Abstract
Purpose
Pharmacogenomics (PGx) studies how inherited genetic variations in individuals affect drug absorption, distribution, and metabolism. PGx panel testing can potentially help improve efficiency and accuracy in individualizing therapy. This study compared the cost-effectiveness between preemptive PGx panel testing, reactive PGx panel testing and usual care (no testing) in cardiovascular disease management.
Methods
We developed a decision analytic model from the US payer’s perspective for a hypothetical cohort of 10,000 patients ≥45 years old, using a short-term decision tree and long-term Markov model. The testing panel included the following gene–drug pairs: CYP2C19–clopidogrel, CYP2C9/VKORC1–warfarin, and SLCO1B1–statins with 30 test-return days. Costs were reported in 2019 US dollars and effectiveness was measured in quality-adjusted life years (QALYs). The primary outcome was incremental cost-effectiveness ratio (ICER = ΔCost/ΔQALY), assuming 3% discount rate for costs and QALYs. Scenario and probabilistic sensitivity analyses were performed to assess the impact of demographics, risk level, and follow-up timeframe.
Results
Preemptive testing was found to be cost-effective compared with usual care (ICER $86,227/QALY) at the willingness-to-pay threshold of $100,000/QALY while reactive testing was not (ICER $148,726/QALY). Sensitivity analyses suggested that our cost-effectiveness results were sensitive to longer follow-up, and the age group 45–64 years.
Conclusion
Compared with usual care, preemptive PGx panel testing was cost-effective in cardiovascular disease management.
Key words: pharmacogenomics, cost-effectiveness, genomic panel testing, cardiovascular diseases management
INTRODUCTION
Pharmacogenomics (PGx) investigates the effect of inherited genetic variations on drug absorption, distribution, and metabolism.1,2 PGx can be used to predict response variability for certain drugs, which in turn can facilitate individualized disease management. The US Food and Drug Administration (FDA) has approved 385 drugs with genomic biomarker information in their labeling to assist in PGx-guided therapy, which has seen increased adoption in clinical practice in recent years.3–6
Cardiovascular diseases (CVD) are among the most prevalent and expensive chronic diseases. In the United States, approximately 630,000 people die from CVDs every year, and the economic burden is projected to increase to $1.1 trillion in 2035.7,8 Given the substantial burden of morbidity and costs associated with CVD, PGx-guided therapy can potentially play an important role in reducing this burden by preventing drug-related adverse events and optimizing treatment effectiveness.2
Having access to PGx testing results can inform a clinician of the presence or absence of specific gene variant(s) that a drug might adversely react to, and thus can help the clinician prescribe the right medication at the right dose. PGx testing can be done preemptively or reactively. Preemptive testing can be conducted before a disease is symptomatic and a drug with PGx information is prescribed. The results can then be integrated into patients’ health records, making this information available at the point of care.9,10 Reactive testing, on the other hand, is conducted before any high-risk medication is prescribed to better understand the effects of medication options and to maximize treatment effectiveness upon receiving the PGx test results.5,9 Panel testing is a method that allows for multiple genes to be analyzed in one assay. Given that patients often have multiple gene variants, panel testing may be significantly more efficient and less expensive than conducting multiple single-gene assays.11–13 In the cardiovascular arena, as suggested by FDA Table of Pharmacogenomic Biomarkers in Drug Labeling, the Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines, as well as a recent comprehensive literature review by Zhu et al., four of the most prominent genes and the related drug choices included in a PGx panel test are CYP2C19 (clopidogrel vs. ticagrelor), CYP2C9/VKORC1 (warfarin vs. novel oral anticoagulants [NOACs]), and SLCO1B1 (statins vs. proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors).14,15 The recent price reduction for PCSK9 inhibitors from $14,000/year to $5850/year (Repatha and Praluent) may substantially change CVD management because while statins are by far the most well-established first-line pharmacotherapy for hyperlipidemia management, PCSK9 inhibitors have been found to have considerable clinical significance in hyperlipidemia management for patients with elevated risk of statin-related adverse events (e.g., myopathy) and/or for whom statins are ineffective.16–19 SLCO1B1 variants have been found to be associated with muscle toxicity from many statins (e.g., simvastatin, rosuvastatin), but the associations between dosage, SLCO1B1 variations, and clinical outcomes are not yet well established.15,20 The emergence of PCSK9 inhibitor as an alternative to statin reshaped lipid treatment.21 However, for many insurers, PCSK9 prescription needs a 3-month statin trial proven to be ineffective and also requires prior authorization.22 PGx testing can flag patients unlikely to benefit from statins, and initiation of PCSK9 therapy instead of statin for them would be a cost-saving and efficient strategy that can avoid statin-related adverse events and circumvent the prior authorization process. Evidence on the cost-effectiveness of implementing PGx panel testing, either preemptively or reactively, compared with usual care for any of the gene–drug combinations is scarce.14
The aim of this study was to compare cost-effectiveness of preemptive and reactive PGx panel testing strategies with usual care (no PGx testing) to guide treatment choices for a population with average CVD risk. The secondary aim of the study was to assess cost-effectiveness of the three strategies in different patient subgroups varied by age, gender, race, CVD risk level, and timeframe for whom experimental studies are either very costly, infeasible, or require significantly longer time to complete.
MATERIALS AND METHODS
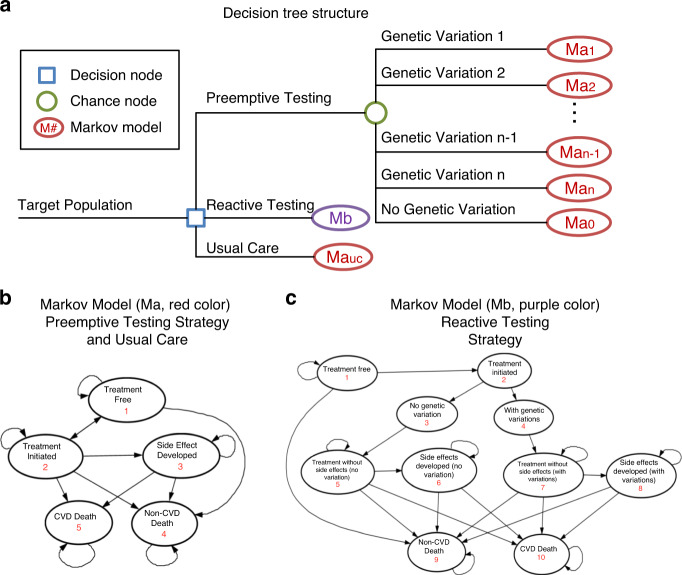
We developed a decision analytic model composed of a decision tree and long-term Markov models (up to 100 years) to evaluate the cost-effectiveness of three treatment strategies in CVD management: (1) preemptive PGx panel testing, (2) reactive PGx panel testing, and (3) usual care for treatment with no PGx testing (Fig. 1a). Health states corresponding to preemptive and reactive PGx panel testing are shown in Fig. 1b, c. Each arrow illustrates a transition pathway from one disease state to another and is assigned a corresponding probability value. All patients start at treatment-free state, which is assumed to be the same as disease-free state. The preemptive group and usual care group share the same model structure because they do not have a time period in which they must wait for the genetic testing results to come back; however, they both have distinct sets of transition probabilities. Usual care group is assigned the probabilities from outcome studies with no genetic information, while preemptive group is assigned the probabilities obtained from clinical trials. In the reactive testing arm, the patient first undergo usual care when diagnosis is established, and after one cycle (one month), patients follow a path similar to those in the preemptive cohort described above. Other disease states include treatment without adverse events, treatment with adverse events, death from CVD, and background deaths, which are adopted as non-CVD deaths. All the adverse events from corresponding medications are considered as different health states from treatments without adverse events, lasting repetitive cycles until the patients transition to another state. The details of disease states, medications, and their adverse events are listed in eFig. 1.
Fig. 1. Model structure for the cost-effectiveness analysis.
(a) Decision tree structure. The simulation begins with study individuals entering the model as the target population, and proceeding into one of three treatment strategies: preemptive pharmacogenomics (PGx) panel testing (genetic testing performed before a disease is diagnosed), reactive PGx panel testing (genetic testing activated when a drug is needed), and usual care (no testing is performed). Ma Markov model a, Mb Markov model b, uc usual care. Subscripts 1 to n indicate n possibilities of genetic variations, with 0 indicating no variation. Preemptive testing and usual care share the same Markov model structure, while reactive testing is under a different model structure. The actual detailed decision tree is listed in eFig. 6. (b, c) Simplified Markov model structures. Preemptive PGx panel testing strategy and usual care share the same model structure for similar transition processes among the disease states (Fig. 1b or Ma), and reactive PGx panel testing strategy has a different model structure considering the different treatment approaches with the known genetic information (Fig. 1c or Mb). The actual disease states used in the simulation are listed in eFig. 7. CVD cardiovascular disease.
Typically, odds ratios (OR), relative ratios, or hazard ratios are reported comparing disease development between patients with and without genetic variation, and are calculated using the following equations:
| 1a |
| 1b |
where pvar is the probability with genetic variation, Pnon_var is the probability with no genetic variation, and OR is the odds ratio of the genetic variation compared with the nonvariation group. Hazard ratios and relative ratios are estimated as OR. Cycle length for the model is one month. We adopted the US health-care payer’s perspective primarily because health insurers will play a major role in the adoption of preemptive versus reactive PGx panel testing in the United States.
Study population
The study population include a hypothetical cohort of 10,000 individuals resembling the US population aged 45 years and older (median: 60 years old, distribution listed in eFig. 2), sex (49% male), and race (80% White, 14% African American, 6% Asian) distribution as per the National Vital Statistics System, Centers for Disease Control and Prevention, and Census Bureau, and is assumed to have average risk of developing a first CVD event (approximately 4% in 10 years).23–25 The model tracks individuals in the cohort through each health state and estimates costs and quality-adjusted life years (QALYs) over 50 years. The specific CVD-related conditions and/or outcomes considered are identified in the systematic review14 as well as from a comprehensive review of guidelines from the American Heart Association, American College of Cardiology, and other cardiology-related societies or organizations (eTable 1) are as follows: myocardial infarction (MI), coronary heart disease (CHD), atrial fibrillation (AF), valvular heart disease (VHD), hyperlipidemia (HLP), peripheral artery disease (PAD), and ischemic stroke (stroke).
Testing strategies
As indicated previously, the PGx panel test for the study include the following genes: CYP2C19, CYP2C9/VKORC1, and SLCO1B1.14,15 The genetic polymorphisms included in the simulation model are listed in eTable 2. Alternative drugs as suggested by extant evidence are used when PGx testing suggests genetic variations associated with potential harm (eTable 2):26–28 ticagrelor is used as an alternative drug to clopidogrel, NOACs are used as alternatives to warfarin, and PCSK9 inhibitors are used as alternative to statins. We model the alternative treatments that are not known to have a drug–gene relationship with the indicated genes (NOAC and PCSK9 inhibitor), or have a trace of drug–gene relationship but are found to be cost-effective under genotype-guided strategy (ticagrelor).15,20,28,29
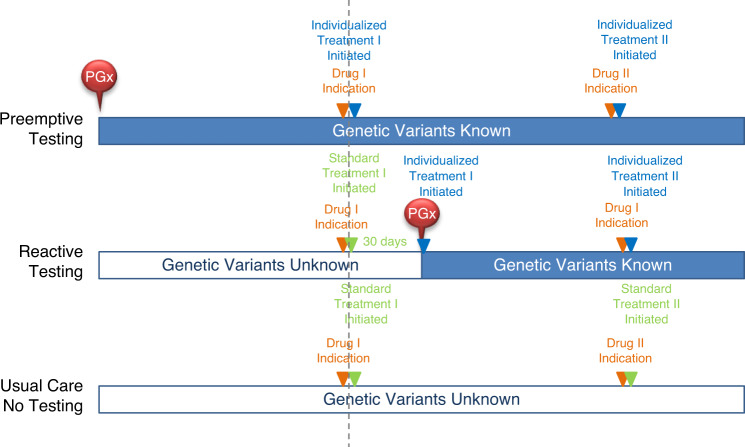
The timelines of the three test strategies are illustrated in Fig. 2. Patients undergo preemptive PGx panel testing before disease develops and drug therapy is indicated. Reactive PGx panel testing refers to tests administered after the first disease is diagnosed and drug therapy is indicated, but before the treatment is initiated. There is a 30-day test turnaround time for reactive testing. In this study, the reactive testing is panel-based instead of single gene–based, which means that all four genes would be tested once one of the index drugs is indicated. The genetic information would be kept in the patient’s health records and would inform appropriate treatment for other diseases developed in the future. In that sense, the reactive testing strategy is “partially preemptive.” Patients with no genetic testing at any point in time are defined as the usual care group, and no alternative drugs are given. The major differences between preemptive and reactive testing are (1) preemptive strategy tests everyone, while reactive strategy only tests patients who develop disease; (2) patients in the reactive testing arm undergo usual care for one month, when all disease probabilities follow those of the usual care arm.
Fig. 2. The timeline for preemptive pharmacogenomics (PGx) panel testing and reactive PGx panel testing strategies vs. usual care.
For the preemptive testing strategy, patients undergo genetic testing before the disease is diagnosed (drug therapy is indicated) and treatment is initiated. With the known genetic variations, treatment can be initiated directly once the patient develops the disease and the drug is indicated. However, not everyone will develop disease and therefore PGx testing may be wasteful in these healthy individuals. For the reactive testing strategy, patients undergo genetic testing after drug therapy is indicated and standard treatment is initiated. Upon receiving the genetic information, individualized treatment is initiated. In this way, genetic testing is only given to patients who are prescribed at least one of the three medications (i.e., clopidogrel, warfarin, or statin). However, once the results are ready, further treatment could be initiated immediately after the disease is diagnosed and the drug is indicated. No genetic testing is performed under the usual care strategy.
Model inputs
The range and distribution of parameters were obtained using currently available results from published studies, including randomized controlled clinical trials, meta-analyses, cohort or cross-sectional studies, and cost-effectiveness analyses (eTable 3). Overall non-CVD death rates were captured by age and race from the CDC’s National Vital Statistics System (eFig. 3).23 Model input values for the parameters were converted to monthly rates and then converted into monthly probabilities to conform to the monthly model cycles (Eq. 2).
| 2 |
where p is the probability for in a time period (e.g., 1 month), r is the disease developing rate, and t is the time period (e.g., 1 month).
The base-case value for each of the variables was derived from the population that closely resembled the modeling population in specific health status. For example, the base-case value for monthly medication cost of NOACs was obtained from the cost-effectiveness study on NOAC in atrial fibrillation patients.27 The base-case analysis was calculated using the base-case values to report a most expected estimation that could happen in a real-world scenario. The ranges of the parameters were derived from multiple studies that exist in the current literature, with the same payer perspective as ours and that focused on US populations. Two major sources were used to identify references: (1) the database of our previous work, a systematic review of cost-effectiveness in CVD patient management (12 references);2 and (2) clinical guidelines and the studies cited by these disease-specific guidelines (21 references) (eTable 3). Assumptions were made on parameters for which pertinent data were not available (eTable 4).
Costs
We included costs associated with medications (e.g., warfarin), genetic panel testing, disease treatment (e.g., monthly costs for post-MI), disease costs by event (i.e., hospitalization for MI and stroke), treatment of drug side effects (e.g., bleeding from warfarin use), and costs of CVD- and non-CVD-related deaths. When available, we captured costs from published literature with the same perspective and converted them to 2019 US dollars using the US gross domestic product deflator (Supplementary Material II).30
Utility
QALYs were used to calculate the effectiveness of the three strategies, including disutility of diseases, their treatments and adverse drug effects from the treatments. Utility values were captured from literature on the US population with the same disease diagnosis (eTable 3). Each disease state was assigned a value that reflected the overall quality of life when living with that disease state. There are two types of death modeled in this study: CVD death and non-CVD death. Both deaths were absorbing states, while the utility value of 0.2355 was used for the final cycle when a patient dies of CVD rather than the health state the patient was in just prior to CVD death.27,31 No other death transition resulted in a lower utility than that of the health state just prior to death.
Analysis
The study reported incremental cost-effectiveness ratios (ICERs) as the primary outcome for the base-case and scenario analyses. We assessed cost-effectiveness of the three treatment strategies under different scenarios to illustrate the hypothetical outcomes that could be achieved for a specific patient population. In each of the scenarios, one parameter was set at a fixed value or range of values while the other parameters still followed the assumed model distributions. Parameters included in the scenario analyses were patient’s age, sex, risk level, race, and study timeframe (Table 1), which were known to be associated with health outcomes, disease rates, and death rates along with the effects of the genetic variants.32 One-way sensitivity analyses were conducted for all the parameters. The ranges of ICERs calculated from the minimum value and maximum value of each parameter were compared and the top 20 parameters with the largest ICER ranges were reported. Probabilistic sensitivity analysis was conducted and cost-effectiveness acceptability curve was reported. Cost and utility values followed gamma distribution and probability values followed beta distribution.33 Tables were generated for parameters (e.g., non-CVD death, probabilities from disease-free to disease development) changing with race, gender, and age (eTable 5). The analysis used a 3% annual discount rate for both costs and QALYs. Cost-effectiveness was examined using the willingness-to-pay (WTP) threshold set at $100,000 per QALY. The model was constructed and analyzed using TreeAge Pro 2019 software (TreeAge Software, Williamstown, MA).
Table 1.
Cost-effectiveness of preemptive testing, reactive testing, or usual care.
| Costs, $ | QALYs, n | Comparison groups | Incremental costs, $ | Incremental QALYs, n | Life years, n | ICER, $/QALY | Conclusion (willingness-to-pay = $100,000) | |
|---|---|---|---|---|---|---|---|---|
| Base-case cohort | ||||||||
| Usual care (UC) | 175,410 | 5.85 | - | - | - | 12.52 | - | - |
| Reactive testing (REA) | 188,754 | 5.94 | REA vs. UC | 13,344 | 0.09 | 12.50 | 148,726 | Not cost-effective |
| Preemptive testing (PRE) | 205,841 | 6.20 | PRE vs. UC | 30,431 | 0.35 | 13.52 | 86,227 | Cost-effective |
| PRE vs. REA | 17,087 | 0.26 | 64,921 | Cost-effective | ||||
| Scenario analysis | ||||||||
| Age | ||||||||
| 45–54 | ||||||||
| UC | 194,588 | 6.74 | - | - | - | 14.89 | - | - |
| REA | 211,294 | 6.86 | REA vs. UC | 16,705 | 0.17 | 14.83 | 95,480 | Cost-effective |
| PRE | 232,635 | 7.22 | PRE vs. UC | 38,047 | 0.45 | 16.24 | 84,398 | Cost-effective |
| 55–64 | ||||||||
| UC | 185,146 | 5.94 | - | - | - | 13.17 | - | - |
| REA | 198,787 | 6.00 | REA vs. UC | 13,641 | 0.07 | 13.17 | 209,616 | Not cost-effective |
| PRE | 218,092 | 6.30 | PRE vs. UC | 32,946 | 0.34 | 14.27 | 97,717 | Cost-effective |
| 65–74 | ||||||||
| UC | 166,155 | 5.12 | - | - | - | 10.74 | - | - |
| REA | 176,460 | 5.12 | REA vs. UC | 10,305 | 0.00 | 10.63 | na | Not effectivea |
| PRE | 188,094 | 5.27 | PRE vs. UC | 21,939 | 0.19 | 11.23 | 112,706 | Not cost-effective |
| 75 and above | ||||||||
| UC | 108,553 | 3.06 | - | - | - | 5.68 | - | - |
| REA | 114,475 | 3.03 | REA vs. UC | 5922 | −0.04 | 5.57 | na | Not effectivea |
| PRE | 119,172 | 3.14 | PRE vs. UC | 10,620 | 0.09 | 5.89 | 123,025 | Not cost-effective |
| Sex | ||||||||
| Male | ||||||||
| UC | 167,470 | 5.60 | - | - | - | 11.68 | - | - |
| REA | 179,764 | 5.67 | REA vs. UC | 12,294 | 0.07 | 11.64 | 180,139 | Not cost-effective |
| PRE | 195,765 | 5.91 | PRE vs. UC | 28,295 | 0.31 | 12.62 | 91,451 | Cost-effective |
| Female | ||||||||
| UC | 171,309 | 6.36 | - | - | - | 12.74 | - | - |
| REA | 186,462 | 6.54 | REA vs. UC | 15,154 | 0.18 | 12.76 | 83,271 | Cost-effective |
| PRE | 204,955 | 6.77 | PRE vs. UC | 33,647 | 0.42 | 13.83 | 80,921 | Cost-effective |
| Race | ||||||||
| White | ||||||||
| UC | 178,971 | 5.96 | - | - | - | 12.62 | - | - |
| REA | 192,414 | 6.07 | REA vs. UC | 13,443 | 0.11 | 12.59 | 122,496 | Not cost-effective |
| PRE | 209,334 | 6.32 | PRE vs. UC | 30,363 | 0.36 | 13.63 | 84,740 | Cost-effective |
| Asian | ||||||||
| UC | 179,465 | 5.72 | - | - | - | 12.62 | - | - |
| REA | 192,853 | 5.79 | REA vs. UC | 13,387 | 0.08 | 12.71 | 172,821 | Not cost-effective |
| PRE | 208,215 | 6.05 | PRE vs. UC | 28,750 | 0.34 | 13.68 | 85,616 | Cost-effective |
| African American | ||||||||
| UC | 150,745 | 5.31 | - | - | - | 11.59 | - | - |
| REA | 164,097 | 5.35 | REA vs. UC | 13,352 | 0.03 | 11.60 | 408,284 | Not cost-effective |
| PRE | 182,529 | 5.63 | PRE vs. UC | 31,784 | 0.32 | 12.65 | 99,814 | Cost-effective |
| Risk level | ||||||||
| Risk 50% higher (6%) | ||||||||
| UC | 176,174 | 5.83 | - | - | - | 12.52 | - | - |
| REA | 189,391 | 5.92 | REA vs. UC | 13,217 | 0.09 | 12.50 | 144,728 | Not cost-effective |
| PRE | 206,731 | 6.19 | PRE vs. UC | 30,557 | 0.36 | 13.52 | 83,986 | Cost-effective |
| PRE vs. REA | 17,340 | 0.27 | 63,630 | Cost-effective | ||||
| Risk 100% higher (8%) | ||||||||
| UC | 176,496 | 5.78 | - | - | - | 12.49 | - | - |
| REA | 189,537 | 5.86 | REA vs. UC | 13,041 | 0.09 | 12.47 | 152,010 | Not cost-effective |
| PRE | 206,707 | 6.13 | PRE vs. UC | 30,211 | 0.35 | 13.46 | 86,040 | Cost-effective |
| PRE vs. REA | 17,170 | 0.27 | 64,710 | Cost-effective | ||||
| Timeframe | ||||||||
| 5 years | ||||||||
| UC | 75,716 | 2.81 | - | - | - | 4.13 | - | - |
| REA | 80,602 | 2.77 | REA vs. UC | 4886 | 0.04 | 4.02 | na | Not effectivea |
| PRE | 83,307 | 2.85 | PRE vs. UC | 7591 | 0.04 | 4.21 | 202,950 | Not cost-effective |
| PRE vs. REA | 2706 | 0.07 | 36,655 | Cost-effective | ||||
| 20 years | ||||||||
| UC | 158,487 | 5.38 | - | - | - | - | - | - |
| REA | 170,125 | 5.42 | REA vs. UC | 11,638 | 0.03 | 10.45 | 365,326 | Not cost-effective |
| PRE | 183,231 | 5.63 | PRE vs. UC | 24,744 | 0.25 | 10.38 | 99,248 | Cost-effective |
| PRE vs. REA | 13,105 | 0.22 | 11.09 | 60,267 | Cost-effective | |||
| 30 years | ||||||||
| UC | 171,945 | 5.74 | - | - | - | - | - | - |
| REA | 184,671 | 5.82 | REA vs. UC | 12,726 | 0.08 | 12.05 | 162,859 | Not cost-effective |
| PRE | 200,496 | 6.05 | PRE vs. UC | 28,551 | 0.30 | 12.02 | 94,260 | Cost-effective |
| PRE vs. REA | 15,825 | 0.22 | 12.94 | 70,411 | Cost-effective | |||
Costs and QALYs were per person over the 50-year timeframe for base-case and scenario analyses for age, sex, race, risk level and indicated timeframe.
The bold values were preemptive testing values.
ICER incremental cost-effectiveness ratio, PRE preemptive testing, QALY quality-adjusted life year, REA reactive testing, UC usual care.
a“Not effective” indicates fewer QALYs gained. It violate the basic assumption of cost-effectiveness that treatment arms must be equal to or more effective than the usual care arm. Therefore, ICER was not reported.
RESULTS
Base-case analysis
The results of the base-case analysis are presented in Table 1. Preemptive PGx panel testing compared with usual care (no PGx testing) resulted in an ICER of $86,227/QALY. Reactive PGx panel testing compared with usual care yielded an ICER of $148,726/QALY. These results suggested that preemptive panel testing was cost-effective compared with usual care at a WTP of $100,000/QALY, while reactive panel testing was not cost-effective. Compared with usual care, preemptive testing resulted in an average of 0.35 additional QALYs for each person, while reactive testing resulted in an average increase of 0.09 QALYs; preemptive testing increased the average costs by $30,431 per person, while reactive testing increased costs by $13,344 per person. Compared with reactive testing, preemptive testing was found to be cost-effective with an ICER of $64,921/QALY. The key outcomes modeled in the analysis are listed in eTable 5, including proportion of patients with each CVD disease, proportion of death and CVD death, and all adverse events.
Scenario analyses
The changes in age, sex, race, and risk level did not change the study conclusions about cost-effectiveness of preemptive testing compared with usual care (column “Conclusion,” Table 1). Preemptive testing changed from ineffective to effective beginning at 26 years, implying that the cost-effectiveness of preemptive testing was sensitive to changes in the study timeframe. On the other hand, reactive testing was found to be cost-effective in certain scenarios.
Compared with usual care, the cost-effectiveness of preemptive PGx testing varied by age (Table 1): the 45–55 and 55–65 year age groups had ICERs of $84,398/QALY and $97,717/QALY, respectively, and were cost-effective. The groups with age ≥65 years old (65–74 years, and 75 years and above) had ICERs higher than WTP and were not cost-effective. Reactive PGx testing was cost-effective for 45–54 years old (ICER of $95,480) and was not cost-effective for age ≥55 years old.
Compared with usual care, preemptive tests had higher ICER ($91,451/QALY) among male patients than female patients ($80,921/QALY) and both groups were cost-effective. Reactive tests were not cost-effective for male patients ($180,139/QALY) but were cost-effective for female patients ($83,271/QALY).
The ICER of PGx testing generally changed slightly with race (Table 1). Compared with usual care, preemptive testing resulted in a lower ICER for African Americans ($99,814/QALY), while the ICERs for Whites ($84,740/QALY) and Asians ($85,616/QALY) were similar. Reactive testing had higher ICERs for all three racial groups and was not cost-effective.
If the risk level was increased by half (to 6%), preemptive testing yielded an ICER of $83,986/QALY. Doubling the risk level from 4% to 8% led to a slightly higher ICER for preemptive testing ($86,040/QALY) compared with usual care. When the risk level increased, the costs for all three strategies changed slightly, while the QALYs declined.
Cost-effectiveness of preemptive PGx testing improved when patients were observed for a longer timeframe. For example, preemptive testing for a 5-year time horizon was not cost-effective (ICER = $202,950/QALY) compared with usual care; however, for a 20-year horizon, preemptive testing became cost-effective (ICER = $99,248/QALY), which went down to $94,260/QALY at 30 years. The key outcomes of CVD disease, death, and adverse events are listed in eTable 5.
Sensitivity analyses: one-way sensitivity analyses
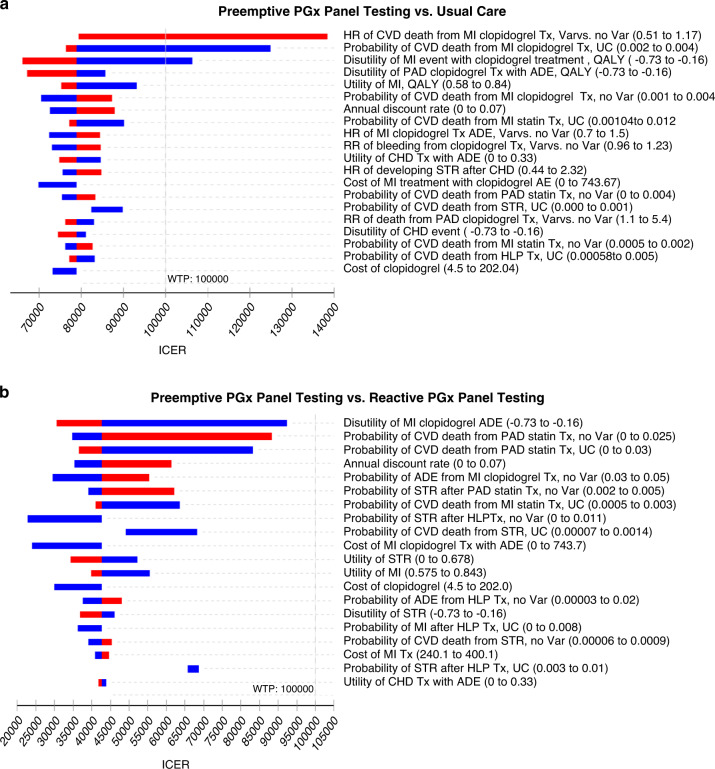
One-way sensitivity analysis was performed on the parameters associated with costs, utilities, and transition probabilities (Fig. 3). We examined how the ICER range changed while varying each of the model parameters in its range. We ascertained that a parameter changed the conclusion of the model’s cost-effectiveness if the ICER increased from below $100,000/QALY to above $100,000/QALY, which suggested that the outcome changed from cost-effective to not cost-effective. We found three parameters that changed the conclusion of cost-effectiveness: hazard ratio of CVD death from MI with clopidogrel treatment for patients with genetic variants versus wild type, probability of CVD death from MI with clopidogrel treatment in the usual care group, and disutility of MI event; other factors did not contribute to the ICERs of preemptive PGx testing versus usual care (Fig. 3a). None of the parameters could change the cost-effectiveness conclusion of preemptive versus reactive PGx test (Fig. 3b) and reactive versus usual care (eFig. 4).
Fig. 3. Tornado diagram for incremental cost-effectiveness ratio (ICER).
(a) Preemptive pharmacogenomics (PGx) panel testing vs. usual care. (b) Preemptive vs. reactive PGx panel testing. Each horizontal bar represents the change in ICER when the value of the corresponding parameter is varied from its lower limit to its upper limit. Red color suggests negative correlation, and blue suggests positive correlation. The top 20 parameters that impacted the ICER values the most are listed. Cost and probability values are reported on a monthly basis, while utility was reported on a yearly basis. ADE adverse drug events, AF atrial fibrillation, CHD coronary heart disease, CVD cardiovascular disease, HR hazard ratio, MI myocardial infarction, PAD peripheral artery disease, RR relative risk, STR stroke, Tx treatment, UC usual care, Var genetic variation, WTP willingness-to-pay.
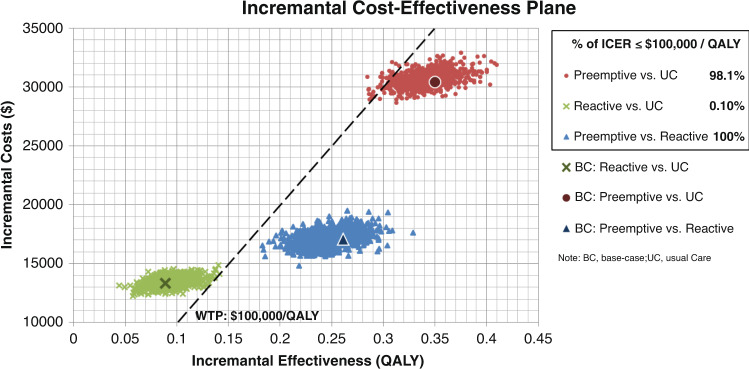
Sensitivity analyses: probabilistic sensitivity analysis
We performed 1,000 samples of 10,000 population where the input parameters were varied simultaneously along with the prespecified distributions for the underlying parameters (eTable 3). This analysis simulated the real-world situation in which each individual could have a set of parameters that varies from one person to another. Figure 4 illustrated the ICER plane from these samples, with each dot representing each of the samples. At a WTP of $100,000/QALY and compared with usual care, preemptive testing was cost-effective 98.1% of the time and reactive testing was cost-effective only 0.10% of the time. Compared with reactive testing, preemptive testing was cost-effective 100% of the time.
Fig. 4. Estimated cost-effectiveness density for the preemptive testing vs. usual care, reactive vs. usual care, and preemptive vs. reactive testing.
Each dot represents the incremental costs and the quality-adjusted life years (QALYs) gained from each sample of the 10,000 microsimulations. The willingness-to-pay (WTP) line was at $100,000/QALY. UC usual care.
Examined through cost-effectiveness acceptability curves (CEACs), preemptive PGx testing became cost-effective compared with usual care at a WTP of $90,000/QALY and above. Reactive PGx testing was not cost-effective for the WTP range $0–$200,000/QALY (eFig. 5).
DISCUSSION
Our study provided four key takeaways for PGx panel testing implementation. First is that identifying candidate groups of patients through PGx panel testing and initiating PGx-guided medication in the first place can result in better outcomes and reduced overall costs.22,34 FOURIER trial estimated that PGx-guided strategy would become cost-effective if the price of PCSK9 inhibitors were to be reduced by 69% (to $4536/year).26 Our results upheld that finding. Our study applied the most updated cost ($487.50/month) for PCSK9 inhibitors and found that preemptive PGx-guided PCSK9 inhibitor use was a cost-effective strategy compared with usual care, which uses statin ($4.17/month) as a first choice treatment for all.16,17,35 We found that the monthly cost of ticagrelor at $190.47 was cost-effective compared with clopidogrel at $34.84, and the monthly cost of NOAC at $231.13 was cost-effective compared with warfarin at $24.60.
Second, we identified that the cost-effectiveness of preemptive PGx testing was affected by age group. It benefits the age groups 45–64 years old, and was not cost-effective if applied to populations older than 65 years old. It implied that for an individual, PGx information should be tested earlier. The cost-effectiveness was only marginally affected by sex, race, and CVD risk level of an individual patient. Actionable insights from applying PGx testing in a system-wide manner have been documented in a few recent cases. For example, Carpenter et al. reported that 7039 unique patients started at least one PGx actionable medication during a 12-month period in a safety net health-care system (Eskenazi Health, Indianapolis, IN).12 Another study from Vanderbilt University reported that 64.8% of its patients were exposed to at least one medication following actionable PGx test results during a 5-year time period.13 Our findings further underscore the idea that rolling out preemptive PGx panel testing in a system-wide manner will benefit individuals 45–64 years old the most.
Third, while comparing preemptive and reactive strategies, preemptive testing was found to be superior to reactive testing with regard to both cost-effectiveness (lower ICER) and patient-centered outcomes (higher additional QALYs gained), when the testing was panel-based. Therefore, if the PGx panel testing was to be implemented, preemptive PGx panel testing would likely be more cost-effective than reactive testing under the study assumptions. This finding was in line with higher uptake rates for preemptive PGx testing than reactive PGx testing among the health systems that have adopted PGx testing in recent years. According to a survey of personnel from known PGx implementation projects, 65% of the projects performed PGx testing preemptively, while 35% were performed reactively.4 However, the study assumed that the turnaround time was 30 days. With advances in technology and shortening of test times, this could overestimate the differences between preemptive and reactive testing.
Fourth, PGx testing may become more cost-effective under a longer timeframe (i.e., longer than 20 years). Very few studies examined the cost-effectiveness of preemptive PGx testing using different timeframes. Lala et al. found PGx-guided reactive testing to be dominant by gaining more QALYs while being cost-saving, and the cost savings increased over a lifetime compared with 15 months in acute coronary syndrome (ACS) patients.36 Our study results reported that both reactive and preemptive testing had higher QALYs and higher costs during a longer timeframe compared with a shorter timeframe. The difference in costs might be due to the partially preemptive design of the reactive testing strategy. Under the shorter timeframe, fewer patients developed disease, so that more preemptive tests were done with no utility, while less reactive testing was done at lower cost, since reactive testing only tested patients who developed disease. Smaller QALY values would be observed during a shorter period of time. Likewise, under a longer timeframe, greater cost-effectiveness of PGx testing would be observed.
This study followed the best practice of cost-effectiveness analysis recommended by the Second Panel on Cost-effectiveness in Health and Medicine.37 Due to the limited availability of data (e.g., indirect costs for lost wages and uncompensated caregiver time) to incorporate other perspectives, only the health-care payer’s perspective was adopted; however, the evidence generated by this study can help inform cost-effectiveness from other perspectives such as a societal perspective that includes payers as stakeholders.37,38 The inputs for the model in our study were from randomized controlled trials, cohort/cross-sectional studies, meta-analyses, and cost-effectiveness analyses conducted on populations similar to the target population considered in our study (eTable 3).
Study limitations
In our simulation sample, the genetic variants were not equally distributed across races. African American populations have a much higher mortality rate from all-cause deaths and higher rate of developing cardiovascular diseases.23–25,39 However, there was a lack of information of treatment effectiveness for minority races, therefore, average values of PGx-guided treatment effectiveness among all the race groups were used in the model.40 This study was also limited by not including other races, such as Native American, since evidence from clinical outcome studies was limited. Therefore, we call for further research on possible race-specific genetic variants and their potential impact on the cost-effectiveness of different treatment strategies.
While our study showed preemptive panel testing was cost-effective compared with reactive testing or standard of care, other implementation barriers still need to be addressed, including translational incentives, health-care professional education, patient acceptance, and ethical challenges.2 The efforts in these expertise areas were not modeled in this study. Our model was designated to provide a very broad view of the value of implementing PGx testing in a system-wide manner, and the simulation results suggested that from the payer’s perspective, preemptive testing is more likely to be beneficial to the US population.
Conclusion
Preemptive PGx panel testing was cost-effective compared with usual care or reactive testing for population 45–64 years old at the WTP threshold of $100,000/QALY. Given its potential for long-term cost-effectiveness as documented in our study, health-care payers might consider rolling out preemptive PGx panel testing in a system-wide manner with efforts from multiple expertise areas.
Supplementary information
Acknowledgements
This research was partially supported by a research grant from Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery and Mayo Clinic Center for Individualized Medicine. The views expressed in the article are those of the authors and do not necessarily reflect the views of Mayo Clinic.
Disclosure
R.W. and L.W. are cofounders of and stockholders in OneOme LLC, a pharmacogenomic decision support company. The other authors declare no conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41436-020-00995-w) contains supplementary material, which is available to authorized users.
References
- 1.Weinshilboum R. Inheritance and drug response. N Engl J Med. 2003;348:529–537. doi: 10.1056/NEJMra020021. [DOI] [PubMed] [Google Scholar]
- 2.Weinshilboum R, Wang L. Pharmacogenomics: bench to bedside. Nat Rev Drug Discov. 2004;3:739. doi: 10.1038/nrd1497. [DOI] [PubMed] [Google Scholar]
- 3.Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern Med. 2016;176:958–968. doi: 10.1001/jamainternmed.2016.1251. [DOI] [PubMed] [Google Scholar]
- 4.Volpi S, Bult CJ, Chisholm RL, et al. Research directions in the clinical implementation of pharmacogenomics: an overview of US programs and projects. Clin Pharmacol Ther. 2018;103:778–786. doi: 10.1002/cpt.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526:343. doi: 10.1038/nature15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voora D, Shah SH, Spasojevic I, et al. The SLCO1B1* 5genetic variant is associated with statin-induced side effects. J Am Coll Cardiol. 2009;54:1609–1616. doi: 10.1016/j.jacc.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunbar SB, Khavjou OA, Bakas T, et al. Projected costs of informal caregiving for cardiovascular disease: 2015 to 2035: a policy statement from the American Heart Association. Circulation. 2018;137:e558–e77. doi: 10.1161/CIR.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 8.Van Dyke M, Greer S, Odom E, et al. Heart disease death rates among blacks and whites aged≥ 35 years—United States, 1968–2015. MMWR Surveill Summ. 2018;67:1. doi: 10.15585/mmwr.ss6705a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keeling NJ, Rosenthal MM, West-Strum D, Patel AS, Haidar CE, Hoffman JM. Preemptive pharmacogenetic testing: exploring the knowledge and perspectives of US payers. Genet Med. 2019;21:1224. doi: 10.1038/gim.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bielinski SJ, Olson JE, Pathak J. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time—using genomic data to individualize treatment protocol. Mayo Clin Proc. 2014;89:25–33. doi: 10.1016/j.mayocp.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji Y, Skierka JM, Blommel JH, et al. Preemptive pharmacogenomic testing for precision medicine: a comprehensive analysis of five actionable pharmacogenomic genes using next-generation DNA sequencing and a customized CYP2D6 genotyping cascade. J Mol Diagn. 2016;18:438–445. doi: 10.1016/j.jmoldx.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter JS, Rosenman MB, Knisely MR, Decker BS, Levy KD, Flockhart DA. Pharmacogenomically actionable medications in a safety net health care system. SAGE Open Med. 2016;4:2050312115624333. doi: 10.1177/2050312115624333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schildcrout JS, Denny JC, Bowton E, et al. Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther. 2012;92:235–242. doi: 10.1038/clpt.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Swanson KM, Rojas RL, et al. Systematic review of the evidence on the cost-effectiveness of pharmacogenomics-guided treatment for cardiovascular diseases. Genet Med. 2020;22:475–486. doi: 10.1038/s41436-019-0667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical Pharmacogenetics Implementation Consortium. Guidelines. 2019. https://cpicpgx.org/guidelines. Accessed 25 April 2019.
- 16.Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed 7 August 2019.
- 17.Wendling P. Second price cut for PCSK9 inhibitor alirocumab (Praluent). 2019. https://www.medscape.com/viewarticle/908990. Accessed 7 August 2019.
- 18.Caraballo PJ, Parkulo M, Blair D, et al. Clinical decision support to implement CYP2D6 drug-gene interaction. Stud Health Technol Inform. 2015;216:946. [PubMed] [Google Scholar]
- 19.Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–2540. doi: 10.1016/j.jacc.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration. Table of pharmacogenomic biomarkers in drug labeling. 2019. https://www.fda.gov/drugs/science-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling. Accessed 11 July 2019.
- 21.Hlatky MA, Kazi DS. PCSK9 inhibitors: economics and policy. J Am Coll Cardiol. 2017;70:2677–2687. doi: 10.1016/j.jacc.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Doshi JA, Puckett JT, Parmacek MS, Rader DJ. Prior authorization requirements for proprotein convertase subtilisin/kexin type 9 inhibitors across US private and public payers. Circ Cardiovasc Qual Outcomes. 2018;11:e003939. doi: 10.1161/CIRCOUTCOMES.117.003939. [DOI] [PubMed] [Google Scholar]
- 23.Powell KP, Christianson CA, Cogswell WA, et al. Educational needs of primary care physicians regarding direct-to-consumer genetic testing. J Genet Couns. 2012;21:469–478. doi: 10.1007/s10897-011-9471-9. [DOI] [PubMed] [Google Scholar]
- 24.Amponsah MK, Benjamin EJ, Magnani JW. Atrial fibrillation and race–a contemporary review. Curr Cardiovasc Risk Rep. 2013;7:336–345. doi: 10.1007/s12170-013-0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez CJ, Soliman EZ, Alonso A, et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25:71–6. e1. doi: 10.1016/j.annepidem.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazi DS, Penko J, Coxson PG, et al. Updated cost-effectiveness analysis of PCSK9 inhibitors based on the results of the FOURIER trial. JAMA. 2017;318:748–750. doi: 10.1001/jama.2017.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You JH. Universal versus genotype-guided use of direct oral anticoagulants in atrial fibrillation patients: a decision analysis. Pharmacogenomics. 2015;16:1089–1100. doi: 10.2217/pgs.15.64. [DOI] [PubMed] [Google Scholar]
- 28.Kazi DS, Garber AM, Shah RU, et al. Cost-effectiveness of genotype-guided and dual antiplatelet therapies in acute coronary syndrome. Ann Intern Med. 2014;160:221–232. doi: 10.7326/M13-1999. [DOI] [PubMed] [Google Scholar]
- 29.Limdi NA, Cavallari LH, Lee CR, et al. Cost-effectiveness of CYP2C19-guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real-world data. Pharmacogenomics J. 2020;20:724–735. doi: 10.1038/s41397-020-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bureau of Economic Analysis. GDP price deflator 2019. https://www.bea.gov/data/prices-inflation/gdp-price-deflator. Accessed 1 May 2019.
- 31.Coleman CI, Limone BL. Cost-effectiveness of universal and platelet reactivity assay-driven antiplatelet therapy in acute coronary syndrome. Am J Cardiol. 2013;112:355–362. doi: 10.1016/j.amjcard.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 32.Kaye JB, Schultz LE, Steiner HE, Kittles RA, Cavallari LH, Karnes JH. Warfarin pharmacogenomics in diverse populations. Pharmacotherapy. 2017;37:1150–1163. doi: 10.1002/phar.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 34.United Healthcare. Clinical pharmacy program guidelines for Brilinta and Effient. 2020. https://www.uhcprovider.com/content/dam/provider/docs/public/commplan/az/pharmacy-clinical-guidelines/AZ-Brilinta-Effient-Clinical-Guidelines.pdf. Accessed 20 February 2020.
- 35.Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med. 2015;162:533–541. doi: 10.7326/M14-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lala A, Berger J, Sharma G, Hochman J, Scott Braithwaite R, Ladapo J. Genetic testing in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a cost‐effectiveness analysis. J Thromb Haemost. 2013;11:81–91. doi: 10.1111/jth.12059. [DOI] [PubMed] [Google Scholar]
- 37.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 38.Neumann PJ, Sanders GD. Cost-effectiveness analysis 2.0. N Engl J Med. 2017;376:203–205. doi: 10.1056/NEJMp1612619. [DOI] [PubMed] [Google Scholar]
- 39.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nugent A, Conatser KR, Turner LL, et al. Reporting of race in genome and exome sequencing studies of cancer: a scoping review of the literature. Genet Med. 2019;21:2676–2680. doi: 10.1038/s41436-019-0558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.