Abstract
Purpose
Opioid addiction is a major public health concern. Chronic opioid use (COU) patterns after radiation for head and neck cancer (HNC) remain poorly understood. The aim of this study was to estimate the prevalence of COU and to identify its risk factors in patients with HNC undergoing curative-intent radiation therapy (RT) or chemoradiotherapy (CRT).
Methods and Materials
We performed a systematic review and meta-analysis using the PubMed (Medline), EMBASE, and Cochrane Library databases, queried from dates of inception until January 2020. COU was defined as persistent use of opioids ≥ 3 months after treatment completion. Meta-analyses were performed using random effects models. Heterogeneity was assessed using the I2 value.
Results
Seven retrospective studies, reporting on 1841 patients, met the inclusion criteria. Median age was 59.4 (range: 56.0-62.0) years with 1343 (72.9%) men and 498 (27.1%) women. Primary tumor locations included oropharynx (n = 891, 48.4%), oral cavity (n = 533, 29.0%), larynx (n = 93, 5.1%), hypopharynx (n = 32, 1.7%), and nasopharynx (n = 29, 1.6%). Eight hundred fifty-four (46.0%) patients had stage I/II and 952 (50.3%) had stage III-IV disease. Three hundred one (16.3%) patients had RT alone, 738 (40.1%) received CRT, and 594 (32.3%) underwent surgery followed by adjuvant RT/CRT. The proportion of patients with HNC who developed COU post-RT/CRT was 40.7% at 3 months (95% confidence interval [CI]: 22.6%-61.7%; I2 = 97.1%) and 15.5% at 6 months (95% CI: 7.3%-29.7%; I2 = 94.3%). Oropharyngeal malignancies had the highest rate of COU based on primary tumor location (46.6%; 95% CI: 30.8%-63.1%; P < .0001). High proportions of COU were found in patients with a history of psychiatric disorder(s) (61.7%), former/current alcohol abuse (53.9%), and opioid requirements before radiation treatment (51.6%; P = .035).
Conclusions
A significant proportion of patients who undergo RT for HNC suffer from COU. High-risk factors for COU include an oropharyngeal primary, history of psychiatric disorder, former/current alcohol abuse, and pre-treatment opioid use. New strategies to mitigate COU are needed.
Introduction
Opioid addiction is a major public health concern. Approximately 14% of patients with cancer experience chronic opioid use (COU) requiring continuous opioid prescriptions for at least 90 days post-diagnosis.1 Despite comprising only 3.7% of new cancer diagnoses, patients with head and neck cancer (HNC) alone account for 11.7% of opioid-related deaths.2 Compared with other cancer types, most patients with HNC receiving radiation therapy (RT) or chemoradiotherapy (CRT) experience radiation-induced mucositis (RIM) (80%)3 and enter a vicious cycle of pain, dysphagia, malnourishment, and reduced quality of life.4, 5, 6 Opioids are commonly used to ensure completion of curative-intent HNC RT, a practice that is not supported by randomized trials and may place patients at risk for COU, opioid addiction, and overdose.7, 8, 9
Since COU patterns in RIM pain remain poorly understood, the objectives of this study were to determine the rate of COU and to identify risk factors for COU in patients with HNC undergoing curative-intent RT or CRT.
Methods and Materials
We performed a systematic review and meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The PubMed (Medline), EMBASE, and Cochrane Library databases were queried from their respective dates of inception until January 2020 for COU, HNC, and RT (see Supplementary Material). COU was defined as persistent use of opioids ≥ 3 months after treatment completion. Peer-reviewed studies in the English language that reported on COU in patients with HNC who received RT/CRT were included. Studies with ≤ 5 patients or insufficient COU information were excluded. Two investigators (S.Z. and C.L.) independently screened titles and abstracts and performed full-text reviews of eligible studies. Meta-analyses of proportion were performed using random effects models for variables for which data were available from at least 3 included studies. Heterogeneity was assessed using the I2 value. All meta-analyses were completed using the Comprehensive Meta-Analysis software version 3.0. A 1-sample test for proportions was performed to compare COU at 3 months between patients with HNC who received RT/CRT and the general population of patients with cancer (14%).1 All statistical tests were 2-sided at the .05 significance level.
Results
A total of 134 studies were identified, with 7 retrospective studies8, 9, 10, 11, 12, 13, 14 (reporting on 1841 patients) meeting inclusion criteria (Fig 1). Median age was 59.4 (range: 56.0-62.0) years with 1343 (72.9%) men and 498 (27.1%) women. Primary tumor locations included oropharynx (n = 891, 48.4%), oral cavity (n = 533, 29.0%), larynx (n = 93, 5.1%), hypopharynx (n = 32, 1.7%), nasopharynx (n = 29, 1.6%), and nasal cavity/paranasal sinuses (n = 17, 0.9%). A total of 854 (46.0%) patients had stage I/II disease and 952 (50.3%) had stage III/IV disease. Three hundred one (16.3%) patients had RT alone, 738 (40.1%) received CRT, and 594 (32.3%) underwent surgery followed by adjuvant RT/CRT (Table 1).
Figure 1.
The article selection process flow diagram.
Table 1.
Studies included in the systematic review on chronic opioid use after radiation for head and neck cancer ∗
| Source† | Sample size | Mean (SD) or median (IQR) age, y | Men, n | Women, n | Oral cavity primary, n | Oropharynx primary, n | Larynx primary, n | Other primary, n | Early stage (I-II), n | Advanced stage (III-IV), n | Surgery & adjuvant RT/CRT, n | RT alone, n | CRT, n | History of psychiatric d/o, n | History of former/current EtOH use or abuse, n | History of former/current smoking, n | Pre-treatment opioid use, n | Chronic pain condition, n | COU at 3 mo, n | COU at 6 mo, n | COU at 12 mo, n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bollig and Jorgensen, 201910 | 122 | 59.8 (SD: 9.1) | 113 | 9 | — | 122 | — | — | 122 | 0 | 25 | 62 ‡ | 18 | 50 | 49 | 23 | — | 56 | — | — | |
| Kallurkar et al, 201911 | 53 | 61.23 (SD: 12.62) | 39 | 14 | 6 | 23 | 17 | 11 | 8 | 26 | 21 | — | 32 | — | — | — | — | — | 16 | — | — |
| Kwon et al, 20139 | 70 | 56 (IQR: 50-64) | 56 | 14 | 1 | 57 | 5 | 5 | 6 | 64 | — | — | — | 9 | 21 | 46 | 10 | — | 44 | 23 | — |
| McDermott et al, 20198 | 811 | — § | 497 | 314 | 393 | 310 | — | — | 564 | 247 | 254 | 54 | 237 | — | 95 | 116 | 337 | — | 150 | 68 | — |
| Schumacher et al, 201913 | 276 | 60 (—) | 218 | 58 | 98 | — | 46 | 14 | 66 | 204 | 116 | 150 | 126 | 21 | — | 159 | 65 | 101 | — | — | 20 |
| Silver et al, 201912 | 198 | 62 (SD: 9) | 177 | 21 | — | 198 | — | — | 32 | 166 | 18 | 35 | 145 | 44 | 131 | 137 | 45 | 57 | 104 | — | — |
| Smith et al, 201914 | 311 | 58.4 (SD: 12.2) | 243 | 68 | 35 | 181 | 25 | 48 | 56 | 245 | 160 | — | 198 | — | 51 | 167 | 47 | — | — | 40 | — |
| Total n (%) | 1841 | — | 1343 (72.9) | 498 (27.1) | 533 (29.0) | 891 (48.4) | 93 (5.1) | 78 (4.2) | 854 (46.0) | 952 (50.3) | 594 (32.3) | 301 (16.3) | 738 (40.1) | 92 (13.8) | 348 (23.0) | 674 (44.6) | 527 (29.5) | 158 (33.3) | 370 (29.5) | 131 (11.0) | 20 (7.0) |
Abbreviations: COU = chronic opioid use; CRT = chemoradiotherapy; d/o = disorder; EtOH = alcohol; HPV = human papilloma virus; IQR = interquartile range; RT = radiation therapy; SD = standard deviation.
—: not reported
All data were extracted independently by 2 authors (S.Z. and C.L.).
All included studies originated from centers in the United States.
This study pooled patients who received RT alone and CRT.
This study reported age categories with no clearly defined mean or median age for all patients included.
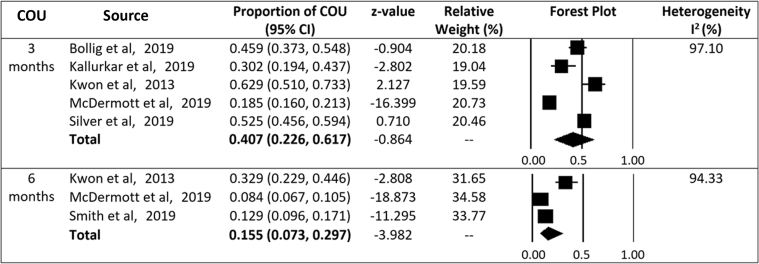
The proportion of patients with HNC who received curative-intent RT and developed COU was 40.7% at 3 months (95% confidence interval [CI]: 22.6%-61.7%; I2 = 97.1%; 5 studies) and 15.5% at 6 months (95% CI: 7.3%-29.7%; I2 = 94.3%; 3 studies; Fig 2). One study reported that 7.2% of patients had persistent COU at 1 year.13 Compared to the general population of patients with cancer, HNC patients have a significantly higher proportion of COU at 3 months (40.7% vs 14.0%; P < .0001; 95% CI: 38.0%-43.5%).
Figure 2.
Chronic opioid use at 3 and 6 months after radiation for head and neck cancer. Abbreviations: CI = confidence interval; COU = chronic opioid use.
There were significant differences in COU based on primary tumor sites (P < .0001), with the highest rate in oropharyngeal malignancies (46.6%; 95% CI: 30.8%-63.1%; I2 = 95.8%). High proportions of COU were found in patients with a history of psychiatric disorder(s) (61.7%; 95% CI: 28.7%-86.5%), former/current alcohol abuse (53.9%; 95% CI: 37.2%-69.9%), and opioid requirements before radiation treatment (51.6%; 95% CI: 24.8%-77.6%; P = .035). Of patients who smoked, 36.3% (95% CI: 19.2%-57.7%) developed COU. There was no significant difference in the proportion of COU by sex (P = .683), disease stage (I/II vs III/IV; P = .443), or treatment received (RT, CRT, or adjuvant RT/CRT; P = .711).
Discussion
The HNC population commonly constitutes patients with a significant history of smoking and/or alcohol abuse, often coupled with a comorbid psychiatric disorder.15 This likely exacerbates their experience of pain during RT. As the cornerstone of cancer pain management,8 opioid therapy may increase their risk for COU.16 Combined with the chronic morbidities of treatment, this may lead to decreased functional status, reduced return to work, and increased rates of suicide. We found that the prevalence of COU is significantly higher in patients with HNC treated with RT compared to the general population of patients with cancer. Risk factors for COU in patients with HNC include an oropharyngeal primary tumor, history of psychiatric disorder, alcohol abuse, and pre-treatment opioid use.
In the non-cancer chronic pain literature, untreated psychiatric disorders and prior or current substance abuse are known drivers for COU, misuse, and addiction.17 To our knowledge, this is the first systematic review and meta-analysis to confirm these as risk factors for COU in patients with HNC who received RT. Identifying high-risk patients with pre-treatment screening would facilitate mobilizing multidisciplinary teams including a pain specialist to curtail the risk of opioid misuse. These patients may also benefit from tailored psychosocial interventions and closer follow-up, with 1 physician prescribing opioids during treatment. COU may decline over time with such initiatives, and with the increasing prevalence of human papilloma virus–related HNC, which is shifting the demographic to patients with fewer risk factors for opioid addiction.
Prophylactic gabapentin has been shown to reduce opioid requirements in patients with HNC receiving CRT.18 This suggests that RIM pain likely requires a multimodal analgesic approach to address its nociceptive and neuropathic components for optimal pain relief.
Several limitations of this study warrant mention. Significant heterogeneity is noted between studies and is accounted for, in part, by the random effects model. Only 2 studies included demographic patient data on chronic pain,12,13 which was insufficient for pooling in a meta-analysis. Discerning between chronic and acute pre-treatment opioid use as high-risk factors for COU was therefore not feasible. All studies were retrospective in design, with no control groups, and subject to selection bias.
Conclusions
Given the location of their malignancy, its treatment, and common risk factors, a significant proportion of patients with HNC who receive RT suffer from COU. Prospective studies are required to evaluate opioid alternatives in managing RIM for effective pain relief in this patient population.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Data sharing statement: All data generated and analyzed during this study are included in this published article and its supplementary information files.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.09.023.
Supplementary Materials
References
- 1.Cuthbert C.A., Xu Y., Kong S., Boyne D.J., Hemmelgarn B.R., Cheung W.Y. Patient-level factors associated with chronic opioid use in cancer: A population-based cohort study. Support Care Cancer. 2020;28:4201–4209. doi: 10.1007/s00520-019-05224-y. [DOI] [PubMed] [Google Scholar]
- 2.Chino F., Kamal A., Chino J. Incidence of opioid-associated deaths in cancer survivors in the United States, 2006-2016: A population study of the opioid epidemic. JAMA Oncol. 2020;6:1100–1102. doi: 10.1001/jamaoncol.2020.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trotti A., Bellm L.A., Epstein J.B. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 4.Mirabile A., Airoldi M., Ripamonti C. Pain management in head and neck cancer patients undergoing chemo-radiotherapy: Clinical practical recommendations. Crit Rev Oncol Hematol. 2016;99:100–106. doi: 10.1016/j.critrevonc.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Alfieri S., Ripamonti C.I., Marceglia S. Temporal course and predictive factors of analgesic opioid requirement for chemoradiation-induced oral mucositis in oropharyngeal cancer. Head Neck. 2016;38(Suppl 1):E1521–E1527. doi: 10.1002/hed.24272. [DOI] [PubMed] [Google Scholar]
- 6.Elad S., Yarom N. The search for an effective therapy and pain relief for oral mucositis. JAMA. 2019;321:1459–1461. doi: 10.1001/jama.2019.3269. [DOI] [PubMed] [Google Scholar]
- 7.Sethi R.K.V., Panth N., Puram S.V., Varvares M.A. Opioid prescription patterns among patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2018;144:382–383. doi: 10.1001/jamaoto.2017.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott J.D., Eguchi M., Stokes W.A. Short- and long-term opioid use in patients with oral and oropharynx cancer. Otolaryngol Head Neck Surg. 2019;160:409–419. doi: 10.1177/0194599818808513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon J.H., Hui D., Chisholm G., Bruera E. Predictors of long-term opioid treatment among patients who receive chemoradiation for head and neck cancer. Oncologist. 2013;18:768–774. doi: 10.1634/theoncologist.2013-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollig C.A., Jorgensen J.B. Effect of treatment modality on chronic opioid use in patients with T1/T2 oropharyngeal cancer. Head Neck. 2019;41:892–898. doi: 10.1002/hed.25482. [DOI] [PubMed] [Google Scholar]
- 11.Kallurkar A., Kulkarni S., Delfino K., Ferraro D., Rao K. Characteristics of chronic pain among head and neck cancer patients treated with radiation therapy: A retrospective study. Pain Res Manag. 2019;2019 doi: 10.1155/2019/9675654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver N., Dourado J., Hitchcock K. Chronic opioid use in patients undergoing treatment for oropharyngeal cancer. Laryngoscope. 2019;129:2087–2093. doi: 10.1002/lary.27791. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher LD, Sargi ZB, Masforroll M, et al. Long-term opioid use in curative-intent radiotherapy: One-year outcomes in head/neck cancer patients. Head Neck. https://doi.org/10.1002/hed.26034 [DOI] [PMC free article] [PubMed]
- 14.Smith W.H., Luskin I., Resende Salgado L. Risk of prolonged opioid use among cancer patients undergoing curative intent radiation therapy for head and neck malignancies. Oral Oncol. 2019;92:1–5. doi: 10.1016/j.oraloncology.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Ing J.W. Head and neck cancer pain. Otolaryngol Clin North Am. 2017;50:793–806. doi: 10.1016/j.otc.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Paice J.A. A delicate balance: Risks vs benefits of opioids in cancer pain. Pain. 2020;161:459–460. doi: 10.1097/j.pain.0000000000001773. [DOI] [PubMed] [Google Scholar]
- 17.Webster L.R. Risk factors for opioid-use disorder and overdose. Anesth Analg. 2017;125:1741–1748. doi: 10.1213/ANE.0000000000002496. [DOI] [PubMed] [Google Scholar]
- 18.Hermann G.M., Iovoli A.J., Platek A.J. A single-institution, randomized, pilot study evaluating the efficacy of gabapentin and methadone for patients undergoing chemoradiation for head and neck squamous cell cancer. Cancer. 2020;126:1480–1491. doi: 10.1002/cncr.32676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.