Abstract
BACKGROUND:
Fractures associated with postmenopausal osteoporosis (PMO) are associated with pain, disability, and increased mortality. A recent, nationwide evaluation of racial difference in outcomes after fracture has not been performed.
OBJECTIVE:
To determine if 1-year death, debility, and destitution rates differ by race.
DESIGN:
Observational cohort study.
SETTING:
US Medicare data from 2010 to 2016.
PARTICIPANTS:
Non-Hispanic black and white women with PMO who have sustained a fragility fracture of interest: hip, pelvis, femur, radius, ulna, humerus, and clinical vertebral.
MEASUREMENTS:
Outcomes included 1-year: (1) mortality, identified by date of death in Medicare vital status information, (2) debility, identified as new placement in long-term nursing facilities, and (3) destitution, identified as becoming newly eligible for Medicaid.
RESULTS:
Among black and white women with PMO (n = 4,523,112), we identified 399,000 (8.8%) women who sustained a major fragility fracture. Black women had a higher prevalence of femur (9.0% vs 3.9%; P < .001) and hip (30.7% vs 28.0%; P < .001) fractures and lower prevalence of radius/ulna (14.7% vs 17.0%; P < .001) and clinical vertebral fractures (28.8% vs 33.5%; P < .001) compared with white women. We observed racial differences in the incidence of 1-year outcomes after fracture. After adjusting for age, black women had significantly higher risk of mortality 1 year after femur, hip, humerus, and radius/ulna fractures; significantly higher risk of debility 1 year after femur and hip fractures; and significantly higher risk of destitution for all fractures types.
CONCLUSIONS:
In a sample of Medicare data from 2010 to 2016, black women with PMO had significantly higher rates of mortality, debility, and destitution after fracture than white women. These findings are a first step toward understanding and reducing disparities in PMO management, fracture prevention, and clinical outcomes after fracture.
Keywords: disparities, epidemiology, fractures, osteoporosis
Osteoporosis, the most common age-related metabolic bone disease, affects more than 11 million adults aged 50 years or older in the United States, of which, more than 8 million are women.1,2 An additional approximate 35 million adults have low bone mass,1,2 which, in the presence of other clinical risk factors, increases the risk for fractures. Hip fractures are the most adverse fracture type associated with osteoporosis. In the United States, a woman’s annual lifetime risk of hip fracture is equal to that of breast cancer, ovarian cancer, and uterine cancer combined.3
Studies have found that 25% of patients die within 1 year of experiencing a hip fracture,4–7 with increases in mortality also present following vertebral and other non-vertebral fractures.4,6–14 It has also been reported that 50% of hip fracture patients do not return to their prior level of functioning, 20% require extended time in skilled nursing facilities, and the medical expenses associated with fractures result in 10% requiring financial assistance.15
The vast majority of studies evaluating outcomes after fragility fracture have been performed in primarily non-Hispanic white populations. The few studies that have stratified results by race and ethnicity have found racial differences in outcomes, particularly mortality.16–18 Data from these studies are relatively old and/or had regional samples, which limits the generalizability of findings, warranting a current national evaluation. There has also been limited research on racial differences in other nonmortality outcomes after fracture.
Given the significant adverse outcomes associated with fragility fractures, and the growing number in older persons of color, it is important to determine whether racial disparities are present in postfracture outcomes. Using a cohort of non-Hispanic black and white women with postmenopausal osteoporosis (PMO) from 2010 to 2016 US Medicare data, the goal of our study was to determine if racial differences exist in the year after a fragility fracture for the following outcomes: death, debility, and destitution.
METHODS
Population
Our cohort of women with PMO was previously assembled as a part of the Food and Drug Administration 10-year, claims-based pharmacovigilance study of denosumab, 60 mg, for the treatment of PMO.19 Women with PMO were identified based on age of 65 years or older and any one of the following: osteoporosis diagnosis code, fracture history, or osteoporosis medication at the time of cohort entry. Women were required to have Medicare Parts A, B, and D for at least 12 continuous months before cohort entry, not be enrolled in a Medicare Advantage Plan (Part C), and not have claims related to cancer (ignoring nonmelanoma skin cancer) or Paget’s disease 12 months preceding cohort entry.19 For our evaluation, we further excluded women from other racial and ethnic groups.
Identifying Fragility Fractures
We used a validated fracture identification algorithm20 to identify the fragility fractures most associated with osteoporosis, including hip, pelvis, femur, radius/ulna, humerus, and clinical vertebral, in women with PMO between 2010 and 2015. The fracture algorithm identifies incident fractures using claims meeting one of the following criteria: (1) fracture International Classification of Disease (ICD) diagnosis on an inpatient claim in the primary (first) position; (2) fracture ICD diagnosis on an inpatient claim in a nonprimary position; or (3) fracture discharge diagnosis on an outpatient physician evaluation and management (E&M) claim paired with a fracture repair code of the same fracture site as the diagnosis code. Incident clinical vertebral fractures can meet criteria 1, 3, or 4. Criterion 4 requires a fracture ICD diagnosis on an outpatient claim paired with a physician E&M code and a spine imaging code within 10 days.20 We utilized both ICD, Ninth Revision (ICD-9), and ICD, Tenth Revision (ICD-10), codes for fracture identification. Based on a validation study of 520 fractures that were clinically adjudicated from a large National Institutes of Health–funded cohort study linked to Medicare claims data, our fracture episode algorithm was found to have positive predictive values to identify the fracture sites of interest ranging from 90.9% to 98.6%.20
We further excluded women whose incident fracture had a trauma indicator (ICD-9 E codes; ICD-10 S, V-Y codes), women with multiple fractures on the same day who had trauma indicators on the nonincident fracture episode, women who did not have Medicare A + B + D − C coverage at least 12 months before the fracture episode start date, and those whose fracture episode start date did not allow for 1-year follow-up (ie, fracture start date and/or outcome date occurring on same day or fracture occurred on the last day of follow-up) (Figure 1).
Figure 1.
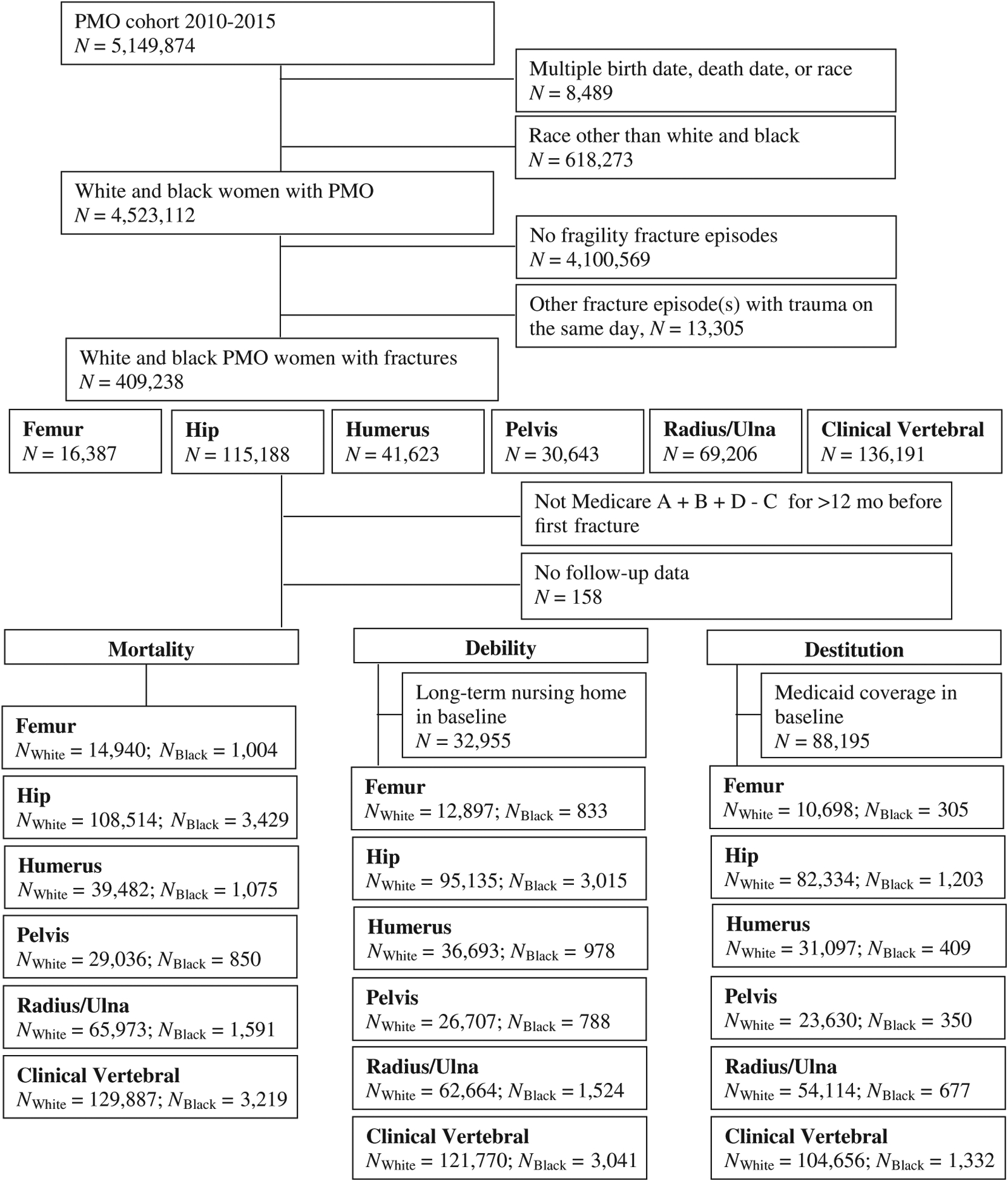
Identification of white and black postmenopausal osteoporosis (PMO) with fractures by outcome.
Identifying Outcomes
The primary outcomes of this study were death, debility, and destitution. Follow-up for each outcome started at episode start date and ended 1 year after fracture start date or December 31, 2016. We used the date of death from the vital status information from the Medicare beneficiary summary file. The death dates reported by the Centers for Medicare and Medicaid Services (CMS) are linked to multiple databases, including the Medicare Common Working File, the Railroad Retirement Board, and the Social Security Administration, with 99% of death dates having been validated.21
We defined debility as new placement in a long-term nursing facility. To identify debility, we used a modified definition of the incident long-term nursing home placement claims-based algorithm developed by Yun and colleagues.22 In our modified definition, the nursing facility claim was required to not be between the admission date and discharge date of a skilled nursing claim.
We defined destitution as becoming newly eligible for Medicaid. We identified Medicaid enrollment by the Medicaid buy-in (payment of Medicare premiums) variable or those who were newly eligible for Part D low-income subsidies.
For both the debility and destitution outcome, we excluded those who either had long-term nursing home stays or Medicaid coverage during the 12-month baseline period (long-term nursing home = 8.3%; Medicaid coverage = 22.1%) (Figure 1).
Statistical Analyses
We described brief demographic characteristics (age, region, Charlson comorbidity index) and PMO indicators of the women with fractures by race using t-test for continuous variables and χ2 test for categorical variables. We calculated the overall incidence of each outcome by race and by fracture site. Next, we calculated the age-standardized 1-year outcome incidence rates (IRs) per 1,000 person-years by race for each fracture site, standardizing the rates to the 2010 Census population aged 65 years or older. We also calculated a composite outcome of all three outcomes. To do so, we used the earliest date of any of the outcomes, censor date (eg, loss of coverage), or 365 days after fracture start date and the follow-up date for the composite outcome. We then used Poisson regression to test if racial differences existed in outcome IRs, with adjustment for age.
We performed a sensitivity analysis in the Medicare 5% sample, a random sample of all Medicare beneficiaries created by CMS for research. The 5% sample was not selected on the basis of osteoporosis, thus allowing us to compare the results between a general sample of older black and white women (who may and may not have osteoporosis) and those selected with PMO. We again calculated outcome incidence and age-standardized IR, and we tested racial differences using Poisson regression.
Last, we evaluated a metric of racial disparity using the number needed to harm principles from clinical trials. Calculated as [1,000/(outcome IR in black women – outcome IR in white women)], this metric summarizes the expected number of black and white women with fracture to follow for 1 year to observe one additional adverse outcome among black women. The smaller the number, the greater the disparity between the races in the outcomes after fracture.23
We used SAS version 9.4 (SAS Institute Inc) and Stata v. 16 (Stata Corp) to conduct all analyses. The research was approved by the University of Alabama at Birmingham Institutional Review Board.
RESULTS
Of the 4,523,112 black and white women with PMO from 2000 to 2015, we identified 422,543 (9.3%) women who sustained a fracture, of whom 409,238 (96.9%) sustained one of the major fragility fractures of interest. After exclusions (Figure 1), our final analytic sample included 399,000 women, of whom 11,168 (2.8%) were non-Hispanic black. There were no differences in age at PMO index date (79.3 years; P = .94); however, there was a larger prevalence of women in the youngest age group (65–74 years) in black women than in white women (24.8% vs 22.3%; P < .001) at the time of fracture. Black women were more likely to have an osteoporosis diagnosis or osteoporosis treatment on the PMO index date compared with white women. The complete descriptive characteristics of the sample are shown in Table 1.
Table 1.
Demographic and Outcome Characteristics of Women With PMO With Fragility Fracture Identified in 2010 to 2015 by Race
| Characteristics | Total (N = 399,000) | White (N = 387,832 [97.2%]) | Black (N = 11,168 [2.8%]) | P Valuea |
|---|---|---|---|---|
| Age on PMO date, mean (SD), y | 79.3 (8.0) | 79.3 (8.0) | 79.3 (8.5) | .94 |
| PMO indicators, No. (%)b | ||||
| OP diagnosis | 37,206 (9.3) | 35,726 (9.2) | 1,480 (13.3) | <.001 |
| Fragility fracture diagnosis | 4,084 (1.0) | 3,928 (1.0) | 156 (1.4) | <.001 |
| OP treatment | 13,836 (3.5) | 13,268 (3.4) | 568 (5.1) | <.001 |
| Age on fracture date, mean (SD), y | 81.5 (8.0) | 81.5 (8.0) | 81.3 (8.6) | .05 |
| Age on fracture date, y, No. (%) | ||||
| 65–74 | 89,326 (22.4) | 86,552 (22.3) | 2,774 (24.8) | <.001 |
| 75–84 | 155,206 (38.9) | 151,087 (39.0) | 4,119 (36.9) | |
| ≥85 | 154,468 (38.7) | 150,193 (38.7) | 4,275 (38.3) | |
| Region, No. (%) | ||||
| Northeast | 82,369 (20.6) | 80,487 (20.8) | 1882 (16.9) | <.001 |
| Midwest | 103,618 (26.0) | 101,541 (26.2) | 2077 (18.6) | |
| South | 160,119 (40.1) | 153,583 (39.6) | 6,536 (58.5) | |
| West | 52,894 (13.3) | 52,221 (13.5) | 673 (6.0) | |
| Charlson score, No. (%)c | ||||
| 0 | 165,757 (41.5) | 163,045 (42.0) | 2,712 (24.3) | <.001 |
| 1 | 50,425 (12.6) | 48,977 (12.6) | 1,448 (13.0) | |
| ≥2 | 182,818 (45.8) | 175,810 (45.3) | 7,008 (62.8) | |
| Fracture site, No. (%) | ||||
| Femur | 15,944 (4.0) | 14,940 (3.9) | 1,004 (9.0) | <.001 |
| Hip | 111,943 (28.1) | 108,514 (28.0) | 3,429 (30.7) | <.001 |
| Humerus | 40,557 (10.2) | 39,482 (10.2) | 1,075 (9.6) | .06 |
| Pelvis | 29,886 (7.5) | 29,036 (7.5) | 850 (7.6) | .62 |
| Radius/ulna | 67,564 (16.9) | 65,973 (17.0) | 1,591 (14.2) | <.001 |
| Clinical vertebral | 133,106 (33.4) | 129,887 (33.5) | 3,219 (28.8) | <.001 |
| Outcomes, No. (%) | ||||
| Death | 61,913 (15.5) | 59,724 (15.4) | 2,189 (19.6) | <.001 |
| Debility | 16,103 (4.0) | 15,642 (4.0) | 461 (4.1) | .62 |
| Destitution | 8,203 (2.1) | 7,933 (2.0) | 270 (2.4) | .01 |
| Composite | 80,944 (20.3) | 78,199 (20.2) | 2,745 (24.6) | <.001 |
Abbreviations: OP, osteoporosis; PMO, postmenopausal osteoporosis.
P value assessed via χ2 test for categorical variables and t-test for continuous variables.
PMO indicators on PMO index date.
Charlson score calculated using Romano version.28
The most prevalent fractures in our sample included clinical vertebral (33.4%), hip (28.1%), and radius/ulna (16.9%) (Table 1). Black women had a significantly higher prevalence of femur (9.0% vs 3.9%; P < .001) and hip (30.7% vs 28.0%; P < .001) fractures than white women; whereas they had a significantly lower prevalence of radius/ulna (14.2% vs 17.0%; P < .001) and clinical vertebral (28.8% vs 33.5%; P < .001) fractures (Table 1).
The prevalence of women who experienced death, debility, and destitution outcomes within 1 year of their fracture was 15.5%, 4.0%, and 2.1%, respectively, with 20.3% of women experiencing any one of the three outcomes. Overall, we observed significant differences by race, with black women having significantly higher 1-year mortality (19.6% vs 15.4%; P < .001), destitution (2.4% vs 2.0%; P = .006), and composite outcome (24.6% vs 20.2%; P < .001) (Table 1). When evaluating crude outcome incidence by fracture site, compared with white women, black women had significantly higher incidence of death 1 year after femur, hip, humerus, and radius/ulna fractures (Table 2). Black women had a significantly higher 1-year incidence of debility following femur fractures (6.6% vs 3.9%) compared with white women (Table 2); however, we did not observe any racial differences in 1-year debility after other fracture types evaluated. Black women had a significantly higher incidence of 1-year destitution following femur, hip, humerus, pelvis, and clinical vertebral fractures compared with white women (Table 2). When considering the combination of any one of the three outcomes, compared with white women, black women had significantly higher 1-year outcome incidence, ranging from 13.7% vs 10.3% for radius/ulna fractures to 33.5% vs 26.1% for femur fractures (Table 2).
Table 2.
Incidence and Age-Standardized IRs of Postfracture Outcomes by Race and Fracture Site
| Femur | ||||||||
|---|---|---|---|---|---|---|---|---|
| White (N = 14,940/12,897/10,698/14,940)a | Black (N = 1,004/833/305/1,004)a | |||||||
| Outcome | NE | PY | I | Age-Standardized IR (95% CI) | NE | PY | I | Age-Standardized IR (95% CI) |
| Death | 3,245 | 12,614 | 21.7 | 185.4 (177.7–193.4) | 285 | 803 | 28.4** | 280.6 (240.8–325.1) |
| Debility | 499 | 10,869 | 3.9 | 39.7 (35.7–44.0) | 55 | 648 | 6.6** | 67.2 (47.3–92.7) |
| Destitution | 368 | 9,068 | 3.4 | 33.7 (29.6–38.2) | 21 | 236 | 6.9** | 83.8 (45.6–141.2) |
| Composite | 3,893 | 12,117 | 26.1 | 240.0 (230.9–249.3) | 336 | 757 | 33.5** | 358.7 (312.3–410.2) |
| Hip | ||||||||
| White (N = 108,514/95,135/82,334/108,514)a | Black (N = 3,429/3,015/1,203/3,429)a | |||||||
| Outcome | NE | PY | I | Age-Standardized IR (95% CI) | NE | PY | I | Age-Standardized IR (95% CI) |
| Death | 24,825 | 90,938 | 22.9 | 190.1 (186.4–193.9) | 957 | 2,775 | 27.9** | 263.5 (238.4–290.5) |
| Debility | 3,041 | 79,461 | 3.2 | 34.2 (32.3–36.1) | 113 | 2,396 | 3.7 | 49.7 (37.4–64.9) |
| Destitution | 3,038 | 68,441 | 3.7 | 36.2 (34.1–38.5) | 107 | 945 | 8.9 | 109.5 (76.3–152.3) |
| Composite | 29,519 | 87,489 | 27.2 | 245.3 (240.9–249.8) | 1,115 | 2,650 | 32.5 | 336.8 (306.9–368.8) |
| Humerus | ||||||||
| White (N = 39,482/36,693/31,097/39,482)a | Black (N = 1,075/978/409/1,075)a | |||||||
| Outcome | NE | PY | I | Age-Standardized IR (95% CI) | NE | PY | I | Age-Standardized IR (95% CI) |
| Death | 4,790 | 36,363 | 12.1 | 101.3 (98.0–104.7) | 184 | 958 | 17.1** | 161.0 (135.8–189.5) |
| Debility | 1,784 | 32,831 | 4.9 | 43.0 (40.8–45.3) | 55 | 849 | 5.6 | 56.5 (41.4–75.4) |
| Destitution | 723 | 28,567 | 2.3 | 21.2 (19.4–23.1) | 29 | 360 | 7.1 | 82.2 (51.5–124.6) |
| Composite | 6,775 | 34,779 | 17.2 | 152.9 (148.8–157.2) | 248 | 910 | 23.1 | 236.7 (204.7–272.2) |
| Pelvis | ||||||||
| White (N = 29,036/26,707/23,630/29,036)a | Black (N = 1,075/978/409/1,075)a | |||||||
| Outcome | NE | PY | I | Age-Standardized IR (95% CI) | NE | PY | I | Age-Standardized IR (95% CI) |
| Death | 5,242 | 25,502 | 18.1 | 145.5 (138.6–152.6) | 175 | 738 | 20.6 | 188.6 (149.9–234.4) |
| Debility | 1,315 | 22,776 | 4.9 | 46.8 (42.5–51.3) | 28 | 671 | 3.6 | 36.2 (21.4–57.2) |
| Destitution | 668 | 20,641 | 2.8 | 23.6 (20.5–27.1) | 20 | 300 | 5.7 | 53.6 (25.5–99.2) |
| Composite | 6,816 | 24,246 | 23.5 | 205.5 (197.0–214.2) | 213 | 708 | 25.1 | 241.3 (197.1–292.3) |
| Radius/Ulna | ||||||||
| White (N = 65,973/62,664/54,114/65,973)a | Black (N = 1,591/1,524/677/1,591)a | |||||||
| Outcome | NE | PY | I | Age-Standardized IR (95% CI) | NE | PY | I | Age-Standardized IR (95% CI) |
| Death | 4,112 | 63,614 | 6.2 | 51.7 (50.0–53.4) | 144 | 1,509 | 9.1** | 80.4 (67.0–95.5) |
| Debility | 2,480 | 59,031 | 4.0 | 34.8 (33.4–36.3) | 61 | 1,412 | 4.0 | 36.0 (27.2–46.8) |
| Destitution | 791 | 52,056 | 1.5 | 13.1 (12.1–14.1) | 27 | 638 | 4.0 | 36.0 (23.2–53.5) |
| Composite | 6,820 | 61,639 | 10.3 | 90.4 (88.1–92.7) | 218 | 1,457 | 13.7 | 126.6 (109.5–145.7) |
| Clinical Vertebral | ||||||||
| White (N = 129,887/121,770/104,656/129,887)a | Black (N = 3,219/3,041/1,332/3,219)a | |||||||
| Outcome | NE | PY | I | Age-Standardized IR (95% CI) | NE | PY | I | Age-Standardized IR (95% CI) |
| Death | 17,510 | 118,980 | 13.5 | 113.2 (111.0–115.3) | 444 | 2,960 | 13.8 | 126.7 (113.6–141.0) |
| Debility | 6,523 | 108,113 | 5.4 | 44.4 (43.1–45.7) | 149 | 2,705 | 4.9 | 48.5 (40.0–58.2) |
| Destitution | 2,345 | 95,417 | 2.2 | 20.1 (19.0–21.2) | 66 | 1,214 | 5.0** | 46.4 (34.6–60.9) |
| Composite | 24,376 | 113,761 | 18.8 | 164.8 (162.2–167.5) | 615 | 2,827 | 19.1 | 187.2 (170.7–204.9) |
Note. I = (NE/NPopulation) * 100; IR = (NE/PY) * 1,000. IRs standardized to 2010 US Census. Incidence P values calculated based on two-sample test of proportions.
Abbreviations: CI, confidence interval; I, incidence; IR, incidence rate; NE, number of events; PY, person-years.
Total population count by outcome: death/debility/destitution/composite.
P < .001.
After accounting for age and standardizing to the 2010 US population aged 65 years or older, we observed similar trends to the crude incidence (Table 2). For example, for hip fracture, the age-standardized IRs per 1,000 person-years in black women were 38.6%, 45.3%, 202.5%, and 37.3% higher for death, debility, destitution, and the composite outcome, respectively, than the IRs in white women (eg, death IR [95% confidence interval {CI}] for IRBlack = 263.5 [238.4–290.5] and for IRWhite = 290.1 [186–193.9]) (Table 2).
To formally test the racial disparity, we used age-adjusted Poisson regression models. Figure 2 displays the IR ratio and the 95% CI by fracture type and outcome. We observed significant racial differences in all outcomes for both femur and hip fractures. For example, for hip fracture, compared with white women, black women had a significant (P < .001) 24%, 22%, 2.5-fold, and 22% higher age-adjusted risk of death, debility, destitution, and the composite outcome, respectively, 1 year following fracture (Figure 2). Black women also had significantly higher risk of death, destitution, and the composite outcome in both humerus and radius/ulna fractures (Figure 2). In pelvis fractures, black women had a lower age-adjusted risk of debility (0.72; 95% CI = 0.54–0.96), but had a 2.1-fold higher risk of destitution (2.09; 95% CI = 1.67–2.62) (Figure 2). For clinical vertebral fractures, black women had a 2.3-fold higher risk in 1-year destitution than white women (2.33; 95% CI = 2.09–2.61), with no observed differences in the IRs of the other outcomes of interest (Figure 2).
Figure 2.
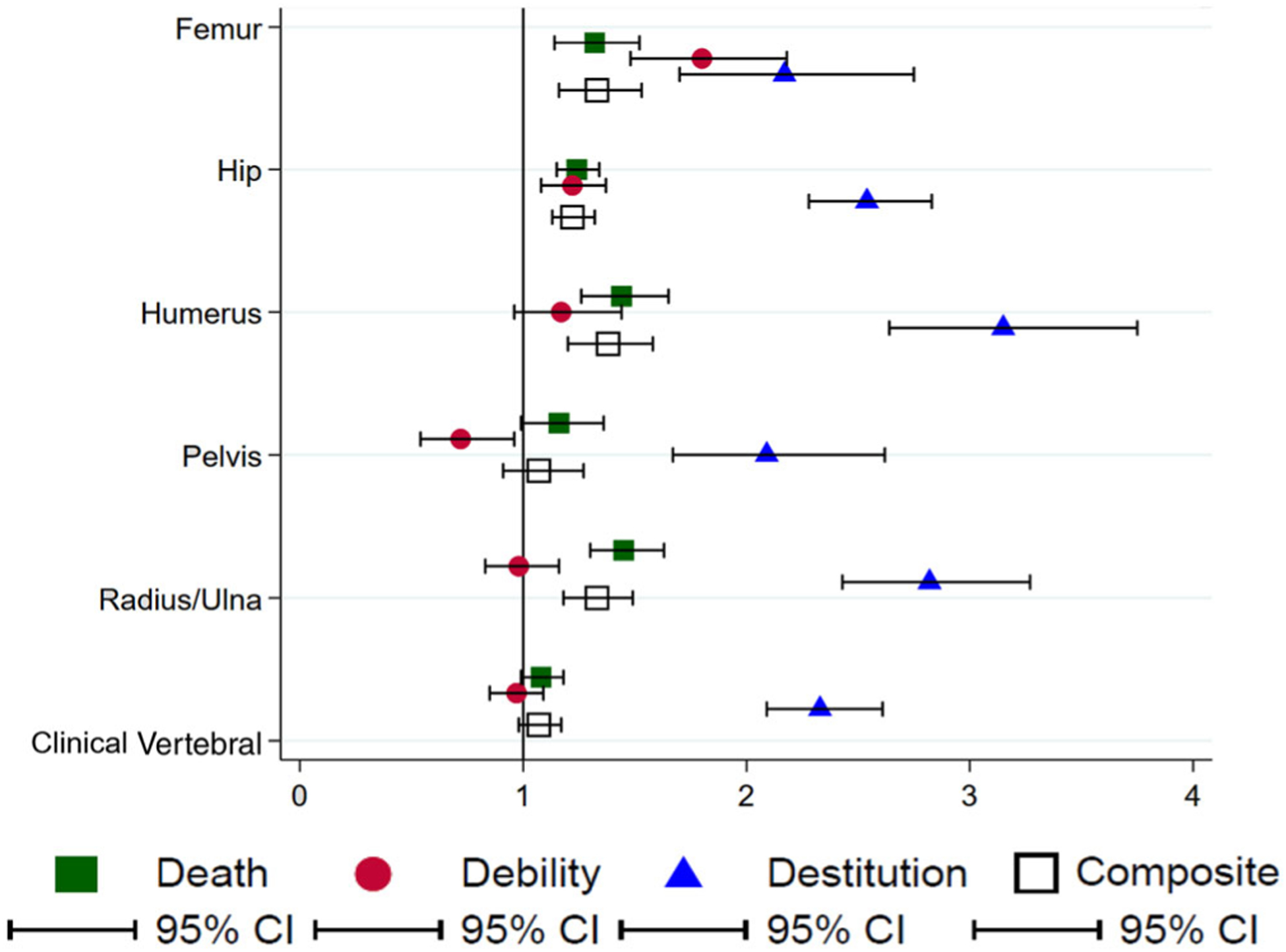
Incidence rate ratio of postfracture outcomes by fracture type in black women compared to white women with postmenopausal osteoporosis. To the right of the line of unity (1), a higher incidence rate of postfracture outcomes in black women compared with white women; to the left of the line, a lower incidence rate of postfracture outcomes in black women compared with white women. If the confidence interval (CI) crosses the line the unity, then no difference in risk is observed between black and white women.
Sensitivity Analysis in 5% Sample
Using the same inclusion and exclusion with the exception of requiring PMO (no osteoporosis diagnosis codes, medications, or fractures), we identified 55,438 women with fragility fractures from the CMS 5% sample, of whom 2,162 (3.9%) were black. Similar to the PMO results, a larger proportion of black women with fractures were in the 65 to 74 years age group, and there was a higher prevalence of femur and hip fractures and a lower prevalence of radius/ulna and clinical vertebral fractures than in white women. Although point estimates differed, the racial differences in outcomes were similar. Black women in the 5% sample, compared with white women, had on average 62.9%, 19.9%, 186.4%, and 54.0% higher age-standardized 1-year death, debility, destitution, and composite IRs per 1,000 person-years, respectively, for all fractures combined. Likewise, using Poisson regression, we observed that black women had significantly 32%, 2.4-fold, and 24% higher risk of death, destitution, and composite when compared with white women for all fractures combined (data not shown).
Racial Disparity
The racial disparity metric or number needed to harm varied by outcome and fracture site (Table 3). For every 14 hip fractures occurring in black and white women with PMO, one additional black woman died; whereas 74 clinical vertebral fractures are needed for one additional death to be experienced in black women (Table 3). Likewise, for every 11 hip fractures in black and white women, one additional black woman experienced any one of the three outcomes. The disparity in outcomes is greater for more adverse fracture types, like femur and hip, and the disparity is greater for more severe outcomes (eg, death).
Table 3.
Racial Disparity in Composite Outcome by Fracture Type
| Outcome | Femur | Hip | Humerus | Pelvis | Radius/Ulna | Clinical Vertebral |
|---|---|---|---|---|---|---|
| Death | 11 | 14 | 17 | 23 | 35 | 74 |
| Debility | 36 | 65 | 74 | −94 | 833 | 244 |
| Destitution | 20 | 14 | 16 | 33 | 44 | 38 |
| Composite | 8 | 11 | 12 | 28 | 28 | 45 |
Note. The racial disparity calculated as [1,000/(IRBlack - IRWhite)] summarizes the expected number of black and white women with fracture to follow for 1 year to observe one additional adverse outcome among black women. The smaller the number, the greater the disparity between the races in the outcomes after fracture.
DISCUSSION
In this analysis of a national sample of US Medicare data comprising all non-Hispanic black and white women with PMO, we found that between 2010 and 2015, approximately 9% of women sustained one of the six fragility fractures of interest. The black women in our sample had a larger prevalence of femur and hip fractures and a lower prevalence of radius/ulna and clinical vertebral fractures compared with white women. Data on prevalence of fractures by race and ethnicity are limited, but a study utilizing 1986 to 1990 data from the Medicare 5% sample found that compared to white women, black women had lower rates of many fragility fractures, including 75% lower rates of proximal humerus, 70% lower rates of distal radius/ulna, 69% lower rates of pelvic, and 62% lower rates of hip fracture; whereas there were no significant differences in rates of femur fractures.24 Similar to the previous study, we found lower prevalence of radius/ulna fractures in black women, but unlike this study, we found higher prevalence of hip and femur fractures in black women. Although both studies utilized Medicare data, the previous study utilized the 5% sample, whereas our study utilized women with PMO, who may have an inherent higher fracture risk given the osteoporosis status.
Our primary objective was to evaluate racial differences in the three postfracture outcomes: death, debility, and destitution. Overall, we found that black women with PMO had significantly higher incidence of death compared with white women with PMO. The same was true when observed by fracture site, clearly indicating a racial disparity in 1-year mortality after fracture. We did not observe a racial difference in the overall incidence in 1-year debility. However, we observed differences in debility incidence by fracture site in black compared with white women with PMO, which was limited to the most adverse fracture types (eg, femur and hip). We observed significant racial differences in the overall incidence of destitution after fracture overall as well as by fracture site. The risk of destitution in black women with PMO was twice to three times higher than white women with PMO. Although it is well documented that black women have a lower prevalence of osteoporosis1,25 and incidence of hip fractures,26 our findings show that compared with white women with PMO, black women with PMO who fracture have significantly worse clinical outcomes after hip and several other types of fragility fractures.
To our knowledge, there has only been one study evaluating the three outcomes we described. In their analysis of the Medicare 5% sample, Tajeu and colleagues at our institution used propensity score matched hip fracture patients to women without hip fractures.15 They identified death, debility, and destitution in 28.6%, 20.1%, and 6.6% of hip fracture patients, respectively, but did not evaluate racial differences in the outcomes.15 Prior studies evaluating racial differences in mortality rates have concentrated on hip fractures, with higher mortality rates observed in black women, ranging from 33% in a study using 1984 to 1987 Medicare data17 to 13% in an analysis of all New York State hip fractures.16 Although our study controlled only for age, whereas other studies have adjusted for potential cofounders other than age, our results with respect to hip fracture mortality are in line with previous studies. The goal of this study was to describe the clinical experience of black and white women with fractures; thus, other confounders were not considered at this time. Our study is the first to evaluate racial differences in other fracture outcomes (debility and destitution) and, to our knowledge, there are no studies evaluating racial differences in mortality outcomes for other fracture types, specifically focusing on black and white populations.
A major strength of our study was the use of a cohort that captures all women with PMO in Medicare; however, this comes with some limitations. These women had to meet coverage requirements and not be enrolled in a Medicare Advantage Plan. Medicare Advantage Plans are not required to submit individual claims, thus making outcome and exposure ascertainment more difficult. Although excluding these individuals is common practice in Medicare-based studies, it does limit the generalizability of findings. Although the use of the PMO cohort is a strength, women with PMO may have higher overall fracture incidence, again limiting our generalizability. To evaluate our findings in a more generalizable cohort of Medicare beneficiaries, we conducted a sensitivity analysis in the Medicare 5% sample. The women in the 5% sample analysis did not have to meet the PMO criteria, and although point estimates were different, we observed similar increases in each of the outcomes among black women in the 5% sample as we did in the women with PMO. For both the debility and destitution analyses, we excluded women with evidence of long-term nursing home and Medicaid coverage in the baseline period, resulting in a reduction in sample size, again limiting generalizability of our study. Although excluding women with a history of long-term nursing home stays reduced our sample size, it provided an unbiased estimate of new debility. Excluding women with Medicaid coverage in the baseline had a more significant impact on our sample size, particularly our black population. According to CMS, nearly 40% of those with dual Medicare-Medicaid coverage are persons of color.27 The exclusion of dual eligible women could have implications of healthcare utilization, medication coverage, and facility quality metrics; however, this was not the goal of our study. One of our goals was to evaluate how a fracture impacts finances in older women, and our findings clearly show that a fracture is associated with detrimental financial effects on black women at greater rates than white women. Last, although our study also utilized a highly valid algorithm to identify fractures, our algorithm was designed to have higher specificity than sensitivity, potentially missing real fractures that were neither hospitalized nor repaired.
CONCLUSIONS
To our knowledge, this is the first comprehensive evaluation of fracture events and novel outcomes after fracture by race. Our study shows that black women with PMO experience worse outcomes after fragility fractures than their white counterparts. Future studies are needed to explain why these disparities exist, with ultimate goals of developing interventions and/or programs to mitigate and reduce disparities in fracture outcomes.
ACKNOWLEDGMENTS
Financial Disclosure: This work was sponsored by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases 1K01AR068400.
Footnotes
Conflict of Interest: N.C.W.: Research grants: Amgen; consultant: NortonRose Fulbright/Pfizer.
L.C.: Research grants: Amgen.
K.G.S.: Consultant: Gilead and Radius; research grants and consultant: Amgen.
C.J.B.: None.
J.M.S.: None.
J.R.C.: Research grants and consultant: Amgen, Lilly, and Radius.
Sponsor’s Role: The National Institute of Arthritis and Musculoskeletal and Skin Diseases did not play a role in the design, methods, recruitment, data collection, analysis, or preparation of this article.
REFERENCES
- 1.Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014. Osteoporos Int. 2017;28(6):1979–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cauley JA, Wampler NS, Barnhart JM, et al. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19(12):1717–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–882. [DOI] [PubMed] [Google Scholar]
- 5.Empana JP, Dargent-Molina P, Breart G, EPIDOS Group. Effect of hip fracture on mortality in elderly women: the EPIDOS prospective study. J Am Geriatr Soc. 2004;52(5):685–690. [DOI] [PubMed] [Google Scholar]
- 6.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513–521. [DOI] [PubMed] [Google Scholar]
- 7.Huntjens KM, Kosar S, van Geel TA, et al. Risk of subsequent fracture and mortality within 5 years after a non-vertebral fracture. Osteoporos Int. 2010; 21(12):2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11(7):556–561. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ 3rd. Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993;137(9):1001–1005. [DOI] [PubMed] [Google Scholar]
- 10.Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int. 1998;8(3):291–297. [DOI] [PubMed] [Google Scholar]
- 11.Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38–42. [DOI] [PubMed] [Google Scholar]
- 12.Kado DM, Duong T, Stone KL, et al. Incident vertebral fractures and mortality in older women: a prospective study. Osteoporos Int. 2003;14(7):589–594. [DOI] [PubMed] [Google Scholar]
- 13.Ong T, Kantachuvesiri P, Sahota O, Gladman JRF. Characteristics and outcomes of hospitalised patients with vertebral fragility fractures: a systematic review. Age Ageing. 2018;47(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shortt NL, Robinson CM. Mortality after low-energy fractures in patients aged at least 45 years old. J Orthop Trauma. 2005;19(6):396–400. [DOI] [PubMed] [Google Scholar]
- 15.Tajeu GS, Delzell E, Smith W, et al. Death, debility, and destitution following hip fracture. J Gerontol A Biol Sci Med Sci. 2014;69(3):346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dy CJ, Lane JM, Pan TJ, Parks ML, Lyman S. Racial and socioeconomic disparities in hip fracture care. J Bone Joint Surg Am. 2016;98(10):858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penrod JD, Litke A, Hawkes WG, et al. The association of race, gender, and comorbidity with mortality and function after hip fracture. J Gerontol A Biol Sci Med Sci. 2008;63(8):867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue F, Ma H, Stehman-Breen C, et al. Design and methods of a post-marketing pharmacoepidemiology study assessing long-term safety of Prolia(R) (denosumab) for the treatment of postmenopausal osteoporosis. Pharmacoepidemiol Drug Saf. 2013;22(10):1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright NC, Daigle SG, Melton ME, Delzell ES, Balasubramanian A, Curtis JR. The design and validation of a new algorithm to identify incident fractures in administrative claims data. J Bone Miner Res. 2019;34(10): 1798–1807. [DOI] [PubMed] [Google Scholar]
- 21.Jarosek S. Death Information in the Research Identifiable Medicare Data. https://www.resdac.org/articles/death-information-research-identifiable-medicare-data. 2018. Accessed February 26, 2019.
- 22.Yun H, Kilgore ML, Curtis JR, et al. Identifying types of nursing facility stays using Medicare claims data: an algorithm and validation. Health Serv Outcomes Res Method. 2010;10:100–110. [Google Scholar]
- 23.Sedgwick P, What is number needed to harm (NNH)? BMJ. 2013;347: f4869. [Google Scholar]
- 24.Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612–618. [DOI] [PubMed] [Google Scholar]
- 25.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20(2):185–194. [DOI] [PubMed] [Google Scholar]
- 26.Wright NC, Saag KG, Curtis JR, et al. Recent trends in hip fracture rates by race/ethnicity among older US adults. J Bone Miner Res. 2012;27(11):2325–2332. [DOI] [PubMed] [Google Scholar]
- 27.Data Analysis Brief: Medicare-Medicaid Dual Enrollment 2006 Through 2016. Baltimore, MD: CMS Medicare-Medicaid Coordination Office; 2017. https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-Coordination/Medicare-Medicaid-Coordination-Office/DataStatisticalResources/Downloads/Eleven-YearEver-EnrolledTrendsReport_2006-2016.pdf. Accessed January 22, 2020. [Google Scholar]
- 28.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]


