Abstract
The separation of C2H2/CO2 is particularly challenging owing to their similarities in physical properties and molecular sizes. Reported here is a mixed metal–organic framework (M’MOF), [Fe(pyz)Ni(CN)4] (FeNi-M’MOF, pyz=pyrazine), with multiple functional sites and compact one-dimensional channels of about 4.0 Å for C2H2/CO2 separation. This MOF shows not only a remarkable volumetric C2H2 uptake of 133 cm3cm−3, but also an excellent C2H2/CO2 selectivity of 24 under ambient conditions, resulting in the second highest C2H2-capture amount of 4.54 molL−1, thus outperforming most previous benchmark materials. The separation performance of this material is driven by π–π stacking and multiple intermolecular interactions between C2H2 molecules and the binding sites of FeNi-M’MOF. This material can be facilely synthesized at room temperature and is water stable, highlighting FeNi-M’MOF as a promising material for C2H2/CO2 separation.
Keywords: acetylene, adsorption, gas separation, iron, metal–organic frameworks
Metal–organic frameworks (MOFs) have emerged as very promising porous materials for adsorptive gas separation because they integrate the merits of tunable pore sizes and functional pore surfaces that can realize not only a molecular sieving effect, but also preferential gas binding.[1] Many MOFs have been explored for simplifying various gas separation and purification schemes ranging from mature ones, such as carbon dioxide capture (CO2) from methane and nitrogen, to more challenging olefin/paraffin and alkyne/alkene separations.[2] For C2H2 and CO2 gas molecules, the similarities in physical properties (differ in boiling point by ca. 3% and ca. 6 K) and identical molecular shapes/sizes (3.3 × 3.3 × 5.7 Å3 for C2H2, 3.2 × 3.3 × 5.4 Å3 for CO2), with kinetic diameters of about 3.3 Å, make it very difficult and challenging to realize efficient porous materials for C2H2/CO2 separation under ambient conditions.[3] A few ultra-microporous MOFs featuring bare oxygen or fluorine base sites have been developed to preferentially bind C2H2 molecules through hydrogen-bonding interactions or bind CO2 molecules through electrostatic interactions, showing high C2H2/CO2 selectivity but low C2H2 uptake.[4] Another approach is to incorporate strong adsorption binding sites, mainly open metal sites, into MOFs with large pore volumes to boost the uptake capacity of the preferred gas molecules.[5] UTSA-74 represents a unique example with open metal centers having two accessible sites which can bind two C2H2 molecules, but only one CO2 molecule, differing from its isomer MOF-74 which adsorbs similar amounts of C2H2 and CO2 under the same conditions.[5c] Though progress has been made over the past several years, the uptake capacity versus selectivity trade-off still poses a daunting challenge for addressing C2H2/CO2 separation.[6]
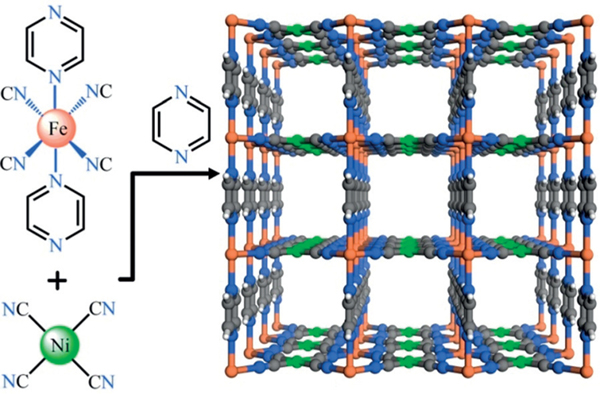
The vast database of reported MOF structures enables comparative analyses to target potential candidates with dual functionalities, featuring moderate pore volumes and accessible functional sites, to realize both high gas uptake and separation selectivities. Among plentiful ligands, cyanide is a short and highly basic ligand that is feasible to construct robust MOFs with modest pore aperture size, such as Prussian blue and Hofmann-type compounds.[7] For those MOFs with metalloligands, the open metal sites on ligands are accessible for gas molecules, whereas expected narrow pore structures originating from compact ligands enforce additional multiple intermolecular interactions to form, as demonstrated by a series of mixed metal–organic frameworks (M’MOFs).[8] In this regard, a Hofmann-type MOF [Fe(pyz)Ni(CN)4] (FeNi-M’MOF, pyz=pyrazine), discovered in 2001, showing open nickel sites and polarized surfaces as well as compact pore channels of about 4.0 Å, is particularly interesting.[9] The high density of functional sites and ultra-micropore would collaboratively enforce gas separation with high gas uptake and separation selectivities. Herein we investigate the mixed iron/nickel MOF FeNi-M’MOF for potential C2H2/CO2 separation. In this MOF, C2H2 molecules are found to preferentially bind the organic moieties and open Ni sites through π–π stacking and multiple intermolecular interactions, respectively, whereas CO2 molecules mainly distribute on the open Ni sites through relatively weak interactions. In this context, FeNi-M’MOF shows a very high C2H2/CO2 selectivity of 24 that is superior to the previous top-performing MOFs while retaining a remarkable C2H2 uptake capacity of 133 cm3cm−3, and thus an excellent C2H2-capture capacity of 4.54 mol L−1 at 298 K and 1 bar for 50:50 C2H2/CO2 separation, which is close to that of the benchmark UTSA-74 and exceeds that of other out-performing MOFs.[5c]
FeNi-M’MOF is a pillared-layer M’MOF, in which the Fe[Ni(CN)4] layer is connected by the pyz pillars. The Ni atoms show square-planar coordination geometry while Fe atoms are octahedrally coordinated. The Ni atoms are coordinated by carbon atoms of four different cyan groups, whereas the Fe atoms are fully coordinated by nitrogen atoms from four different cyan groups and two pyz linkers. Fe[Ni(CN)4] layers are then connected by pyz linkers into a three-dimensional network with one-dimensional channels of about 4.15 × 4.27 or 3.94 × 4.58 Å2. The open metal site density of FeNi-M’MOF is about 9.2 mmolcm−3, which is higher than that of most MOFs, as shown in Table S2 (see the Supporting Information).
FeNi-M’MOF was synthesized at room temperature in water and methanol (Figure 1).[10] By adding the solution of K2[Ni(CN)4] into the mixed methanol and water solution of Fe2+ and pyz, the FeNi-M’MOF microcrystalline powders were obtained after stirring for 30 minutes. The powder X-ray diffraction (PXRD) of products indicated that those products have a good crystallinity and match well with the simulated XRD pattern, indicating the purity of FeNi-M’MOF. The resultant FeNi-M’MOF was further validated by elemental analysis (EA), thermogravimetry analysis (TGA), energy dispersive spectroscopy (EDS), and X-ray photoelectron spectroscopy (XPS) analysis (see the Supporting Information). This MOF also exhibits excellent water stability as shown in Figure S2. After soaking in water for 30 days, the crystallinity of FeNi-M’MOF is still retained. The TGA curve indicated that FeNi-M’MOF exhibits a considerable thermal stability up to 200°C (see Figure S4). The thermal stability of FeNi-M’MOF was also confirmed by variable-temperature PXRD (see Figure S5), indicating that FeNi-M’MOF can maintain its crystalline structure up to about 200°C. The fast and facile synthesis method, excellent water stability, and good thermal stability indicate FeNi-M’MOF is a promising separation material for scale-up synthesis.
Figure 1.
The crystal structure of FeNi-M’MOF viewed along the a/b axis. Fe, Ni, C, N, and H in FeNi-M’MOF are represented by orange, green, gray, blue, and white, respectively.
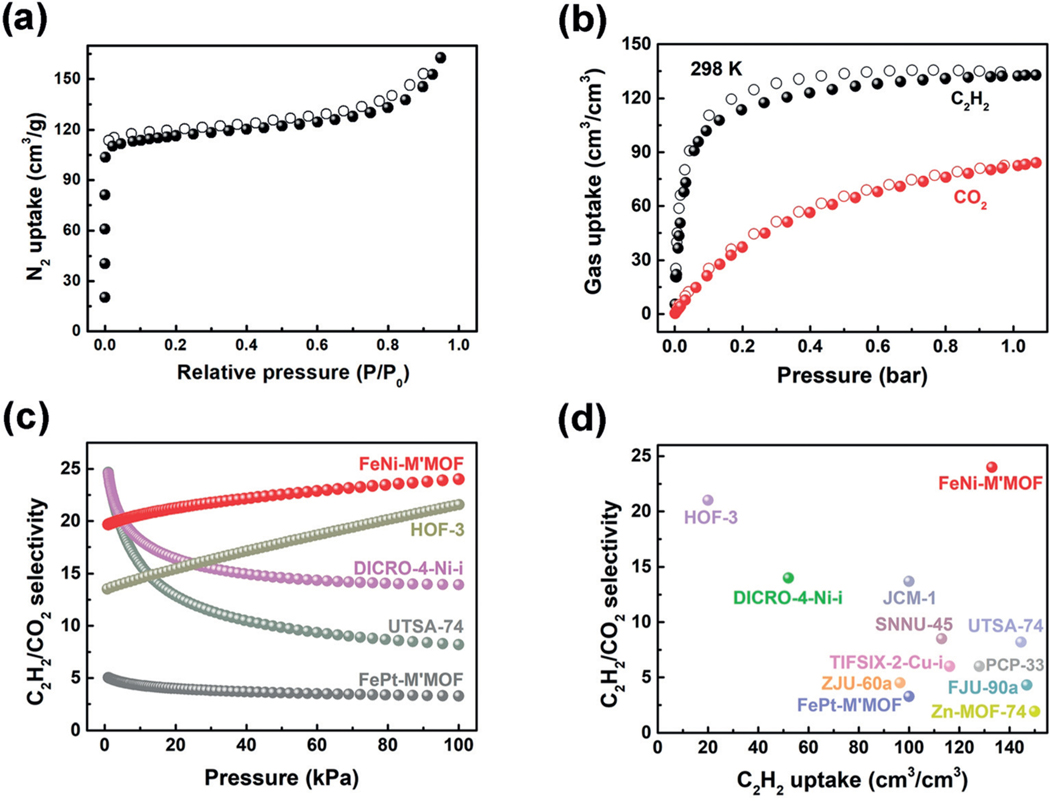
The Brunauer-Emmett-Teller (BET) surface area of FeNi-M’MOF was measured to be 383 m2g−1 by an N2 sorption experiment at 77 K as shown in Figure 2a. The experimental total pore volume is about 0.25 cm3g−1, and slightly smaller than the theoretical one calculated from the crystal structure (0.30 cm3g−1), which can be attributed to the insufficient filling of N2 molecules in the ultramicroporous pore channels.
Figure 2.
a) N2 sorption isotherms for FeNi-M’MOF at 77 K. b) C2H2 and CO2 sorption isotherms for FeNi-M’MOF at 298 K. c) Comparison of IAST selectivities for equimolar C2H2/CO2 mixtures in FeNi-M’MOF, FePt-M’MOF and other materials in the range of 0–1 bar at 298 K. d) Comparison of C2H2/CO2 adsorption selectivity and volumetric C2H2 uptake at 1 bar in FeNi-M’MOF, FePt-M’MOF and other porous materials.
The C2H2 and CO2 gas adsorption isotherms of FeNi-M’MOF were measured at both 273 and 298 K. As shown in Figure 2b, the volumetric C2H2 uptake capacity of FeNi-M’MOF is 133 cm3cm−3 (4.29 mmolg−1) at 1 bar and 298 K, which is higher than those of many other MOFs, such as DICRO-4-Ni-i (52 cm3cm−3),[4e] ZJU-60a (96 cm3cm−3),[11] Cu[Ni(pdt)2] (108 cm3cm−3),[6a] SNNU-45 (113 cm3cm−3),[6b] TIFSIX-2-Cu-i (116 cm3cm−3),[4f] PCP-33 (128 cm3cm−3),[12] and comparable to those of UTSA-74 (144 cm3cm−3),[5c] FJU-90a (146 cm3cm−3),[6c] and Zn-MOF-74 (150 cm3cm−3).[13] The CO2 uptake of FeNi-M’MOF is 84 cm3cm−3 (2.72 mmolg−1) at 1 bar and 298 K. At 1 bar and 273 K, C2H2 and CO2 uptakes of FeNi-M’MOF are up to 145 and 102 cm3cm−3 respectively, as shown in Figure S8. Interestingly, the Pt analogue [Fe(pyz)Pt(CN)4] (FePt-M’MOF; see Figures S10–S12) shows much lower uptake capacities for C2H2 and CO2 (100 and 105 cm3cm−3, respectively), indicating the potential binding contribution of Ni sites in this type of MOF for C2H2 molecules. To evaluate the separation performance of this material, ideal adsorbed solution theory (IAST) was employed to calculate the adsorption selectivity. As shown in Figure 2c, at 100 kPa and 298 K, the C2H2/CO2 (50:50) selectivity of FeNi-M’MOF is 24. The selectivity of FeNi-M’MOF is higher than those of most MOFs, such as Zn-MOF-74 (1.92),[5c] FJU-90a (4.3),[6c] UTSA-74a (8.2),[5c] JCM-1 (13.4),[4b] DICRO-4-Ni-i (13.9),[4e] and benchmark HOF-3a (21).[14] It should be noted that both the uptake capacity and separation selectivity can significantly affect the practical performance of an adsorbent. HOF-3a has a high selectivity, but the low uptake of C2H2 reduced its separation performance. In contrast, FeNi-M’MOF can address such trade-offs between the adsorption capacity and selectivity as shown in Figure 2d. The high selectivity and high C2H2 adsorption capacity of FeNi-M’MOF jointly reveal its useful separation potential for C2H2/CO2.
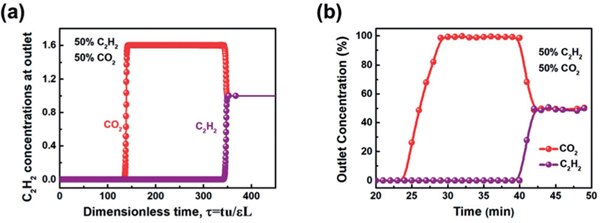
Transient breakthrough simulations were conducted to demonstrate the C2H2/CO2 separation performance of FeNi-M’MOF. The simulations in Figure 3a demonstrate the FeNi-M’MOF is of potential use for this challenging separation of C2H2/CO2 mixtures. The C2H2/CO2 mixtures (50:50) were used as feeds to mimic the industrial process conditions. Pure CO2 first eluted through the bed, where the CO2 purity was 99.95%, followed by the breakthrough of C2H2 after a certain time, τbreak, during which FeNi-M’MOF was saturated by C2H2. The C2H2-capture amount of FeNi-M’MOF is 4.54 molL−1 based on the simulated column breakthrough, which is close to that of the benchmark UTSA-74 (4.86 molL−1)[5c] and higher than those of most out-performing MOFs, such as Zn-MOF-74 (4.06 molL−1),[5c] FJU-90a (4.16 molL−1),[6c] and PCP-33 (4.16 molL−1).[12] Accordingly, FeNi-M’MOF shows not only a high C2H2/CO2 selectivity and high C2H2 uptake but also high C2H2-capture capability from gas mixtures, endowing this material with a useful C2H2/CO2 separation potential. Based on experimental breakthrough studies, we further evaluated the performance of FeNi-M’MOF in near practical separation processes for a C2H2/CO2 mixture (50:50 v/v) as shown in Figure 3b. Indeed, FeNi-M’MOF exhibits excellent C2H2/CO2 mixture separation performance at 298 K. CO2 was first eluted through the adsorption bed without any detectable C2H2, whereas the latter was retained in the MOF column for a remarkable period prior to saturate the MOF. The retention time of pure CO2 and C2H2 for C2H2/CO2 (50:50 v/v) mixture on FeNi-M’MOF are up to 24 and 40 min, respectively. Accordingly, the captured C2H2 was calculated to be 4.10 molL−1 with a separation factor of 1.7.
Figure 3.
a) Transient breakthrough simulations for separation of equimolar C2H2/CO2 mixture using FeNi-M’MOF at 298 K, with a partial pressure of 50 kPa for each. b) Experiment breakthrough curves for equimolar C2H2/CO2 mixture in a packed column with FeNi-M’MOF at 298 K and 1 bar.
The isosteric heat of adsorption (Qst) has been used to evaluate the strength of interaction between the adsorbent and the adsorbate, which is calculated (see Figure S13) from the adsorption isotherms at 273 and 298 K. The Qst values are 27–32.8 and about 24.5 kJmol−1 of FeNi-M’MOF for C2H2 and CO2, respectively. The Qst value of C2H2 in FeNi-M’MOF is lower than those of other MOFs such as HKUST-1 (39 kJmol−1),[15] FeMOF-74 (47.5 kJmol−1),[16] and SIFSIX-2-Cu-i (41.9 kJmol−1),[1e] and is comparable to that of UTSA-74 (31 kJmol−1).[5c] These data indicate FeNi-M’MOF has a lower regeneration energy for C2H2 production, which would be beneficial for practical applications.
To understand the separation performance of FeNi-M’MOF, the adsorption modes of C2H2 in FeNi-M’MOF were established by DFT-D calculations (see Figure S14). The modeling structures indicated that there are two binding sites for C2H2 in FeNi-M’MOF: Site I, located in the middle of two adjacent pyz rings, where C2H2 was adsorbed through the π–π interactions between C2H2 and the pyz rings (see Figure S14a). The C2H2 static binding energy in site I is up to 41.4 kJmol−1. Site II, located in the middle of two adjacent Ni open metal sites, where C2H2 a molecule is adsorbed through the interactions between C≡C and Ni open metal sites and is perpendicular to c axis. The C2H2 static binding energy in this site is 29.9 kJmol−1, which is smaller than that of site I (see Figure S14b).
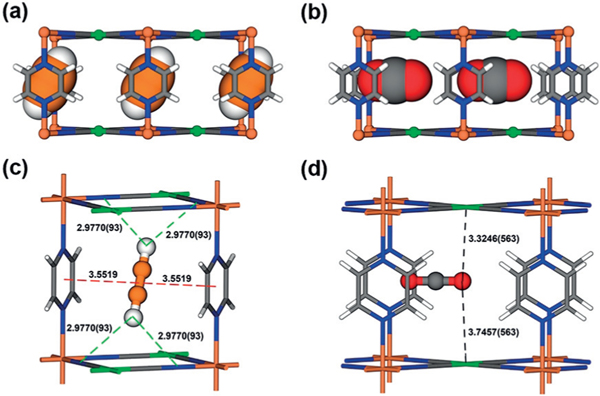
Further visualization of these host–guest interactions was carried out through high-resolution neutron powder diffraction experiments. The crystal structure under low C2D2 loading was measured first (Figure 4a). As expected, C2D2 molecules preferentially distribute on site I. C2D2 molecules were identified between the two pyz rings through π–π stacking (3.552 Å). The C2D2 molecules show a titling angle of 27.4° from the [001] direction (crystallographic c axis; see Figure S15a). In addition, multiple intermolecular interactions were also observed between C2D2 and FeNi-M’MOF (Dδ+···Nδ−: 2.977 Å, Cδ−···Nδ−: 3.808 Å, Figure 4c; see Figure S15b). In contrast, the preferential CO2 binding site is located at the open Ni site (Figure 4b). The electronegative Oδ− atoms of CO2 interact with the positive open-metal site Niδ+. However, the distance across the channel is insufficient for favorable Niδ+···Oδ−=C=Oδ−···Niδ+ interactions to form in the structure. Thus, CO2 molecules were adsorbed near the center of the channel and parallel to the channel. Oδ− atom of CO2 inserts between the adjacent two Niδ+ atoms from different layers and the distance of Oδ−···Niδ+ are 3.746 and 3.325 Å, respectively (Figure 4d). This type interaction is relatively weak, consistent with the gentle adsorption isotherm and low Qst value of CO2 in FeNi-M’MOF. The multiple binding sites of FeNi-M’MOF for gas molecules and its different binding modes toward C2H2 and CO2 enable FeNi-M’MOF to selectively adsorb C2H2 from CO2 with both high C2H2 uptake and remarkable C2H2/CO2 selectivity.
Figure 4.
Neutron diffraction crystal structure of a) FeNi-M’MOF⊃C2D2 and b) FeNi-M’MOF⊃CO2, viewed from the a/b axis. Adsorption binding sites of c) C2D2 and c) CO2 for FeNi-M’MOF. Fe, Ni, C, N, O, H in FeNi-M’MOF and CO2 are represented by orange, green, gray, blue, red, and white, respectively; C and D in C2D2 are represented by orange and white, respectively. The labelled distance is measured in Å.
In summary, highly selective C2H2/CO2 separation has been successfully realized by a mixed iron/nickel MOF FeNi-M’MOF using a metalloligand approach. The structural features the of cyanonickelate and optimal pore channels in this MOFallow C2H2 molecules to interact at multiple binding sites, with both very high C2H2 uptake and C2H2/CO2 selectivity in volumetric ratio. The so-called dual functionality in this material enables this MOF to serve as one of the best materials for C2H2/CO2 separation in terms of C2H2-capture capability. This work also illustrates an outstanding example to further reveal the huge separation potential of MOF adsorbents, especially for challenging gas separation and purification. The active ongoing research affords tremendous opportunities for energy-efficient separation.
Supplementary Material
Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LY20E020001), National Natural Science Foundation of China (51602301 and 51672251), and the Welch Foundation (AX-1730). J.G. acknowledges the Fundamental Research Funds of Zhejiang Sci-Tech University (2019Q007).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/anie.202000323.
Contributor Information
Junkuo Gao, Institute of Functional Porous Materials, The Key laboratory of Advanced Textile Materials and Manufacturing Technology of Ministry of Education, School of Materials Science and Engineering, Zhejiang Sci-Tech University, Hangzhou 310018 (China); Department of Chemistry, University of Texas at San Antonio One UTSA Circle, San Antonio, (USA).
Xuefeng Qian, Institute of Functional Porous Materials, The Key laboratory of Advanced Textile Materials and Manufacturing Technology of Ministry of Education, School of Materials Science and Engineering, Zhejiang Sci-Tech University, Hangzhou 310018 (China).
Rui-Biao Lin, Department of Chemistry, University of Texas at San Antonio One UTSA Circle, San Antonio, TX 78249-0698 (USA).
Rajamani Krishna, Van’t Hoff Institute of Molecular Sciences, University of Amsterdam Science Park 904, 1098 XH Amsterdam (The Netherlands).
Hui Wu, NIST Center for Neutron Research, National Institute of Standards and Technology, Gaithersburg, MD 20899-6102 (USA).
Wei Zhou, NIST Center for Neutron Research, National Institute of Standards and Technology, Gaithersburg, MD 20899-6102 (USA).
Banglin Chen, Department of Chemistry, University of Texas at San Antonio One UTSA Circle, San Antonio, TX 78249-0698 (USA).
References
- [1].a) Li H, Li L, Lin R-B, Zhou W, Xiang S, Chen B, Zhang Z, EnergyChem 2019, 1, 100006; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ding M, Flaig RW, Jiang H-L, Yaghi OM, Chem. Soc. Rev 2019, 48, 2783–2828; [DOI] [PubMed] [Google Scholar]; c) Lin R-B, Li L, Zhou H-L, Wu H, He C, Li S, Krishna R, Li J, Zhou W, Chen B, Nat. Mater 2018, 17, 1128–1133; [DOI] [PubMed] [Google Scholar]; d) Taylor MK, Runčevski T, Oktawiec J, Bachman JE, Siegelman RL, Jiang H, Mason JA, Tarver JD, Long JR, J. Am. Chem. Soc 2018, 140, 10324–10331; [DOI] [PubMed] [Google Scholar]; e) Cui X, Chen K, Xing H, Yang Q, Krishna R, Bao Z, Wu H, Zhou W, Dong X, Han Y, Li B, Ren Q, Zaworotko MJ, Chen B, Science 2016, 353, 141–144; [DOI] [PubMed] [Google Scholar]; f) Cadiau A, Adil K, Bhatt PM, Belmabkhout Y, Eddaoudi M, Science 2016, 353, 137–140; [DOI] [PubMed] [Google Scholar]; g) Lin R-B, Xiang S, Zhou W, Chen B, Chem 2019, 10.1016/j.chempr.2019.1010.1012. [DOI] [Google Scholar]
- [2].a) Chen K-J, Madden DG, Mukherjee S, Pham T, Forrest KA, Kumar A, Space B, Kong J, Zhang Q-Y, Zaworotko MJ, Science 2019, 366, 241–246; [DOI] [PubMed] [Google Scholar]; b) Liu Y, Chen Z, Liu G, Belmabkhout Y, Adil K, Eddaoudi M, Koros W, Adv. Mater 2019, 31, 1807513; [DOI] [PubMed] [Google Scholar]; c) Cui WG, Hu TL, Bu XH, Adv. Mater 2019, 31, 1806445; [DOI] [PubMed] [Google Scholar]; d) Siegelman RL, Milner PJ, Kim EJ, Weston SC, Long JR, Energy Environ. Sci 2019, 12, 2161–2173; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Li L, Lin R-B, Krishna R, Li H, Xiang S, Wu H, Li J, Zhou W, Chen B, Science 2018, 362, 443–446; [DOI] [PubMed] [Google Scholar]; f) Liao P-Q, Huang N-Y, Zhang W-X, Zhang J-P, Chen X-M, Science 2017, 356, 1193–1196; [DOI] [PubMed] [Google Scholar]; g) Yang S, Ramirez-Cuesta AJ, Newby R, Garcia-Sakai V, Manuel P, Callear SK, Campbell SI, Tang CC, Schröder M, Nat. Chem 2014, 7, 121–129; [DOI] [PubMed] [Google Scholar]; h) Aubrey ML, Kapelewski MT, Melville JF, Oktawiec J, Presti D, Gagliardi L, Long JR, J. Am. Chem. Soc 2019, 141, 5005–5013. [DOI] [PubMed] [Google Scholar]
- [3].Reid CR, Thomas KM, J. Phys. Chem. B 2001, 105, 10619–10629. [Google Scholar]
- [4].a) Yang H, Trieu TX, Zhao X, Wang Y, Wang Y, Feng P, Bu X, Angew. Chem. Int. Ed 2019, 58, 11757–11762; Angew. Chem. 2019, 131, 11883–11888; [DOI] [PubMed] [Google Scholar]; b) Lee J, Chuah CY, Kim J, Kim Y, Ko N, Seo Y, Kim K, Bae TH, Lee E, Angew. Chem. Int. Ed 2018, 57, 7869–7873; Angew. Chem. 2018, 130, 7995–7999; [DOI] [PubMed] [Google Scholar]; c) Lin R-B, Li L, Wu H, Arman H, Li B, Lin R-G, Zhou W, Chen B, J. Am. Chem. Soc 2017, 139, 8022–8028; [DOI] [PubMed] [Google Scholar]; d) Jiang M, Cui X, Yang L, Yang Q, Zhang Z, Yang Y, Xing H, Chem. Eng. J 2018, 352, 803–810; [Google Scholar]; e) Scott HS, Shivanna M, Bajpai A, Madden DG, Chen K-J, Pham T, Forrest KA, Hogan A, Space B, Perry IV JJ, Zaworotko MJ, ACS Appl. Mater. Interfaces 2017, 9, 33395–33400; [DOI] [PubMed] [Google Scholar]; f) Chen K-J, Scott HS, Madden DG, Pham T, Kumar A, Bajpai A, Lusi M, Forrest KA, Space B, Perry IV JJ, Zaworotko MJ, Chem 2016, 1, 753–765; [Google Scholar]; g) Qazvini OT, Babarao R, Shi Z-L, Zhang Y-B, Telfer SG, J. Am. Chem. Soc 2019, 141, 5014–5020. [DOI] [PubMed] [Google Scholar]
- [5].a) Zeng H, Xie M, Huang Y-L, Zhao Y, Xie X-J, Bai J-P, Wan M-Y, Krishna R, Lu W, Li D, Angew. Chem. Int. Ed 2019, 58, 8515–8519; Angew. Chem. 2019, 131, 8603–8607; [DOI] [PubMed] [Google Scholar]; b) Duan J, Higuchi M, Zheng J, Noro S-I, Chang I-Y, Hyeon-Deuk K, Mathew S, Kusaka S, Sivaniah E, Matsuda R, J. Am. Chem. Soc 2017, 139, 11576–11583; [DOI] [PubMed] [Google Scholar]; c) Luo F, Yan C, Dang L, Krishna R, Zhou W, Wu H, Dong X, Han Y, Hu T-L, OKeeffe M, Wang L, Luo M, Lin R-B, Chen B, J. Am. Chem. Soc 2016, 138, 5678–5684. [DOI] [PubMed] [Google Scholar]
- [6].a) Peng Y-L, Pham T, Li P, Wang T, Chen Y, Chen K-J, Forrest KA, Space B, Cheng P, Zaworotko MJ, Zhang Z, Angew. Chem. Int. Ed 2018, 57, 10971–10975; Angew. Chem. 2018, 130, 11137–11141; [DOI] [PubMed] [Google Scholar]; b) Li Y-P, Wang Y, Xue Y-Y, Li H-P, Zhai Q-G, Li S-N, Jiang Y-C, Hu M-C, Bu X, Angew. Chem. Int. Ed 2019, 58, 13590–13595; Angew. Chem. 2019, 131, 13724–13729; [DOI] [PubMed] [Google Scholar]; c) Ye Y, Ma Z, Lin R-B, Krishna R, Zhou W, Lin Q, Zhang Z, Xiang S, Chen B, J. Am. Chem. Soc 2019, 141, 4130–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Aguilà D, Prado Y, Koumousi ES, Mathoniere C, Clérac R, Chem. Soc. Rev 2016, 45, 203–224; [DOI] [PubMed] [Google Scholar]; b) Zakaria MB, Chikyow T, Coord. Chem. Rev 2017, 352, 328–345; [Google Scholar]; c) Otsubo K, Haraguchi T, Kitagawa H, Coord. Chem. Rev 2017, 346, 123–138; [Google Scholar]; d) Sakaida S, Otsubo K, Sakata O, Song C, Fujiwara A, Takata M, Kitagawa H, Nat. Chem 2016, 8, 377–383; [DOI] [PubMed] [Google Scholar]; e) Deshmukh MM, Ohba M, Kitagawa S, Sakaki S, J. Am. Chem. Soc 2013, 135, 4840–4849; [DOI] [PubMed] [Google Scholar]; f) Culp JT, Smith MR, Bittner E, Bockrath B, J. Am. Chem. Soc 2008, 130, 12427–12434. [DOI] [PubMed] [Google Scholar]
- [8].a) Das MC, Xiang S, Zhang Z, Chen B, Angew. Chem. Int. Ed 2011, 50, 10510–10520; Angew. Chem. 2011, 123, 10696–10707; [DOI] [PubMed] [Google Scholar]; b) Xiang S-C, Zhang Z, Zhao C-G, Hong K, Zhao X, Ding D-R, Xie M-H, Wu C-D, Das MC, Gill R, Tomas K, Chen B, Nat. Commun 2011, 2, 204. [DOI] [PubMed] [Google Scholar]
- [9].Niel V, Martinez-Agudo JM, Muñoz MC, Gaspar AB, Real JA, Inorg. Chem 2001, 40, 3838–3839. [DOI] [PubMed] [Google Scholar]
- [10].Gao J, Cong J, Wu Y, Sun L, Yao J, Chen B, ACS Appl. Energy Mater 2018, 1, 5140–5144. [Google Scholar]
- [11].Duan X, Zhang Q, Cai J, Yang Y, Cui Y, He Y, Wu C, Krishna R, Chen B, Qian G, J. Mater. Chem. A 2014, 2, 2628–2633. [Google Scholar]
- [12].Duan J, Jin W, Krishna R, Inorg. Chem 2015, 54, 4279–4284. [DOI] [PubMed] [Google Scholar]
- [13].Xiang S, Zhou W, Zhang Z, Green MA, Liu Y, Chen B, Angew. Chem. Int. Ed 2010, 49, 4615–4618; Angew. Chem. 2010, 122, 4719–4722. [DOI] [PubMed] [Google Scholar]
- [14].Li P, He Y, Zhao Y, Weng L, Wang H, Krishna R, Wu H, Zhou W, O’Keeffe M, Han Y, Chen B, Angew. Chem. Int. Ed 2015, 54, 574–577; Angew. Chem. 2015, 127, 584–587. [DOI] [PubMed] [Google Scholar]
- [15].He Y, Krishna R, Chen B, Energy Environ. Sci 2012, 5, 9107–9120. [Google Scholar]
- [16].Bloch ED, Queen WL, Krishna R, Zadrozny JM, Brown CM, Long JR, Science 2012, 335, 1606–1610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.