Abstract
Background
Diabetes mellitus (DM) aggravates symptoms and prognosis of acute ischemic stroke (AIS), and inflammation plays an important role therein. Resolvin D2 (RvD2) is one of the specialized pro-resolving mediators (SPMs), while leukotriene B4 (LTB4) is a classic proinflammatory mediator. The ratio of RvD2 to LTB4 is an index of pro-resolving/proinflammatory balance. We aim to explore the role of RvD2/LTB4 ratio in ischemic stroke complicated with DM.
Methods
The plasma levels of RvD2 and LTB4 were analyzed by enzyme immunoassay in stroke patients with DM (DM + AIS group) or without DM (nonDM+AIS group). Patients were followed up at 90 days after stroke onset, and modified Rankin Score (mRS) was assessed. The association of RvD2/LTB4 ratio with stroke severity and prognosis was also analyzed.
Results
The plasma levels of RvD2 were positively correlated to LTB4. The RvD2/LTB4 ratio in DM + AIS group was lower than that in the nonDM+AIS group. No correlation was found between the RvD2/LTB4 ratio and infarct size or NIHSS score. The RvD2/LTB4 ratio at baseline was significantly lower in the poor prognosis group (mRS ≥ 3) than that in the good prognosis group (mRS ≤ 2).
Conclusions
Our study indicated that the balance between pro-resolving and proinflammatory mediators was impaired by diabetes in ischemic stroke. The RvD2/LTB4 ratio may serve as a biomarker of prognosis for ischemic stroke.
1. Introduction
Diabetes mellitus (DM) is an independent risk factor for ischemic stroke and can treble the risk of cardiovascular and cerebrovascular diseases [1]. Inflammation plays an important role in the pathogenesis of diabetes. Pickup showed that cytokines mediate inflammatory response in type 2 diabetes and are directly involved in the development of DM and its vascular complications [2]. The use of salicylic acid or interleukin (IL-) 1 receptor antagonists in DM patients can lower blood glucose levels, further supporting the hypothesis that DM is an inflammatory disease [3]. Inflammation also plays a key role in the pathophysiology of ischemic stroke complicated with DM [4]. For example, matrix metalloproteinase-2 (MMP-2) activity, cyclooxygenase-2 (COX-2), IL-1β, and IL-6 were increased in the diabetic mouse brain after stroke [5–7].
Resolution of inflammation is the key program that regulates inflammation and ensures proinflammatory responses are not overactive. Uncontrolled inflammatory response and impaired resolution contribute to the pathological process of many diseases. In the past decade, specialized pro-resolving mediators (SPMs) have been identified as the central controllers of inflammation resolution. These small lipid molecules, including, e.g., lipoxins (LXs), resolvins (Rvs), protectins (PDs), and maresins (MaRs), monitor inflammation of resolution in a receptor-dependent manner [8]. The balance between proinflammatory mediators and SPMs regulates the duration of inflammatory response and the time course of homeostasis recovery. Imbalance between these two forces leads to the occurrence of inflammatory diseases. Leukotriene B4 (LTB4) is a classical proinflammatory lipid mediator derived from arachidonic acid (AA). The ratio of SPMs to LTB4 reflects the degree of the resolving-proinflammatory balance and has been studied in a variety of disease models. For example, it has been reported that the LXA4/LTB4 ratio decreased in alveolar lavage fluid of patients with cystic fibrosis, suggesting that disturbed resolution of inflammation contributes to the pathological changes of cystic fibrosis in the airway [9]. The ratio of RvD1/LTB4 and MaR1/LTB4 was reduced in preeclampsia compared to normal pregnancy, indicating that insufficient production of SPMs may be involved in the occurrence of preeclampsia [10]. Resolvin D2 (RvD2) is derived from docosahexaenoic acid (DHA). It has been shown that RvD2 can reduce infarct volume and dampen inflammation in experimental models of stroke [11, 12].
The RvD2/LTB4 ratio has been used as a biomarker for carotid intima thickness and plaque stability in cardiovascular disease [13, 14].
The aim of this study is to characterize the changes of RvD2/LTB4 ratio in DM patients with acute ischemic stroke. We also explored possible associations between the RvD2/LTB4 ratio and clinical characteristics of the patients, as well as the relationship of RvD2/LTB4 ratio and stroke prognosis.
2. Methods
2.1. Study Population and Clinical Data Collection
We enrolled 57 patients with acute ischemic stroke from the Department of Neurology, Shanghai Jiao Tong University Affiliated Sixth People's Hospital. According to the combination of DM, the patients were divided into two groups: non-DM patients with acute ischemic stroke (nonDM+AIS group) and DM patients with acute ischemic stroke (DM + AIS group). Patients with coma, severe cardiac/hepatic/renal dysfunction, mental illness, history of other endocrine diseases, tumor, or autoimmune disease were excluded from the study. Patients who had acute systematic inflammatory disease within 1 month or received fibrinolytic thrombolysis were also excluded. All stroke patients met the diagnostic criteria of Chinese guidelines for the diagnosis and treatment of acute ischemic stroke 2014 [15] and were confirmed by computed tomography (CT) or magnetic resonance imaging (MRI). All DM patients met the diagnostic criteria made by the European Diabetes Policy Group for type 2 diabetes [16]. The patients were followed up at 90 days after stroke onset, and the modified Rankin Score (mRS) was assessed.
General information was collected covering age, gender, smoking/drinking history, and medical history. Routine laboratory analyses including glycated hemoglobin (HbA1c), fasting blood glucose, triglyceride, cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), serum uric acid, white blood cell count, and neutrophil count were performed following routine procedures in the Department of Laboratory Medicine of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. All participants or their authorized caregivers signed an informed consent. The study was approved by the ethical committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital.
2.2. Blood Sampling and Enzyme Immunoassay Analysis
Fasting blood samples were collected in EDTA anticoagulant tubes by venipuncture from all participants. All samples were taken within 72 h after disease onset. The samples were then centrifuged immediately, and the resulting plasma was stored at -80°C until further processing. Endogenous concentrations of RvD2 and LTB4 in plasma were determined by enzyme immunoassay (EIA) kits (Cayman Chemical, MI, USA). The collected plasma samples underwent a purification procedure according to the instructions of the EIA kits. Briefly, C18 Sep-Pak® light columns (Waters Corporation, MA, USA) were conditioned by methanol and water. The plasma samples were acidified to pH 4.0 (for LTB4) or pH 5.0 (for RvD2) and injected into the preconditioned C18 column. Subsequently, the columns were washed with distilled water, and the purified samples were eluted with 1% methanol in ethyl acetate (for LTB4) or methanol (for RvD2). The samples were then dried by N2 gas and resuspended in EIA buffer for assessment of RvD2 and LTB4 according to the specific instructions for the kits.
2.3. Statistical Analysis
All statistical analyses were performed using the IBM SPSS software (version 22, SPSS Inc., USA). Continuous and parametric variables were expressed as mean ± standard deviation (SD), and nonparametric distributed variables were expressed as median (IQR) and 25th-75th percentiles. Categorical variables were expressed as percentages. Differences between the two groups were compared by Mann–Whitney U test for nonparametric variables, and χ2 or Fisher's exact test was used for categorical variables. Spearman correlation analysis was performed to evaluate the possible associations of lipid mediator with clinical or laboratory parameters. Receiver operating characteristic (ROC) curves and area under the curves (AUC) were calculated to evaluate the predictive power of the RvD2/LTB4 ratio for stroke outcome. p < 0.05 was considered as significant in all statistical analyses.
3. Results
3.1. Clinical Characteristics
A total of 57 cases were enrolled, including 25 nondiabetic patients with acute ischemic stroke (nonDM+AIS group) and 32 diabetic patients with acute ischemic stroke (DM + AIS group). There was no significant difference in age, history of smoking, drinking, hypertension, and atrial fibrillation between the two groups. The levels of HbA1c and fasting blood glucose were higher in the diabetic group than in the nondiabetic group (Table 1). There was no significant difference with regard to other laboratory tests, including triglycerides, cholesterol, HDL, LDL, serum uric acid, white blood cell count, and neutrophil count.
Table 1.
Baseline characteristics of the patients.
| nonDM+AIS (n = 25) | DM + AIS (n = 32) | p | |
|---|---|---|---|
| Age | 66.36 ± 9.50 | 69.31 ± 9.89 | 0.260 |
| Gender (male) | 19 (76) | 24 (75) | 0.931 |
| Smoking | 8 (32) | 6 (18.8) | 0.249 |
| Alcohol use | 4 (16) | 4 (12.5) | 0.706 |
| Hypertension | 17 (68) | 26 (81.3) | 0.249 |
| Atrial fibrillation | 3 (12) | 5 (15.6) | 0.995 |
| HbA1c | 5.80 (5.50-6.05) | 7.55 (6.90-9.23) | <0.01 |
| Fasting blood glucose/mmol/L | 5.31 (4.88-6.31) | 7.82 (6.67-9.58) | <0.01 |
| Triglyceride/mmol/L | 4.63 (3.48-5.58) | 4.50 (3.44-5.75) | 0.803 |
| Cholesterol/mmol/L | 1.63 (1.21-2.17) | 1.59 (1.02-3.08) | 0.923 |
| HDL/mmol/L | 1.13 (0.81-1.31) | 0.98 (0.87-1.20) | 0.705 |
| LDL/mmol/L | 3.08 ± 1.01 | 3.09 ± 0.86 | 0.975 |
| Uric acid/umol/L | 346 (326-361) | 321 (259-354) | 0.061 |
| White blood cell count × 109/L | 7.20 (5.75-9.05) | 6.95 (5.95-8.68) | 0.994 |
| Neutrophils cell count × 109/L | 4.20 (3.10-5.90) | 4.90 (3.83-6.88) | 0.228 |
Data are presented as mean ± SD or median ± IQR or n (%). DM: diabetes mellitus group; HDL: high-density lipoprotein; AIS: acute ischemic stroke; LDL: low-density lipoprotein.
3.2. Plasma Levels of RvD2, LTB4, and Their Ratio in DM + AIS and nonDM+AIS Groups
There was no significant difference between the two groups with regard to plasma RvD2 concentration (Figure 1(a)). LTB4 levels in the DM + AIS group were higher than that in the nonDM+AIS group (Figure 1(b)). To evaluate the balance of pro-resolving lipid mediators and pro-inflammation lipid mediators, we calculated the RvD2/LTB4 ratio. The DM + AIS group had a lower RvD2/LTB4 ratio than the nonDM+AIS group (Figure 1(c)). A positive correlation was found between RvD2 and LTB4 levels (r = 0.262, p = 0.049) (Figure 1(d)).
Figure 1.
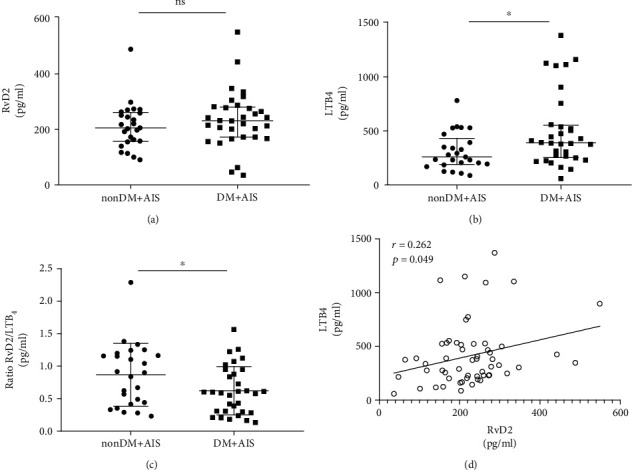
Plasma levels of RvD2, LTB4, and RvD2/LTB4 ratio in acute ischemic stroke. (a) Comparison of the plasma concentrations of RvD2 shows no difference in the nonDM+AIS group and DM + AIS group. (b) Plasma concentrations of LTB4 were increased in the nonDM+AIS group and nonDM+AIS group. (c) The RvD2/LTB4 ratio of the DM + AIS group was lower compared to that in the nonDM+AIS group. (d) Correlation analysis shows a positive association between RvD2 and LTB4 (Spearman rho test, r = 0.262, p = 0.049). Error bars represent IQR. ns: none significant. ∗p < 0.05. DM: diabetes mellitus; AIS: acute ischemic stroke; LTB4: leukotriene B4; RvD2: resolvin D2.
3.3. Association of Infarct Volume/NIHSS Score with RvD2/LTB4 Ratio
Diffusion-weighted MRI was used to assess infarct volume. The volume of cerebral infarction was calculated according to the Pullicino formula (length × width × layer/2, cm3) [17]. The stroke patients were divided into small infarct (<5 cm3, n = 29), medium infarct (5-10 cm3, n = 7), and large infarct (>10 cm3, n = 19). There was no significant difference in RvD2/LTB4 ratio between the three groups (Figure 2(a)). The NIHSS score was used to evaluate the degree of neurological impairment on the first day of admission. The patients were divided into three groups, including mild neurological impairment (NIHSS score < 4, n = 28), moderate neurological impairment (NIHSS score 4-15, n = 25), and severe neurological impairment (NIHSS score > 15, n = 4). We found that there was a gradient trend of decrease of RVD2/LTB4 ratio from mild to severe neurological impairment, though not statistically significant (Figure 2(b)).
Figure 2.
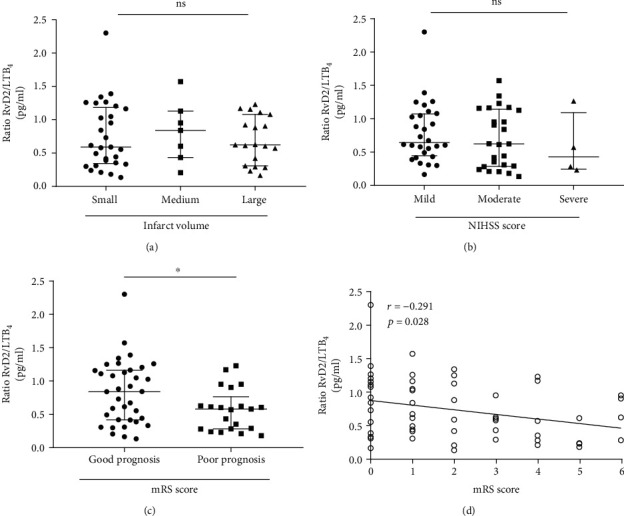
The association of RvD2/LTB4 ratio with infarct volume, NIHSS score, and 90-day prognosis. (a) Ratios of plasma RvD2/LTB4 were not different in the small, medium, and large infarct volume group. (b) Ratios of plasma RvD2/LTB4 were not different in mild, moderate, and severe neurological deficit groups. (c) Ratios of plasma RvD2/LTB4 in good prognosis were higher compared with that in the poor prognosis group. (d) There was a negative association of RvD2/LTB4 ratio with 90-day mRS (Spearman rho test, r = −0.291, p = 0.028). Error bars represent IQR. ns: none significant. ∗p < 0.05. LTB4: leukotriene B4; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; RvD2: resolvin D2.
3.4. Relationship between the Ratio of RvD2/LTB4 and Stroke Prognosis
According to the 90-day mRS score, the patients were divided into the good prognosis group (mRS ≤ 2, n = 36) and poor prognosis group (mRS ≥ 3, n = 21). We found that baseline RvD2/LTB4 ratio in the good prognosis group was higher than that in the poor prognosis (0.85[0.42–1.17] vs. 0.58 [0.28–0.77], p = 0.040) (Figure 2(c)). Correlation analysis showed that the RvD2/LTB4 ratio was negatively correlated with the mRS score (Spearman's rho test, r = −0.291, p = 0.028) (Figure 2(d)). ROC curve analysis revealed that RvD2/LTB4 produced an unsatisfactory area under the curve (AUC = 0.664) for predicting stroke prognosis with a 95% confidence interval of 0.527–0.784, p = 0.025. The associated criterion was 0.63 with 76.2% specificity and 58.3% sensitivity (Figure 3).
Figure 3.
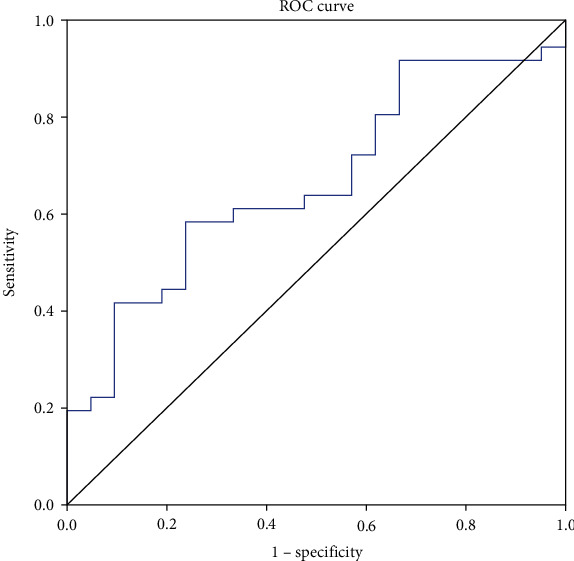
Possible predictive role of RvD2/LTB4 in stroke prognosis. ROC curve analysis showed AUC = 0.664 with a 95% confidence interval of 0.527–0.784, p = 0.025. The associated criterion was 0.63 with 76.2% specificity and 58.3% sensitivity. AUC: area under the curve; LTB4: leukotriene B4; ROC: receiver operating characteristic curves; RvD2: resolvin D2.
4. Discussion
In the present study, the RvD2/LTB4 ratio was significantly reduced in DM stroke patients compared to non-DM stroke diabetic patients, indicating that DM impairs the balance between pro-resolving and proinflammatory signals in ischemic stroke. Furthermore, the RvD2/LTB4 ratio in the acute stage of ischemic stroke was correlated with patient prognosis. These results provided novel potential mechanisms in excessive inflammation in DM patients with ischemic stroke.
Inflammation is a key program that mediates pathogenic and pathological effects of DM on ischemic stroke [4, 18]. The levels of circulating proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and IL-1, are increased in DM patients [19]. Sitagliptin was applied in the treatment of diabetes mellitus type 2. Studies have shown that sitagliptin has a neuroprotective effect by reducing neuroinflammation [20]. Atorvastatin could decrease the concentration of IL-6 and TNF-α and improved the outcome of ventilator-associated pneumonia in patients with ischemic stroke [21]. Herrera et al. demonstrated that RvE1 increased the neutrophil phagocytosis of P. gingivalis in WT animals but had no impact in diabetes animals. In RvE1 receptor-transgenic diabetic mice, the impaired neutrophil phagocytosis ability was rescued by RvE1 [22]. Above all, the proresolution system appears to be compromised in DM. This may explain why diabetic patients with ischemic stroke have more severe symptoms and prognosis. Parlapiano et al. demonstrated that diabetic patients have increased levels of LTB4 and activity of polymorphonuclear leukocyte, and this activation is correlated to glycated hemoglobin level [23].
Overactivated inflammation has been reported in the etiology of exaggerated brain damage in diabetic stroke models [24, 25]. Effective resolution of inflammation is essential for balancing poststroke inflammation to restore homeostasis of the brain. SPMs promote the resolution of inflammation and the recovery of tissue homeostasis [8]. RvD2 has been shown to exert anti-inflammatory and pro-resolving properties in various disease models [12, 26, 27]. The levels of endogenous RvD2 were inconsistent in different disease models and at different stages of the disease. Zuo et al. reported that the experimental stroke led to a decrease of RvD2 in the brain [12]. In epilepsy, the levels of brain RvD2 were reduced after seizure [28]. Conversely, in a mouse model of acute lung injury, the levels of RvD2 were increased within 24 h of LPS-induced lung inflammation [29]. In a murine model of hind limb ischemia, RvD2 and 17-HDHA were generated robustly in the bone marrow as early as 24 h postsurgery [27]. Thus, it is valuable to evaluate the balance of pro-resolving and proinflammatory signals in a comprehensive way. LTB4 is a classic inflammatory lipid mediator and has been implicated in ischemic stroke pathology [30, 31]. The levels of LTB4 in plasma increased rapidly after stroke in humans [30]. Early and sustained increase of LTB4 is associated with poor functional recovery of stroke [30]. High LTB4 levels were also associated with larger nonhealing lesion areas and increased bacterial load in skin wound of diabetic mice [32]. The ratio of SPMs/LTB4 has been considered as an index of the balance between pro-resolving and proinflammatory signals. For example, the ratios of plasma RvD1/LTB4 and MaR1/LTB4 were associated with inflammation status in preeclampsia [10]. In atherosclerosis, reduced salivary RvD1/LTB4 ratio was found to reflect an imbalance of pro-resolving and proinflammatory forces and could predict thicker carotid intima [13]. Moreover, an abnormal ratio of SPM/LTB4 was observed in the instable plaques derived from atherosclerosis patients [14].
In the present study, we utilized the plasma RvD2/LTB4 ratio as a possible indicator for poststroke inflammation. We found lower RvD2/LTB4 ratio in DM patients compared to non-DM patients. With ischemic stroke compared to nondiabetic patients. This may indicate that DM impairs the resolution of inflammation in acute ischemic stroke. Such an effect of DM in the resolution of inflammation has been shown in other diseases. For example, an imbalance of proinflammatory and pro-resolving mediators was observed in DM patients with tuberculosis [33]. A study on DM-associated retina damage showed that hyperglycemia may decrease RvD1 in the retina [34]. Furthermore, DM has been shown to impair the biosynthesis of PD1 and thus result in the overactivation of macrophages in wound healing [35]. These studies supported our finding that DM may disturb the resolution of inflammation in ischemic stroke.
We also explored the association of RvD2/LTB4 ratio with stroke severity and prognosis. We found no correlation between the RvD2/LTB4 ratio and infarct volume nor NIHSS score. This finding is consistent with previous publications showing that inflammation is not linearly associated with the degree of brain damage in stroke [31, 36, 37].
On the other hand, it has been shown that neuroendocrine biomarkers, such as copeptin and cortisol, are correlated with short-term outcome and mortality of ischemic stroke [38]. We found an association between plasma RvD2/LTB4 ratio and 90-day prognosis. Patients with poor prognosis had lower levels of RvD2/LTB4 ratio at baseline. The levels of pro-resolving activities at the acute phase may represent an overall capacity of the body to restore homeostasis after stroke. This is consistent with the previous report that high levels of circulating TNF-α, IL-6, and MMP-9 were associated with a poor prognosis in acute ischemic stroke [39–41]. The ROC curve analysis of the RvD2/LTB4 ratio for prognosis revealed an AUC of 0.664. Although the RvD2/LTB4 ratio may serve as a biomarker of stroke prognosis, the predictive power, specificity, and sensitivity are relatively low.
In summary, our data indicate that DM may disturb the balance between pro-resolving and proinflammatory mediators in ischemic stroke. Moreover, the reduced RvD2/LTB4 ratio in the acute phase of stroke may predict a poor prognosis. These findings provide new insights into the failure of resolution of inflammation in DM patients with ischemic stroke. Limitation of this study includes the small sample size, single center design, and no validation cohort. Larger sample sizes of patients from multiple centers are needed to validate our findings in future studies. In addition, studies involving animal and cellular models are essential to further elucidate the intrinsic mechanisms of resolution failure in DM patients with acute ischemic stroke.
Acknowledgments
We thank all the patients who participated in this study and the staff of our stroke unit. The authors received the following financial support for the research, authorship, and/or publication of this article: the National Nature Science Foundation of China (grant numbers 31771185, 81974158, and 31811530007) and the Swedish Foundation for International Cooperation in Research and Higher Education (Grant No. CH2017-7308).
Contributor Information
Yuwu Zhao, Email: zhaoyuwu2005@126.com.
Xiuzhe Wang, Email: xiuzhewang@hotmail.com.
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Ethical Approval
The study protocols were approved by local ethics committee boards.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
ZM and XT performed the experiment and analyzed the data. MS gave important suggestion in methodology and was involved in manuscript preparation. YZ and XW designed and monitored the study. All the authors read through the manuscript and approved the final version.
References
- 1.Emerging Risk Factors Collaboration, Sarwar N., Gao P., et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. The Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickup J. C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 3.Donath M. Y., Shoelson S. E. Type 2 diabetes as an inflammatory disease. Nature Reviews Immunology. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 4.Fann D. Y., Lee S. Y., Manzanero S., Chunduri P., Sobey C. G., Arumugam T. V. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Research Reviews. 2013;12(4):941–966. doi: 10.1016/j.arr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Ergul A., Elgebaly M. M., Middlemore M. L., et al. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurology. 2007;7(1):p. 33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tureyen K., Bowen K., Liang J., Dempsey R. J., Vemuganti R. Exacerbated brain damage, edema and inflammation in type-2 diabetic mice subjected to focal ischemia. Journal of Neurochemistry. 2011;116(4):499–507. doi: 10.1111/j.1471-4159.2010.07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bémeur C., Ste-Marie L., Desjardins P., et al. Dehydroascorbic acid normalizes several markers of oxidative stress and inflammation in acute hyperglycemic focal cerebral ischemia in the rat. Neurochemistry International. 2005;46(5):399–407. doi: 10.1016/j.neuint.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Serhan C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringholz F. C., Buchanan P. J., Clarke D. T., et al. Reduced 15-lipoxygenase 2 and lipoxin A4/leukotriene B4 ratio in children with cystic fibrosis. The European Respiratory Journal. 2014;44(2):394–404. doi: 10.1183/09031936.00106013. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira Perucci L., Santos T. A. P., Santos P. C. Pre-eclampsia is associated with reduced resolvin D1 and maresin 1 to leukotriene B4 ratios in the plasma. American Journal of Reproductive Immunology. 2020;83(2, article e13206) doi: 10.1111/aji.13206. [DOI] [PubMed] [Google Scholar]
- 11.Dong X., Gao J., Zhang C. Y., Hayworth C., Frank M., Wang Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano. 2019;13(2):1272–1283. doi: 10.1021/acsnano.8b06572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo G., Zhang D., Mu R., et al. Resolvin D2 protects against cerebral ischemia/reperfusion injury in rats. Molecular Brain. 2018;11(1):p. 9. doi: 10.1186/s13041-018-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thul S., Labat C., Temmar M., Benetos A., Bäck M. Low salivary resolvin D1 to leukotriene B4 ratio predicts carotid intima media thickness: a novel biomarker of non-resolving vascular inflammation. European Journal of Preventive Cardiology. 2017;24(9):903–906. doi: 10.1177/2047487317694464. [DOI] [PubMed] [Google Scholar]
- 14.Fredman G., Hellmann J., Proto J. D., et al. An imbalance between specialized pro-resolving lipid mediators and pro- inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nature Communications. 2016;7(1, article 12859) doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M., He M. L. Chinese guidelines for the diagnosis and treatment of acute ischemic stroke 2014. Zhong Hua Shen Jing Ke Za Zhi. 2015;48(4):246–257. [Google Scholar]
- 16.1999 European Diabetes Policy Group. A desktop guide to Type 2 diabetes mellitus. Diabetic Medicine. 1999;16(9):716–730. doi: 10.1046/j.1464-5491.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- 17.Sims J. R., Gharai L. R., Schaefer P. W., et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72(24):2104–2110. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shukla V., Shakya A. K., Perez-Pinzon M. A., Dave K. R. Cerebral ischemic damage in diabetes: an inflammatory perspective. Journal of Neuroinflammation. 2017;14(1):p. 21. doi: 10.1186/s12974-016-0774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Temelkova-Kurktschiev T., Henkel E., Koehler C., Karrei K., Hanefeld M. Subclinical inflammation in newly detected type II diabetes and impaired glucose tolerance. Diabetologia. 2002;45(1):p. 151. doi: 10.1007/s125-002-8256-1. [DOI] [PubMed] [Google Scholar]
- 20.Wiciński M., Wódkiewicz E., Słupski M., et al. Neuroprotective activity of sitagliptin via reduction of neuroinflammation beyond the incretin effect: focus on alzheimer’s disease. BioMed Research International. 2018;2018:9. doi: 10.1155/2018/6091014.6091014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y., Zhu C., Liu C., Gao Y. Effect of prior atorvastatin treatment on the frequency of hospital acquired pneumonia and evolution of biomarkers in patients with acute ischemic stroke: a multicenter prospective study. BioMed Research International. 2017;2017:8. doi: 10.1155/2017/5642704.5642704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrera B. S., Hasturk H., Kantarci A., et al. Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infection and Immunity. 2015;83(2):792–801. doi: 10.1128/IAI.02444-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parlapiano C., Danese C., Marangi M., et al. The relationship between glycated hemoglobin and polymorphonuclear leukocyte leukotriene B4 release in people with diabetes mellitus. Diabetes Research and Clinical Practice. 1999;46(1):43–45. doi: 10.1016/S0168-8227(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 24.Kumari R., Willing L. B., Krady J. K., Vannucci S. J., Simpson I. A. Impaired wound healing after cerebral hypoxia—ischemia in the diabetic mouse. Journal of Cerebral Blood Flow and Metabolism. 2007;27(4):710–718. doi: 10.1038/sj.jcbfm.9600382. [DOI] [PubMed] [Google Scholar]
- 25.Kumari R., Willing L. B., Patel S. D., et al. The PPAR-gamma agonist, darglitazone, restores acute inflammatory responses to cerebral hypoxia-ischemia in the diabetic Ob/Ob mouse. Journal of Cerebral Blood Flow and Metabolism. 2010;30(2):352–360. doi: 10.1038/jcbfm.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascoal L. B., Bombassaro B., Ramalho A. F., et al. Resolvin RvD2 reduces hypothalamic inflammation and rescues mice from diet-induced obesity. Journal of Neuroinflammation. 2017;14(1):p. 5. doi: 10.1186/s12974-016-0777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M. J., Sansbury B. E., Hellmann J., et al. Resolvin D2 enhances postischemic revascularization while resolving inflammation. Circulation. 2016;134(9):666–680. doi: 10.1161/CIRCULATIONAHA.116.021894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frigerio F., Pasqualini G., Craparotta I., et al. n-3 Docosapentaenoic acid-derived protectin D1 promotes resolution of neuroinflammation and arrests epileptogenesis. Brain. 2018;141(11):3130–3143. doi: 10.1093/brain/awy247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sham H. P., Walker K. H., Abdulnour R. E., et al. 15-epi-Lipoxin A4, resolvin D2, and resolvin D3 induce NF-κB regulators in bacterial pneumonia. The Journal of Immunology. 2018;200(8):2757–2766. doi: 10.4049/jimmunol.1602090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan S. J., Ng M. P. E., Zhao H., et al. Early and sustained increases in leukotriene B4 levels are associated with poor clinical outcome in ischemic stroke patients. Neurotherapeutics. 2020;17(1):282–293. doi: 10.1007/s13311-019-00787-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X., Liu J., Zhao S., et al. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke. 2016;47(2):498–504. doi: 10.1161/STROKEAHA.115.012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandt S. L., Wang S., Dejani N. N., et al. Excessive localized leukotriene B4 levels dictate poor skin host defense in diabetic mice. JCI Insight. 2018;3(17) doi: 10.1172/jci.insight.120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shivakoti R., Dalli J., Kadam D., et al. Lipid mediators of inflammation and resolution in individuals with tuberculosis and tuberculosis-diabetes. Prostaglandins & Other Lipid Mediators. 2020;147, article 106398 doi: 10.1016/j.prostaglandins.2019.106398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi H., Carion T. W., Jiang Y., Chahine A., Steinle J. J., Berger E. A. A regulatory role for β-adrenergic receptors regarding the resolvin D1 (RvD1) pathway in the diabetic retina. PLoS One. 2017;12(11, article e0185383) doi: 10.1371/journal.pone.0185383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong S., Tian H., Lu Y., et al. Neuroprotectin/protectin D1: endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. American Journal of Physiology Cell Physiology. 2014;307(11):C1058–C1067. doi: 10.1152/ajpcell.00270.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grønhøj M. H., Clausen B. H., Fenger C. D., Lambertsen K. L., Finsen B. Beneficial potential of intravenously administered IL-6 in improving outcome after murine experimental stroke. Brain, Behavior, and Immunity. 2017;65:296–311. doi: 10.1016/j.bbi.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 37.Montaner J., Alvarez-Sabín J., Molina C., et al. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke. 2001;32(8):1759–1766. doi: 10.1161/01.STR.32.8.1759. [DOI] [PubMed] [Google Scholar]
- 38.Tu W.-J., Dong X., Zhao S.-J., Yang D.-G., Chen H. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. Journal of Neuroendocrinology. 2013;25(9):771–778. doi: 10.1111/jne.12052. [DOI] [PubMed] [Google Scholar]
- 39.Ramiro L., Simats A., García-Berrocoso T., Montaner J. Inflammatory molecules might become both biomarkers and therapeutic targets for stroke management. Therapeutic Advances in Neurological Disorders. 2018;11 doi: 10.1177/1756286418789340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bustamante A., Sobrino T., Giralt D., et al. Prognostic value of blood interleukin-6 in the prediction of functional outcome after stroke: a systematic review and meta-analysis. Journal of Neuroimmunology. 2014;274(1-2):215–224. doi: 10.1016/j.jneuroim.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Mazzotta G., Sarchielli P., Caso V., et al. Different cytokine levels in thrombolysis patients as predictors for clinical outcome. European Journal of Neurology. 2004;11(6):377–381. doi: 10.1111/j.1468-1331.2004.00798.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.


