Introduction
Stevens-Johnson syndrome (SJS) and its more severe variant, toxic epidermal necrolysis (TEN), belong to the same spectrum of immunological disorders affecting the skin and the mucous membranes.1 These disorders affect multiple organ systems in the acute phase and can be fatal. In the acute phase of SJS/TEN, 50–88% of patients have been reported to have ocular involvement, with signs ranging from conjunctival hyperemia to complete sloughing of the ocular surface and eyelid skin.2–5 Up to 41% may have moderate-severe ocular involvement in the acute phase.6 If not appropriately treated in the acute phase, a keratinized eyelid margin and limbal stem cell deficiency (LSCD) can lead to potentially vision-threatening complications such as corneal neovascularization and conjunctivalization, persistent epithelial defects, and keratinization of the entire ocular surface in the chronic phase.7 Chronic ocular sequelae are the most disabling long-term complications occurring in survivors of SJS/TEN.8 In a recently published multi-centre study from two large tertiary care eye hospitals, SJS/TEN constituted the third most common etiology for bilateral LSCD.9 Treatment options employed in unilateral LSCD often cannot be used in this bilateral disease. Thus, a missed window of opportunity for acute treatment can result in bilateral blindness with limited and invasive remaining treatment options.10
In the past decade, management strategies involving concomitant use of amniotic membrane and corticosteroids have been employed in the acute phase, resulting in a reduction of complications in the chronic phase.11 The use of amniotic membrane transplantation (AMT) within the first week of diagnosis of SJS/TEN in patients with moderate-severe ocular involvement has shown a reduction in chronic ocular disease, although long-term outcomes have not been reported.6, 12–15 In this study, we evaluated the impact of a treatment protocol for acute SJS/TEN that was instituted at Massachusetts Eye and Ear (MEE) in January 2008. We studied the long-term ocular outcomes in this group of patients who were treated as part of this protocol and compared them to a group of SJS/TEN patients who were treated at MEE before January 2008, prior to the institution of this protocol.
Methods
This study was approved by the institutional review board of the Massachusetts Eye and Ear and the Massachusetts General Hospital. The study was conducted under Health Insurance Portability and Accountability Act (HIPAA) compliance and adhered to the tenets of the Declaration of Helsinki.
Patient selection
A retrospective chart review was performed on all patients who underwent an ophthalmological examination between January 2000 and September 2017 during an admission for acute SJS/TEN at two hospitals where MEE physicians see such patients in consultation. The inclusion criteria for the study were as follows: 1) Diagnosis of SJS/TEN by a dermatologist or burn surgeon based on onset of high fever, typical skin eruptions and involvement of at least two mucosal sites including the ocular surface, +/− skin biopsy at the physician’s discretion; 2) Follow up care at MEE for at least 6 months post discharge after acute SJS/TEN. Exclusion criteria included any patient with 1) less than 6 months follow-up from the date of hospital discharge; 2) a cause of vision loss other than ocular surface disease from SJS/TEN.
Since the treatment protocol was instituted in January 2008, SJS/TEN patients were divided into two groups: those admitted for acute SJS/TEN between January 2000 and December 2007 (Group I) and those admitted between January 2008 and September 2017 (Group II). The details of the acute care protocol used in the ocular management of patients with SJS/TEN have been described previously10, 16 and are mentioned in Figure 1.
Figure 1.
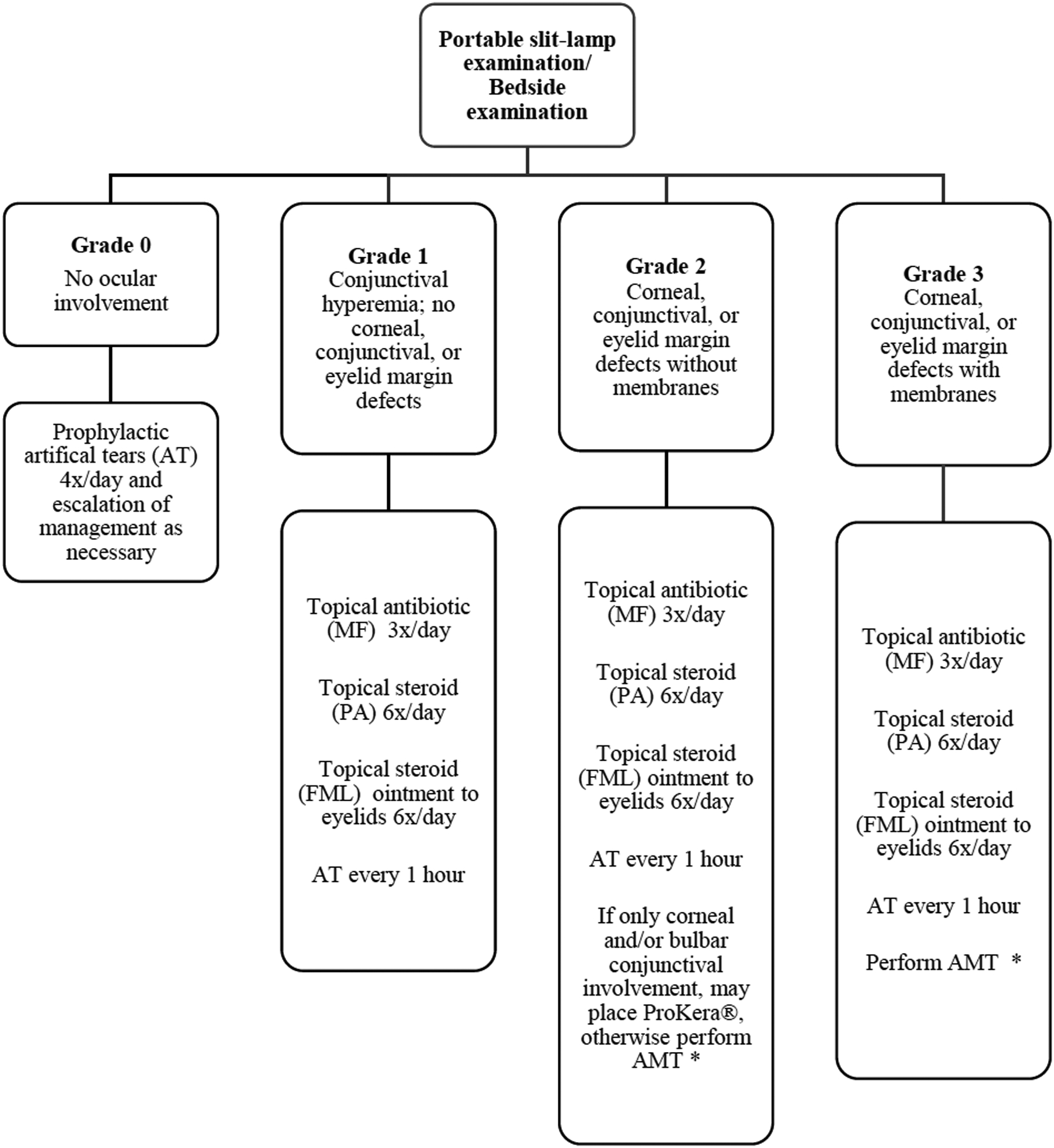
Flow diagram outlining protocol for management of ocular manifestations in acute Stevens-Johnson syndrome/toxic epidermal necrolysis. (MF = moxifloxacin 0.5%; PA = prednisolone acetate 1%; FML= fluorometholone 0.1%; AT = artificial tears; AMT = amniotic membrane transplantation)
*Decision to perform AMT based on feasibility (intubation status, cooperation, etc.). ProKera is acceptable if only bulbar conjunctival or corneal involvement is present or when AMT is not feasible.
Grade of acute involvement adapted from Sotozono’s classification (Sotozono C, Ueta M, Nakatani E, et al. Predictive factors associated with acute ocular involvement in Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol 2015;160:228–37.e2)
Data collection and statistical analysis
Patients were characterized as having SJS, SJS-TEN overlap, or TEN based on the percentage of total body surface area (TBSA) with epidermal detachment (SJS: <10% TBSA; SJS-TEN overlap: 10–30% TBSA; TEN: >30% TBSA).17 The acute phase was characterized as the period between symptom onset of SJS/TEN up to 2 months later; the sub-acute phase was defined as 2 to 6 months after symptom onset, while the chronic phase was defined as > 6 months after symptom onset. Ocular involvement in the acute phase was retrospectively graded based on clinical exam notes for each eye using the grading system proposed by Sotozono et al (Figure 1).18 Ocular examination was carried out on a daily basis for the first week in the acute stage of the disease and every 2–3 days thereafter until the patient was discharged, and the eye was treated accordingly. The highest grade of severity that was observed during admission was used for this study. After discharge, every patient was advised to follow-up on a monthly basis for the first 3 months. After the first 3 months, follow-up visits occurred every 4–6 months for the subsequent 3 years and at least yearly thereafter. Frequency of follow-up visits was adjusted as needed based on active pathology. The presumed etiology of SJS/TEN, and the ocular treatment for every eye of every patient was recorded. Additionally, the number of eyes which underwent surgical AMT (AmnioGraft; Bio-Tissue, Inc., Miami, FL) or self-retained AMT (ProKera® ; Bio-Tissue, Inc., Miami, FL) was documented. Surgical techniques for AMT have been detailed in previous reports, with a newer technique that allows rapid, sutureless application of AMT recently published.13, 19 For the chronic phase, the duration of follow-up, complications, subsequent surgical procedures and best-corrected visual acuity (BCVA) at final follow-up were documented. The follow-up for each patient was calculated from the day of discharge from the hospital. The primary outcome measures were BCVA at final follow-up and complications in the chronic phase.
Statistical analyses were conducted using SAS software (SAS Institute Inc., Cary, NC, Version 9.4), with a p value < 0.05 considered statistically significant. Continuous parametric data were reported as mean (± standard deviation) and non-parametric data were reported as median with inter-quartile range (IQR). To compare variables between individuals and not eyes, for e.g. continuous variables such as age and duration of follow-up between patients in the two groups, the Mann-Whitney U test was utilized, while the categorical variables were compared using the Fisher’s exact test and the chi-square test whenever appropriate. Because both eyes of all study participants were used for analyses, in order to independently compare variables for two eyes of the same patient, generalized estimating equations (GEE) was used to fit the repeated measurements model to account for the correlation between paired eyes within individuals. However, with GEE, when one of the variables in the two groups is 0% or 100%, the p-value does not converge. Hence, in such circumstances, we used the Fisher’s exact test and included only one eye per patient based on a random number generator. (For patients with the same acute stage grade in both eyes, we used the Microsoft Excel random number generator to choose 1 eye per patient and for patients with different acute stage grades between eyes, we chose the worse eye.) Kaplan–Meier survival plots were performed for time-to-event analyses (event being BCVA < 20/200), and differences between the two groups were compared using the log-rank test. For the Kaplan-Meier survival plot, we used only 1 eye per patient using the same method as above. Cox proportional-hazards regression analysis was used to identify independent risk factors associated with BCVA < 20/200 in the chronic phase of SJS/TEN. This was done using both eyes of every patient and accounting for the correlation between paired eyes within individuals.
Results
Demographics and baseline characteristics
A total of 48 patients who met our inclusion criteria were included in our study (18 eyes of 9 patients in Group I and 78 eyes of 39 patients in Group II). The baseline characteristics of patients in both groups and further details of the acute SJS/TEN episode are given in Table 1. There was no significant difference between the two groups regarding age (p=0.41) or sex (p=0.44). There was a significant difference in the median follow-up between the two groups (p<0.001) with Group I having longer follow-up. There was no significant difference between the two groups with regard to the proportion of patients having SJS, SJS-TEN Overlap, or TEN. Additionally, there was no significant difference in the proportion of patients who received systemic immunosuppression in the acute phase between both groups.
Table 1.
Characteristics of patients with ocular involvement in the acute phase of Stevens- Johnson syndrome/toxic epidermal necrolysis
| Category | Group I: SJS/TEN onset before January 2008, n = 9 patients | Group II: SJS/TEN onset after January 2008, n = 39 patients | P value |
|---|---|---|---|
| Mean age in years at the time of acute SJS/TEN (range) | 34.2 ± 19 (5–58) | 29.1 ± 18.7 (1.5–71) | 0.41 |
| Number of adults/children* at the time of acute SJS/TEN | 7/2 | 26/13 | 0.52 |
| Sex (female; male) | 7; 2 | 25; 14 | 0.44 |
| Median follow-up in years (IQR) | 12.95 (10.3 – 14.56) | 2.6 (1– 4.3) | < 0.001 |
| Number of patients, n (%) | |||
| with SJS | 1/9 (11) | 11/39 (28) | 0.67 |
| with SJS-TEN overlap | 1/9 (11) | 9/39 (23) | 0.67 |
| with TEN | 7/9 (78) | 19/39 (49) | 0.55 |
| Presumed etiology, n (%) | |||
| Antibiotics | |||
| Sulfonamides | 1/9 (11) | 16/39 (41) | 0.27 |
| Other antibiotics | 2/9 (22) | 1/39 (3) | 0.11 |
| Anti-epileptics | 1/9 (11) | 6/39 (15) | 0.65 |
| NSAIDs | 4/9 (44) | 5/39 (13) | 0.19 |
| Allopurinol | 0/9 (0) | 1/39 (3) | 1.0 |
| Other drugs | 1/9 (11) | 5/39 (13) | 1.0 |
| Infectious etiology | 0/9 (0) | 3/39 (8) | 1.0 |
| (Mycoplasma pneumoniae) | |||
| Unknown etiology | 0/9 (0) | 2/39 (5) | 1.0 |
| Systemic immunosuppression in the acute phase | |||
| None | 4/9 (44) | 7/39 (18) | 0.24 |
| Systemic steroids | 5/9 (56) | 21/39 (54) | 1.0 |
| IVIG | 1/9 (11) | 15/39 (38) | 0.43 |
| Cyclosporine | 0/9 (0) | 7/39 (18) | 0.58 |
| Etanercept | 0/9 (0) | 2/39 (5) | 1.0 |
SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis; IQR = inter-quartile range; NSAIDs = non-steroidal anti-inflammatory drugs; IVIG = intravenous immunoglobulin
Children defined as younger than 18 years
Ocular involvement and treatment during acute SJS/TEN
In patients in whom visual acuity could be measured, 100% of eyes in Group I (14/14) and Group II (68/68) had BCVA ≥ 20/40 at time of first ophthalmologic consultation during acute SJS/TEN admission. This vision was measured at the bedside. Vision could not be measured in two patients in Group I and five in Group II secondary to intubation and sedation status at the time of the first ophthalmological examination. There was no significant difference in the grade of acute involvement between the two groups. However, there was a difference in the ocular treatment given in the acute phase. All eyes in Group I received topical treatment alone (topical steroids, topical antibiotics and topical lubricants) across all grades of acute ocular involvement. Eyes in Group II which belonged to the grade 1 acute severity group received topical treatment alone as described in Figure 1. Within the first month of SJS/TEN onset, none of the eyes in Group I that had grade 2 or grade 3 acute ocular involvement received AMT/ProKera, while 53/61 (87%) eyes in Group II with the same grades of involvement received AMT/ProKera. Eight out of 61 eyes did not receive AMT/ProKera, most commonly secondary to refusal by the patient or family. Forty-five eyes of 24 patients received AMT at the bedside while eight eyes of four patients received AMT in the operating room. Seventeen eyes of 11 patients received repeat AMT/ProKera after dissolution of the initial amniotic membrane. Further details on each cohort’s ocular manifestations and treatment in the acute phase are presented in Table 2.
Table 2.
Characteristics of eyes of patients with ocular involvement in the acute phase of Stevens-Johnson syndrome/toxic epidermal necrolysis
| Category | Group I: | Group II: | P value |
|---|---|---|---|
| SJS/TEN onset before January 2008 (total n = 9 patients, 18 eyes) | SJS/TEN onset after January 2008 (total n = 39 patients, 78 eyes) | ||
| Number of eyes with BCVA at onset of SJS/TEN, n (%) | |||
| ≥ 20/40 | 14/18 (78) | 68/78 (87) | 0.84 |
| Could not be measured | 4/18 (22) | 10/78 (13) | 0.48 |
| Number of eyes in acute phase with, n (%) | |||
| Grade 1 involvement (mild) | 4/18 (22) | 17/78 (22) | 0.98 |
| Grade 2 involvement (severe) | 6/18 (33) | 42/78 (54) | 0.27 |
| Grade 3 involvement (very severe) | 8/18 (44) | 19/78 (24) | 0.23 |
| Number of eyes which received topical treatment alone in the acute phase*, n (%) | |||
| Total | 18/18 (100) | 25/78 (32) | 0.002 |
| With grade 1 involvement | 4/4 (100) | 17/17 (100) | 1.00 |
| With grade 2 or 3 involvement | 14/14 (100) | 8/61 (13) | <0.0001 |
| Number of eyes with grade 2 or 3 involvement which received AMT/ProKera*, n (%) | |||
| Total | 0/14 (0) | 53/61 (87) | <0.0001 |
| Within 7 days of onset | 0/14 (0) | 40/61 (66) | 0.02 |
| From 7–14 days of onset | 0/14 (0) | 10/61 (16) | 0.2 |
| From 14–28 days of onset | 0/14 (0) | 3/61 (5) | 1.00 |
SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis; BCVA = best-corrected visual acuity; AMT = amniotic membrane transplantation
Generalized estimated equations (GEE) were used to calculate p values where possible as explained in the text. When GEE could not be used, Fisher’s exact test was done using one eye of each patient for statistical purposes. For ease of the reader, each eye of every patient is included here.
Complications in the chronic phase of SJS/TEN
The percentage of eyes with vision-threatening complications in the chronic phase was significantly higher in Group I (67%, 12/18) versus Group II (17%, 13/78, p = 0.002), and most complications occurred within the first two years in both groups (Figure 2). Certain complications in the sub-acute and chronic phases which can cause acute loss of vision, for example, persistent epithelial defect, infectious keratitis, and sterile corneal perforation, were significantly more common in eyes in Group I. The complications in the chronic phase which cause a gradual, irreversible loss of vision, including moderate-severe eyelid margin keratinization, conjunctivalization of the entire corneal surface, central corneal neovascularization, and opacification of the central cornea were also significantly higher in eyes in Group I. We also performed a sub-group analysis on all eyes of patients with > 4 years of follow-up in both groups. We analyzed the visual outcomes of these eyes considering 4 years as an end-point (Table 3). The proportion of eyes with BCVA ≥ 20/40 at 4 years was significantly higher in Group II (p = 0.008) and those < 20/200 was significantly higher in Group I (p = 0.026). Additionally, none of the 22 eyes in Group II with > 4 years of follow-up required a corneal surgical intervention such as tectonic penetrating keratoplasty or keratoprosthesis implantation while 4 eyes in Group I required at least one of these interventions.
Figure 2.
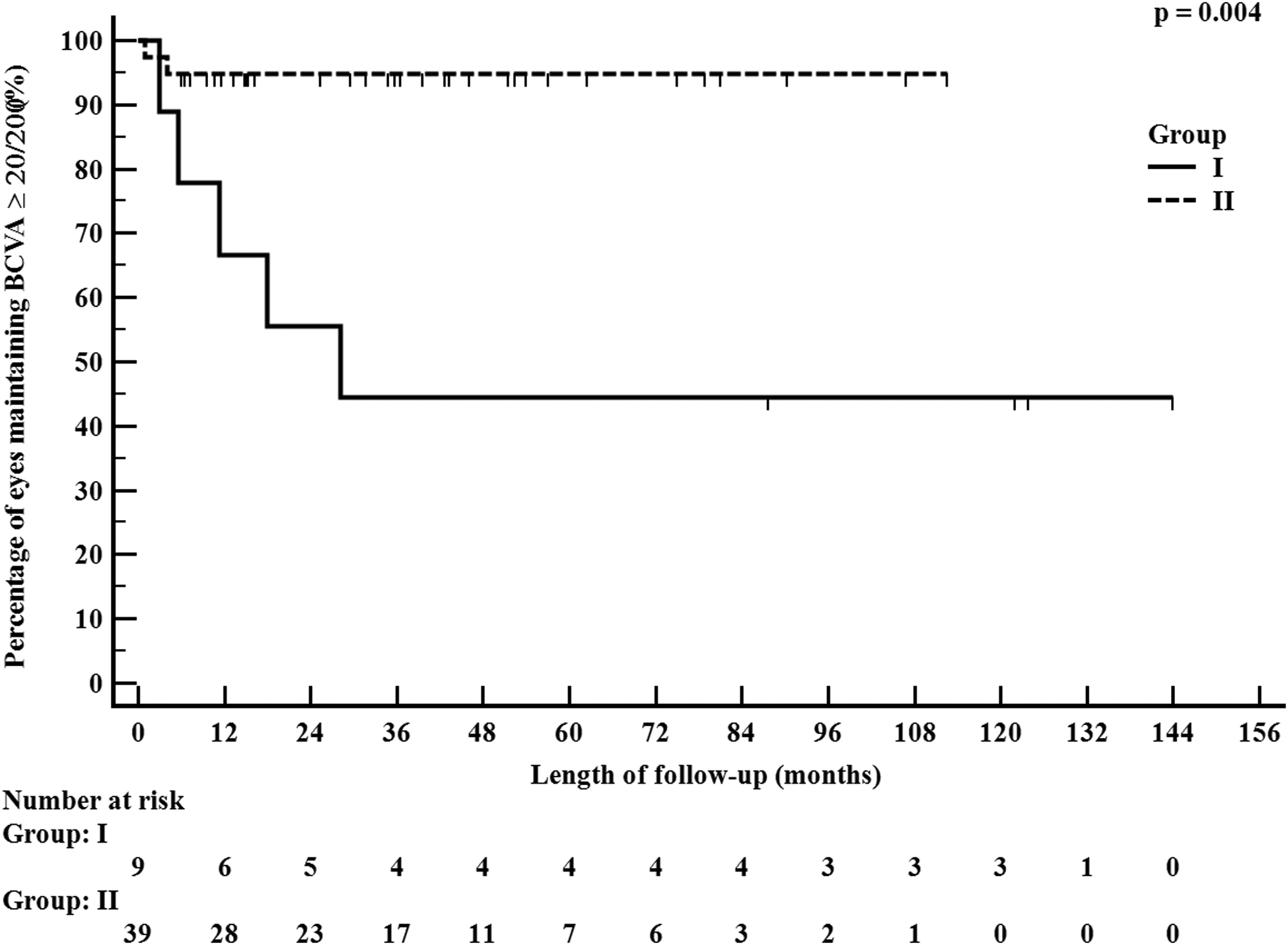
Kaplan-Meier analysis demonstrating time to blindness (≤20/200) in the chronic phase of Stevens-Johnson syndrome/toxic epidermal necrolysis in eyes treated before January 2008 (Group I – 9 eyes) versus eyes treated after January 2008 (Group II – 39 eyes), considering one eye per patient. The survival curves for the two groups are significantly different (p = 0.004).
Table 3.
Details of eyes with complications in the chronic phase of Stevens-Johnson syndrome/toxic epidermal necrolysis
| Group I: | Group II: | P value | |
|---|---|---|---|
| Complications secondary to ocular involvement during acute SJS/TEN | SJS/TEN onset before January 2008, n (%), (total n = 18 eyes of 9 patients) | SJS/TEN onset after January 2008, n (%), (total n = 78 eyes of 39 patients) | |
| Persistent epithelial defect | 11/18 (61) | 6/78 (8) | <0.001 |
| Sterile corneal perforation* | 6/18 (33) | 0/78 (0) | 0.01 |
| Infectious keratitis | 5/18 (28) | 2/78 (3) | 0.007 |
| Meibomian gland disease* | 18/18 (100) | 54/78 (69) | 0.06 |
| Punctal closure by scarring | 12/18 (67) | 16/78 (21) | 0.01 |
| Epiphora due to punctal closure by scarring* | 0/18 (0) | 4/78 (5) | 1.0 |
| Acquired NLDO* | 0/18 (0) | 2/78 (3) | 1.0 |
| Chronic dacryocystitis* | 2/18 (11) | 0/78 (0) | 0.19 |
| Lid margin keratinization | |||
| Mild* | 0/18 (0) | 20/78 (26) | 0.17 |
| Moderate - Severe | 12/18 (67) | 13/78 (17) | 0.002 |
| Trichiasis / Distichiasis | 16/18 (89) | 29/78 (37) | 0.02 |
| Entropion | 8/18 (44) | 9/78 (12) | 0.02 |
| Ectropion* | 0/18 (0) | 2/78 (3) | 1.0 |
| Tarsal conjunctival scarring | 12/18 (67) | 31/78 (40) | 0.11 |
| Dry eye | |||
| Mild | 4/18 (22) | 17/78 (22) | 0.98 |
| Moderate-severe | 14/18 (78) | 13/78 (17) | 0.002 |
| Symblepharon | 13/18 (72) | 18/78 (23) | 0.02 |
| Conjunctivalization of cornea | |||
| Peripheral corneal surface* | 0/18 (0) | 2/78 (3) | 1.0 |
| Involving central cornea | 12/18 (67) | 1/78 (1) | 0.001 |
| Corneal vascularization | |||
| Peripheral | 2/18 (11) | 9/78 (12) | 0.97 |
| Central | 12/18 (67) | 3/78 (4) | <0.001 |
| Corneal opacity | |||
| Peripheral | 1/18 (6) | 2/78 (3) | 0.51 |
| Central | 12/18 (67) | 4/78 (5) | <0.001 |
| Total LSCD | 12/18 (67) | 3/78 (4) | < 0.001 |
| BCVA at last follow-up | |||
| ≥ 20/40 | 6/18 (33) | 72/78 (92) | <0.001 |
| 20/50 to 20/200 | 3/18 (17) | 4/78 (5) | 0.08 |
| < 20/200 | 9/18 (50) | 2/78 (3) | < 0.001 |
SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis; NLDO = nasolacrimal duct obstruction; LSCD = limbal stem cell deficiency; BCVA = best-corrected visual acuity; CF = counting fingers; LP = light perception; NLP = no light perception
Generalized estimated equations (GEE) were used to calculate p values where possible as explained in the text. When GEE could not be used, Fisher’s exact test was done using one eye of each patient for statistical purposes. For ease of the reader, each eye of every patient is included here.
The details of eyes with complications in the chronic phase of SJS/TEN are shown in Table 4. A significantly higher proportion of eyes retained BCVA ≥ 20/40 in Group II compared to Group I at last follow-up (p < 0.001). Additionally, a significantly higher proportion of eyes in Group I had BCVA < 20/200 as compared to eyes in Group II at last follow-up (p < 0.001). Additionally, the Kaplan-Meier survival curves over the study period depicting time to BCVA < 20/200 were significantly different for the two groups (p = 0.004, Figure 2). The difference in outcomes between the two groups was fully evident before 36 months of follow-up, and therefore not a function of longer follow-up times for those in Group 1. Five study patients in Group I were bilaterally blind secondary to chronic complications of SJS/TEN. Out of these five, two retained BCVA ≥ 20/200 in one eye at the last follow-up visit secondary to a keratoprosthesis implantation. In Group II, there were no patients who were bilaterally blind and all maintained BCVA > 20/200 in at least one eye without any surgical intervention. Four representative cases of eyes in the chronic phase, two each from Groups I and II, are presented in Figure 3.
Table 4.
Sub-group analysis of all patients with follow-up > 4 years
| BCVA at 4 years | Eyes belonging to Group I with SJS/TEN onset before January 2008 with more than 4 years of follow-up, n (%), n = 18 eyes of 9 patients | Eyes belonging to Group II with SJS/TEN onset after January 2008 with more than 4 years of follow-up, n (%), n = 22 eyes of 11 patients | P value |
|---|---|---|---|
| ≥ 20/40 | 7/18 (39)* | 22/22 (100)† | 0.008 |
| 20/50 to 20/200 | 4/18 (22)‡ | 0/22 (0) | 0.2 |
| < 20/200 | 7/18 (39) | 0/22 (0) | 0.026 |
| < 20/200 to 20/400 | 2/18 (11)§ | 0/22 (0) | 0.48 |
| CF | 3/18 (17) | 0/22 (0) | 0.16 |
| LP | 2/18 (11) | 0/22 (0) | 0.48 |
| NLP | 0/18 (0) | 0/22 (0) | 1.00 |
SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis; BCVA = best-corrected visual acuity; CF = counting fingers; LP = light perception; NLP = no light perception
One eye with BCVA after undergoing Boston keratoprosthesis type 2
Six eyes with BCVA with PROSE scleral lenses
One eye with BCVA after undergoing Boston keratoprosthesis type 1
Both eyes with BCVA after undergoing tectonic penetrating keratoplasty
Figure 3.
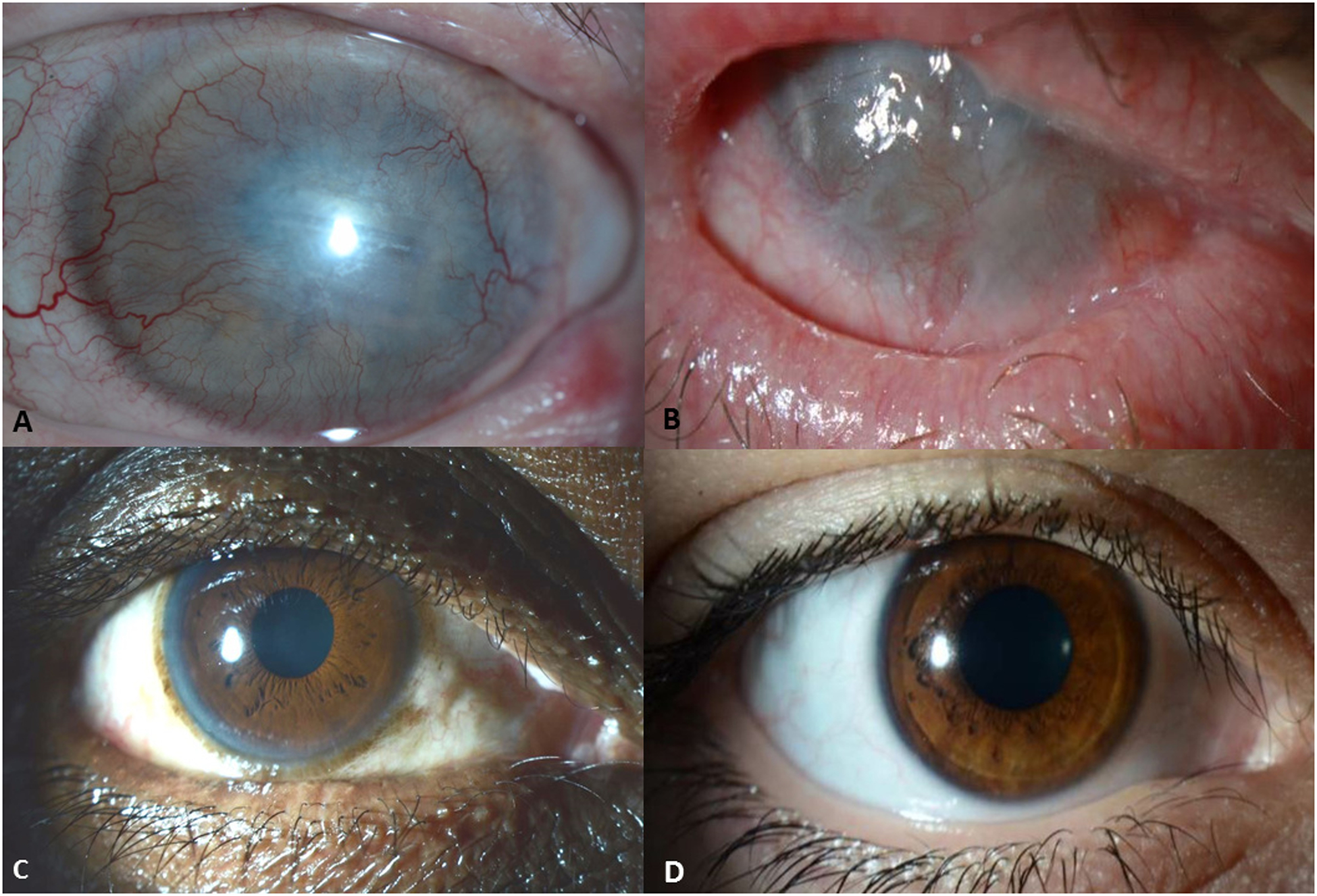
All of the eyes in the panel above had grade 3 ocular involvement in the acute phase of SJS/TEN. A) Right eye of a patient in Group I, 10 years after acute SJS/TEN showing corneal neovascularization with patient now using PROSE devices. B) Right eye of a patient in Group I, three years after acute SJS/TEN showing total limbal stem cell deficiency, conjunctivalization of the ocular surface, ankyloblepharon, and superior and inferior symblepharon causing loss of fornices. C) Right eye of a patient in Group II, three years after acute SJS/TEN. The patient received amniotic membrane transplantation in the acute phase and retained a clear cornea. D) Right eye of a patient in Group II, four years after acute SJS/TEN. The patient received amniotic membrane transplantation in the acute phase and retained a clear cornea.
Management of complications in the chronic phase of SJS/TEN
The total number of procedures needed in Group I in the chronic phase was greater than in Group II. Five patients in Group I underwent keratoprosthesis implantation while none in Group II required this intervention. Two eyes in Group I underwent enucleation secondary to a painful blind eye. The procedures required for managing the complications of chronic SJS/TEN are presented in Table 5.
Table 5.
Details of subsequent procedures in eyes with complications in the chronic phase of Stevens-Johnson syndrome/toxic epidermal necrolysis
| Group I: | Group II: | P value | |
|---|---|---|---|
| Procedures in the chronic phase of SJS/TEN | Eyes with acute SJS/TEN before January 2008, n (%), (total n = 18 eyes of 9 patients) | Eyes with acute SJS/TEN after January 2008, n (%), (total n = 78 eyes of 39 patients) |
|
| PROSE lenses | 7/18 (39) | 22/78 (28) | 0.51 |
| Punctal plugs | 2/18 (11) | 7/78 (9) | 0.84 |
| Surgical procedures | |||
| Punctal cautery | 2/18 (11) | 6/78 (8) | 0.74 |
| Cyanoacrylate glue* | 3/18 (17) | 0/78 (0) | 0.01 |
| AMT in chronic phase | |||
| Number of eyes | 8/18 (44) | 1/78 (1) | <0.001 |
| Number of procedures | 22 | 2 | |
| Fornix reconstruction with AM | |||
| Number of eyes* | 3/18 (17) | 0/78 (0) | 0.01 |
| Number of procedures | 5 | 0 | |
| Lid margin mucous membrane graft | 2/18 (11) | 8/78 (10) | 0.92 |
| Surgery for entropion | 8/18 (44) | 4/78 (5) | 0.006 |
| Tectonic PK | |||
| Number of eyes* | 5/18 (28) | 0/78 (0) | 0.003 |
| Number of procedures | 9 | 0 | |
| Patch graft* | 1/18 (6) | 0/78 (0) | 0.19 |
| KLAL* | 2/18 (11) | 0/78 (0) | 0.04 |
| Lr-CLAL* | 1/18 (6) | 0/78 (0) | 0.19 |
| Boston type 1 keratoprosthesis | |||
| Number of eyes* | 4/18 (22) | 0/78 (0) | 0.01 |
| Number of procedures | 9 | 0 | |
| Boston type 2 keratoprosthesis | |||
| Number of eyes* | 1/18 (6) | 0/78 (0) | 0.19 |
| Number of procedures | 3 | 0 | |
| Subsequent glaucoma procedures | |||
| Number of eyes* | 5/18 (28) | 0/78 (0) | 0.003 |
| Number of procedures | 8 | 0 | |
| Subsequent retinal procedures | |||
| Number of eyes* | 4/18 (22) | 0/78 (0) | 0.01 |
| Number of procedures | 5 | 0 | |
| Enucleation* | 2/18 (11) | 0/78 (0) | 0.04 |
SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis; PROSE = prosthetic replacement of the ocular surface ecosystem; AMT = amniotic membrane transplantation; PK = penetrating keratoplasty; KLAL = keratolimbal allograft; lr-CLAL = living-related conjunctival-limbal allograft
Generalized estimated equations (GEE) were used to calculate p values where possible as explained in the text. When GEE could not be used, Fisher’s exact test was done using one eye of each patient for statistical purposes. For ease of the reader, each eye of every patient is included here.
Cox proportional-hazards regression analysis was performed to analyze risk factors associated with BCVA < 20/200 in the chronic phase of SJS/TEN. The grade of ocular involvement in the acute phase was associated with a hazard ratio (HR) of 5.49 [95% confidence interval (CI) 2.33 – 12.94; p < 0.001]. The absence of AMT in the acute phase of SJS/TEN was associated with a HR of 16.14 (95% CI 3.52 – 73.91; p < 0.001).
Discussion
Although there have been several case-reports and case-series highlighting the importance of acute-stage ocular management in SJS/TEN, there have been no studies examining long-term outcomes in this cohort of patients. The findings of this study show that with timely ocular examination, and appropriate management based on grading of ocular involvement in the acute phase, the incidence of corneal blindness as a result of SJS/TEN may be substantially reduced.
Loss of visual acuity after SJS/TEN is thought to occur because of chronic or episodic ocular surface inflammation, predisposing the ocular surface to persistent epithelial defect and sterile keratolysis, and to progressive ocular surface disease secondary to moderate to severe eyelid margin and tarsal conjunctival keratinization.7, 20, 21 In a study which described the natural ocular history of SJS/TEN in children, the authors noted that 66% of patients, 99% of whom did not receive prior AMT, presented more than a year after acute SJS with low vision or blindness.22 In a randomized control trial by Sharma et al. the addition of AMT to medical treatment was superior to medical treatment alone in regards to ocular outcomes, though follow up was limited at 6 months. Authors in another study also concluded that eyes with moderate to severe ocular surface inflammation in the acute phase, who received medical management in the form of topical medications alone, fared worse in the chronic phase compared to patients who received AMT in conjunction with topical medication.6 However, the mean follow-up was 13.6 months in patients treated with AMT. Similarly, most other reports that describe acute ophthalmic care in SJS/TEN have limited follow-up, precluding accurate evaluation of long-term outcomes of acute treatment as complications can occur years after disease onset.3, 12–15, 18, 24–26 Our data show that a decrease in BCVA to < 20/200 primarily occurred up to three years after disease onset. Our study has the longest follow up of this cohort of patients in the published literature.
The results of our study demonstrate that a treatment protocol that includes the use of AMT appears to be highly successful in reducing ocular morbidity. Instituting such a protocol requires timely cooperation between the burn or dermatology service and ophthalmology. Ophthalmology should be consulted by the primary service upon admission. We believe an ocular examination should be performed on all SJS/TEN patients within 24 hours of admission given the short window of opportunity to treat acute disease. However, a previous study has shown that only 66% of burn intensive care units in the United States consult ophthalmology for every SJS/TEN patient.23 With our current data demonstrating that an acute treatment protocol can significantly affect outcomes, it is critical that ophthalmology be consulted immediately upon suspicion of SJS/TEN. Additionally, it is imperative for an ophthalmologist experienced in treating the ocular manifestations of SJS/TEN to examine the patient daily for at least the first week after onset as signs and symptoms can rapidly change, altering the severity grading and therefore the treatment.
Nursing and ancillary staff are critical to patient care. They must be aware of the necessity of ocular care and the management plan so that they can help plan for visits by the ophthalmology team as well as ensure timely administration of ocular medications. Nursing staff must also be taught how to place medication on top of the AMT so as not to manipulate the delicate membrane.
Current data suggest that AMT should be performed within the first week of SJS/TEN onset and that each missed treatment opportunity can lead to irreversible damage.10 To treat bilateral blindness in end-stage ocular disease in SJS/TEN, measures like keratoprosthesis implantation may be the only option. However, keratoprosthesis implantation in an eye with SJS/TEN is fraught with its own serious complications.27 Considering the number of surgical procedures that were required in Group I to treat complications in the chronic phase, coupled with significantly poorer visual outcomes, the best management strategy is one that prevents or reduces the need for later procedures. Furthermore, the grade of acute ocular involvement was found to be a significant risk factor for progressing to BCVA < 20/200 in the chronic phase, consistent with a previous report in which the prevalence of visual disturbance and ocular surface dryness was associated with the ocular severity grade in the acute phase.18 Our data suggest that a treatment protocol such as that we describe for the acute phase of SJS/TEN can reduce visual morbidity.
Our study has limitations. Due to the retrospective nature of the study, we had to rely on hospital records of ophthalmological examinations in the acute phase and it is possible that the records did not accurately reflect the extent of acute ocular involvement. Visual acuities at the time of consultation could not be reported in some patients given sedation status. Additionally, we had fewer patients in Group I compared to Group II. This was secondary to exclusion of some patients from Group I due to missing data and/or inadequate follow-up. Referral and consultation patterns may have also affected the differences between groups.
Although most eyes in Group II received AMT or ProKera within the first 7 days after SJS/TEN onset, in a small proportion of eyes, this treatment was delayed, either due to a delayed diagnosis of SJS/TEN or lack of continuity of care in the acute phase. Some patients were initially treated elsewhere and then transferred from surrounding hospitals to the burn service at MEE affiliated hospitals, thus delaying the initial ophthalmology consultation. Lastly, although the follow-up period in Group I was significantly longer compared to Group II, as demonstrated in the Kaplan-Meier curve in Figure 2, the vision threatening complications all occurred in the first three years after onset of disease. When we confined comparisons of outcomes to only patients with at least four years of follow-up in each group, patients in Group I still had significantly worse visual outcomes and also underwent more surgical interventions. In spite of the disparity in sample size between groups and the smaller numbers in Group I, the differences between the two groups were significant.
In conclusion, we believe that every patient in the acute phase of SJS/TEN should receive a consultation by an ophthalmologist experienced in the management of SJS/TEN within the first week of onset. Examination should be performed daily during the first week after admission and/or disease onset and then managed on a per case basis. Medical management in the form of topical lubricants, antibiotics, and corticosteroids medications should be started upon any sign of ocular inflammation and AMT performed in moderate to severe disease. The risk to benefit ratio for AMT is very low, and a low threshold for performing AMT is supported by the data. The treatment protocol described herein (Figure 1) resulted in a significant reduction in corneal blindness from SJS/TEN in our patients. Further, a rapid, sutureless AMT technique can reduce the time and resources necessary for this procedure and should increase implementation of AMT by ophthalmologists.19 AMT should be performed as soon as possible within the first week of SJS/TEN onset, when indicated (Darren Gregory, MD, personal communication). After the patient is discharged from the hospital, regular follow up with an ophthalmologist is critical to detect and treat any complications of the disorder. We believe that implementation of this protocol by those that treat acute SJS/TEN patients, can result in a significant reduction of visual loss in those affected by this severe and debilitating disorder.
Table 6.
Cox proportional-hazards regression analysis of factors associated with decrease of best-corrected visual acuity to < 20/200 in the chronic phase of acute Stevens-Johnson syndrome/toxic epidermal necrolysis
| Factors | Hazard ratio | P value | 95% Confidence Interval |
|---|---|---|---|
| Increased ocular severity grade in the acute phase | 5.49 | < 0.001 | 2.33 – 12.94 |
| Absence of amniotic membrane transplantation in in the acute phase | 16.14 | < 0.001 | 3.52 – 73.91 |
Acknowledgements/Disclosure:
Funding/support: Research reported in this publication was supported by the National Eye Institute of the National Institutes of Health under Award Number K23EY028230. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsor or funding organization had no role in the design or conduct of this research.
Financial disclosures: Authors SSS, RR, and JC have no financial disclosures. Author HNS has received monetary compensation for expert testimony in a case of SJS/TEN.
Other acknowledgements: The authors thank Hui Zheng, PhD, MGH Biostatistics Center for advising on statistical methods. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Each of the coauthors has seen and agrees with the changes made to this revised manuscript and to the way his or her name is listed.
References
- 1.Kohanim S, Palioura S, Saeed HN, et al. Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis--A Comprehensive Review and Guide to Therapy. I. Systemic Disease. Ocul Surf 2016;14:2–19. [DOI] [PubMed] [Google Scholar]
- 2.Power WJ, Ghoraishi M, Merayo-Lloves J,J, Neves RA, Foster CS. Analysis of the acute ophthalmic manifestations of the erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis disease spectrum. Ophthalmology 1995;102:1669–76. [DOI] [PubMed] [Google Scholar]
- 3.Chang YS, Huang FC, Tseng SH, Hsu CK, Ho CL, Sheu HM. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: acute ocular manifestations, causes, and management. Cornea 2007;26:123–9. [DOI] [PubMed] [Google Scholar]
- 4.Revuz J, Penso D, Roujeau JC, et al. Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch Dermatol 1987;123:1160–5. [DOI] [PubMed] [Google Scholar]
- 5.Oplatek A, Brown K, Sen S, Halerz M, Supple K, Gamelli RL. Long-term follow-up of patients treated for toxic epidermal necrolysis. J Burn Care Res 2006;27:26–33. [DOI] [PubMed] [Google Scholar]
- 6.Hsu M, Jayaram A, Verner R, Lin A, Bouchard C. Indications and outcomes of amniotic membrane transplantation in the management of acute stevens-johnson syndrome and toxic epidermal necrolysis: a case-control study. Cornea 2012;31:1394–402. [DOI] [PubMed] [Google Scholar]
- 7.Iyer G, Srinivasan B, Agarwal S, Kamala Muralidharan S, Arumugam S. Comprehensive approach to ocular consequences of Stevens Johnson Syndrome - the aftermath of a systemic condition. Graefes Arch Clin Exp Ophthalmol 2014;252:457–67. [DOI] [PubMed] [Google Scholar]
- 8.Lee HY, Walsh SA, Creamer D. Long-term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow-up. Br J Dermatol 2017;177:924–35. [DOI] [PubMed] [Google Scholar]
- 9.Vazirani J, Nair D, Shanbhag S, Wurity S, Ranjan A, Sangwan V. Limbal stem cell deficiency-demography and underlying causes. Am J Ophthalmol 2018;188:99–103. [DOI] [PubMed] [Google Scholar]
- 10.Kohanim S, Palioura S, Saeed HN, et al. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis - A comprehensive review and guide to therapy. II. Ophthalmic Disease. Ocul Surf 2016;14:168–88. [DOI] [PubMed] [Google Scholar]
- 11.Ciralsky JB, Sippel KC, Gregory DG. Current ophthalmologic treatment strategies for acute and chronic Stevens-Johnson syndrome and toxic epidermal necrolysis. Curr Opin Ophthalmol 2013;24:321–8. [DOI] [PubMed] [Google Scholar]
- 12.Gregory DG. Treatment of acute Stevens-Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases. Ophthalmology 2011;118:908–14. [DOI] [PubMed] [Google Scholar]
- 13.Ma KN, Thanos A, Chodosh J, Shah AS, Mantagos IS. A novel technique for amniotic membrane transplantation in patients with acute Stevens-Johnson syndrome. Ocul Surf 2016;14:31–6. [DOI] [PubMed] [Google Scholar]
- 14.Shammas MC, Lai EC, Sarkar JS, Yang J, Starr CE, Sippel KC. Management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis utilizing amniotic membrane and topical corticosteroids. Am J Ophthalmol 2010;149:203–13.e2. [DOI] [PubMed] [Google Scholar]
- 15.Sharma N, Thenarasun SA, Kaur M, et al. Adjuvant role of amniotic membrane transplantation in acute ocular Stevens-Johnson syndrome: a randomized control trial. Ophthalmology 2016;123:484–91. [DOI] [PubMed] [Google Scholar]
- 16.Saeed HN, Chodosh J. Ocular manifestations of Stevens-Johnson syndrome and their management. Curr Opin Ophthalmol 2016;27:522–9. [DOI] [PubMed] [Google Scholar]
- 17.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993;129:92–6. [PubMed] [Google Scholar]
- 18.Sotozono C, Ueta M, Nakatani E, et al. Predictive factors associated with acute ocular involvement in Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol 2015;160:228–37.e2. [DOI] [PubMed] [Google Scholar]
- 19.Shanbhag SS, Chodosh J, Saeed HN. Sutureless amniotic membrane transplantation with cyanoacrylate glue for acute Stevens-Johnson syndrome/toxic epidermal necrolysis. Ocul Surf 2019. March 11. pii: S1542–0124(19)30010–2. doi: 10.1016/j.jtos.2019.03.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rojas MV, Dart JK, Saw VP. The natural history of Stevens Johnson syndrome: patterns of chronic ocular disease and the role of systemic immunosuppressive therapy. Br J Ophthalmol 2007;91:1048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Pascuale MA, Espana EM, Liu DT, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology 2005;112:904–12. [DOI] [PubMed] [Google Scholar]
- 22.Basu S, Shanbhag SS, Gokani A, Kedar R, Bahuguna C, Sangwan VS. Chronic ocular sequelae of Stevens-Johnson syndrome in children: Long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol 2018;189:17–28. [DOI] [PubMed] [Google Scholar]
- 23.Le HG, Saeed H, Mantagos IS, Mitchell CM, Goverman J, Chodosh J. Burn unit care of Stevens Johnson syndrome/toxic epidermal necrolysis: A survey. Burns 2016;42:830–5. [DOI] [PubMed] [Google Scholar]
- 24.Shay E, Kheirkhah A, Liang L, Sheha H, Gregory DG, Tseng SC. Amniotic membrane transplantation as a new therapy for the acute ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis. Surv Ophthalmol 2009;54:686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yip LW, Thong BY, Lim J, et al. Ocular manifestations and complications of Stevens-Johnson syndrome and toxic epidermal necrolysis: an Asian series. Allergy 2007;62:527–31. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad MS, Frank GS, Hink EM, Palestine AG, Gregory DG, McCourt EA. Amniotic membrane transplants in the pediatric population. J AAPOS 2017;21:215–8. [DOI] [PubMed] [Google Scholar]
- 27.Sayegh RR, Ang LP, Foster CS, Dohlman CH. The Boston keratoprosthesis in Stevens-Johnson syndrome. Am J Ophthalmol 2008;145:438–44. [DOI] [PubMed] [Google Scholar]


