 118 patients had concomitant autoimmune diseases. The trial was stopped when at least 30 relapses had occurred.
Table 1:
Baseline characteristics of all randomly assigned patients
| Tocilizumab (n=59) | Azathioprine (n=59) | |
|---|---|---|
| Sex | ||
| Women | 55 (93%) | 53 (90%) |
| Men | 4 (7%) | 6 (10%) |
| Age, years | 48·1 (13·4) | 45·3 (14·5) |
| Disease history, years | 6·0 (2·9) | 6·2 (3·1) |
| AQP4-IgG positivity | 50 (85%) | 53 (90%) |
| Annualised relapse rate during previous 24 months | 1·71 (0·60) | 1·68 (0·68) |
| EDSS score at randomisation* | 4·5 (4·0–5·5) | 4·5 (4·0–6·0) |
| Time from last relapse to randomisation, days | 118 (71) | 92 (61) |
| Type of relapse during previous 24 monthst† | ||
| Optic neuritis | 46 (78%) | 47 (80%) |
| Acute myelitis | 56 (95%) | 55 (93%) |
| Area postrema syndrome | 11 (19%) | 14 (24%) |
| Acute brainstem symptom | 16 (27%) | 20 (34%) |
| Diencephalic clinical syndrome | 2 (3%) | 3 (5%) |
| Symptomatic cerebral syndrome | 8 (14%) | 6 (10%) |
| Immunosuppressant therapy at randomisation‡ | ||
| Oral corticosteroids | 10 (17%) | 5 (8%) |
| Mycophenolate mofetil | 7 (12%) | 8 (14%) |
| Azathioprine | 4 (7%) | 6 (10%) |
| Tacrolimus | 2 (3%) | 0 |
| Cyclophosphamide | 0 | 2 (3%) |
| Methotrexate | 0 | 2 (3%) |
| Oral corticosteroids and azathioprine | 12 (20%) | 20 (34%) |
| Oral corticosteroids and mycophenolate mofetil | 11 (19%) | 8 (14%) |
| Oral corticosteroids and methotrexate | 3 (5%) | 3 (5%) |
| Oral corticosteroids and cyclophosphamide | 4 (7%) | 2 (3%) |
| Oral corticosteroids and tacrolimus | 2 (3%) | 1 (2%) |
| Azathioprine and cyclophosphamide | 0 | 2 (3%) |
| Oral corticosteroids and cyclosporin | 1 (2%) | 0 |
| Oral corticosteroids and IVIG | 1 (2%) | 0 |
| IVIG | 1 (2%) | 0 |
| None | 1 (2%) | 0 |
| Concomitant autoimmune diseases | ||
| Any | 25 (42%) | 22 (37%) |
| Sjögren’s syndrome | 7 (12%) | 7 (12%) |
| Urticarial vasculitis | 0 | 1 (2%) |
| Autoimmune thyroiditis | 4 (7%) | 2 (3%) |
| Systemic lupus erythematosus | 1 (2%) | 2 (3%) |
| Rheumatoid arthritis | 6 (10%) | 4 (7%) |
| Autoimmune haemolytic anaemia | 0 | 1 (2%) |
| Myasthenia gravis | 1 (2%) | 1 (2%) |
| Ankylosing spondylitis | 1 (2%) | 0 |
| Immune thrombocytopenic purpura | 1 (2%) | 0 |
| Mixed connective tissue disease | 4 (7%) | 2 (3%) |
| Primary hyperthyroidism | 2 (3%) | 2 (3%) |
Data are n (%), mean (SD), or median (IQR). AQP4-IgG=aquaporin-4 autoantibody. EDSS=Expanded Disability Status Scale. IVIG=intravenous immunoglobulin.
Scores range from 0 (no disability) to 10 (death).
Relapses defined according to the 2015 International Panel for Neuromyelitis Optica Diagnosis criteria.15
Oral corticosteroids denoted prednisolone (15–20 mg per day) or methylprednisolone (12–16 mg per day), used alone or in combination with other immunosuppressants.
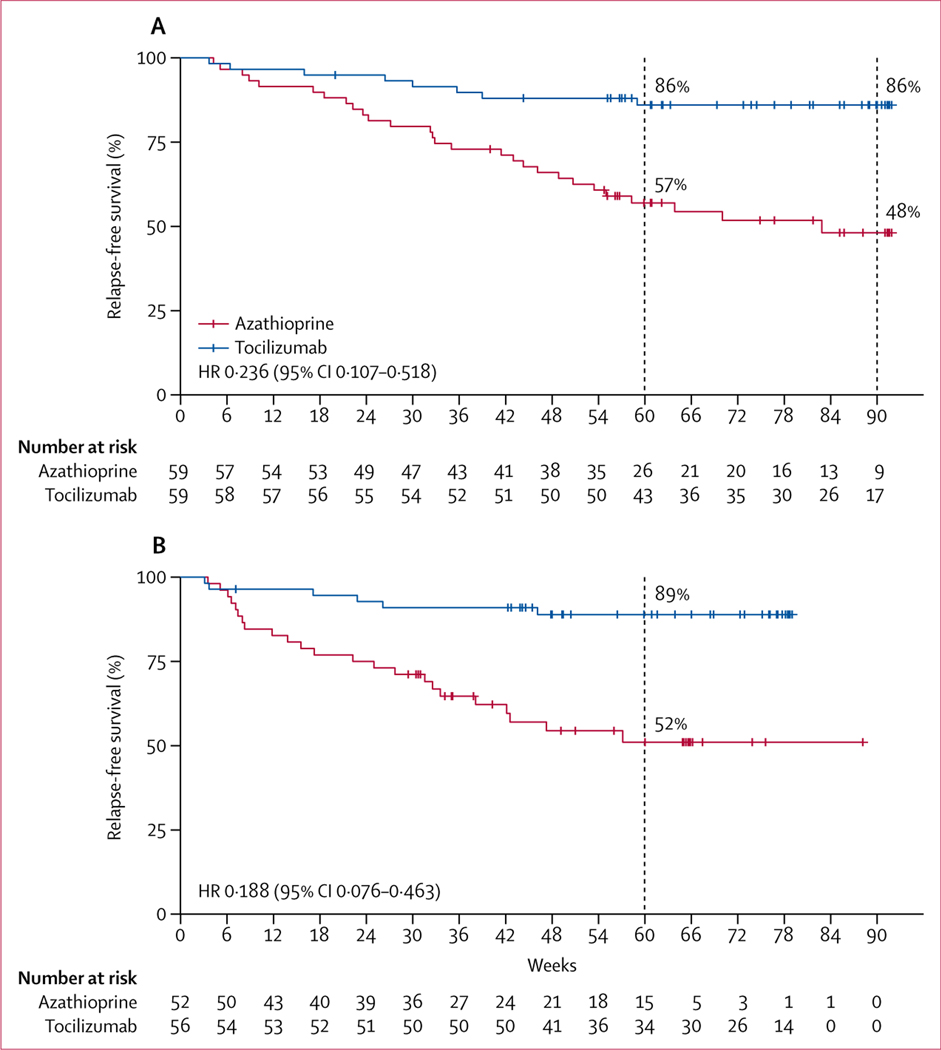
At 60 weeks, the risk of relapse was significantly lower in the tocilizumab group than the azathioprine group (HR 0·274 [95% CI 0·123–0·607]; p=0·0006). 43 (73%) of 59 patients in the tocilizumab group and 25 (42%) of 59 patients in the azathioprine group were followed up for 90 weeks. This 90-week follow-up duration for a large proportion of patients was not derived from amendment of the trial protocol, but as a result of time needed to execute this trial—ie, when the final group of patients was recruited, the first group of patients had already been in the trial for around 30 weeks. Among patients who did not relapse, the longest follow-up duration was 92 weeks.
Overall, a total of 36 relapses were recorded in the study. In the full analysis set, the median time to the first relapse was longer in the tocilizumab group than the azathioprine group (78·9 weeks [IQR 58·3–90·6] vs 56·7 weeks [32·9–81·7]; p=0·0026). By the end of the study, eight (14%) of 59 patients in the tocilizumab group had a relapse compared with 28 (47%) of 59 in the azathioprine group (HR 0·236 [95% CI 0·107–0·518]; p<0·0001; table 2, figure 2A). 25 (70%) of relapses (six patients in the tocilizumab group and 19 patients in the azathioprine group) met definite clinical criteria for relapse. Two (25%) of eight patients in the tocilizumab group and nine (32%) of 28 patients in the azathioprine group who had minor changes in EDSS score and did not meet clinical criteria for relapse had their relapses confirmed by MRI, indicated by new or enlarging T2-weighted MRI lesions or T1-weighted gadolinum-enhancing lesions (appendix p 20). In the per-protocol analysis, at the end of the study, 50 (89%) of 56 patients in the tocilizumab group and 29 (56%) of 52 patients in the azathioprine group were relapse-free (HR 0·188 [95% CI 0·076–0·463]; p<0·0001; figure 2B). Median time to first relapse was longer in the tocilizumab group than the azathioprine group (67·2 weeks [47·9–77·9] vs 38·0 [23·6–64·9]; p<0·0001). Details of relapses are shown in the appendix (p 20).
Table 2:
Efficacy outcomes of all randomly assigned patients
| Tocilizumab (n=59) | Azathioprine (n=59) | HR (95% CI)* | p value | |
|---|---|---|---|---|
| Primary outcome | ||||
| First relapse | 8 (14%) | 28 (47%) | 0·236 (0·107 to 0·518) | <0·0001 |
| Time to first relapse, weeks | 78·9 (58·3 to 90·6) | 56·7 (32·9 to 81·7) | −14–3 (−26·7 to −3·4) | 0·0026 |
| Secondary outcomes | ||||
| Confirmed disease progression at 12 weeks | 5 (8%) | 15 (25%) | 0·288 (0·105 to 0·795) | 0·0087 |
| Serum AQP4-IgG titres† | ||||
| Change from baseline | −240 (−720 to −240) | 0 (−240 to 0) | −240 (−480 to −240) | <0·0001 |
| Percentage change | −50% (−75 to −25) | 0 (−33 to 0) | −33% (−50 to −17) | <0·0001 |
| Exploratory outcome | ||||
| Confirmed disease progression at 24 weeks | 2 (3%) | 6 (10%) | 0·221 (0·047 to 1·042) | 0·0004 |
Data are n (%) or median (IQR). HR=hazard ratio. AQP-IgG=AQP-4 autoantibody.
The difference is for the tocilizumab group compared with the azathioprine group.
The cutoff for AQP4-IgG seropositivity and seronegativity by live cell-based assay was 1:10.
Figure 2: Kaplan-Meier plots of time to first relapse.
(A) Full analysis set. (B) Per-protocol population. Data shown includes censored patients. The minimum planned follow-up period from randomisation was 60 weeks. Patients were censored at first relapse, discontinuation of the study, or when the trial was ended according to the protocol (ie, when at least 30 relapses had occurred), whichever came first. p values were calculated using the log-rank test. Short red and blue vertical lines show censored data.
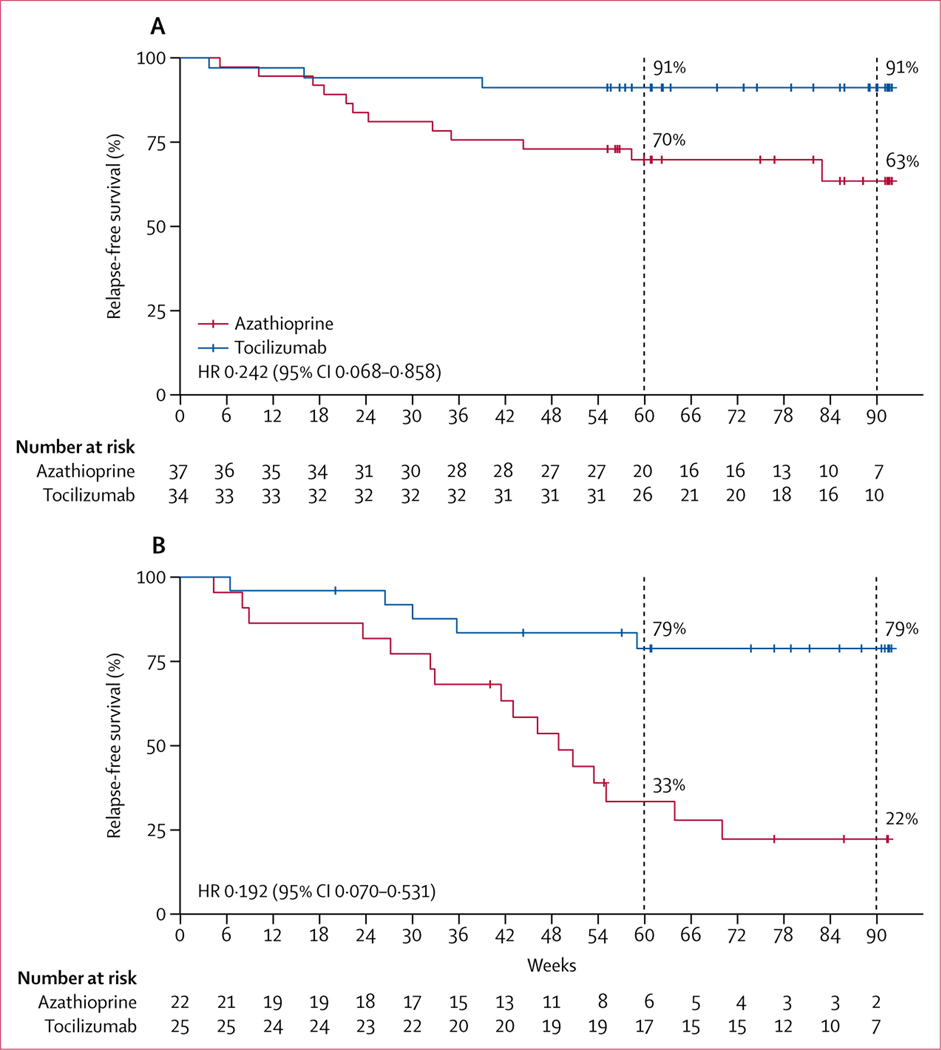
In the prespecified subgroup analysis of patients without concomitant autoimmune diseases, at the end of the study, 13 (35%) of 37 patients in the azathioprine group and three (9%) of 34 patients in the tocilizumab group had relapsed (HR 0·242 [95% CI 0·068–0·858]; p=0·0100; figure 3A). The median time to the first relapse was 80·3 weeks (IQR 60·7–91·0) in the tocilizumab group and 60·9 weeks (44·3–85·1) in the azathioprine group (p=0·0706). At the end of the study, among patients with concomitant autoimmune diseases, 15 (68%) of 22 patients in the azathioprine group and five (20%) of 25 patients in the tocilizumab group had relapsed (HR 0·192 [95% CI 0·070–0·531]; p=0·0004; figure 3B). The median time to first relapse was longer for the tocilizumab group than the azathioprine group (76·7 weeks [IQR 57·0–90·6] vs 47·5 weeks [32·3–63·9]; p=0·0122). In the azathioprine group, the risk of relapse was higher among patients with concomitant autoimmune disease than those without concomitant autoimmune disease (HR 0·349 [95% CI 0·164–0·742]; p=0·0058; appendix p 10). In the tocilizumab group, no differences in risk of relapse were identified between patients with and without concomitant autoimmune diseases (HR 0·419 [95% CI 0·100–1·755]; p=0·2134; appendix p 11). The median time to first relapse for each subgroup suggested a treatment effect consistent with that of the overall study population.
Figure 3: Kaplan-Meier plots of time to first relapse by concomitant autoimmune disease status in the full analysis set.
(A) Patients with NMOSD without concomitant autoimmune diseases. (B) Patients with NMOSD with concomitant autoimmune diseases. Data shown includes censored patients. Short red and blue vertical lines show censored data. NMOSD=neuromyelitis optica spectrum disorder.
In the per-protocol analysis of patients with concomitant autoimmune diseases, risk of relapse was significantly lower in the tocilizumab group than the azathioprine group (HR 0·104 [95% CI 0·030–0·363]; p<0·0001; appendix p 12) and median time to the first relapse was significantly longer for the tocilizumab group than the azathioprine group (67·2 weeks [IQR 47·9–77·7] vs 31·6 weeks [8·3–47·3]; p=0·0003); however, among patients without concomitant autoimmune diseases, no differences were identified between the treatment groups with regard to risk of relapse (HR 0·332 [95% CI 0·088–1·254]; p=0·104; appendix p 13), although the median time to first relapse was longer for the tocilizumab group than the azathioprine group (67·4 weeks [IQR 47·9–78·1] vs 49·1 weeks [30·7–65·3] weeks; p=0·0030).
Subgroup analysis stratified by age, disease duration, AQP4-IgG status, baseline disease activity, (ie, number of relapses 1 and 2 years before randomisation), baseline disability, and total number of previous relapses indicated treatment benefit of tocilizumab (appendix p 14). No difference in the proportion of patients who relapsed was identified between the treatment groups in AQP4-IgG seronegative patients and patients with low disease activity in the year before enrolment. Among AQP4-IgG-seronegative patients, two (22%) of nine patients in the tocilizumab group and three (50%) of six patients in the azathioprine group had a relapse (p=0·4087). Kaplan-Meier plots for the subgroup analyses by AQP4-IgG status are shown in appendix (p 15). Of the 15 AQP4-IgG seronegative patients, three (3%) were myelin oligodendrocyte glycoprotein-IgG positive (one patient in the tocilizumab group and two patients in the azathioprine group). The patient in the tocilizumab group was relapse-free at the end of the study; one of the two patients in the azathioprine group had a single relapse on day 580 and the other patient was relapse-free at the end of the trial. Among patients who had only one relapse in the year before randomisation, we found no differences between treatment groups in risk of relapse; however, for patients with more than one relapse in the year before randomisation, the risk of relapse was lower in the tocilizumab group than the azathioprine group (appendix p 14). Tocilizumab also reduced the risk of relapses significantly compared with azathioprine in patients who had at least three relapses in the 2 years before randomisation (appendix p 14). No differences were identified between the treatment groups in change in EDSS score between baseline and the end of the trial, although fewer participants in the tocilizumab group than the azathioprine group had an increase in EDSS score between baseline and the end of the trial (relative risk 3·667 [95% CI 1·603–8·387]; p=0·0005; appendix p 19).
At 12 weeks, five (8%) of 59 patients in the tocilizumab group and 15 (25%) of 59 patients in the azathioprine group had confirmed disability progression (cumulative probability 9·1% vs 29·5%; HR 0·288 [95% CI 0·105–0·795]; relative risk reduction 71·2%; p=0·0087; table 2; appendix p 17). Among AQP4-IgG positive patients, AQP4-IgG titres remained unchanged in patients in the azathioprine group, but decreased significantly in the tocilizumab group between baseline and the end of the study. The median reduction in AQP4-IgG titres was 50% (IQR 75 to 25) in the tocilizumab group (table 2).
We implemented vigorous measures, including frequent visits by investigators, periodical conferences, and careful review of data obtained from each centre, to minimise variation between centres. For the primary and secondary outcomes, we present the statistical analyses that accounted for centre effect (appendix p 9). All p values were less than 0·05 and the same conclusions were obtained for the outcomes compared with the original analyses, with no significant differences observed between centres.
The incidence of adverse events was similar in the tocilizumab group (57 [97%] of 59 patients) and azathioprine group (56 [95%] of 59 patients) groups (table 3). The most commonly reported adverse events were increased alanine transaminase concentrations (18 [31%] of 59 patients in the tocilizumab group vs 27 [46%] of 59 patients in the azathioprine group), upper respiratory tract infections (17 [29%] vs 23 [39%]), and urinary tract infections (17 [29%] vs 21 [36%]). Most adverse events were mild.
Table 3:
Adverse events in the safety population
| Tocilizumab (n=59) | Azathioprine (n=59) | |
|---|---|---|
| Any adverse event | 57 (97%) | 56 (95%) |
| Total adverse events | 437 | 544 |
| Any treatment-related adverse events, as determined by investigator* | 36 (61%) | 49 (83%) |
| Adverse event severity | ||
| Grade 1 | 53 (90%) | 55 (93%) |
| Grade 2 | 28 (47%) | 32 (54%) |
| Grade 3† | 8 (14%) | 19 (32%) |
| Grade 4 | 1 (2%) | 2 (3%) |
| Grade 5 | 1 (2%) | 1 (2%) |
| Adverse event leading to discontinuation of agent | 2 (3%) | 3 (5%) |
| Adverse event occurring in ≥10% of patients | ||
| Hepatotoxicity | 18 (31%) | 27 (46%) |
| Upper respiratory tract infection | 17 (29%) | 23 (39%) |
| Urinary tract infection | 17 (29%) | 21 (36%) |
| Anaemia | 16 (27%) | 21 (36%) |
| Leukopenia | 4 (7%) | 23 (39%) |
| Nausea | 8 (14%) | 19 (32%) |
| Fatigue | 13 (22%) | 6 (10%) |
| Adverse events by system organ class occurring in ≥10% of all patients | ||
| Investigations | 43 (73%) | 46 (78%) |
| Infections and infestations | 39 (66%) | 43 (73%) |
| Gastrointestinal disorders‡ | 27 (46%) | 34 (58%) |
| Nervous system disorders | 13 (22%) | 18 (31%) |
| Musculoskeletal and connective tissue disorders | 11 (19%) | 6 (10%) |
| General disorders and administration site conditions | 9 (15%) | 5 (8%) |
| Serious adverse events | ||
| Pneumonia | 2 (3%) | 1 (2%) |
| Herpes zoster | 1 (2%) | 2 (3%) |
| Deep vein thrombosis | 1 (2%) | 1 (2%) |
| Basal ganglia haemorrhage | 1 (2%) | 0 |
| Myelitis | 1 (2%) | 0 |
| Bacterial urinary tract infection | 0 | 1 (2%) |
| Influenza | 0 | 1 (2%) |
| Neutropenia | 0 | 1 (2%) |
| Bacterial bronchitis | 0 | 1 (2%) |
| Brain stem stroke | 0 | 1 (2%) |
| Multiple myeloma | 0 | 1 (2%) |
| Meningitis listeria | 0 | 1 (2%) |
Data are n (%) or n. Some patients were included in more than one category of adverse event. Severity of adverse events was graded according to the Common Terminology Criteria for Adverse Events (version 5.0) and were coded according to the preferred terms in the Medical Dictionary for Regulatory Activities (version 22.0). Patients were counted once for each preferred term, regardless of the number of events.
Treatment-related adverse events were categorised by investigators as either not related or unlikely, possibly, probably, or definitely related to the study treatment.
Grade 3 (severe) adverse events were those that interrupted a patient’s usual daily activities and required systemic drug therapy or other treatment.
No gastrointestinal perforations were reported.
The incidence of serious adverse events was higher in the azathioprine group than the tocilizumab group (nine [15%] of 59 patients in the tocilizumab group vs five [8%] of 59 patients in the azathioprine group; table 3). Two cerebral vascular events (one haemorrhage in the tocilizumab group and one ischaemic stroke in the azathioprine group) were reported. Multiple myeloma was reported in one patient in the azathioprine group. These events were deemed to be unrelated to study treatment by the investigators.
Two patients died (one in the azathioprine group and one in the tocilizumab group); neither death was considered treatment-related. The patient who died in the azathioprine group had a new relapse of thoracic myelitis on day 156. High-dose intravenous methylprednisolone was given to the patient and neurological deficits marginally improved. Ten days later, the patient had an abrupt fever (40·5°C) followed by sudden coma. Neurological examination found meningeal irritation. CSF bacterial culture confirmed acute meningoencephalitis caused by Listeria monocytogenes. Despite aggressive treatment with intravenous ampicillin and moxifloxacin, the patient died of brain herniation caused by severe intracranial infection and cerebral oedema. The death in the tocilizumab group occurred in an individual with a history of multiple severe relapses of longitudinal extensive transverse myelitis, Sjögren’s syndrome, and interstitial lung disease. The patient had an episode of gastroenteritis, shortness of breath, hiccups, and quadriplegia on study day 179. Arterial blood gas analysis indicated type 2 respiratory failure and the patient was immediately placed on a mechanical ventilator. An acute relapse of myelitis ascending to the medulla oblongata was diagnosed on the basis of clinical examination; MRI was not available. Intravenous methylprednisolone 1 g per day was administered. The patient died of cardiopulmonary failure 3 days after the onset of relapse. Autopsy was not done. The cause of death was attributed to central respiratory failure secondary to myelitis involving the high cervical spine and medulla oblongata.
An exploratory analysis showed that the risk of 24-week confirmed disability progression was lower in the tocilizumab group than the azathioprine group (HR 0·221 [95% CI 0·047–1·042]; relative risk reduction 77·9%; p=0·0309; appendix p 18).
Patients in the tocilizumab group had a lower risk of optic neuritis than patients in the azathioprine group (HR 0·182 [95% CI 0·049–0·677]; p=0·0110). Both treatment groups had reduced pRNFL thickness and P100 amplitude between baseline and the end of the study in the affected eyes (appendix p 23); however, no significant differences were identified between the two groups. P100 latency was prolonged significantly in the affected eyes of patients in the azathioprine group compared with those in the tocilizumab group (p=0·0091). No significant differences in these visual assessments in the unaffected eyes were identified between the two groups (appendix p 23). Furthermore, in both affected eyes and unaffected eyes, no significant differences were identified in logMAR visual acuity, high-contrast visual acuity, or low-contrast visual acuity between the two groups (appendix p 23).
Discussion
To our knowledge, the TANGO study is the first trial to compare tocilizumab with azathioprine for the treatment of NMOSD. This study showed that patients given tocilizumab had a lower risk of NMOSD relapse, 12-week confirmed disability progression, and lower serum AQP4-IgG titres than did patients given azathioprine.
NMOSD is a rare, and severe disabling CNS autoimmune disorder, which at present is treated with a variety of immunosuppressive and biologic drugs.18 Most clinical investigations have been limited to retrospective analyses; however, prospective phase 3 clinical trials have reported treatment efficacy using complement pathway inhibitors, B lymphocyte depletion, and IL-6 signalling blockade. Future efforts to optimise treatment for patients with NMOSD will require the head-to-head comparison of long-term therapeutic benefits and adverse events associated with such drugs.
Previous trials have demonstrated the efficacy of the anti-complement C5 monoclonal antibody eculizumab,19 anti-CD19 monoclonal antibody inebilizumab,20 and anti-IL-6 receptor monoclonal antibody satralizumab13 for the treatment of patients with NMOSD. Eculizumab has been approved in the USA, European Union, and Japan for the treatment of patients with AQP4-IgG seropositive NMOSD, and the other monoclonal antibodies are likely to be approved in the near future. Satralizumab is a novel anti-IL-6 receptor monoclonal antibody that uses antibody recycling technology to enhance serum half-life. 21 Satralizumab has been shown to reduce the risk of NMOSD relapse by 62% compared with placebo when used in combination with baseline immunosuppressants.13 A similar result was observed using satralizumab as monotherapy.22 The efficacy observed in the tocilizumab group of the TANGO trial is similar to that reported for satralizumab. Distinct from the satralizumab and other phase 3 NMOSD trials, the TANGO trial provides comparative data for the commonly used drug azathioprine in NMOSD. The significant decrease in risk of relapse and improvement in disability observed with tocilizumab treatment indicates that IL-6 signalling blockade is more efficacious than azathioprine in patients with highly relapsing NMOSD. Furthermore, the likely lower cost of tocilizumab versus satralizumab might render a potential economic benefit.
Compared with tocilizumab, azathioprine had lower therapeutic efficacy in patients with NMOSD, especially among those with concurrent autoimmune diseases. In the azathioprine group, 65% of patients with NMOSD without comorbid autoimmune diseases remained relapse-free at 1 year; however, only 32% of patients in the azathioprine group with comorbid autoimmune diseases were relapse-free at the same timepoint. A similar distinction was not observed in the tocilizumab group, in which efficacy was evident for patients with NMOSD regardless of autoimmune disease status. The differences in efficacy observed between azathioprine and tocilizumab in patients with NMOSD with comorbid autoimmune diseases might be due to differences in the mechanism of action of the medications or alterations in disease pathophysiology. In most systemic autoimmune conditions, immunosuppressive drugs, such as azathioprine, often require the concurrent use of glucocorticosteroids to control disease activity. For example, in patients with Sjögren’s syndrome, azathioprine and cyclosporine monotherapy have minimal benefit and a high prevalence of adverse events.23 At present, no compelling evidence exists to indicate that combining immunosuppressants (ie, corticosteroids, mycophenolate mofetil, or methotrexate) with azathioprine results in improved efficacy for patients with NMOSD who have concurrent autoimmune diseases. By contrast, IL-6 signalling blockade might have improved therapeutic effect across a broad range of autoimmune disorders due to its pleiotropic effects. Tocilizumab has been used as a steroid-sparing biologic in a variety of autoimmune diseases.24
For AQP4-IgG seropositive patients, a significant decrease in serum AQP4-IgG titres was associated with the use of tocilizumab. Considering the prominent role of AQP4-IgG in NMOSD lesion formation,25 the reduction in serum AQP4-IgG titres might represent an important mechanism by which tocilizumab exerts its therapeutic effect.
Our exploratory analyses on visual function showed that between baseline and the end of the study, patients in both treatment groups had decreases in pRNFL thickness. Tocilizumab resulted in a lower rate of atrophy, but no significant differences were observed when compared with azathioprine. The lower rate of atrophy of pRNFLs is probably attributed to the lower risk of relapse of optic neuritis in patients receiving tocilizumab. The results indicate that patients might not benefit from optic nerve recovery after treatment with tocilizumab or azathioprine.
Our trial has several limitations. First, study participants and investigators were not masked to the assigned treatment. To mitigate any potential influence on relapse assessment or severity, EDSS raters and the relapse adjudication expert panel were masked to the treatment. Second, since most patients with NMOSD in the trial were AQP4-IgG seropositive; the number of AQP4-IgG seronegative patients was insufficient to assess differences in efficacy between the two groups. A retrospective study demonstrated that switching patients with highly-active myelin oligodendrocyte glycoprotein-IgG positive optic neuritis to tocilizumab after rituximab failure reduced relapse activity.26 In our study, one patient in the tocilizumab group was single myelin oligodendrocyte glycoprotein-IgG positive, but had not relapsed by the end of the trial. Since myelin oligodendrocyte glycoprotein-IgG related disorders compose a distinct clinical and pathological condition from AQP-IgG positive NMOSD, any potential benefit of tocilizumab for myelin oligodendrocyte glycoprotein-IgG related disorders will require further investigation. Third, similar to other NMOSD clinical trials,19 patients in the TANGO study had a high rate of previous relapses. Therefore, the results might not be generalisable to patients with NMOSD who have a less aggressive disease course. Fourth, although both tocilizumab and azathioprine have been reported to have beneficial effects on disease activity in multiple small, cross-sectional studies,6,12,27 the absence of efficacy of the selected dosing regimens in some patients who relapsed in the TANGO study might skew the analysis of comparative efficacy. Lastly, all the patients in this trial were of Han-Chinese ethnicity. Therefore, the generalisability of the study to non-Chinese populations is unclear.
In conclusion, tocilizumab was associated with a significantly lower risk of relapse than azathioprine in patients with NMOSD. However, the long-term effect of tocilizumab in patients with NMOSD warrants further study.
Supplementary Material
Research in context
Evidence before this study
We searched PubMed and Web of Science from inception to Nov 30, 2017, without language restrictions, using the search terms “azathioprine in neuromyelitis optica”, “azathioprine in NMO”, “azathioprine in neuromyelitis optica spectrum disorder (NMOSD)”, “azathioprine in NMOSD”, “tocilizumab in neuromyelitis optica”, “tocilizumab in NMO”, “tocilizumab in neuromyelitis optica spectrum disorder”, and “tocilizumab in NMOSD”. Our search yielded 83 studies investigating azathioprine or tocilizumab for the treatment of NMOSD.
82 of the studies were observational cohort studies reporting reductions in annualised relapse rate and improvement on the Expanded Disability Status Scale after treatment with azathioprine or tocilizumab. However, the results could be attributed to regression towards the mean or selection bias as a result of inappropriate sampling and patient characteristics (eg, disability severity, aquaporin-4 autoantibody serostatus). Although azathioprine has been recommended as a first-line off-label drug for neuromyelitis optica spectrum disorder (NMOSD) by the 2010 European Federation of Neurological Societies guideline, 69 of 75 studies associated with azathioprine were retrospective and therefore limited by reporting bias. Only one randomised controlled study has compared the efficacy of azathioprine with other immunotherapeutic drugs. This single centre, open-label, randomised controlled study from Iran compared the efficacy of azathioprine and rituximab in 68 patients with NMOSD. Azathioprine treatment was not found to be superior to rituximab; however, compliance measures, including regular visits and telephone follow-up, were not implemented. Furthermore, the participants enrolled in each of these studies were not diagnosed on the basis of 2015 International Panel for Neuromyelitis Optica Diagnosis criteria.
Eight observational studies have assessed the efficacy of tocilizumab with or without other immunosuppressants in patients with NMOSD, which included a total of 22 patients, some of whom were refractory to rituximab treatment.
Although disease activity was controlled, dosing regimens and duration of therapy varied substantially between these studies. Because of the small sample sizes of these studies, benefit–risk assessments were not possible. Whether tocilizumab is superior to commonly used immunosuppressants, such as azathioprine and mycophenolate mofetil, in large populations remains unknown.
Added value of this study
Placebo-controlled trials have shown the efficacy of monoclonal antibodies in patients with NMOSD: anti-complement C5 (eculizumab in PREVENT), anti-CD19 (inebilizumab in N-MOMENTUM), and anti-interleukin (IL)-6 receptor (satralizumab in SAkuraSky). To our knowledge, TANGO is the first randomised, controlled study comparing the safety and efficacy of tocilizumab and azathioprine in patients with highly relapsing NMOSD. We found evidence of superior efficacy of tocilizumab when compared with azathioprine. TANGO provides evidence for another safe and effective treatment that inhibits the IL-6 signalling pathway to prevent relapses in patients with NMOSD.
Implications of all the available evidence
TANGO shows that tocilizumab reduces the risk of relapses and lowers probability of disability progression compared with azathioprine in patients with highly relapsing NMOSD. Larger-scale and longer-term studies in non-Chinese patients are required to further validate tocilizumab and other anti-IL-6 therapies in patients with NMOSD.
Acknowledgments
This Article is dedicated to friends and colleagues from Tianjin Medical University General Hospital and Beijing Tiantan Hospital, and to all medical professionals in China, for their professionalism throughout the COVID-19 outbreak. We thank patients and their families for participation, and Kaibin Shi, Qiang Liu, Ye Liu, Rui Zhang, and other members of the neuroimmunology team at Tianjin Medical University General Hospital (Tianjin, China) for their support, and Samuel X Shi (Barrow Neurological Institute, Phoenix, AZ, USA) for editorial input. This study was funded by the Tianjin Medical University Clinical Research Project (2017kylc005), the Advanced Innovation Center for Human Brain Protection (Neuroimmune 01), the National Key Research and Development Program of China (2018YFC1312200), and the National Science Foundation of China (91642205, 81830038, and 81601019).
Funding Tianjin Medical University, Advanced Innovation Center for Human Brain Protection, National Key Research and Development Program of China, National Science Foundation of China.
Footnotes
Declaration of interests
JLB reports personal fees from Chugai, Genentech, Genzyme, AbbVie, Roche, Clene Nanomedicine, Equillium, Frequency Therapeutics, and Alexion; grants and personal fees from EMD Serono and Novartis; and grants from the Guthy Jackson Charitable Foundation, outside the submitted work; and has a patent “Compositions and Methods for the Treatment of Neuromyelitis Optica” issued. All other authors declare no competing interests.
Data sharing
Data collected for this study including individual participant data and study protocol will be available to others upon publication of the TANGO trial. All of the individual participant data collected during the trial, after de-identification, will be shared. Data will be available after approval of a proposal with a signed data access agreement to achieve aims in the approved prospectus. Proposals should be directed to chaozhang@tmu.edu.cn.
References
- 1.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004; 364: 2106–12. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007; 6: 805–15. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos MC, Bennett JL, Verkman AS. Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurol 2014; 10: 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol 2014; 261: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sellner J, Boggild M, Clanet M, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol 2010; 17: 1019–32. [DOI] [PubMed] [Google Scholar]
- 6.Elsone L, Kitley J, Luppe S, et al. Long-term efficacy, tolerability and retention rate of azathioprine in 103 aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder patients: a multicentre retrospective observational study from the UK. Mult Scler 2014; 20: 1533–40. [DOI] [PubMed] [Google Scholar]
- 7.Mealy MA, Wingerchuk DM, Palace J, Greenberg BM, Levy M. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol 2014; 71: 324–30. [DOI] [PubMed] [Google Scholar]
- 8.Bichuetti DB, Perin MMM, Souza NA, Oliveira EML. Treating neuromyelitis optica with azathioprine: 20-year clinical practice. Mult Scler 2019; 25: 1150–61. [DOI] [PubMed] [Google Scholar]
- 9.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity 2019; 50: 1007–23. [DOI] [PubMed] [Google Scholar]
- 10.Ayzenberg I, Kleiter I, Schröder A, et al. Interleukin 6 receptor blockade in patients with neuromyelitis optica nonresponsive to anti-CD20 therapy. JAMA Neurol 2013; 70: 394–97. [DOI] [PubMed] [Google Scholar]
- 11.Araki M, Matsuoka T, Miyamoto K, et al. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology 2014; 82: 1302–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ringelstein M, Ayzenberg I, Harmel J, et al. Long-term therapy with interleukin 6 receptor blockade in highly active neuromyelitis optica spectrum disorder. JAMA Neurol 2015; 72: 756–63. [DOI] [PubMed] [Google Scholar]
- 13.Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med 2019; 381: 2114–24. [DOI] [PubMed] [Google Scholar]
- 14.Shahmohammadi S, Doosti R, Shahmohammadi A, et al. Autoimmune diseases associated with Neuromyelitis Optica Spectrum Disorders: a literature review. Mult Scler Relat Disord 2019; 27: 350–63. [DOI] [PubMed] [Google Scholar]
- 15.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics 1946; 2: 110–14. [PubMed] [Google Scholar]
- 17.Bretz F, Maurer W, Brannath W, Posch M. A graphical approach to sequentially rejective multiple test procedures. Stat Med 2009;28: 586–604. [DOI] [PubMed] [Google Scholar]
- 18.Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol 2016; 18: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med 2019; 381: 614–25. [DOI] [PubMed] [Google Scholar]
- 20.Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019; 394: 1352–63. [DOI] [PubMed] [Google Scholar]
- 21.Collongues N, Ayme-Dietrich E, Monassier L, de Seze J. Pharmacotherapy for neuromyelitis optica spectrum disorders: current management and future Options. Drugs 2019; 79: 125–42. [DOI] [PubMed] [Google Scholar]
- 22.Traboulsee A, Greenberg BM, Bennett JL, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol 2020; 19: 402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos-Casals M, Brito-Zerón P, Bombardieri S, et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann Rheum Dis 2020; 79: 3–18. [DOI] [PubMed] [Google Scholar]
- 24.Dougados M, Kissel K, Sheeran T, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis 2013; 72: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratelade J, Asavapanumas N, Ritchie AM, Wemlinger S, Bennett JL, Verkman AS. Involvement of antibody-dependent cell-mediated cytotoxicity in inflammatory demyelination in a mouse model of neuromyelitis optica. Acta Neuropathol 2013; 126: 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayward-Koennecke H, Reindl M, Martin R, Schippling S. Tocilizumab treatment in severe recurrent anti-MOG-associated optic neuritis. Neurology 2019; 92: 765–67. [DOI] [PubMed] [Google Scholar]
- 27.Costanzi C, Matiello M, Lucchinetti CF, et al. Azathioprine: tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology 2011; 77: 659–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.