Abstract
Background
SARS-CoV-2 caused the COVID-19 pandemic in 2020. The virus is likely to show seasonal dynamics in European climates as other respiratory viruses and coronaviruses do. Analysing the association with meteorological factors might be helpful to anticipate how cases will develop with changing seasons.
Methods
Routinely measured ambient daily mean temperature, absolute humidity, and relative humidity were the explanatory variables of this analysis. Test-positive COVID-19 cases represented the outcome variable. The analysis included 54 English cities. A two-stage meta-regression was conducted. At the first stage, we used a quasi-Poisson generalized linear model including distributed lag non-linear elements. Thereby, we investigate the explanatory variables’ non-linear effects as well as the non-linear effects across lags.
Results
This study found a non-linear association of COVID-19 cases with temperature. At 11.9°C there was 1.62-times (95%-CI: 1.44; 1.81) the risk of cases compared to the temperature-level with the smallest risk (21.8°C). Absolute humidity exhibited a 1.61-times (95%-CI: 1.41; 1.83) elevated risk at 6.6 g/m3 compared to the centering at 15.1 g/m3. When adjusting for temperature RH shows a 1.41-fold increase in risk of COVID-19 incidence (95%-CI: 1.09; 1.81) at 60.7% in respect to 87.6%.
Conclusion
The analysis suggests that in England meteorological variables likely influence COVID-19 case development. These results reinforce the importance of non-pharmaceutical interventions (e.g., social distancing and mask use) during all seasons, especially with cold and dry weather conditions.
Keywords: Temperature, Humidity, COVID-19, Distributed lag non-linear model, Meta-analysis
Abbreviations
- AH
Absolute Humidity
- CI
Confidence Interval
- df
degrees of freedom
- DLNM
Distributed Lag Non-linear Model
- R
Pearson correlation coefficient
- REML
Restricted Maximum Likelihood
- RH
Relative Humidity
- RR
Risk Ratio
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus-2
- TSR
Time-series Regression
1. Introduction
In 2020 a novel coronavirus, called severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), caused a pandemic that had spread from Wuhan around the globe (Valencia, 2020). Infection with SARS-CoV-2 can induce a severe disease (COVID-19), especially in older patients or people with underlying conditions (Wu et al., 2020a; Emami et al., 2020). Until October 31st 2020 45.6 million COVID-19 cases and 1,189,183 deaths had been reported worldwide by WHO (WHO, 2020). In England, 989,749 COVID-19 infections were reported, 46,229 of which were deathly (WHO, 2020).
For other respiratory viruses, environmental factors play a determinant role in transmission as a biological or behavioural catalyst, leading to seasonality of disease outbreaks (Dowell and Shang Ho, 2004). Possible explanations for the causal pathway are that ambient temperature and humidity affect the droplet size and dynamics (Thomas, 2013). Also, the virus stability in aerosols and on surfaces might be affected by temperature, and moistness (Casanova et al., 2010; Chan et al., 2011; Aboubakr et al., 2020). Other mechanisms by which temperature affects transmission are lowered host-immunity levels at cold temperature (Foxman et al., 2015; Fares, 2013), and behaviour changes in winter, such as spending more time indoors with other people (Fares, 2013).
Epidemiological studies have shown that ambient air temperature could be important in transmission of coronaviruses and is likely to show a non-linear association (Xie and Zhu, 2020; Tan et al., 2005; Mecenas et al., 2020). Some studies report that low temperatures (Mecenas et al., 2020; Guo et al., 2020; Lin et al., 2020; Zhu et al., 2020; Liu et al., 2020a; Qi et al., 2020; Meyer et al., 2020; Pequeno et al., 2020; Wu et al., 2020b; Hoang and Tran, 2020a) and low humidity (Guo et al., 2020; Zhu et al., 2020; Qi et al., 2020; Wu et al., 2020b; Şahin, 2020) enhance the spread of SARS-CoV-2. Additionally, earlier laboratory tests confirmed that temperature and humidity influence the survival time of SARS-CoV (which is structurally similar to SARS-CoV-2) on surfaces (Casanova et al., 2010; Chan et al., 2011). Studies on the ecological influence of humidity showed heterogeneous results (Pani et al., 2020; Meo et al., 2020; Runkle et al., 2020; Tosepu et al., 2020; Briz-Redón and Serrano-Aroca, 2020). A systematic review about this association concluded that hot and wet climates have a protective effect (Mecenas et al., 2020).
A recent review highlights data-related and methodological concern related to studies evaluating the association between meteorological variables and COVID-19 spread (Dong, 2021). In particular the authors of the review pointed out that differences on previous results can be due to short observation period, lack of controlling for non-pharmaceutical interventions, an inappropriate size of the geographical unit and incorrect statistical methods. Moreover, it is likely that the influence of temperature as well as humidity differ amongst geographies, cultural contexts (e.g. due to differing behaviour, city planning, etc.), and climate conditions (Auler et al., 2020). It is then important to evaluate the effect of meteorological variables in different geographical areas. To our knowledge no previous study has specifically evaluated the association between meteorological variables and COVID-19 spread in England.
In this study, we overcome the methodological issues of previous approaches by using a time-series modelling approach to examine the impact of meteorological variables on SARS-CoV-2 transmission, considering city-level data in England accounting for confounding of non-pharmaceutical interventions, time trends and other city-level covariates. Moreover, we used appropriate statistical methods to analyse time-series studies for infectious diseases taking into account non-linear and lagged effects along with auto-correlation (Imai et al., 2015).
The aim of this study is to investigate the daily mean outdoor air temperature, and absolute and relative humidity as predictors of daily confirmed COVID-19 incidence in England.
2. Methods
2.1. Study data
The study analysed 54 English cities from 30th January to October 31, 2020. We defined as outcome the daily number of cases using the UK coronavirus data provided by the government (UK, 2020). The cases data included all positive lab confirmed virus test results. D Dates in the dataset refer to the date at which specimens were taken from the tested individuals. The COVID-19 time-series was aggregated at lower-tier local authority resolution and linked to the cities.
For the exposure, we used temperature and dew point temperature time-series from the ERA5 dataset. These are published by Copernicus on a regular latitude/longitude grid of 25 km × 25 km in NetCDF format (Hersbach et al., 2018). The bi-hourly mean temperatures and dew point temperatures 2 m above surface were averaged for each day at the closest data point available to the centroid coordinate of every city. From dew point temperature and the corresponding temperature we obtained relative humidity (RH) using the R “humidity” package (Jun Cai, 2019). Additionally, the following formula was used to calculate the absolute humidity (AH) which represents the mass of water vapour in the air mixture (Shi et al., 2020a):
As an indicator for general mobility we extract time-series for England from the Google Mobility indices (residential mobility, mobility in parks, retail, transport, groceries and at work) (Google, 2020). These indices represent the change in duration spent at home or the change in visitor counts at different places (e.g parks, retail, transport, groceries, work) on a given day compared to that weekday's median in the period from 3rd January and February 6, 2020, before the pandemic reached England (according to reported cases) (Google, 2020).
For each city, demographic background information such as population size, population density, and age proportion above 65 years were retrieved from the OECD Regional and Metropolitan database (OECD, 2016).
2.2. Statistical analysis
In this study, we investigate short-term associations between meteorological exposures and COVID-19 incidence using a two-stage design. In the first stage, we estimated the location specific exposure-response association through time-series regression (TSR) analysis adjusting for time-varying confounders. In the second stage, the association parameter, i.e. the model coefficients of the TSR, were pooled using a meta-analytic model. Meta-regression considers the precision of the estimate and can incorporate location-specific predictors (e.g. population density). The described two-stage statistical modelling approach was used for estimations of temperature, RH, and AH effects separately.
2.3. First stage analysis
Only points in time from 10 days before the initial 10 confirmed daily cases were included in this analysis. The aim was to eliminate initial imported cases and only account for local transmission while having enough exposure data to consider a lagged effect of up to 10 days before the observed outcome. This was changed in the sensitivity analysis considering different lags.
For each location, a generalized linear model with quasi-Poisson family and log link was used to model the observed COVID-19 case counts. The meteorological exposure variables ambient temperature, RH, and AH were modelled using distributed lag non-linear model (DLNM) terms. In this way, possible non-linear exposure-outcome relationships were described together with non-linear delayed lag-response effects (Gasparrini, 2013).
The DLNM terms (crossbasis) act as basis function predictors in two dimensions: the exposure space and the lag space. For the exposure space we chose as basis function the natural cubic spline function with 3 knots respectively corresponding to the 25th, the 50th, and the 75th percentile of the exposure distribution of all cities. As the current literature suggests, an approximate incubation period between 5 and 12 days for COVID-19, we considered 0–10 days as the default lag, accordingly (Lauer et al., 2020). In the lag dimension we considered as basis function a natural spline with one internal knot.
The crossbasis terms were reduced by the lag or exposure dimensions to analyse the association on the exposure or lag dimension, respectively. The R package “dlnm” allowed to include the crossbasis-element and fit the generalized linear model of the TSR (Gasparrini, 2011).
Confounding by season and long-term trends was modelled by a natural spline function of time in dateswith 4 degrees of freedom (df) (Imai et al., 2015).
Autocorrelation of residuals in the case of infectious disease is pathogen-specific and needs to be accounted for (Imai et al., 2015). Lagged autocorrelation due to “true contagion” was incorporated for all days up to 5 days before. The logarithm of lagged outcome counts was used instead of past counts. Previous analyses suggested that including past cases could lead to overadjustment, and using logarithm scale better matches the mechanisms of disease transmission (Imai et al., 2015). As time-series contained days with no cases, these values were replaced by one. Previous models for influenza have been shown not to be sensitive to this replacement (Imai et al., 2015).
Residential mobility was considered as a possible confounder as it is associated with the outcome of COVID-19 cases (via affecting population mixing) as well as the exposures (e.g. weather-dependent free time outdoor mobility), and is not on the causal pathway between the exposures and the outcome. The mobility-term was modelled by a distributed linear term with a lag of 0–14 days, where the lag dimension was modelled with a natural cubic spline with two internal knots equally spaced on log scale. Weekends and weekdays influence the mixing differently. Thus, incorporation of a weekend-indicator was assumed to minimise the overdispersion of the case data.
Firstly, we fitted the first stage models considering each meteorological variable (outdoor temperature, absolute humidity, relative humidity) as main exposure adjusting by trend, residential mobility and autocorrelation (univariable models). In a second step, we fitted a model considering outdoor temperature and relative humidity as main exposure adjusting by trend, residential mobility and autocorrelation (multivariable model).
2.4. Second stage analysis
The obtained TSR coefficients and corresponding covariance matrices from the first-stage modelling at city level were pooled via multivariate meta-regression models using a restricted maximum likelihood (REML) method for estimation (Sera et al., 2019). The pooled set of spline coefficients were used to obtain the exposure-response function, which was expressed in terms of relative risk (RR) and confidence intervals (CIs) in respect to the observed minimal risk. The exposure response functions were centered to the exposure level with minimum RR within the 5th-95th percentile of the exposure distribution. For the lag-response plots, we used the 5th lag day as reference (i.e. the middle of the 10 lag days included). We used the I2-statistic which measures the relative excess in heterogeneity that cannot be explained by the sampling error (Gasparrini et al., 2012). For fitting the multivariate meta-analysis, we used the R package “mixmeta” (Sera et al., 2019).
2.5. Sensitivity analysis
The main model was tested for sensitivity considering the following modelling alterations. Firstly, an alteration of the date-term from a 4 df to 6 df natural spline function was conducted. Secondly, we changed the lagged effect of the crossbasis from 0-10 days to 0–5 days or 0–14 days. Moreover, we included population statistics such as population density and proportion of above 65-years-olds, as well as different pollution levels in the different cities during the study period at the second stage analysis as variables in the meta-regression. Finally, we analysed the influence of using other Google Mobility indicators considering each indicator as alternative confounding term in the first-stage models.
3. Results
3.1. Descriptive analysis
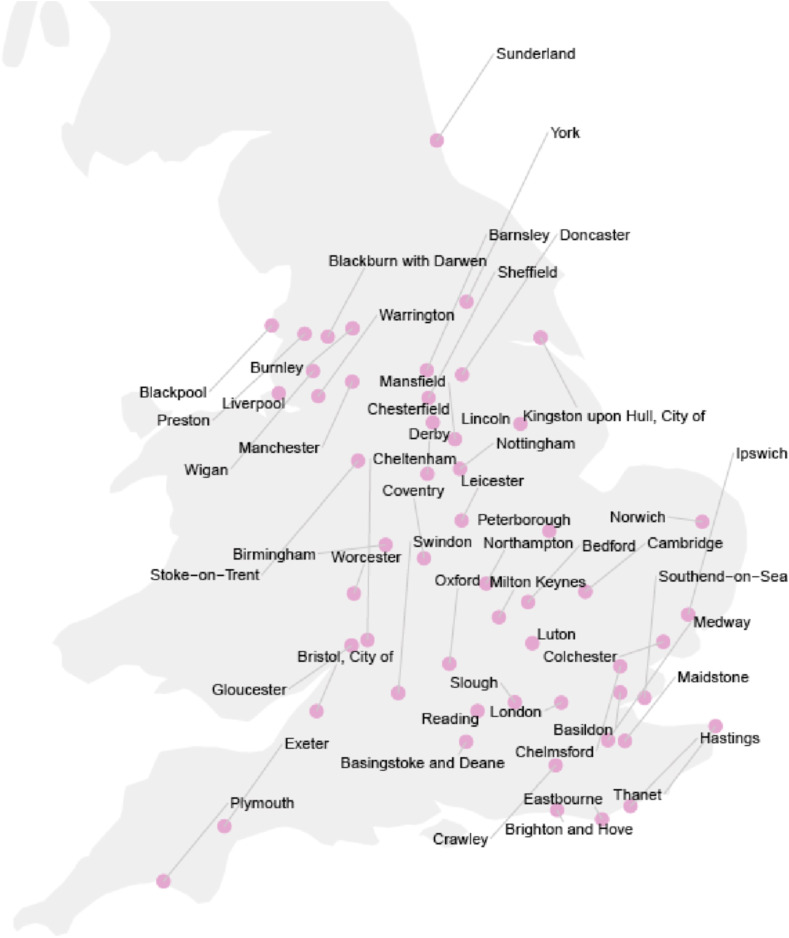
Overall, 394,863 COVID-19 cases were confirmed during the 281-day study period in the 54 English cities considered (Fig. 1 ).
Fig. 1.
53 English cities that were included. The map shows the location as well as the names of the cities that were included into this study.
There were huge differences in cumulative cases spanning from 432 cases in Hastings up to 112,822 in London (Table 1 ). The cities’ case incidence showed similar patterns as their population densities (Appendix A). The proportion of over 65-year-olds varied between approximately 10% to 23% and pollution levels (PM2.5) in the cities lied between 0.2 and 40.2 μg/m3.
Table 1.
City characteristics. Median, minimum, and maximum values amongst the included cities are displayed for COVID-19 cases, city demographics and pollution levels.
| City parameter | Median | Minimum | Maximum |
|---|---|---|---|
| Total confirmed cases (#) | 2,644 | 432 | 112,822 |
| City population sizes (#) | 669,924 | 138,214 | 10,491,206 |
| Density (# per km2 ) | 1,106.47 | 4,268.20 | 1,161.41 |
| Proportion aged above 65 years (%) | 16.1 | 10.0 | 22.8 |
| PM2.5 (μg/m3) | 7.3 | 0.2 | 43.5 |
Average temperature, RH, and AH-levels varied between the cities. The overall range of observed exposure values varied between approximately −6 and 36°C for temperature, 8 and 99% for RH, and 2 and 24 g/m3 for AH. More details are displayed in Table 2 . Residential mobility was, on average, 13.8% higher than before the pandemic.
Table 2.
Summary table of observed cases, environmental data, and mobility.
| Parameter | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Cases per day (#) | 27.6 | 55.8 | 0.00 | 2,515 |
| Temperature (°C) | 15.9 | 3.9 | −6.6 | 36.2 |
| RH (%) | 71.1 | 9.7 | 7.7 | 99.3 |
| AH (g/m3) | 10.1 | 2.1 | 1.7 | 23.8 |
| Residential Mobility Increase vs “normal” (Δ%) | 13.8 | 1.1 | −0.7 | 31.6 |
COVID-19 incidence showed a two-waves epidemic curve with a first peak at the beginning of April 2020 and the second wave taking off from September 2020 onwards (Appendix B). Both, case counts, and residential mobility, showed dependency on the day of the week. Residential mobility rose in accordance to the first wave to a level of over 25–33% above usual levels during the week and dropped towards a level around 10% in September 2020 . Averaging the mean exposures among all cities daily, temperatures had a positive trend from spring to summer with a peak in August. RH was lowest in spring (May and April) and increased towards the winter season with a high at end of October. AH exhibited a similar trend as observed for temperature (Appendix C).
Looking at temporal Pearson correlation, RH showed a moderate correlation (r = −0.21) with absolute humidity. COVID-19 cases were negatively correlated with relative humidity (r = −0.34) and temperature (r = −0.17) (Appendix D).
3.2. Temperature analysis
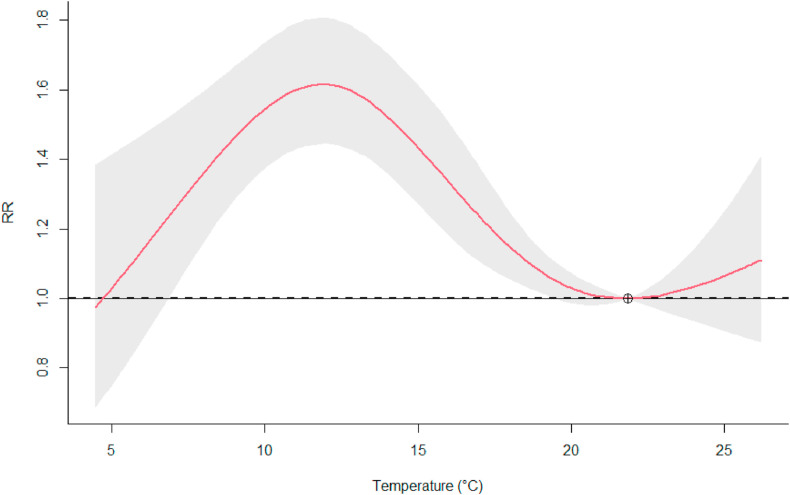
The association of temperature with the COVID-19 is visualised in Fig. 2 by showing the cumulative RR over the lags (0–10 days). The highest risk was observed at 11.9°C compared to the minimum risk at 21.8°C, showing 1.62-times the risk (95%-CI: 1.44; 1.81). The meta-model provided evidence for elevated risk levels at all lags between 1 and 8 days (Appendix E.A).
Fig. 2.
Temperature-RR association. The graph represents the overall cumulative RR of observing COVID-19 test-positives in the English cities (lag 0–10) during the observation period. The red line represents the model estimate and the grey area the modelling uncertainty with a 5% significance level.
3.3. Humidity analysis
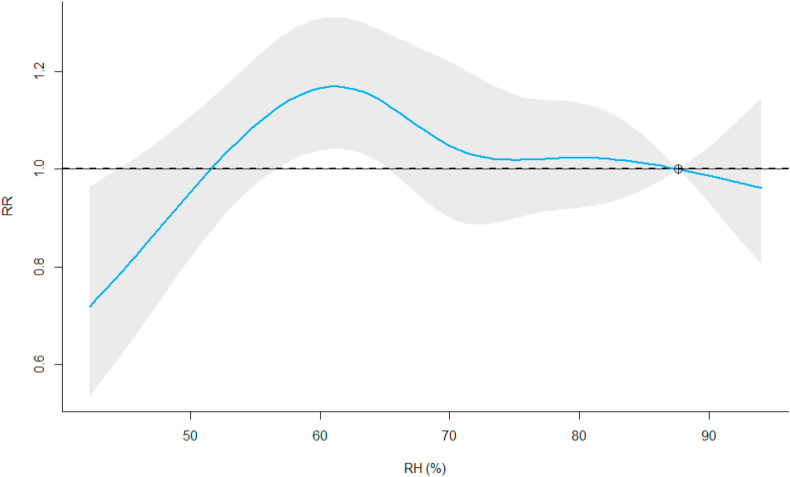
The univariate analysis shows low COVID-19 risk at low level of relative humidity. The risk increases with relative humidity reaching the highest risk (RRmax = 1.17, 95%-CI: 1.04; 1.31) at 61.1% compared to the centering level set to 87.6% (Fig. 3 ). The associated lag-plot at RH-levels of 61.1% does show some evidence for a elevated risk associated with a lag between the 5th and the 10th day (Appendix E.B).
Fig. 3.
RH-RR association. The graph shows the overall cumulative (lag 0–10) RR of observing COVID-19 test-positives in the English cities. The blue line represents the model estimate and the grey area the modelling uncertainty with a 5% significance level.
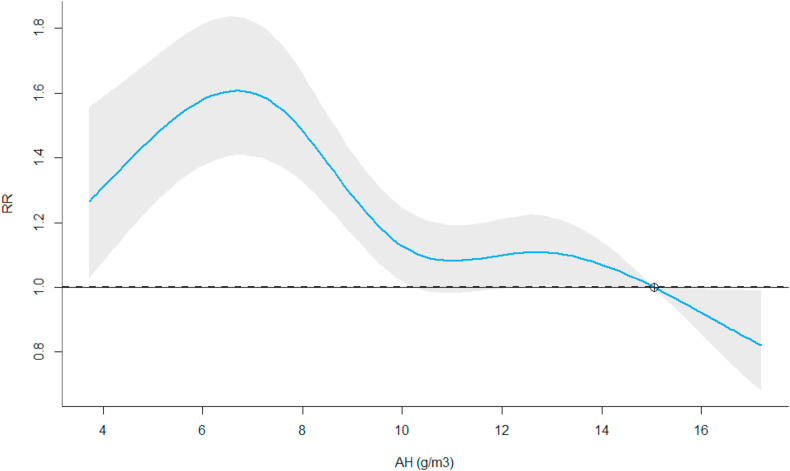
For AH, a local peak in risk was observed at 6.6 g/m3 (Fig. 4 ). Compared to the centering point at 15.1 g/m3 we estimated a 1.61-times higher risk for COVID-19 cases (95%-CI: 1.41; 1.83). Over the lag dimension, the increased risk remain elevated between 1 and 10 days (Appendix E.C).
Fig. 4.
AH-RR association. The graph shows the overall cumulative (lag 0–10) RR of observing COVID-19 test-positives in the English cities. The blue line represents the model estimate and the grey area the modelling uncertainty with a 5% significance level.
3.4. Multivariate model of temperature and RH
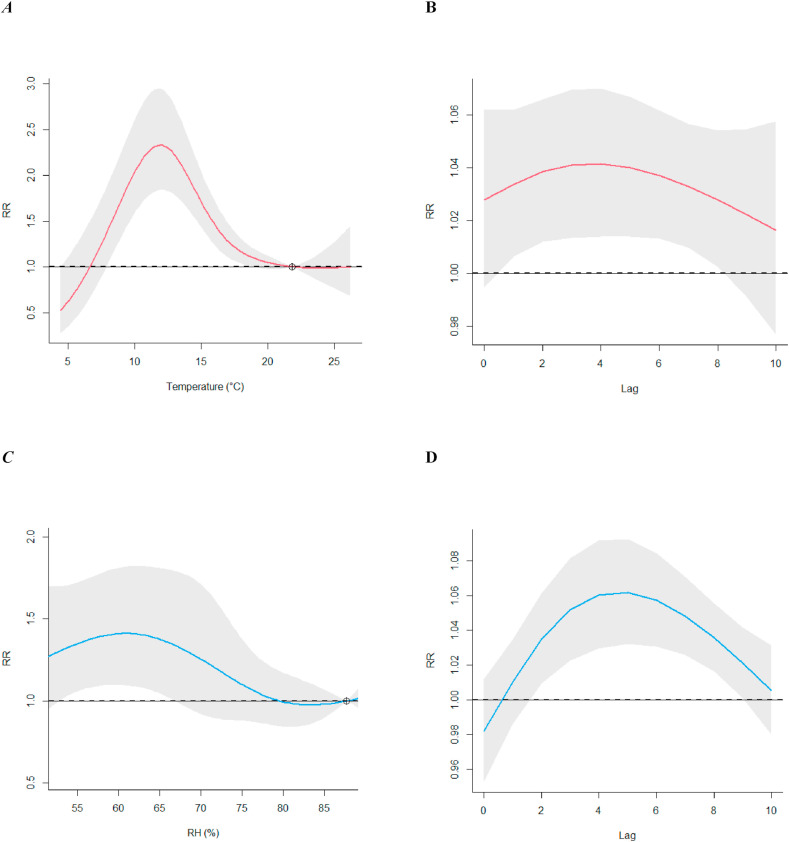
When adjusting for relative humidity, the shape of temperature COVID-19 risk curve is similar to the univariate analysis with a maximum RR of 2.33 (95% CI:1.84; 2.95) at 11.9°C when comparing 22.8°C (Fig. 5 ). The trend for association between relative humidity and COVID-19, however, changes after adjusting for temperature. The maximal RR was observed at 60.7% with a 1.41-times increased associated risk (95% CI: 1.09; 1.81) in respect to the centering point equal to 87.6% (Fig. 5).
Fig. 5.
Multivariate temperature-RH model. (A) shows the RR associated with temperatures if adjusted for RH and (B) the according RR-lag association. (C) Shows the RR-RH trends adjusted for temperature and (D) the according RR-lag association.
3.5. Sensitivity analysis
Increasing the degrees of freedoms in the natural spline for long-term trends from 3 to 6 df leads to a loss of precision for the estimates (Appendix F). For temperature and AH, changing the incorporation of 0–10 lag days down to 0–5 lag days and up to 0–14 lag days, showed that the observed effect on the COVID-19 risk is robust over the different parametrisations (Appendix G and I). RH trends were less robust over different lag configurations (Appendix H). Adding the population density, age proportion above 65 years and the pollution levels of the cities into the meta-regression, produced similar exposure-response associations (Appendix J). Adjusting for social mixing effects using different indices than the google mobility has little influence on the exposure risk relationship (Appendix K, L and M).
4. Discussion
4.1. Main findings
The analysis showed evidence for a non-linear association with an higher risk at outdoor mean temperatures around 12°C (RRmax = 1.62, 95%-CI: 1.44; 1.81). This was robust in the sensitivity analysis since in all analysed models. Regarding relative humidity, adjusting by temperature, we found evidence of increased risk at low relative humidity levels with a peak of risk at 61% RH (RRmax = 1.45, 95% CI: 1.10; 1.90). AH shows a tendency of higher risks at lower humidity, with an RRmax between 6 and 8 g/m3.
4.2. Possible mechanisms explaining the main results
The non-linear effects of temperature and humidity can generally be explained by three mechanisms. Firstly, there is the aspect of human behaviour changing with meteorological conditions. Human mobility and time spent indoors or outdoors depend on weather conditions. Very hot and very cold conditions can lead to more time spent in closed rooms. Similarly, very humid conditions might lead to less time spent outdoors. This has complex implications on the effect of ambient temperature and humidity.
Secondly, the droplet behaviour in aerosols changes with different temperature and humidity levels. High temperature and low humidity promote the accumulation of aerosol particles (since evaporation leaves behind floating droplet nuclei) increasing the likelihood to be respired. Low temperature and high humidity favour contact transmission (Zhao et al., 2020; Lowen et al., 2007). This ambiguity makes non-linear associations plausible. Moreover, research on immunity-related effects showed, amongst other findings, that cold temperatures affect the blood circulation which impairs the adaptive immunity, and dry air hinders the ability of cilia cells to secrete mucus and remove viral particles (innate response) (Lowen et al., 2007; Sun et al., 2020). These phenomena mostly support a negative association with temperature and humidity.
4.3. Comparison with existing literature
Interestingly, the observed tendency of a maximum risk of COVID-19 cases in this study was reflected by a Chinese study (Shi et al., 2020b). This study analysed the non-linear relationship using DLNM-models showing that low temperatures also can reduce the risk of COVID-19 daily incidence (lowest at −10 °C). The highest COVID-19 incidence was predicted at 10°C across 344 cities in mainland China (Shi et al., 2020b). In addition, a South Korean study (Hoang and Tran, 2020b) which used a generalized linear modelling approach observed a positive trend below and a negative trend above 8°C. These findings are in line with what the final model of this study suggests. Other ecological studies in Brazil (Pequeno et al., 2020), and China (Liu et al., 2020b) used generalized linear mixed models and polynomial non-linear regression, respectively. Their results, in contrast, showed a general negative linear association between daily COVID-19 risk and temperature: each 1°C increase in temperature was related to a decrease of 10–20% in COVID-19 cases.
Regarding humidity the evidence basis in the literature is quite mixed. An ecological study of 8 different US cities (Runkle et al., 2020) which used DLNM models had a maximum risk of COVID-19 cases at higher AH-levels than we observed in this study (8–11 g/m3 vs. 6–8 g/m3). Similar to our UK analysis, a maximal twofold increase in risk was found (Runkle et al., 2020). Further, a descriptive analysis of all US states (Gupta et al., 2020), showed that states with an AH range between 4 and 6 g/m3 had the highest numbers of cases within 10 days. A negative association was observed in some South American (Zhu et al., 2020) and Chinese cities (Liu et al., 2020a) by use of correlation and linear regression.
Wu et al. (analysing 166 countries) (Wu et al., 2020b) and Qi et al. (analysing 30 Chinese provinces)(Qi et al., 2020) report a negative association between RH and COVID-19 cases where each 1% increase results in an 0.85% and 22% increase in cases, respectively. In contrast, most ecological studies (e.g. as conducted in Iran (Ahmadi et al., 2020), New York (Bashir et al., 2020), Indonesia, (Tosepu et al., 2020), China and analysing 377 worldwide cities (Meyer et al., 2020)), did not find any significant association between COVID-19 cases and RH.
4.4. Strengths and limitations
Our study has several strengths. The time-series design allows to consider time-varying confounders as non-pharmaceutical interventions and changes in cases definition over the observed period. We used complex DLNM parametrisation to model potential non-linearity and lagged effects of the exposures. By using a meta-regression two-stage principle allows to consider potential differences between cities and increasing the statistical power for the overall analysis.
It is important to highlight also some limitations of our study. The mobility-term were country level and this choice could have introduce some measuring error in this important cofounding variable. Ecologic biases due to cross-regional variation in the “background” risk distributions for COVID-19 cases were accounted for in the sensitivity analysis at meta-regression level. However, more parameters could be included in further analysis. Moreover, spatial dependencies in terms of the cases being imported were not accounted for.
The use of reported COVID-19 test-positive cases only in clinical settings excludes a vast majority of cases from the analysis. Hence, the RR in this study cannot be interpreted as the risk of COVID-19 spread or incidence but only the risk of observing confirmed cases. The latter is not only dependent on the rate at which the virus spreads, but also on symptom severity and availability of health infrastructure, as only sufficiently severe cases would have presented themselves and been tested. This duality must be considered when interpreting the outcome of this analysis.
The advantage of the ERA5 that the data is reported reliably (without missing values) for all the locations, they are available in the public domain, and they have been shown to be useful in analysing the association with communicable disease transmission in previous other studies (Scortichini, 2020; Urban et al., 2019). However, the resolution of 25 km square grid could lead to non-systematic errors.
5. Conclusion
The study suggests that daily ambient mean temperatures of around 11°C–13°C pose a higher risk for COVID-19 cases in England compared to the risk-minimum at 22°C. This implies that typical temperature conditions for England in colder months pose a higher risk (WorldData.info, 2020). A tendency for an increased COVID-19 risk at lower AH around 6–8 g/m3, could be observed in this analysis. Regarding expected year-round AH levels in England, this result would indicate that there is a more than 1.5-times elevated risk from November to April (WorldData.info, 2020). RH as well showed highest RRs at comparatively low RH-levels (around 61%). An overall trend of lower RH-levels being associated with higher risks was only observed when adjusting for temperature. These results confirm the importance of non-pharmaceutical interventions (e.g., social distancing and mask use) during all seasons, especially during cold and dry meteorological conditions.
Author information
LN and FS designed the study and processed the data. FS defined the analysis plan and LN performed the statistical analysis. LN wrote the draft and FS made comments and revised the manuscript.
Credit roles
Luise Nottmeyer: Conceptualization, Formal analysis, Writing – original draft, Visualization. Francesco Sera: Conceptualization, Methodology, Writing – review & editing, Supervision.
Funding sources
The authors were funded by the Medical Research Council-UK [grant ID: MR/R013349/1], the Natural Environment Research Council UK [grant ID: NE/R009384/1], and the European Union Horizon 2020 programme [grant ID: 820655].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors obtained professional assistance to this project by Ben Armstrong and the Multi-Country Multi-City Collaborative Research Network.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.110977.
Appendix A - M. Supplementary data
The following is the Supplementary data to this article:
References
- Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transboundary and Emerging Diseases. 2020;1–17 doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi M., Sharifi A., Dorosti S., Jafarzadeh Ghoushchi S., Ghanbari N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Sci. Total Environ. 2020;729:138705. doi: 10.1016/j.scitotenv.2020.138705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auler A.C., Cássaro F.A.M., da Silva V.O., Pires L.F. Evidence that high temperatures and intermediate relative humidity might favor the spread of COVID-19 in tropical climate: a case study for the most affected Brazilian cities. Sci. Total Environ. 2020;729:139090. doi: 10.1016/j.scitotenv.2020.139090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir M.F., et al. Correlation between climate indicators and COVID-19 pandemic in New York, USA. Sci. Total Environ. 2020;728:138835. doi: 10.1016/j.scitotenv.2020.138835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz-Redón Á., Serrano-Aroca Á. A spatio-temporal analysis for exploring the effect of temperature on COVID-19 early evolution in Spain. Sci. Total Environ. 2020;728:138811. doi: 10.1016/j.scitotenv.2020.138811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010;76:2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., et al. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011;2011 doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z., et al. Data-related and methodological obstacles to determining associations between temperature and COVID-19 transmission. Environ. Res. Lett. 2021;16(3) [Google Scholar]
- Dowell S.F., Shang Ho M. Seasonality of infectious diseases and severe acute respiratory syndrome - what we don't know can hurt us. Lancet Infect. Dis. 2004;4:704–708. doi: 10.1016/S1473-3099(04)01177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch. Acad. Emerg. Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- Fares A. Factors influencing the seasonal patterns of infectious diseases. Int. J. Prev. Med. 2013;4:128–132. [PMC free article] [PubMed] [Google Scholar]
- Foxman E.F., et al. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc. Natl. Acad. Sci. U. S. A. 2015;112:827–832. doi: 10.1073/pnas.1411030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. Distributed lag linear and non-linear models in R : the package dlnm. J. Stat. Software. 2011;43:1–20. [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. 5th. Vol. 33. Wiley Online Library; 2013. Modeling Exposure-Lag-Response Associations with Distributed Lag Non-linear Models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A., Armstrong B., Kenward M.G. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat. Med. 2012;31:3821–3839. doi: 10.1002/sim.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Google LLC. 2020. Google COVID-19 Community Mobility Report.https://www.google.com/covid19/mobility/ Accessed in November 2020. [Google Scholar]
- Guo X.J., Zhang H., Zeng Y.P. Transmissibility of COVID-19 in 11 major cities in China and its association with temperature and humidity in Beijing, Shanghai, Guangzhou, and Chengdu. Infect. Dis. Poverty. 2020;9:87. doi: 10.1186/s40249-020-00708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Raghuwanshi G.S., Chanda A. Effect of weather on COVID-19 spread in the US: a prediction model for India in 2020. Sci. Total Environ. 2020;728:138860. doi: 10.1016/j.scitotenv.2020.138860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersbach H., Bell B., Berrisford P., Biavati G., Horányi A., Muñoz Sabater J., Nicolas J., Peubey C., Radu R., Rozum I., Schepers D., Simmons A., Soci C., Dee D., Thépaut J-N. ERA5 hourly data on single levels from 1979 to present. Copernicus Climate Change Service (C3S) Climate Data Store (CDS). Accessed October 2020. 2018 doi: 10.24381/cds.adbb2d47. [DOI] [Google Scholar]
- Hoang T., Tran T.T.A. Ambient air pollution, meteorology, and COVID‐19 infection in Korea. J. Med. Virol. jmv. 2020;26325 doi: 10.1002/jmv.26325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T., Tran T.T.A. Ambient air pollution, meteorology, and COVID‐19 infection in Korea. J. Med. Virol. jmv. 2020;26325 doi: 10.1002/jmv.26325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai C., Armstrong B., Chalabi Z., Mangtani P., Hashizume M. Time series regression model for infectious disease and weather. Environ. Res. 2015;142:319–327. doi: 10.1016/j.envres.2015.06.040. [DOI] [PubMed] [Google Scholar]
- Jun Cai M. 2019. Type Package Title Calculate Water Vapor Measures from Temperature and Dew Point. [Google Scholar]
- Lauer S.A., et al. The incubation period of coronavirus disease 2019 (CoVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., et al. A mechanism-based parameterisation scheme to investigate the association between transmission rate of COVID-19 and meteorological factors on plains in China. Sci. Total Environ. 2020;737:140348. doi: 10.1016/j.scitotenv.2020.140348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., et al. Impact of meteorological factors on the COVID-19 transmission: a multi-city study in China. Sci. Total Environ. 2020;726:138513. doi: 10.1016/j.scitotenv.2020.138513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Lin X., Qin S. arXiv; 2020. The Short-Term Seasonal Analyses between Atmospheric Environment and COVID-19 in Epidemic Areas of Cities in Australia, South Korea, and Italy. [Google Scholar]
- Lowen A.C., Mubareka S., Steel J., Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:e151. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecenas P., Bastos R., Vallinoto A., Normando D. 9th. Vol. 15. PLoS ONE; 2020. Effects of Temperature and Humidity on the Spread of COVID-19: A Systematic Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meo S.A., et al. Effect of temperature and humidity on the dynamics of daily new cases and deaths due to COVID-19 outbreak in Gulf countries in Middle East Region. Eur. Rev. Med. Pharmacol. Sci. 2020;24:7524–7533. doi: 10.26355/eurrev_202007_21927. [DOI] [PubMed] [Google Scholar]
- Meyer A., Sadler R., Faverjon C., Cameron A.R., Bannister-Tyrrell M. Evidence that higher temperatures are associated with a marginally lower incidence of COVID-19 cases. Front. Public Heal. 2020;8:367. doi: 10.3389/fpubh.2020.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oecd Metropolitan areas - regions at a glance. 2016. https://stats.oecd.org/Index.aspx?Datasetcode=CITIES
- Pani S.K., Lin N.H., RavindraBabu S. Association of COVID-19 pandemic with meteorological parameters over Singapore. Sci. Total Environ. 2020;740:140112. doi: 10.1016/j.scitotenv.2020.140112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pequeno P., et al. Air transportation, population density and temperature predict the spread of COVID-19 in Brazil. PeerJ. 2020;8 doi: 10.7717/peerj.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., et al. COVID-19 transmission in Mainland China is associated with temperature and humidity: a time-series analysis. Sci. Total Environ. 2020;728:138778. doi: 10.1016/j.scitotenv.2020.138778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runkle J.D., et al. Short-term effects of specific humidity and temperature on COVID-19 morbidity in select US cities. Sci. Total Environ. 2020;740:140093. doi: 10.1016/j.scitotenv.2020.140093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şahin M. Impact of weather on COVID-19 pandemic in Turkey. Sci. Total Environ. 2020;728:138810. doi: 10.1016/j.scitotenv.2020.138810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scortichini M., et al. Excess mortality during the COVID-19 outbreak in Italy: a two-stage interrupted time series analysis. International Journal of Epidemiology. 2020;49(6) doi: 10.1101/2020.07.22.20159632. 07.22.20159632 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sera F., Armstrong B., Blangiardo M., Gasparrini A. An extended mixed‐effects framework for meta‐analysis. Stat. Med. 2019;38:5429–5444. doi: 10.1002/sim.8362. [DOI] [PubMed] [Google Scholar]
- Shi P., et al. The impact of temperature and absolute humidity on the coronavirus disease 2019 (COVID-19) outbreak - evidence from China. medRxiv. 2020 doi: 10.1101/2020.03.22.20038919. 03.22.20038919 (2020) [DOI] [Google Scholar]
- Shi P., et al. The impact of temperature and absolute humidity on the coronavirus disease 2019 (COVID-19) outbreak - evidence from China. medRxiv. 2020 doi: 10.1101/2020.03.22.20038919. 03.22.20038919 (2020) [DOI] [Google Scholar]
- Sun Z., Thilakavathy K., Kumar S.S., He G., Liu S.V. Potential factors influencing repeated SARS outbreaks in China. Int. J. Environ. Res. Publ. Health. 2020;17:1633. doi: 10.3390/ijerph17051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., et al. An initial investigation of the association between the SARS outbreak and weather: with the view of the environmental temperature and its variation. J. Epidemiol. Community Health. 2005;59:186–192. doi: 10.1136/jech.2004.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R.J. Particle size and pathogenicity in the respiratory tract. Virulence. 2013;4 847 doi: 10.4161/viru.27172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosepu R., et al. Correlation between weather and covid-19 pandemic in Jakarta, Indonesia. Sci. Total Environ. 2020;725:138436. doi: 10.1016/j.scitotenv.2020.138436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban A., Napoli D., Aquaotta F., Cloke H., Kyselý J., Pappenberger F., et al. Evaluation of the ERA5-based UTCI on mortality data in Europe. Environ. Epidemiol. 2019;3:403. [Google Scholar]
- Valencia D.N. Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Cureus. 2020;12 doi: 10.7759/cureus.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO coronavirus disease (COVID-19) dashboard. World Health Organization; Geneva: 2020. https://covid19.who.int/ [Google Scholar]
- WorldData.info . Climate of England - United Kingdom. German Weather service Database. World Data Info. 2020; 2020. https://www.worlddata.info/europe/united-kingdom/climate-england.php [Google Scholar]
- Wu C., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., et al. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci. Total Environ. 2020;729:139051. doi: 10.1016/j.scitotenv.2020.139051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Zhu Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724:138201. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Qi Y., Luzzatto-Fegiz P., Cui Y., Zhu Y. COVID-19: effects of weather conditions on the propagation of respiratory droplets. Nano Lett. 2020;20(10):7744–7750. doi: 10.1101/2020.05.24.20111963. [DOI] [PubMed] [Google Scholar]
- Zhu L., et al. Meteorological impact on the COVID-19 pandemic: a study across eight severely affected regions in South America. Sci. Total Environ. 2020;744:140881. doi: 10.1016/j.scitotenv.2020.140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK, 2020. UK Coronavirus data. UK Government. Accessed November 2020. https://coronavirus.data.gov.uk/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.