Abstract
Empyema or infection of the pleural space is a well described complication of pneumonia, however knowledge of culprit pathogens is still evolving. We report a novel case of empyema due to Actinomyces turicensis, a commensal of the oropharynx and female urogenital tract but previously undescribed cause of empyema. We additionally review general pathogenesis of Actinomyces species within the pleural space. Familiarity with this unique pleural infection pathogen is important for selection of adequate antimicrobial therapy given the propensity of anaerobes such as Actinomyces species to disobey anatomic boundaries and recrudescence of infection in the absence of appropriate therapy.
1. Introduction
Anaerobes are commonly associated with empyema. Novel anaerobes are emerging. Familiarity with this expanding pool of culprit pathogens is important to ensure adequate treatment. We present a previously undescribed case of Actinomyces turicensis causing empyema.
2. Case report
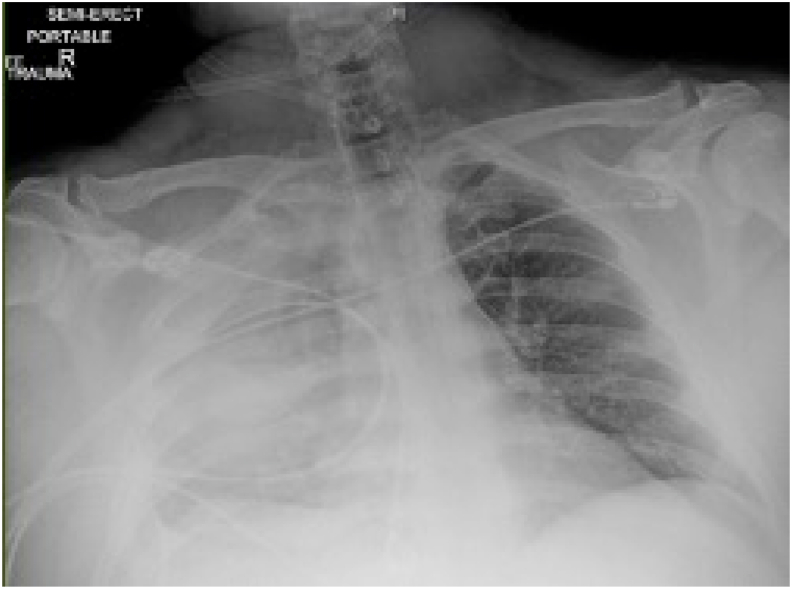
A 33 year old male with a history of obesity (BMI 39.2kg/m2), well controlled, non-insulin dependent, type 2 diabetes (Hgb A1C 6.4%) and obstructive sleep apnea presented to the emergency room with one week of severe, progressive, right sided chest pain, worse with deep inspiration and cough. The patient worked in elevator construction but denied recent musculoskeletal strain. On physical exam he was febrile to 101.9 °F, tachycardic to 128, and hypoxic with oxygenation saturation of 82% on room air for which he was placed on a non-rebreather. He was tachypneic and uncomfortable, speaking in partial sentences. Lung exam was limited by splinting with inspiration. Oropharynx revealed normal dentition without obvious caries though the patient reported rare dental care. Cardiac exam was normal without jugular venous distension, murmur, lower extremity asymmetry, or edema. Laboratory results were notable for a mild leukocytosis, unremarkable basic metabolic panel, CRP and ESR elevated to 202.5mg/L and 66mm/hr respectively, troponin <0.006ng/mL and normal procalcitonin of 0.31ng/mL. Chest x-ray revealed complete opacification of the right hemithorax (Fig. 1).
Fig. 1.
Chest x-ray with complete opacification of the right hemithorax
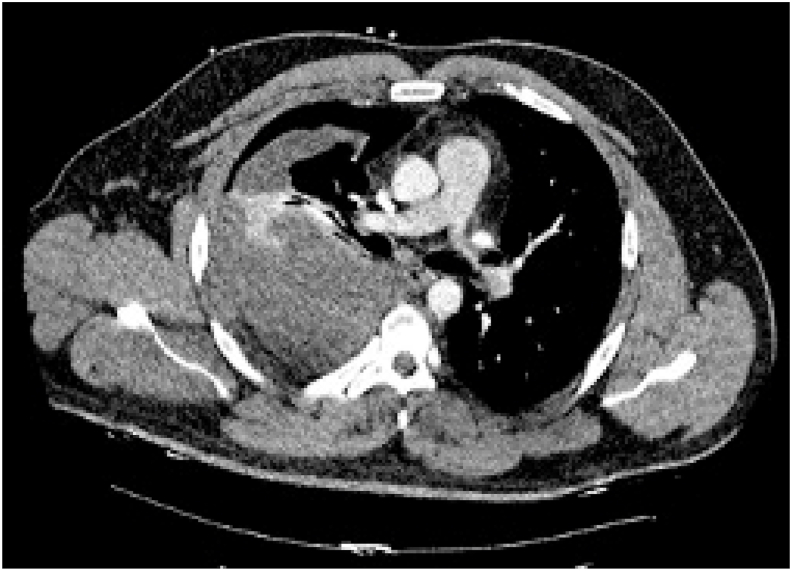
CT pulmonary angiogram was negative for pulmonary embolism but demonstrated a large, loculated pleural effusion with partial collapse of the right upper and lower lobes (Fig. 2).
Fig. 2.
CT chest revealing loculated pleural effusion and lobar collapse.
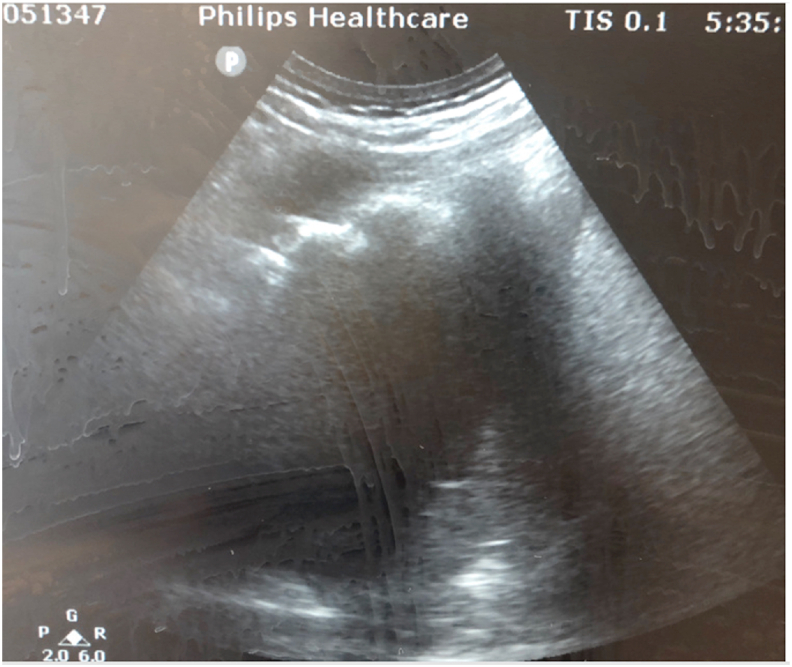
Fluid was visualized under ultrasound (Fig. 3) and a 12 French chest tube was placed posteriorly with immediate return of purulent and foul smelling brown fluid.
Fig. 3.
Fluid pocket identified under ultrasound, located medially to the right scapula.
Pleural fluid studies were consistent with empyema with pH 6.48, glucose <5 mg/dL, LDH 2681 U/L (serum 190), protein 4.1g/dL (serum 6.5) and cholesterol 117mg/dL. Gram stain revealed 4+ gram negative rods which subsequently speciated to Fusobacterium nucleatum. Six days later, an additional pathogen, the gram positive anaerobe Actinomyces turicensis, was isolated. The remel RapID ANA II system, a qualitative method to identify anaerobic pathogens, was used to identify this species [1].
The pleural space was treated with 6 doses of tPA and DNase, allowing for a total of 3.2L of chest tube drainage and rapid improvement in the patient's oxygenation down to 3L nasal cannula within the first 24 hours of admission. Initial treatment with ampicillin/sulbactam was narrowed to amoxicillin/clavulanic acid on discharge with plan to complete 6 months of therapy. In follow up 2 months after discharge, chest pain and dyspnea had resolved. Repeat chest x-ray demonstrated complete resolution of right sided effusion.
3. Discussion
Oropharyngeal colonizers, including anaerobic pathogens, are common empyema culprits [2]. Fetid, or foul smelling samples, are highly predictive of anaerobic pathogens in empyema [3]. While Fusobacterium nucleatum is a common anaerobic pulmonary pathogen, Actinomyces turicensis is a novel and, to our knowledge, previously undescribed cause of empyema. Awareness of Actinomyces subspecies has implications for selection of adequate antimicrobial therapy.
In general, thoracic actinomycosis is uncommon and a high degree of suspicion is required to identify this pathogen. With the capacity to infect the bronchopulmonary tree as well as the mediastinal and hilar lymph nodes, pleura and chest wall, radiographic presentations can be variable, ranging from lobar consolidation to lung abscess to adenopathy and pleural effusion. As a result of these nonspecific radiographic findings, the thoracic consequences of this pathogen are often mistaken for lung cancer, lymphoma or pulmonary infections due to common bacterial organisms, mycobacteria or aspergillosis [4,5].
A. Israelii is the most common Actinomyces species responsible for human disease. This species typically causes cervicofacial infection, with only 15% of known disease resulting in pulmonary pathology. When pulmonary infection does occur, it is thought to be the consequence of aspiration of oropharyngeal secretions with subsequent atelectasis, pneumonitis and potential invasion into the pleural space [6]. Despite propensity to disregard anatomic boundaries, a review of cases of pulmonary A. israelii over the past century confirms a rare association with pleural effusion (8 of 94 cases (9.6%)) and empyema (2 patients) [7]. In addition to A. israelii, a review of nearly 500 clinical isolates of Actinomyces demonstrated the importance of A. meyeri, A. gravenitizii and A. odontolyticus as the primary Actinomyces sub-species isolated within the thoracic cavity to date [8]. These species have been associated with case reports of empyema, organizing pneumonia and cavitary lesions, respectively [9,10,11].
In 1995, the novel actinomyces species, Actinomyces turicensis, was described as a gram positive, predominately anaerobic commensal of the female urogenital tract. Causally associated with oral malodor, this pathogen has additionally been isolated within the tongue microbiome [12]. In fact, Actinomyces infection in general, is two to four times more common in men, partly attributed to poorer oral hygiene [13].
Due to overgrowth of synergistic bacteria and air exposure during processing of broncheolalveolar lavage samples, Actinomyces is notoriously difficult to culture. Subspecies identification is difficult [14]. When possible, identification of branching filaments and typical yellow sulfur granules on culture is pathognomonic for the overall genus [15]. However, more contemporary biochemical testing not only improves genus identification when culture is limited but provides species discrimination. In this case, the remel RapID ANA II system was used to identify A. Turicensis. This biochemical test evaluates the interaction of anaerobic species of interest with 20 biochemicals. A profile of specific biochemical interactions then provides a probability of a panel of potential anaerobic pathogens. While misidentification is possible, this test had the ability to discriminate between various Actinomyces species, including israelli and myeri. Results correlate with a 97% specificity for A. turicensis [16].
As our knowledge of the Actinomyces genus expands, clinical familiarity with this pathogen is critical for expedited diagnosis and treatment. Furthermore, capacity to accurately identify sub-species is important given variable susceptibility to antimicrobial agents and a goal to avoid surgical intervention. For example, a study of 87 Actinomyces species demonstrated reduced susceptibility of A. turicensis to piperacillin/tazobactam as well as erythromycin. As compared to all other species of Actinomyces tested, including A. israelli, A. turicensis additionally had a notably higher minimum inhibitory concentration for linezolid suggesting impaired efficacy of this antibiotic when selected for this species. Reduced susceptibility may relate to poor penetration of antimicrobial agents into actinomycotic lesions [17]. Differences in antimicrobial response are of particular importance given 6 months of recommended antimicrobial therapy for thoracic infections, characterized by propensity for recrudescence [18].
4. Conclusion
To our knowledge, this is the first report of Actinomyces turicensis causing polymicrobial empyema. In conjunction with presumed dental caries as a result of lack of routine dental care and diabetes, increased weekend alcoholic consumption may have increased his susceptibility to aspiration and polymicrobial anaerobic infection. For polymicrobial empyema, attention towards slow growing and novel pathogens should be prioritized to ensure adequate antimicrobial therapy and avoidance of unnecessary surgical intervention.
Consent
Permission for publication of this case report was obtained from the patient described in this case report.
Declaration of competing interest
Neither Dr. Ehab Billatos, MD nor myself, Dr. Shelsey Johnson, MD have conflicts of interest to disclose for this case report submission. We did not have funding or sponsors for this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2021.101365.
Contributor Information
Shelsey W. Johnson, Email: shelsey.johnson@bmc.org.
Ehab Billatos, Email: ehab.billatos@bmc.org.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.https://www.thermofisher.com/document-connect/document-connect.html?url=https%3A%2F%2Fassets.thermofisher.com%2FTFS-Assets%2FMBD%2FInstructions%2FIFU8311002.pdf&title=UmFwSUQgQU5BIElJIFN5c3RlbQ==.
- 2.Brook I., Frazier E.H. Aerobic and anaerobic microbiology of empyema. Retrospective review in two military hospitals. Chest. 1993;103(5):1502–1507. doi: 10.1378/chest.103.5.1502. [DOI] [PubMed] [Google Scholar]
- 3.Alfageme I., Munoz F., Pena N., Umbria S. Empyema of the thorax in adults: etiology, microbiologic findings, and management. Chest. 1993;103(3):839–843. doi: 10.1378/chest.103.3.839. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh M.-J., Liu H.-P., Chang J.-P., Chang C.-H. Thoracic actinomycosis. Chest. 1993;104:366–370. doi: 10.1378/chest.104.2.366. [DOI] [PubMed] [Google Scholar]
- 5.Kim S., Jung L., Oh I., Young-Chul K. Pulmonary Actinomyces during the first decade of 21st century: cases of 94 patients. BMC Infect. Dis. 2013;13:216. doi: 10.1186/1471-2334-13-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mabeza G.F., Macfarlane J. Pulmonary actinomyces. Eur. Respir. J. 2003;21:545–551. doi: 10.1183/09031936.03.00089103. [DOI] [PubMed] [Google Scholar]
- 7.Kim, S et al., 2013.
- 8.Val H., Talbot P.R., Stubbs S.L., Duerden B.I. Identification of clinical isolates of actinomyces species by amplified 16S ribosomal DNA restriction analysis. J. Clin. Microbiol. 2001:3555–3562. doi: 10.1128/JCM.39.10.3555-3562.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazili T., Blair D., Riddell S., Kiska D., Sagra S. Actinomyces meyeri infection: case report and review of the literature. J. Infect. 2012;65(4):357–361. doi: 10.1016/j.jinf.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Fujita Y., Ikura M., Horio Y., Ohkusu K., Kobayashi N. Pulmonary actinomyces graevenitzii infection presenting as organizing pneumonia diagnosed by PCR analysis. J. Med. Microbiol. 2012;61:1156–1158. doi: 10.1099/jmm.0.040394-0. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto T., Kusakabe Y., Enomoto M., Yamamoto N., Aihara K., Yamaoka S., Mishima M. Drastically progressive lung cavity lesion caused by Actinomyces odontolyticus in a patient undergoing chemoradiotherapy: a case report and literature review. Respir Med Case Rep. 2019;28 doi: 10.1016/j.rmcr.2019.100950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyrrell K., Citron D., Warren Y., Nachnani S., Goldstein E. Anaerobic bacteria cultured from the tongue dorsum of subjects with oral malodor. Anaerobe. 2003;9(5):243–246. doi: 10.1016/S1075-9964(03)00109-4. [DOI] [PubMed] [Google Scholar]
- 13.Mabeza and Macfarlane, 2003. [DOI] [PubMed]
- 14.Mabeza and Macfarlane, 2003. [DOI] [PubMed]
- 15.Hsieh, M-J et al., 1993.
- 16.Remel RapID ANA II System Package Insert. June 01, 2017. [Google Scholar]
- 17.Smith A.J., Hall V., Thakker B., Gemmell C.G. Antimicrobial susceptibility testing of Actinomyces species with 12 antimicrobial agents. J. Antimicrob. Chemother. 2005;56:407–409. doi: 10.1093/jac/dki206. [DOI] [PubMed] [Google Scholar]
- 18.Kolditz M., Bickhardt J., Matthiessen W. Medical management of pulmonary actinomyces: data from 49 consecutive cases. J. Antimicrob. Chemother. 2009;63(4):839–841. doi: 10.1093/jac/dkp016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.