Abstract
Purpose:
Macular imaging with optical coherence tomography (OCT) measures the most critical retinal ganglion cells (RGC) in the human eye. The goal of this perspective is to review the challenges to detection of glaucoma progression with macular OCT imaging and propose ways to enhance its performance.
Design:
Perspective with review of relevant literature.
Methods:
Review of challenges and issues related to detection of change on macular OCT images in glaucoma eyes.
Setting:
NA.
Patient or Study Population:
NA.
Intervention or Observation Procedure(s):
NA.
Main Outcome Measure:
Confounding factors affecting detection of change on macular OCT images.
Results:
The main challenges to detection of structural progression in the macula include the magnitude of and the variable amount of test-retest variability among patients, the confounding effect of aging, lack of a reliable and easy-to-measure functional outcome or external standard, confounding effect of macular conditions including myopia, and the measurement floor of macular structural outcomes. Potential solutions to these challenges include controlling head tilt or torsion during imaging, estimating within-eye variability for individual patients, improved data visualization, use of artificial intelligence methods, and implementation of statistical approaches suitable for multidimensional longitudinal data.
Conclusions:
Macular OCT imaging is a crucial structural imaging modality for assessing the central RGCs. Addressing the current shortcomings in acquisition and analysis of macular volume scans can enhance its utility for measuring the health of central RGCs and hence assist clinicians with timely institution of appropriate treatment.
Keywords: Optical Coherence Tomography, OCT, Macula, glaucoma, progression, GCIPL, GCC, GCL, retinal ganglion cells
Table of Contents statement
This perspective addresses the challenges in detection of glaucoma progression using macular imaging with optical coherence tomography and provides potential solutions for optimizing identification of structural change in the central retina in glaucoma patients.
Introduction
Structural biomarkers can now be measured in glaucoma patients in the peripapillary region and macula with an unprecedented accuracy and precision with optical coherence tomography.1 Disease progression in glaucoma manifests itself mainly as thinning of neural tissues although other markers such as schisis of the inner retinal layers or changes in tissue density have been reported.2 Detection of progressive tissue thinning seems a simpler endeavor compared to detection of pathological changes in macular retinal disorders. However, clinicians are still grappling with detecting change at all stages of glaucoma in a consistent and timely manner. Also, a direct correlation of progressive structural thinning as measured with OCT imaging with functional loss is yet to be established longitudinally.3 We review the challenges involved in timely detection of structural decay in glaucoma with an emphasis on macular OCT imaging and propose steps to potentially facilitate this task for monitoring of glaucoma patients.
The challenges
The variable amount of noise among patients
Test-retest variability of macular OCT measurements tends to be low but varies in individual eyes.4, 5 Between-visit variability of OCT measures is the main determinant of how accurately and quickly we can detect changes in macular thickness over time. Detection of the smallest structural changes could be of significant practical interest especially in eyes with advanced glaucoma.6, 7 Visit to visit variability within local areas of the macula can be large enough to make detection of change within an eye problematic. Estimating individual measurement variability may be of particular interest as sampling variability may vary from person to person.
Sources of test-retest variability include procedural factors, such as head tilt/turn, image centration, and focusing among others. Patient factors consist of poor ocular surface, media opacity, presence of high myopia, and other anatomical issues. Technical factors include incorrect segmentation due to poor image quality or ocular conditions such as peripapillary atrophy, and confounding retinal disorders; the latter specifically affect macular OCT measurements.
The slow and variable amount of change in glaucoma patients
Ledolter and Kardon recently provided an elegant review on using repeated measures random effects models in ophthalmology, illustrating their methods on longitudinal data from the Variability in Perimetry (VIP) study.8 Such models can estimate population baseline levels and rates of change over time, which is important when we wish to compare populations distinguished by some characteristic such as demographic variables or by different treatments. More importantly for individual patients, repeated measures random effects models can estimate subject-specific measures including an individual’s baseline level and the individual’s rate of change over time for structural and functional outcomes measures. Rates of change estimates from repeated measures random effects models will be more accurately estimated than from regression models that only use data from an individual patient. Importantly, these models are able to provide estimates on the uncertainty in the estimation of these subject-specific parameters. These models can be potentially used for prediction purposes to assign individual eyes to stable or progressing groups based on one or more features such as rates of change in various structural domains. Ledolter and Kardon found no significant difference in the rates of change between the normal and glaucoma cohorts although the glaucoma group had a slightly faster average rate of change for the global ganglion cell/inner plexiform layer (GCIPL) and a somewhat slower mean rate of change for the average retinal nerve fiber layer (RNFL). Random effects models are but one of many classes of models for modeling the covariances between the repeated measures within a person.9 It is important to properly model the covariances between the repeated measures as this is what provides appropriate inferences about individual slopes. Adding autocorrelation between consecutive observations to a random effects model is an important and sometimes useful elaboration of random effects models.9 Interestingly, in the VIP dataset, the autocorrelations of the residuals were small (ρ =0.19 for RNFL and =0.15 for GCIPL for a lag of 1.0 year) and therefore, the added model complexity may not warrant considering autocorrelation of residuals.
The confounding effect of aging
Age-related loss of retinal ganglion cells (RGCs) is not uniform among subjects nor across the macula. Current evidence suggests that it is a function of the existing number of RGCs at any given time.10 Leung et al. estimated rates of change for the inner retina, outer retina, GCIPL, and full macular thickness measurements over time with a linear mixed model.11 They used a fixed-effect interaction term (baseline thickness × follow-up duration) for adjusting the estimated rates of change for the influence of baseline thickness measurements. However, they used the 95% prediction intervals from their normal cohort to correct the rates of change in glaucoma eyes. If one were to accept that the rates of change are affected by the baseline thickness for most structural parameters, which is a plausible assumption based on available data12, using the rates of change in normal eyes for correcting age-related decay in glaucoma eyes would be expected to overestimate the influence of aging and underestimate true rates of disease progression in eyes with established glaucoma. Zhang et al. reported a slowing of ganglion cell complex (GCC) loss above 65 years of age, which amounted to a third of that seen in their youngest age group (40–55 years, −0.31 vs. −0.11 μm/year).13 The authors could not rule out variations due to chance given the magnitude of the reduction in the rates of change. They also did not consider a random slope along with the random intercept which means that they likely underestimated the uncertainty in the slopes.
Tong and coworkers described clusters in the central macula based on age-related rates of ganglion cell layer (GCL) thinning in a cross-sectional group of 254 normal subjects.14 An average GCL thinning rate of 0.26% per year was reported within the central 20 degrees of the macula beyond the late 30s. Their work is based on the macular volume scans from Spectralis OCT, which provides an 8×8 array of 3°×3° superpixels within the central 24° of the macula (Figure 1). They defined clusters of superpixel based on the age-related rates of change. Regardless of the clustering method(s) used, the superpixels demonstrating similar age-related rates of change were clustered in a pericentral pattern with thicker regions (superpixels) displaying larger absolute age-related rates of change. This is supported by Yoshioka and coworkers’ findings, in which the normalized rates of change were found to be uniform; the investigators normalized rates of change to the highest thickness measurements for the superpixels of interest.10 A recent study by Chauhan et al. on White normal subjects confirms that age-related changes seem to be a function of average thickness in the central macula (Figures 2 and 3).15 The overall smaller confidence intervals for prediction of functional outcomes from structural measurements with the defined clusters in Tong and collaborators study supports the potential utility of clustering superpixels for prediction of functional deterioration from structural changes.14 More than likely, baseline levels and time trends of superpixels belonging to the same cluster are more highly correlated than superpixels from different clusters; but the superpixels from different clusters are not uncorrelated, rather the correlations are low.
Figure 1.
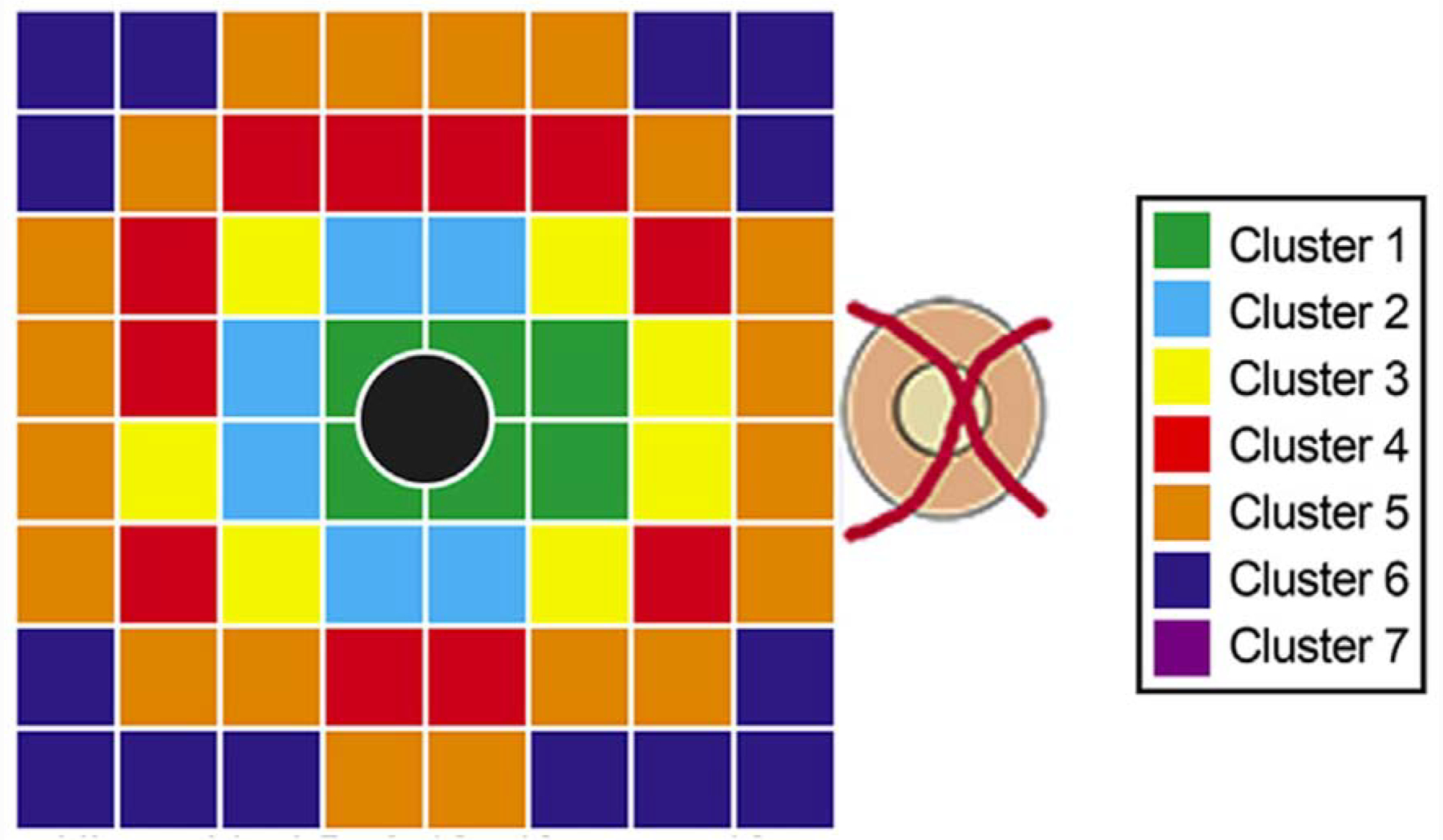
An example of clustering of the superpixels from the macular volume scans of the Spectralis OCT based on age-related rates of change in normal subjects. The Posterior Pole Algorithm of the Spectralis OCT provides an 8×8 array of 3°×3° superpixels within the central 24° of the macula. In this example, the superpixels demonstrating similar age-related rates of change in the ganglion cell layer were clustered in a pericentral pattern with thicker superpixels displaying larger absolute age-related rates of change. This particular set of clusters was derived from hierarchical clustering using 5- and 10-year age brackets and k-means clustering using 10-year age brackets. Data from the foveal pit were excluded from analysis (black circle) since there is no retinal ganglion cells in this area. (Reprinted from Tong J, Phu J, Khuu SK, et al. Development of a Spatial Model of Age-Related Change in the Macular Ganglion Cell Layer to Predict Function From Structural Changes. Am J Ophthalmol. 2019, with permission from Elsevier.)
Figure 2.
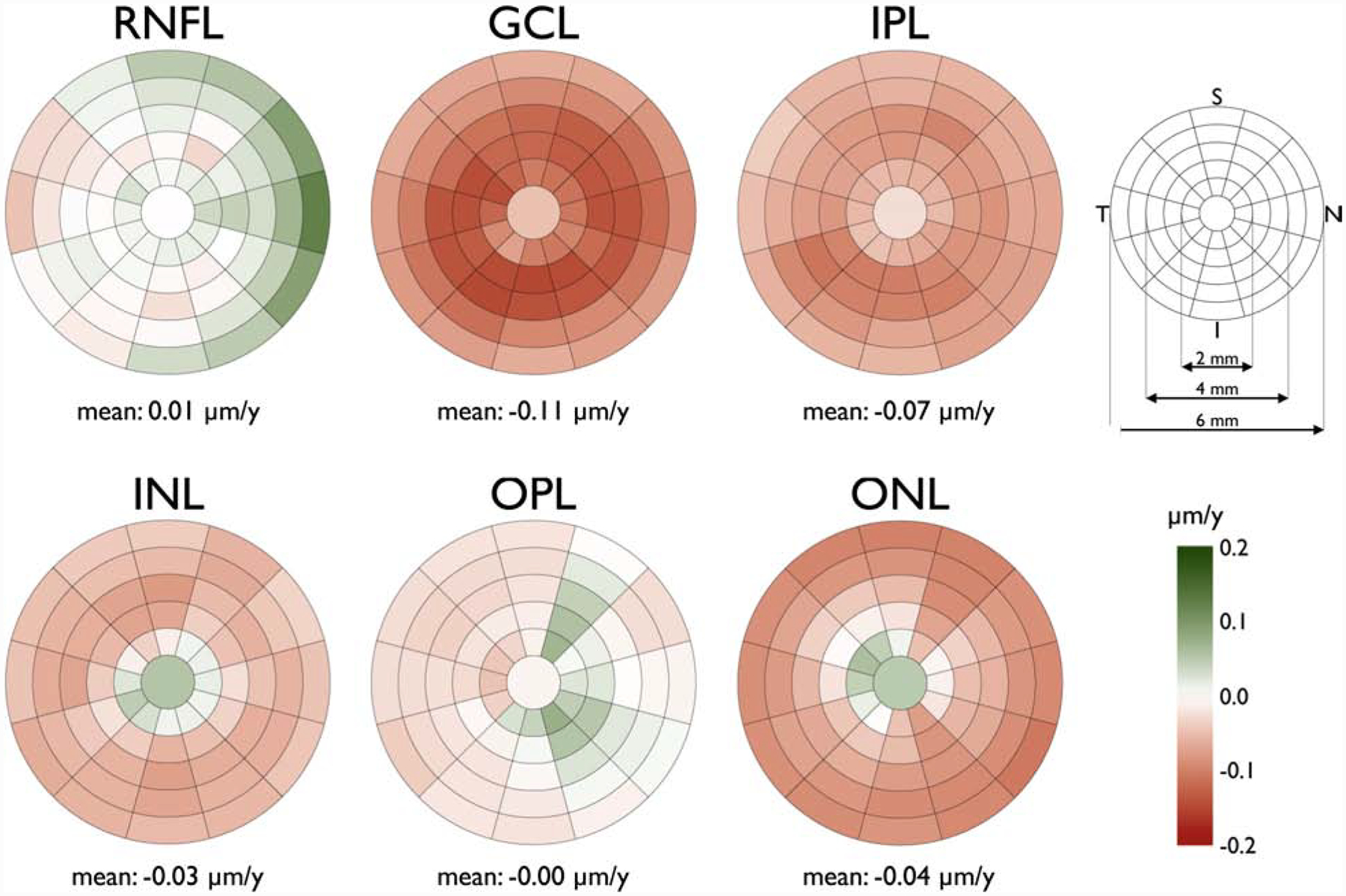
Sectoral absolute mean age-related rate change of the macular retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), and outer nuclear layer (ONL) thickness (right eye format). The innermost circle has a diameter of 1 mm with an incremental increase of 1 mm in the diameter of each concentric circle (key, upper right). Each circle, except the most central one, is divided into twelve 30° sectors. The darker red colors denote more negative rates and darker green sectors show more positive rates (key, bottom right). I: inferior; N: nasal; S: superior; T: temporal. The rates were calculated based on a cross-sectional group of 246 White subjects. (Reprinted from Chauhan BC, Vianna JR, Sharpe GP, et al. Differential Effects of Aging in the Macular Retinal Layers, Neuroretinal Rim, and Peripapillary Retinal Nerve Fiber Layer. Ophthalmology. 2019, with permission from Elsevier.)
Figure 3.
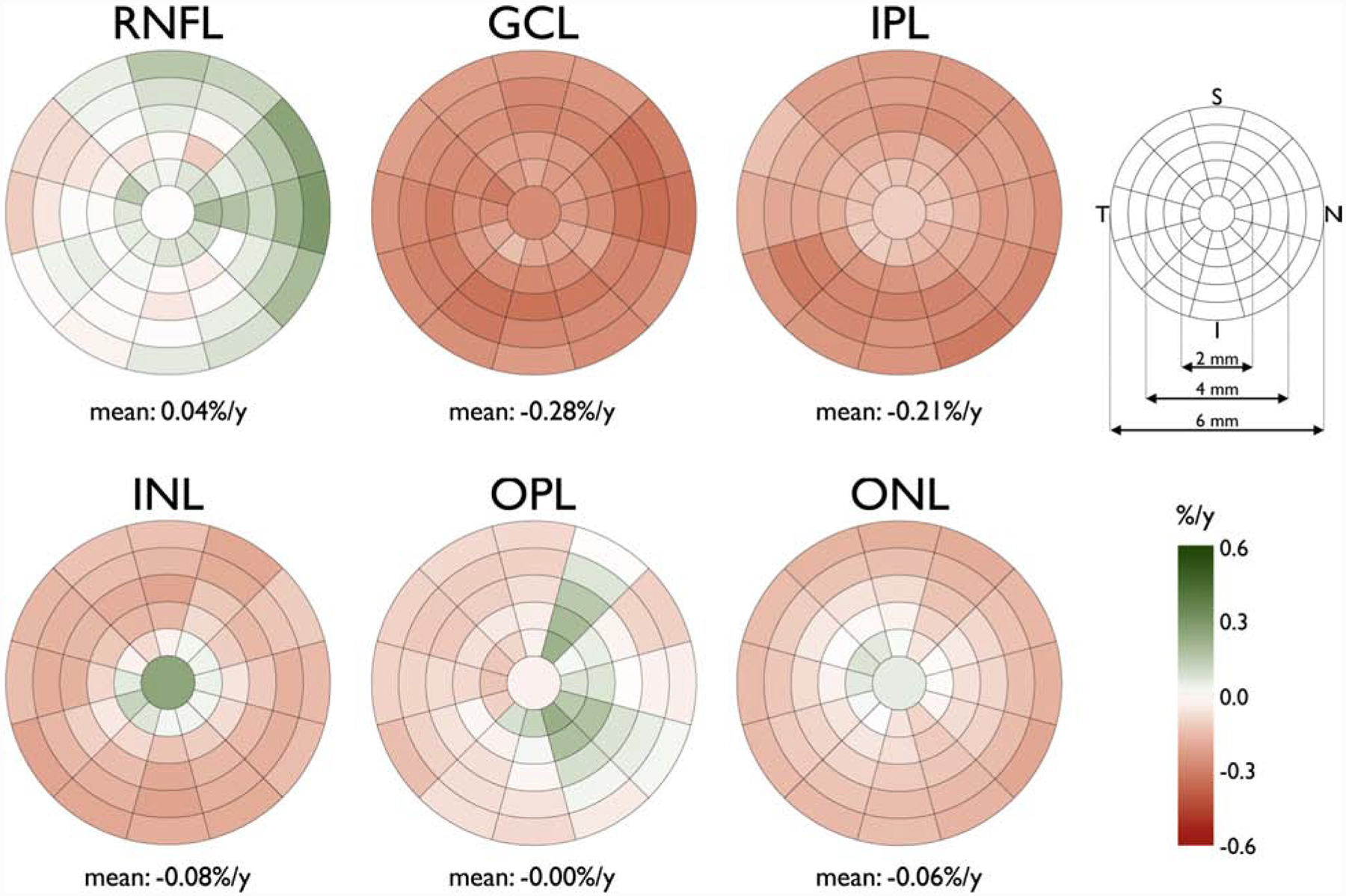
Sectoral relative mean age-related rate change of the macular retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), and outer nuclear layer (ONL) thickness expressed in percent loss (right eye format). The innermost circle has a diameter of 1 mm with an incremental increase of 1 mm in the diameter of each concentric circle (key, upper right). Each circle, except the most central one, is divided into twelve 30° sectors. The darker red colors denote more negative rates and darker green sectors show more positive rates (key, bottom right). I: inferior; N: nasal; S: superior; T: temporal. The rates were calculated based on a cross-sectional group of 246 White subjects. (Reprinted from Chauhan BC, Vianna JR, Sharpe GP, et al. Differential Effects of Aging in the Macular Retinal Layers, Neuroretinal Rim, and Peripapillary Retinal Nerve Fiber Layer. Ophthalmology. 2019, with permission from Elsevier.)
Evidence from histological studies-
Some histological studies have provided evidence for age-related decay in the number of RGCs16–20, whereas others have not.21, 22 The number of eyes included have been generally small (12–22 eyes) and therefore, these studies had low power to detect age-related changes. As an example, when 12 out of 16 eyes reported by Balazsi and colleagues were re-analyzed with automated methods, the influence of age became nonsignificant.16, 21 Other confounding factors may have influenced the results of histological studies. Repka and Quigley reported signs of autolysis in up to 80% of 19 eyes they studies.22 Also, in their study, the average axonal count was roughly 700,000, which is significantly below the average reported in most other studies. Jonas and coauthors reported an age-related loss of optic nerve fibers of about 0.5% per year.23 Gao and Hollyfield found that macular RGCs decreased by about 0.3% per year, amounting to about 16% total RGC loss from the second through the sixth decades. However, the results were based only on 6 eyes and specific to a localized region nasal to the fovea.24
Patterns of change for the inner macular layers have not been well defined
Most if not all studies reporting structural rates of change have assumed a linear decay for structural measures including macular thickness outcomes. While this may be a practical and convenient assumption, and indeed reasonable over short time periods, it needs to be formally verified, especially in untreated cohorts of patients with suspected (or established) glaucoma. The effect of treatment remains a significant confounding factor in published studies of treated glaucoma cohorts.
Lack of established region of interest (ROI) approaches for macular OCT images
There has been a lack of automated and customized approaches for defining and delineating regions of interest in the macula or elsewhere for detection of glaucoma deterioration. Hood et al. reported that a manual ROI approach was better able to detect the extent of progression in the macular region.25 The task of defining the ROI would be potentially useful so as to outline regions where structural measurements are still above their measurement floor and therefore, identification of change would be possible and detected change is plausible. The measurement floor may vary among different eyes and this has to be taken into account when defining ROIs.
Lack of a reliable and easy-to-measure functional outcome
The lengthy testing time and fairly sparse sampling of the central retina with the 10–2 visual field test along with the observed high test-retest variability remain important barriers for reliable assessment of the central visual function in a time-efficient manner. Additionally, no clinically established methods are yet widely available for measuring central functional progression although significant advances have been reported.26–28
Lack of optimized data visualization
To date, clinicians still have to rely on proprietary software developed by OCT manufacturers for visualizing changes of macular thickness measures over time. The complexity and user friendliness of such software and the macular outcome measure used vary among different OCT devices. None provides the ability to rapidly sort through series of images and almost all are still limited to a single thickness measure, i.e., GCC, GCIPL, or GCL. Our preliminary studies have shown that macular outcome measures are not equal with regard to identification of progression. Rabiolo and colleagues recently demonstrated that GCC thickness measurements were more likely to identify statistically significant rates of change in eyes with central or advanced damage at baseline compared to GCIPL or GCL regardless of the level of damage at baseline.29 This is likely related to the less demanding segmentation task involved in delineating GCC as compared to GCIPL or GCL. In addition, our team has recently provided evidence that trend analysis of GCC thickness within macular superpixels can detect statistically significant change over time earlier than the same technique applied to total deviation values at coresponding 10–2 visual field test locations on the.3
Lack of alternative outcome measures other than tissue thickness
There have been reports on the potential utility of changes in tissue density in the retinal nerve fiber layer;30 however, no other biomarker has proven to be superior to macular thickness measures and the utility of tissue density within the macular retina has yet to be explored. Optical coherence tomography angiography (OCTA) is a promising modality for measuring vascular density as a proxy for blood flow both in the circumpapillary and macular regions. It has provided researchers with new potential biomarkers for measuring the health of the macular retinal ganglion cells. Findings from diagnostic studies using macular OCTA to discriminate between healthy eyes and eyes with suspected or established glaucoma and the association of vessel density, the main outcome of interest, with functional findings are promising.31–33 Shoji et al. recently reported that macular OCTA was able to detect longitudinal changes in macular vessel density after an average follow-up period of 14 months in eyes with established glaucoma, whereas no changes in GGC thickness could be detected during the same time interval.34 Validation of these findings in larger cohorts with longer follow-up is needed before OCTA can be recognized as a clinical tool for detection of disease progression in the macula.
The confounding effect of macular diseases including myopia
One major limitation of macular OCT imaging is that various retinal macular pathologies can disrupt inner retinal structural integrity and affect measurements of the inner retina. The influence of milder degrees of outer retinal pathologies such as drusen or retinal pigment epithelial atrophy on inner retinal measurements has yet to be studied. Progressive thinning of the posterior eye wall is a common feature observed in myopia of varying severity. Enlargement and expansion of the posterior pole leads to thinning of the retina including the inner retinal layers. Increasing axial length has been consistently associated with thinning of the inner or full retinal thickness measurements in the macula.35, 36 Part of this apparent thinning of the retinal layers is related to the optical magnification observed in myopic eyes.37 Macular inner thickness measurements have been shown to perform as well as cpRNFL measurements for detection of glaucoma in highly myopic eyes.38, 39 The main challenges in detection of disease progression with macular OCT imaging are potentially inadequate quality of the OCT images due to signal roll off in eyes with longer axial lengths, presence of significant retinal atrophy confounding macular segmentation and thickness measurements, and possible progression of myopia during the follow-up period especially in younger patients. Because of tilting of the posterior pole in some myopic eyes, measuring retinal thickness consistently over time may also present a problem in some eyes.40
Joint modeling of structure and function
Recent work from Hood’s laboratory and collaborators has provided important insight on the correspondence of structural and functional damage in glaucoma patients.41 Medeiros and colleagues and other investigators have explored Bayesian models for enhancing detection of glaucoma using OCT RNFL thickness measurements.42–44 Various Bayesian approaches have been proposed, ranging from combining event and trend analyses for visual fields to using structural change as prior information for modeling of visual field progression. Bayesian methods may allow for inferential quantities of interest that clinicians could find useful. For example, Bayesian methods can allow for inferences such as the probability that progression (or no progression) is occurring in a given time frame in a given superpixel or clusters of superpixels. While Bayesian approaches seem promising, none have been fully developed and hence, are not available routinely to clinicians. Further work on this topic is needed to bring such approaches into routine clinical use.
The floor effect
All OCT structural thickness measures are known to reach their floor after about an 8–10 dB decline in perimetric threshold sensitivity has occurred at corresponding test locations or regions. Macular measures are not an exception.45–48 Central RGC damage can occur early during the course of glaucoma at the same time as the earliest sign of RNFL or neuroretinal rim loss are identified; however, due to the redundancy of the central macular RGCs, the central macula is the only region of the retina where the RGC mass can be measured in later stages of the disease.49
The relationship between macular rates of change and baseline thickness
Rates of change for any structural outcome measure, such as macular OCT thickness measurements, typically depend on the initial value of the outcome of interest for two reasons: First, there is frequently a measurement floor below which such measures cannot fall. The closer the initial measurement is to the floor, the smaller any detected change would be. Measurement of percent change from baseline has been proposed to address this issue. Percent change also allows comparison of rates of change among competing structural measures in glaucoma.50 One of the problems of using percent change in this setting is that smaller amounts of change can be magnified as any given outcome measure approaches its measurement floor. Another problem is that where one starts measuring determines the denominator of the percent change calculation; people who enter a study after significant macular damage have a different reference point than people who enter prior to any decrease. A third issue is that the baseline measurement is usually not informative about the floor level; the floor level will be a different percentage change from initial values for different people. Exponential decay models with random effects are more difficult to fit than linear random effects models. Linear random effects models can be adapted to approximate nonlinear time trends using splines and similar nonlinear curve fitting techniques. Which is a better approach for modeling time trends is still to be determined.
Analysis of covariance has been proposed to account for the influence of initial values of a physiological variable and to compare rates of change across groups.51 Analysis of covariance is flawed, however, as a baseline measure is likely to have different predictive ability for follow-up observations depending on the time lag of prediction, region of interest and progression of the disease in the cohort under study.
Potential solutions
Head tilt or torsion-
Controlling head tilt and turn at every imaging session would be expected to decrease between-visit variability for OCT measurements. While device software handles smaller degrees of head tilt or turn, larger changes in these factors can potentially introduce significant noise and variability into the measurements. A head- or spectacles-mounted sensor-based device could potentially be used for this purpose and is under development.
Estimating within-eye variability-
Although our prior work has demonstrated that the amount of measurement variability is fairly uniform among subjects and within the central region of the macula4, some patients manifest higher variability for various reasons. On the other hand, other eyes demonstrate very low variability and hence, estimating variability in individual eyes could improve detection of change over time. Our team has shown that variability in inner macular thickness measurements over time was similar to within-session repeated measurement variability.7 Hence, repeat imaging of a given eye during the initial one or two visits could provide a good estimate of test-retest variability in thickness measurements and individualized prediction intervals could then be created to optimize detection of change.
Software and access to raw data -
Despite significant advances in OCT imaging over the last 2 decades, available software algorithms on OCT devices remain inaccessible and are designed with limited input from clinicians and statisticians. Also, options for modifying data visualization in real time are restricted. The ideal software would be able to estimate 95% prediction limits for within eye variability, so that true change can be distinguished from noise, would allow visualization of the change on macular OCT images at different resolutions, and would provide sliding scale cutoff points for flagging change from baseline by flipping through images or as a short animated series. It would also perform trend analysis on superpixel sizes of choice or clusters with appropriate predictions so that localized information would not be lost. Real time hierarchical analyses and data visualization based on more sophisticated statistical models and large data sets could be potentially useful in this setting.
Any change analysis algorithm should also allow for the possibility of tissue thickening. Microcystic macular changes in the inner nuclear layer can occur in advanced glaucoma especially in younger patients.52, 53 Also, the possibility of an improvement in macular thickness after marked reduction of the intraocular pressure should be considered. Improvements in the extent of visual field damage and optic nerve appearance after surgical treatment of glaucoma are well established.54, 55
Use of artificial intelligence methods-
Use of artificial intelligence (AI) in glaucoma diagnostics is still in its infancy but results from early investigations have been very promising. Berchuck and colleagues recently found that deep learning models could improve estimation of future visual field rates and trajectories from OCT RNFL thickness measurements compared to traditional statistical methods.56 Unpublished data from our lab have also provided evidence that use of AI approaches could enhance our understanding of linking of structure and function in the macula, improve prediction of functional from structural measurements, and potentially pave the way for improvements in detection of progression (Mohammadzadeh V, Sahin S, Hassan O, Mylavarapu A, Martynian J, Fu Q, Mahmoudinezhad G, Coleman AL, Law SK, Caprioli J, Scalzo F, Nouri-Mahdavi K. Prediction of Central Visual Field Measures from Macular OCT Images with Deep Learning. Oral presentation at the Annual Meeting of the American Glaucoma Society, February 27-March 1, 2020, Washington DC).
In summary, while macular OCT imaging has significantly advanced the field of glaucoma diagnostics, further optimization of imaging procedures, analyses, and data visualization could move the field ahead and enhance clinicians’ ability to detect glaucoma deterioration in a timely manner and facilitate clinical research by shortening time to detection of progression endpoints.
Supplementary Material
Acknowledgments
This work was supported by an NIH R01 grant (R01-EY029792), an unrestricted Departmental Grant from Research to Prevent Blindness (Stein Eye Institute) and an unrestricted grant from Heidelberg Engineering (KNM).
Biographies
Kouros Nouri-Mahdavi, MD, MSc
Kouros Nouri-Mahdavi is currently Associate Professor in Residence and Director of the Glaucoma Advanced Imaging Laboratory in the Department of Ophthalmology at the David Geffen School of Medicine and UCLA’s Stein Eye Institute’s Glaucoma Division. Dr. Nouri-Mahdavi’s research focuses on functional and structural measurements for optimizing diagnosis of glaucoma or its progression, use of machine learning approaches in glaucoma diagnostics, and study of glaucoma surgical outcomes.
Robert Weiss, PhD
Robert E. Weiss is Professor of Biostatistics in the UCLA Fielding School of Public Health. He is an expert in theory and applications of Bayesian methods, longitudinal data analysis, and hierarchical models. He specializes in model specification and model selection for complex data sets.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raza AS, Hood DC. Evaluation of the Structure-Function Relationship in Glaucoma Using a Novel Method for Estimating the Number of Retinal Ganglion Cells in the Human Retina. Invest Ophthalmol Vis Sci. August 2015;56(9):5548–56. doi: 10.1167/iovs.14-16366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee EJ, Kim TW, Kim M, Choi YJ. Peripapillary retinoschisis in glaucomatous eyes. PLoS One. 2014;9(2):e90129. doi: 10.1371/journal.pone.0090129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammadzadeh V, Rabiolo A, Fu Q, et al. Longitudinal Macular Structure-Function Relationships in Glaucoma. Ophthalmology. January 2020;doi: 10.1016/j.ophtha.2020.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miraftabi A, Amini N, Gornbein J, et al. Local Variability of Macular Thickness Measurements With SD-OCT and Influencing Factors. Transl Vis Sci Technol. July 2016;5(4):5. doi: 10.1167/tvst.5.4.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim KE, Yoo BW, Jeoung JW, Park KH. Long-Term Reproducibility of Macular Ganglion Cell Analysis in Clinically Stable Glaucoma Patients. Invest Ophthalmol Vis Sci. July 2015;56(8):4857–64. doi: 10.1167/iovs.14-16350 [DOI] [PubMed] [Google Scholar]
- 6.Asrani S, Rosdahl JA, Allingham RR. Novel software strategy for glaucoma diagnosis: asymmetry analysis of retinal thickness. Arch Ophthalmol. Sep 2011;129(9):1205–11. doi: 10.1001/archophthalmol.2011.242 [DOI] [PubMed] [Google Scholar]
- 7.Nouri-Mahdavi K, Fatehi N, Caprioli J. Longitudinal Macular Structure-Function Relationships in Glaucoma and Their Sources of Variability. Am J Ophthalmol. 11 2019;207:18–36. doi: 10.1016/j.ajo.2019.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledolter J, Kardon RH. Assessing Trends in Functional and Structural Characteristics: A Survey of Statistical Methods With an Example From Ophthalmology. Transl Vis Sci Technol. September 2018;7(5):34. doi: 10.1167/tvst.7.5.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss R. Modeling Longitudinal Data. 1st ed. Springer Texts in Statistics. Springer Science+Business Media; 2005. [Google Scholar]
- 10.Yoshioka N, Zangerl B, Nivison-Smith L, et al. Pattern Recognition Analysis of Age-Related Retinal Ganglion Cell Signatures in the Human Eye. Invest Ophthalmol Vis Sci. June 1 2017;58(7):3086–3099. doi: 10.1167/iovs.17-21450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung CK, Ye C, Weinreb RN, Yu M, Lai G, Lam DS. Impact of age-related change of retinal nerve fiber layer and macular thicknesses on evaluation of glaucoma progression. Ophthalmology. December 2013;120(12):2485–92. doi: 10.1016/j.ophtha.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 12.Nassiri N, Nilforushan N, Coleman AL, Law SK, Caprioli J, Nouri-Mahdavi K. Longitudinal Structure-Function Relationships With Scanning Laser Ophthalmoscopy and Standard Achromatic Perimetry. Arch Ophthalmol. July 2012. 2012;130(7):826–832. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Francis BA, Dastiridou A, et al. Longitudinal and Cross-Sectional Analyses of Age Effects on Retinal Nerve Fiber Layer and Ganglion Cell Complex Thickness by Fourier-Domain OCT. Transl Vis Sci Technol. March 2016;5(2):1. doi: 10.1167/tvst.5.2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong J, Phu J, Khuu SK, et al. Development of a Spatial Model of Age-Related Change in the Macular Ganglion Cell Layer to Predict Function From Structural Changes. Am J Ophthalmol. 12 2019;208:166–177. doi: 10.1016/j.ajo.2019.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauhan BC, Vianna JR, Sharpe GP, et al. Differential Effects of Aging in the Macular Retinal Layers, Neuroretinal Rim, and Peripapillary Retinal Nerve Fiber Layer. Ophthalmology. February 2020;127(2):177–185. doi: 10.1016/j.ophtha.2019.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balazsi AG, Rootman J, Drance SM, Schulzer M, Douglas GR. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol. June 1984;97(6):760–6. doi: 10.1016/0002-9394(84)90509-9 [DOI] [PubMed] [Google Scholar]
- 17.Johnson BM, Miao M, Sadun AA. Age-Related Decline of Human Optic-Nerve Axon Populations. Age. January 1987;10(1):5–9. doi:Doi 10.1007/Bf02431765 [DOI] [Google Scholar]
- 18.Morrison JC, Cork LC, Dunkelberger GR, Brown A, Quigley HA. Aging changes of the rhesus monkey optic nerve. Invest Ophthalmol Vis Sci. August 1990;31(8):1623–7. [PubMed] [Google Scholar]
- 19.Harman A, Abrahams B, Moore S, Hoskins R. Neuronal density in the human retinal ganglion cell layer from 16–77 years. Anat Rec. 10 2000;260(2):124–31. doi: [DOI] [PubMed] [Google Scholar]
- 20.Varma R, Skaf M, Barron E. Retinal nerve fiber layer thickness in normal human eyes. Ophthalmology. December 1996;103(12):2114–9. doi: 10.1016/s0161-6420(96)30381-3 [DOI] [PubMed] [Google Scholar]
- 21.Mikelberg FS, Drance SM, Schulzer M, Yidegiligne HM, Weis MM. The normal human optic nerve. Axon count and axon diameter distribution. Ophthalmology. September 1989;96(9):1325–8. doi: 10.1016/s0161-6420(89)32718-7 [DOI] [PubMed] [Google Scholar]
- 22.Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. January 1989;96(1):26–32. doi: 10.1016/s0161-6420(89)32928-9 [DOI] [PubMed] [Google Scholar]
- 23.Jonas JB, Muller-Bergh JA, Schlotzer-Schrehardt UM, Naumann GO. Histomorphometry of the human optic nerve. Invest Ophthalmol Vis Sci. April 1990;31(4):736–44. [PubMed] [Google Scholar]
- 24.Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. January 1992;33(1):1–17. [PubMed] [Google Scholar]
- 25.Hood DC, Xin D, Wang D, et al. A Region-of-Interest Approach for Detecting Progression of Glaucomatous Damage With Optical Coherence Tomography. JAMA Ophthalmol. December 2015;133(12):1438–44. doi: 10.1001/jamaophthalmol.2015.3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Moraes CG, Furlanetto RL, Ritch R, Liebmann JM. A new index to monitor central visual field progression in glaucoma. Ophthalmology. August 2014;121(8):1531–8. doi: 10.1016/j.ophtha.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 27.de Moraes CG, Song C, Liebmann JM, Simonson JL, Furlanetto RL, Ritch R. Defining 10–2 visual field progression criteria: exploratory and confirmatory factor analysis using pointwise linear regression. Ophthalmology. Mar 2014;121(3):741–9. doi: 10.1016/j.ophtha.2013.10.018 [DOI] [PubMed] [Google Scholar]
- 28.De Moraes CG, Paula JS, Blumberg DM, et al. Detection of progression with 10–2 standard automated perimetry: Development and validation of an event-based algorithm. Am J Ophthalmol. April 2020;doi: 10.1016/j.ajo.2020.03.046 [DOI] [PubMed] [Google Scholar]
- 29.Rabiolo A, Mohammadzadeh V, Fatehi N, et al. Comparison of Rates of Progression of Macular OCT Measures in Glaucoma. Transl Vis Sci Technol. 2020;(In Press.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thepass G, Lemij HG, Vermeer KA. Attenuation Coefficients From SD-OCT Data: Structural Information Beyond Morphology on RNFL Integrity in Glaucoma. J Glaucoma. November 2017;26(11):1001–1009. doi: 10.1097/IJG.0000000000000764 [DOI] [PubMed] [Google Scholar]
- 31.Kwon J, Choi J, Shin JW, Lee J, Kook MS. Alterations of the Foveal Avascular Zone Measured by Optical Coherence Tomography Angiography in Glaucoma Patients With Central Visual Field Defects. Invest Ophthalmol Vis Sci. March 01 2017;58(3):1637–1645. doi: 10.1167/iovs.16-21079 [DOI] [PubMed] [Google Scholar]
- 32.Ghahari E, Bowd C, Zangwill LM, et al. Association of Macular and Circumpapillary Microvasculature with Visual Field Sensitivity in Advanced Glaucoma. Am J Ophthalmol. August 2019;204:51–61. doi: 10.1016/j.ajo.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou H, Moghimi S, Zangwill LM, et al. Macula Vessel Density and Thickness in Early Primary Open-Angle Glaucoma. Am J Ophthalmol. March 2019;199:120–132. doi: 10.1016/j.ajo.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoji T, Zangwill LM, Akagi T, et al. Progressive Macula Vessel Density Loss in Primary Open-Angle Glaucoma: A Longitudinal Study. Am J Ophthalmol. October 2017;182:107–117. doi: 10.1016/j.ajo.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim NR, Kim JH, Lee J, Lee ES, Seong GJ, Kim CY. Determinants of perimacular inner retinal layer thickness in normal eyes measured by Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. May 18 2011;52(6):3413–8. doi: 10.1167/iovs.10-6648 [DOI] [PubMed] [Google Scholar]
- 36.Mwanza J-C, Durbin MK, Budenz DL, et al. Profile and predictors of normal ganglion cell–inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Investigative ophthalmology & visual science. 2011;52(11):7872–7879. [DOI] [PubMed] [Google Scholar]
- 37.Higashide T, Ohkubo S, Hangai M, et al. Influence of Clinical Factors and Magnification Correction on Normal Thickness Profiles of Macular Retinal Layers Using Optical Coherence Tomography. PLoS One. 2016;11(1):e0147782. doi: 10.1371/journal.pone.0147782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akashi A, Kanamori A, Ueda K, Inoue Y, Yamada Y, Nakamura M. The Ability of SD-OCT to Differentiate Early Glaucoma With High Myopia From Highly Myopic Controls and Nonhighly Myopic Controls. Invest Ophthalmol Vis Sci. October 2015;56(11):6573–80. doi: 10.1167/iovs.15-17635 [DOI] [PubMed] [Google Scholar]
- 39.Choi YJ, Jeoung JW, Park KH, Kim DM. Glaucoma detection ability of ganglion cell-inner plexiform layer thickness by spectral-domain optical coherence tomography in high myopia. Invest Ophthalmol Vis Sci. March 28 2013;54(3):2296–304. doi: 10.1167/iovs.12-10530 [DOI] [PubMed] [Google Scholar]
- 40.Hariri A, Lee SY, Ruiz-Garcia H, Nittala MG, Heussen FM, Sadda SR. Effect of angle of incidence on macular thickness and volume measurements obtained by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. August 2012;53(9):5287–91. doi: 10.1167/iovs.12-9767 [DOI] [PubMed] [Google Scholar]
- 41.Hood DC, Tsamis E, Bommakanti NK, et al. Structure-Function Agreement Is Better Than Commonly Thought in Eyes With Early Glaucoma. Invest Ophthalmol Vis Sci. 10 2019;60(13):4241–4248. doi: 10.1167/iovs.19-27920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medeiros FA, Leite MT, Zangwill LM, Weinreb RN. Combining structural and functional measurements to improve detection of glaucoma progression using Bayesian hierarchical models. Invest Ophthalmol Vis Sci. July 2011;52(8):5794–803. doi: 10.1167/iovs.10-7111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell RA, Malik R, Chauhan BC, Crabb DP, Garway-Heath DF. Improved estimates of visual field progression using bayesian linear regression to integrate structural information in patients with ocular hypertension. Invest Ophthalmol Vis Sci. May 2012;53(6):2760–9. doi: 10.1167/iovs.11-7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medeiros FA, Zangwill LM, Girkin CA, Liebmann JM, Weinreb RN. Combining structural and functional measurements to improve estimates of rates of glaucomatous progression. Am J Ophthalmol. June 2012;153(6):1197–205 e1. doi: 10.1016/j.ajo.2011.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mwanza JC, Kim HY, Budenz DL, et al. Residual and dynamic range of retinal nerve fiber layer thickness in glaucoma: Comparison of three OCT platforms. Invest Ophthalmol Vis Sci. October 2015;56(11):6344–51. doi: 10.1167/iovs.15-17248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mwanza JC, Budenz DL, Warren JL, et al. Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. Br J Ophthalmol. June 2015;99(6):732–7. doi: 10.1136/bjophthalmol-2014-305745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miraftabi A, Amini N, Morales E, et al. Macular SD-OCT Outcome Measures: Comparison of Local Structure-Function Relationships and Dynamic Range. Invest Ophthalmol Vis Sci. September 01 2016;57(11):4815–23. doi: 10.1167/iovs.16-19648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amini N, Daneshvar R, Sharifipour F, et al. Structure-Function Relationships in Perimetric Glaucoma: Comparison of Minimum-Rim Width and Retinal Nerve Fiber Layer Parameters. Invest Ophthalmol Vis Sci. September 1 2017;58(11):4623–4631. doi: 10.1167/iovs.17-21936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belghith A, Medeiros FA, Bowd C, et al. Structural Change Can Be Detected in Advanced-Glaucoma Eyes. Invest Ophthalmol Vis Sci. July 01 2016;57(9):Oct511–8. doi: 10.1167/iovs.15-18929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammel N, Belghith A, Weinreb RN, Medeiros FA, Mendoza N, Zangwill LM. Comparing the Rates of Retinal Nerve Fiber Layer and Ganglion Cell-Inner Plexiform Layer Loss in Healthy Eyes and in Glaucoma Eyes. Am J Ophthalmol. June 2017;178:38–50. doi: 10.1016/j.ajo.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 51.Kaiser L Adjusting for baseline: change or percentage change? Stat Med. October 1989;8(10):1183–90. doi: 10.1002/sim.4780081002 [DOI] [PubMed] [Google Scholar]
- 52.Murata N, Togano T, Miyamoto D, Ochiai S, Fukuchi T. Clinical evaluation of microcystic macular edema in patients with glaucoma. Eye (Lond). November 2016;30(11):1502–1508. doi: 10.1038/eye.2016.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brazerol J, Iliev ME, Hohn R, Frankl S, Grabe H, Abegg M. Retrograde Maculopathy in Patients With Glaucoma. J Glaucoma. May 2017;26(5):423–429. doi: 10.1097/IJG.0000000000000633 [DOI] [PubMed] [Google Scholar]
- 54.Parrish RK 2nd, Feuer WJ, Schiffman JC, Lichter PR, Musch DC. Five-year follow-up optic disc findings of the Collaborative Initial Glaucoma Treatment Study. Am J Ophthalmol. April 2009;147(4):717–724 e1. doi:S0002–9394(08)00781–2 [pii] 10.1016/j.ajo.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caprioli J, de Leon JM, Azarbod P, et al. Trabeculectomy Can Improve Long-Term Visual Function in Glaucoma. Ophthalmology. January 2016;123(1):117–28. doi: 10.1016/j.ophtha.2015.09.027 [DOI] [PubMed] [Google Scholar]
- 56.Berchuck SI, Mukherjee S, Medeiros FA. Estimating Rates of Progression and Predicting Future Visual Fields in Glaucoma Using a Deep Variational Autoencoder. Sci Rep. 12 2019;9(1):18113. doi: 10.1038/s41598-019-54653-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


