Abstract
BACKGROUND:
How corticosteroid-use affects treatment response to chemotherapy and immune-checkpoint-inhibitors (CICPIs) remains unknown. We assessed how systemic corticosteroid exposure before CICPI modifies the effect of CICPI on outcomes among patients with metastatic-non-small-cell-lung-cancer (mNSCLC) or extensive-stage-small-cell-lung-cancer (ES-SCLC).
METHODS:
We conducted a retrospective cohort study using electronic health records to examine patients with mNSCLC or ES-SCLC who received chemotherapy (CT) between April 1, 2015 and January 31, 2018 or CICPI between February 1, 2018 and August 31, 2019. We excluded those with an actionable driver mutation. Baseline corticosteroid use was defined as systemic corticosteroids within 28-days before the initiation of CT or CICPI, not including premedications. Coprimary outcomes included overall survival (OS), real-world-progression (rwP), and real-world-progression-free-survival (rwPFS) in CICPI-treated corticosteroid users versus nonusers. We used inverse-probability-of-treatment-weighting (IPW) to adjust for potential confounding.
RESULTS:
The cohort of 316 patients (median [IQR] age, 67 [61–73] years; 156 [49%] male) included 228 CT-treated and 88 CICPI-treated patients. After applying IPW, characteristics were well-balanced between the CT and CICPI groups, and steroid users and nonusers. Using CT-treated steroid nonusers as a common comparator, CICPI-treated steroid users were as likely as CICPI-treated steroid nonusers to die (users IPW-hazard ratio [HR] 0.67, 95%CI 0.35–1.28 versus nonusers IPW-HR 0.88, 95%CI 0.55–1.42; p=0.49), have rwP (IPW-HR 0.35, 95%CI 0.12–0.99 versus IPW-HR 0.41, 95%CI 0.24–0.70; p=0.77), or experience rwPFS (IPW-HR 0.56, 95%CI 0.29–1.09 versus IPW-HR 0.69, 95%CI 0.46–1.03; p=0.59).
CONCLUSION:
Corticosteroid use prior to CICPI was not associated with worse outcomes, suggesting that corticosteroids should be used with CICPIs when indicated.
Keywords: non small cell lung cancer, small cell lung cancer, programmed cell death 1 ligand 1, programmed cell death 1 protein, corticosteroids
INTRODUCTION
Immune-checkpoint-inhibitors (ICPIs) are widely beneficial in cancer treatment, and are routinely used as initial therapy for non-small-cell lung cancer (NSCLC) and small cell lung cancer (SCLC).1–4 Since corticosteroids reduce T-cell activity and could theoretically affect response to ICPI, large phase-III ICPI randomized controlled (RCTs) trials excluded patients using baseline corticosteroids.1,5 Corticosteroids are essential in cancer care,6 and there are limited therapeutic alternatives for some indications such as symptomatic metastases.7
Emerging data suggest corticosteroid use prior to monotherapy ICPI initiation may worsen survival, though use during therapy has not shown to be detrimental.8,9 This may be because corticosteroids suppress the CD8+ T-cell cancer-targeting ability, which normally would undergo stimulation and expansion after initial ICPI exposure.10 However, these findings may also be due to insufficient statistical adjustment for confounding between corticosteroid users and nonusers since corticosteroid users are more likely to have symptomatic metastases and a more aggressive disease. A recent observational study in NSCLC patients found that only the use of corticosteroids for palliative indications prior to ICPIs was associated with reduced survival, further indicating that previous data may be confounded by populations with poor prognostic factors, although this study also did not use modern approaches to adjust for potential confounding.11 Additionally, all previous studies have excluded SCLC patients. Small-cell tumors have shown to have locoregional effects on regulatory T-cells,12 so studies examining immunotherapy response in SCLC are imperative.
Most patients with advanced lung cancer receive combined chemotherapy and ICPI (CICPI) as initial treatment.2–4 Combination CICPI is theorized to boost the effect of ICPI-promoted T-cell priming,13 although it is currently unknown how corticosteroids may modify the effect of CICPI therapy on mNSCLC and ES-SCLC, as the CICPI phase-III trials also excluded patients on baseline corticosteroids.2–4 Studies examining the interaction between corticosteroids and combination chemotherapy are necessary, and national organizations have recently highlighted that identifying strategies that predict response and resistance to immunotherapies is a top research priority.14 Without this information, clinicians may limit the use of corticosteroids, extrapolating from limited single-agent ICPI data. Withholding corticosteroids may increase adverse events and reduce cancer-directed treatment tolerability or quality-of-life. Conversely, liberal corticosteroid use prior to CICPI may jeopardize survival if corticosteroids do indeed negatively impact treatment efficacy.
We assessed how systemic corticosteroid use prior to treatment initiation modifies the effect of CICPI regimens on survival outcomes. We hypothesized that corticosteroid use would reduce treatment response, thus attenuating the effect estimate for CICPI versus chemotherapy (CT) toward the null.
MATERIALS AND METHODS
Study Design and Participants
We conducted a retrospective cohort study using electronic medical record data from a multi-site health system. Our observational study was designed to emulate existing RCTs15 of CICPI versus CT with the key distinction that corticosteroid use was not an exclusion criterion.2–4 This study was designed using the target trial framework (i.e. designed to emulate a hypothetical prospective randomized trial), which respects the temporality between baseline characteristics, exposure, and outcome, thereby reducing potential bias.15 Patients with mNSCLC or metastatic ES-SCLC who received any CT between April 1, 2015 and January 31, 2018 or CICPI between February 1, 2018 (initial publication of phase-III CICPI studies) and August 31, 2019 were included. CICPI regimens included any platinum doublet combined with an ICPI. Patients were excluded if there was the presence of an actionable driver mutation where a first-line targeted therapy would be indicated (e.g. EGFR mutation), prior treatment for mNSCLC or ES-SCLC, active and/or chronic infections (e.g. tuberculosis, hepatitis C, etc.) while initiating CT or CICPI therapy or the use of immunosuppressive medications such as anti-TNF, cyclosporine, tacrolimus, or any other chemotherapy agents. The study protocol was approved by the Lifespan Institutional Review Board.
Exposure and Effect Modifier
Inherent differences exist between patients for whom steroids are indicated versus not indicated, so examining steroid users versus nonusers among CICPI-treated patients presents an important risk of measured and unmeasured confounding bias. Use of CICPI versus CT as study exposures helps minimize both measured and unmeasured confounding bias through an active comparator (CT) group among which steroids were similarly used. Corticosteroid use, defined as any systemic corticosteroid use, not including premedications, within 28 days prior to CT or CICPI initiation, was an effect modifier because it occurred before treatment (i.e. conceptualized as a baseline characteristic). Our primary interest was whether prior corticosteroid use modified the effect of CICPI on outcomes. CT-treated steroid nonusers was the comparator group for estimates, which made identifying relative differences between CICPI-treated steroid users and nonusers possible (i.e., because they share a common comparator).
Outcomes
Outcomes included overall survival (OS), real-world-progression (rwP; a proxy measure of progression defined as the time to the first administration of a subsequent cancer-directed therapy for any reason)16, and real-world-progression-free-survival (rwPFS; the composite of death or progression to second-line therapy).16 Challenges exist with the retrospective assessment of disease progression (i.e. worsening tumor burden).16 When using electronic health record data, information regarding disease progression may need to be collected from unstructured documents such as radiology reports and clinician notes.16 Differences in patient-specific factors may also affect the frequency of imaging or assessment of disease progression, potentially resulting in differential measurement of disease progression.16 The use of rwP and rwPFS helps to mitigate these limitations by using discrete measurable events documented within the medical record.11
We also measured immune-related adverse events (irAEs) among CICPI-treated patients as exploratory outcomes. Most adverse event data was collected from unstructured areas of the electronic medical record (e.g. clinician notes), which limited our ability to accurately document the severity grade of the adverse event. Because milder adverse events are less likely to affect treatment decisions, and thus are less likely to be adequately documented, the grade of the adverse events were not recorded, and irAEs were not considered a primary outcome. For the same reason, we also did not document all (immune- and non-immune-related) adverse events. The treatment of (and the decision to treat) irAEs are primarily dependent on the severity grade of the irAE,17 however this is not always the case for adverse events attributed to chemotherapy alone. These differences may have differentially increased the surveillance and reporting of any adverse events (irAEs or otherwise) in the CICPI group, compared to the CT group overall.
Follow-up was from the first administration of CT or CICPI to an outcome event (death, rwP, or rwPFS), loss-to-follow-up, 866 days of follow-up (the maximum follow-up time in the CICPI-treated population), or the end of study period, whichever occurred first. Outcome events were each examined separately, with death as an additional censoring event for rwP and rwPFS.
Statistical Analyses and Covariates
We estimated propensity scores (PS) for CICPI versus CT treatment using logistic regression models and calculated stabilized inverse probability of treatment weights (IPW) to correct for any potential confounding by measured covariates. Measured covariates included corticosteroid use, age, sex, cancer type, comorbidities and comorbidity burden, performance status, and presence of brain metastases.18–22 To permit assessment of effect measure modification by corticosteroid use, the logistic regression model to estimate the PS for the numerator of the IPW included corticosteroid use as the only independent variable, while the denominator PS model included corticosteroid use, age, sex, cancer type, comorbidities and comorbidity burden, performance status, and presence of brain metastases.23–25
We used IPW Cox proportional hazards regression models to estimate hazard ratios (HR) and 95% robust confidence intervals (95%CIs) for OS, rwP, and rwPFS outcomes with CT-treated steroid nonusers as the reference group for all estimates. IPW Kaplan Meier survival curves were plotted for OS, rwP, and rwPFS. Immune-related adverse events (irAEs) were reported using descriptive statistics. Data were analyzed between March 20, 2020 and April 17, 2020 using Stata, version 16.0 (Stata Corp., College Station, TX).
Sensitivity Analyses
To assess how robust our findings were to potential unmeasured or residual confounding, we conducted sensitivity analyses using the E-value, which is the minimum strength of association, on the risk ratio scale, that an unmeasured confounder would need to have with both treatment and an outcome to fully explain away the observed treatment effect estimate (i.e., if there truly was no effect).26 We calculated the E-value only for those estimates that were significant at the alpha=0.05 significance level.
Stability Analyses
We assessed alternative a priori measures of corticosteroid use based on prednisone-equivalent total-daily-dose and duration of use. Subgroups were defined by tertiles of dose and duration because the extent of corticosteroid exposure required to affect outcomes (i.e., whether certain thresholds of risk exist) is unknown.
RESULTS
Study Cohort
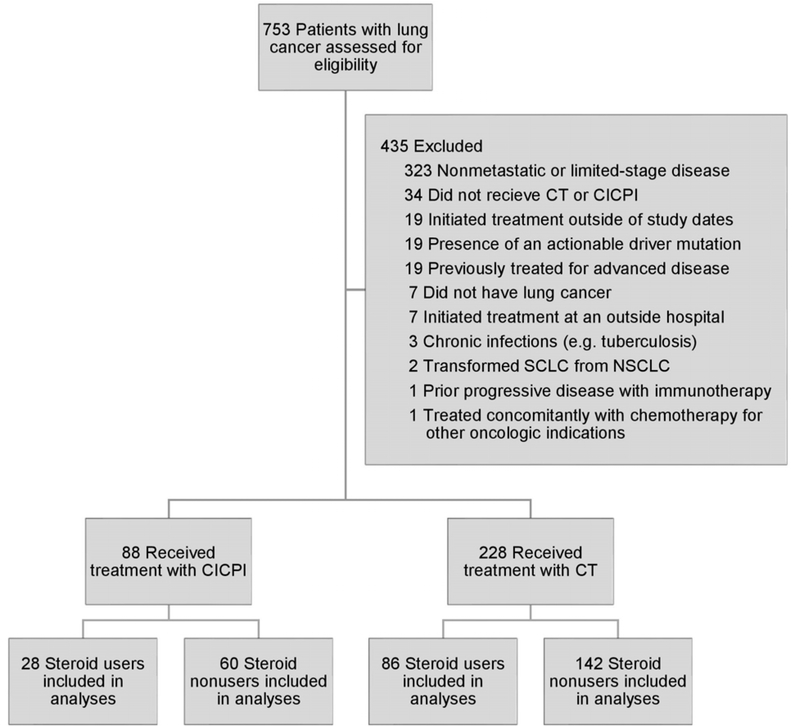
The cohort of 316 patients included 228 CT-initiators and 88 CICPI-initiators, of which 86 (38%) and 28 (32%) were prior corticosteroid-users, respectively (Table 1, Figure 1, Supplemental Table S1). The median (IQR) age was 67 (61–73) years, and 156 (49%) were male. One-hundred-and-forty-five (46%) had NSCLC adenocarcinoma and 112 (35.4%) had SCLC. After applying IPW, characteristics were well-balanced between the CT and CICPI groups, and steroid users and nonusers (Supplemental Tables S2 and S3).
Table 1:
Baseline characteristics of patients initiating chemotherapy with immune checkpoint inhibitors or chemotherapy alone prior to inverse-probability-weighting
| No. (%)a | Overall (n=316) | CT (n=228) | CICPI (n=88) |
|---|---|---|---|
| Age (median [IQR]; years) | 67 (61 – 73) | 67 (61 – 73) | 67 (60 – 74) |
| Male Sex | 156 (49) | 110 (48) | 46 (52) |
| ECOG Performance Statusb | |||
| 0 | 56 (18) | 44 (19) | 12 (14) |
| 1 | 192 (61) | 126 (55) | 66 (75) |
| 2 or greater | 47 (15) | 39 (17) | 8 (9) |
| Not documented | 21 (7) | 19 (8) | 2 (2) |
| Charlson Comorbidity Index Score | |||
| 7 or less | 45 (14) | 33 (15) | 12 (14) |
| 8–10 | 224 (71) | 159 (70) | 65 (74) |
| 11 or more | 47 (15) | 36 (16) | 11 (13) |
| Current or former smoker | 305 (97) | 222 (97) | 83 (94) |
| Histology | |||
| Adenocarcinoma | 145 (46) | 90 (40) | 55 (63) |
| Squamous | 27 (9) | 19 (8) | 8 (9) |
| Small Cell | 112 (35) | 95 (42) | 17 (19) |
| Otherc | 32 (10) | 24 (11) | 8 (9) |
| Chemotherapy Regimen | |||
| Platinum/pemetrexed ± pembrolizumab | 147 (47) | 90 (40) | 57 (65) |
| Platinum/paclitaxel ± pembrolizumab | 18 (6) | 13 (6) | 5 (6) |
| Platinum/nab-paclitaxel ± pembrolizumab | 20 (6) | 11 (5) | 9 (10) |
| Platinum/etoposide ± atezolizumabd | 131 (42) | 114 (50) | 17 (19) |
| Brain metastases prior to therapy | 135 (43) | 99 (43) | 36 (41) |
| Tumor PD-L1% | |||
| <1% | 50 (16) | 24 (11) | 26 (30) |
| 1% or greater | 54 (17) | 13 (6) | 41 (47) |
| Unknown (NSCLC)e | 100 (32) | 96 (42) | 4 (5) |
| Unknown (SCLC)f | 112 (35) | 95 (42) | 17 (19) |
| Patients with an identified mutation | 64 (20) | 33 (15) | 31 (35) |
| KRAS/NRAS | 34 (11) | 16 (7) | 18 (21) |
| Other | 30 (10) | 17 (8) | 13 (15) |
| Not tested – NSCLCf | 56 (18) | 43 (19) | 13 (15) |
| Not tested – SCLCf | 110 (35) | 93 (41) | 17 (19) |
| Prior therapy (prior nonadvanced disease) | 43 (14) | 28 (11) | 18 (21) |
| Chemotherapy or chemoradiationg | 20 (6) | 10 (4) | 10 (11) |
| Radiotherapy | 8 (3) | 5 (2) | 3 (3) |
| Surgery | 15 (5) | 10 (4) | 5 (6) |
| Steroids within 28 days prior to therapy | 114 (36) | 86 (38) | 28 (32) |
| Steroid indicationh | |||
| Symptomatic brain metastases | 64 (56) | 44 (51) | 20 (71) |
| Cancer or treatment-related symptoms | 30 (26) | 24 (28) | 6 (21) |
| Noncancer indication or unknown | 20 (18) | 18 (21) | 2 (7) |
| Total steroid daily dose, in prednisone equivalents (median [IQR]; mg)h | 53 (27, 100) | 53 (27, 107) | 33 (27, 53) |
Abbreviations: CT, chemotherapy; CICPI, chemotherapy and immune checkpoint inhibitors; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1; NSCLC, nonsmall cell lung cancer; SCLC, small cell lung cancer.
ECOG performance score is defined as the following: 0, fully active, able to carry on all pre-disease performance without restriction; 1, restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature; 2, ambulatory and capable of all selfcare but unable to carry out any work activities; 3, capable of only limited selfcare; 4, completely disabled; 5, dead.
Percentages may not add to 100% due to rounding.
Other histologies included large cell neuroendocrine carcinoma, NSCLC with sarcomatoid features, pleiomorphic histology, and mixed histology.
The platinum/etoposide doublet with atezolizumab was the most recently approved regimen of the therapies examined. This may have contributed to the low proportion included in the CICPI group.
Includes patients who were treated before PD-L1 expression assays were commercially available and used clinically as standard of care in the first line setting.
Not routinely tested for in SCLC or neuroendocrine tumor histology.
Includes patients who received surgery in addition to systemic therapy with or without radiation.
Information and percentages are in relation to patients with corticosteroid use.
Figure 1. Flow diagram of lung cancer patients included in the cohort and final analyses, 2015 to 2019.
Abbreviations: CT, chemotherapy; CICPI, chemotherapy and immune checkpoint inhibitor; SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer.
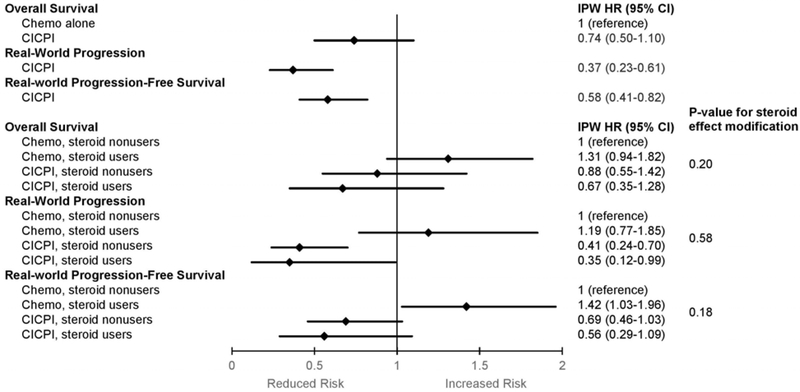
As expected, the overall CICPI group had a lower risk of having rwP (IPW-HR 0.37, 95%CI 0.23–0.61) or rwPFS (IPW-HR 0.58, 95%CI 0.41–0.82), compared to the overall CT group (Figures 2 and 3). CICPI-treated patients overall had a similar risk of death compared to CT-treated patients overall (IPW-HR 0.74, 95%CI 0.50–1.10; Figure 2, Supplemental Table S4).
Figure 2. Outcomes of chemotherapy with immune checkpoint inhibitors versus chemotherapy alone stratified by systemic corticosteroid exposure among patients with advanced lung cancer, 2015 to 2019.
Abbreviations: CICPI, chemotherapy and immune checkpoint inhibitors; IPW, inverse-probability-weighting; HR, hazard ratio; 95%CI, 95% confidence interval.
Hazard ratios and 95% confidence intervals are depicted as diamonds and lines for comparisons between groups overall and stratified by corticosteroid use.
Chemotherapy-treated steroid nonusers were the comparator group for all estimations.
Figure 3. Kaplan Meier curves of overall survival, real-world progression, and real-world progression free survival among patients with advanced lung cancer, 2015 to 2019.
Abbreviations: CT, chemotherapy; CICPI, chemotherapy and immune checkpoint inhibitors.
Kaplan Meier curves for overall survival (A), real-world progression (B), and real-world progression-free-survival (C) of patients with advanced lung cancer treated with chemotherapy and immune checkpoint inhibitors compared to chemotherapy alone, stratified by corticosteroid use. Kaplan Meier plots represent the inverse-probability-weighted estimates. Risk tables listed below the plots include both crude (before weighting) and inverse-probability-weighted populations at each time point.
Outcomes and Modification by Steroids
Using CT-treated steroid nonusers as a common comparator, CICPI-treated steroid users and steroid nonusers had similar OS (steroid users IPW-HR 0.67, 95%CI 0.35–1.28 versus nonusers IPW-HR 0.88, 95%CI 0.55–1.42; p=0.49), rwP (IPW-HR 0.35, 95%CI 0.12–0.99 versus IPW-HR 0.41, 95%CI 0.24–0.70; p=0.77), and rwPFS (IPW-HR 0.56, 95%CI 0.29–1.09 versus IPW-HR 0.69, 95%CI 0.46–1.03; p=0.59; Figures 2 and 3, Supplemental Table S4).
Sensitivity and Stability Analyses
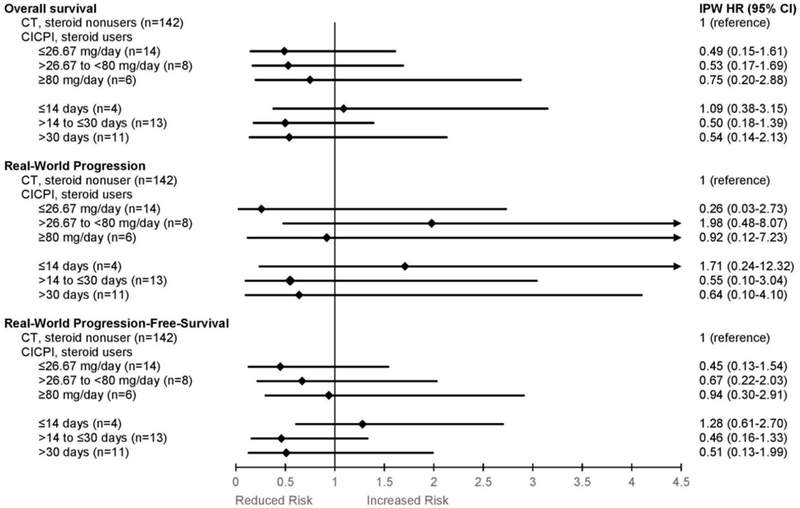
In CICPI-treated versus CT-treated patients overall, the E-value was 3.37 for the rwP estimate and 2.27 for the rwPFS estimate, suggesting our results were moderately robust to potential unmeasured confounding. E-values were not calculated for any other outcome estimates because the confidence interval for estimates included 1 (i.e. the null). No marked differences were observed between corticosteroid subgroups stratified by daily dose or duration of corticosteroid use (Figure 4).
Figure 4: Subgroup analyses (i.e. effect modification analyses) of advanced lung cancer corticosteroid-users stratified by total daily prednisone-equivalents or duration of corticosteroid use, 2015 to 2019.
Abbreviations: IPW, inverse-probability-weighted; HR, hazard ratio; 95% CI, 95% confidence interval; CT, chemotherapy; CICPI, chemotherapy and immune checkpoint inhibitor.
Hazard ratios and 95% confidence intervals are depicted as diamonds and lines for comparisons between groups stratified by total daily dose (prednisone milligram equivalent) or duration of corticosteroid use. Chemotherapy-treated steroid nonusers were the comparator group for all estimations. Patients were considered steroid-users if they had systemic corticosteroid exposure within the 28-day period prior to initiation of CICPI, however the total duration of corticosteroid use was documented and patients may have used corticosteroids before, and during, this 28-day period. Patients without corticosteroid exposure during this 28-day period preceding CT or CICPI were considered corticosteroid nonusers, irrespective of any systemic corticosteroid exposure prior to this 28-day period.
Immune-related Adverse Events
Among CICPI-treated patients, 36 (60%) steroid nonusers and 11 (39%) steroid users had an irAE of any grade (Table 2). The most common irAE seen in both groups was rash or dermatitis occurring in 20% steroid nonusers and 14% steroid users.
Table 2:
Rates of immune-related adverse events among advanced lung cancer patients treated with combination chemotherapy and immune checkpoint inhibitors stratified by corticosteroid use, 2015 to 2019
| Number (%) | CICPI-Treated Patients (n=88) | |
|---|---|---|
| Steroid Nonusers (n=60) | Steroid Users (n=28) | |
| Total patients with irAEs | 36 (60) | 11 (39) |
| Rash/dermatitis | 12 (20) | 4 (14) |
| Colitis | 5 (8) | 2 (7) |
| Hepatitis | 4 (7) | 3 (11) |
| Thyroiditis | 1 (2) | 0 |
| Pneumonitis | 8 (13) | 1 (4) |
| Adrenal insufficiency | 1 (2) | 0 |
| Nephritis | 1 (2) | 0 |
| Temporal Arteritis | 1 (2) | 0 |
| Posterior reversible encephalopathy syndrome | 1 (2) | 0 |
| Autoimmune Hemolytic Anemia | 1 (2) | 0 |
| Ocular Myasthenia gravis | 1 (2) | 0 |
| Bell’s Palsy flair | 0 | 1 (4) |
Abbreviations: CICPI, chemotherapy and immune checkpoint inhibitor; irAEs, immune-related adverse events.
DISCUSSION
In this retrospective cohort study, we examined how corticosteroid use prior to CICPI initiation modifies treatment effects in advanced lung cancer patients. We observed that corticosteroid use prior to CICPI did not jeopardize survival outcomes or treatment response. Withholding corticosteroids could deprive patients of important benefits without impacting CICPI effectiveness, especially because CICPI-treated steroid nonusers had numerically higher rates of irAEs versus steroid users. Our results are especially impactful for patients with symptomatic metastases to which corticosteroids largely improve quality-of-life. Expeditious initiation of tumor-directed therapy in this population is crucial however rapid titration or discontinuation of corticosteroids to facilitate CICPI initiation may lead to adverse events or undertreated symptoms. Corticosteroids should be used when indicated in patients with lung cancer receiving CICPI, particularly in symptomatic patients for which corticosteroids have a beneficial role.
RCTs examining the effect modification of CICPI by corticosteroid use are unlikely to occur due to a lack of equipoise between corticosteroid users and nonusers. Our rigorous observational study emulating such a trial using modern causal inference methods helps fill this knowledge gap. This study, to the best of our knowledge, is the first to examine the effect of corticosteroid use prior to CICPI. The most comparable prior studies examined single-agent ICPIs and found that prior corticosteroid use was associated with decreased survival8,27,28 likely due to T-cell downregulation and apoptosis, subsequently counteracting ICPI-induced T-cell stimulation.5
Two possible explanations may exist for our findings. First, we adjusted for confounding with IPW and used an active comparator design, whereas previous studies did not. This likely reduced bias substantially because corticosteroid use is more common among patients with aggressive or symptomatic disease.11 Second, the apoptotic signal from the glucocorticoid receptor on the T-cell may be overridden by a stronger stimulatory signal from the antigen-bound T-cell receptor.5 Direct chemotherapy-induced tumor lysis increases circulating antigens, improving T-cell priming and activation at therapy initiation, which is potentiated by concomitant ICPIs13, possibly preserving treatment efficacy regardless of corticosteroid use.
Our study is unique in its inclusion in our cohort of both ES-SCLC and mNSCLC patients. To date, the potential treatment effect modification of immunotherapy by corticosteroids has only been evaluated in NSCLC patients, highlighting another important evidence gap. Small-cell tumors are often more aggressive than non-small-cell histologies and many patients present with acutely symptomatic metastases which require corticosteroids.29 While small-cell histology is generally associated with a poorer prognosis,29 it is unlikely to affect our results because the populations of each histology type were well balanced among all cohorts after IPW. The various treatment regimens are also unlikely to have any effect on the results because the choice of CICPI regimen is highly dependent on the tumor histology. With well-balanced histologic populations, the proportion of each of the CICPI regimens is also likely well-balanced across all cohorts. Furthermore, the potential biologic mechanism of combination CICPI would not be unique to any particular platinum doublet or ICPI agent.13 The inclusion of various tumor histologies and CICPI therapies, therefore, improves the generalizability of this study while having a minimal effect on the internal validity of the results.
The primary limitation of this study was its modest sample size, especially in the CICPI group, limiting statistical power for the steroid dose and duration analyses. Larger studies that use similarly detailed data are necessary to reassess the impact of steroid dose and duration with greater precision, as well as the potential impacts of steroid indication (e.g. cancer vs noncancer indications) and tumor type (e.g. SCLC vs NSCLC) as clinically meaningful differences may exist. Furthermore, only the largest corticosteroid total-daily-dose within the 28-day period prior to therapy was documented. Due to frequent corticosteroid dose changes (e.g. tapers), more work is necessary to define exposure with greater nuance, which will be a continued challenge. Additionally, the use of rwP and rwPFS may have misclassified patients who did not truly experience progression in the following two scenarios: 1) patients with disease progression who do not initiate a subsequent therapy, or 2) patients who initiate a subsequent therapy due to reasons other than disease progression (e.g. tolerability). It is unlikely that these misclassifications would occur differentially across treatment groups, and thus they would be unlikely to have a meaningful impact on the treatment effect estimates if they do indeed exist. Using treatment-based rwPFS (defined as the composite outcome of treatment-based rwP or OS) may help to overcome any potential issues related to misclassification. Finally, because our study was observational, we cannot entirely rule-out the possibility of residual confounding by unmeasured covariates. However, our overall CICPI versus CT results closely resembled those from prior RCTs, suggesting that residual confounding is unlikely or minimal.
Conclusions and Implications
The use of corticosteroids prior to CICPI was not associated with reduced survival or treatment response. Corticosteroids should be used with CICPIs when indicated and should not be withheld due to concerns about worse outcomes.
Supplementary Material
HIGHLIGHTS.
One-third of patients receiving chemoimmunotherapy used prior corticosteroids.
Prior corticosteroids did not affect survival or response with chemoimmunotherapy.
Corticosteroids should not be withheld in patients receiving chemoimmunotherapy.
ACKNOWLEDGMENTS
At the time of submission of this manuscript, Dr. Sorial’s affiliation changed to Brigham and Women’s Hospital. Dr. Sorial was previously affiliated with Lifespan-Rhode Island Hospital where this work was completed, and University of Rhode Island. Drs. Sorial and Zullo had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
Funding
Role of the Funders
STUDY SUPPORT: This study was supported by the Rhode Island Hospital Department of Pharmacy. Dr. Zullo is also supported, in part, by grants from the National Institute on Aging (grant number R21AG061632); and from the National Institute of General Medical Sciences (grant number U54GM115677). Dr. Zullo is also supported by a U.S. Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship in Health Services Research and Development. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosures
All authors have no conflicts of interest to report.
Prior Presentation
Parts of this work were presented as a poster presentation at the Vizient/ASHP Midyear Conference in Las Vegas, NV, on December 7, 2019.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 2.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 3.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 4.Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 5.Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cell Mol Life Sci. 2006;63(1):60–72. doi: 10.1007/s00018-005-5390-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulsen O, Klepstad P, Rosland JH, et al. Efficacy of methylprednisolone on pain, fatigue, and appetite loss in patients with advanced cancer using opioids: a randomized, placebo-controlled, double-blind trial. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(29):3221–3228. doi: 10.1200/JCO.2013.54.3926 [DOI] [PubMed] [Google Scholar]
- 7.Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103–114. doi: 10.1007/s11060-009-0057-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbour KC, Mezquita L, Long N, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(28):2872–2878. doi: 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 9.Horvat TZ, Adel NG, Dang T-O, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(28):3193–3198. doi: 10.1200/JCO.2015.60.8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. doi: 10.1038/nature19330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune Checkpoint Inhibitor Outcomes for Patients With Non–Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol. 2019;37(22):1927–1934. doi: 10.1200/JCO.19.00189 [DOI] [PubMed] [Google Scholar]
- 12.Becker JC, Andersen MH, Schrama D, Thor Straten P. Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother CII. 2013;62(7):1137–1148. doi: 10.1007/s00262-013-1434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. doi: 10.1038/cdd.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Research Priorities to Accelerate Progress Against Cancer. ASCO. Published January 24, 2020. Accessed June 16, 2020. https://www.asco.org/research-guidelines/reports-studies/clinical-cancer-advances-2020/research-priorities-accelerate
- 15.Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol. 2016;183(8):758–764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffith SD, Miksad RA, Calkins G, et al. Characterizing the Feasibility and Performance of Real-World Tumor Progression End Points and Their Association With Overall Survival in a Large Advanced Non-Small-Cell Lung Cancer Data Set. JCO Clin Cancer Inform. 2019;3:1–13. doi: 10.1200/CCI.19.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole SR, Hernán MA. Constructing Inverse Probability Weights for Marginal Structural Models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danaei G, García Rodríguez LA, Cantero OF, Logan R, Hernán MA. Observational data for comparative effectiveness research: an emulation of randomised trials to estimate the effect of statins on primary prevention of coronary heart disease. Stat Methods Med Res. 2013;22(1):70–96. doi: 10.1177/0962280211403603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernán MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiol Camb Mass. 2008;19(6):766–779. doi: 10.1097/EDE.0b013e3181875e61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiol Camb Mass. 2000;11(5):561–570. doi: 10.1097/00001648-200009000-00012 [DOI] [PubMed] [Google Scholar]
- 22.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiol Camb Mass. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 23.VanderWeele TJ. On the distinction between interaction and effect modification. Epidemiol Camb Mass. 2009;20(6):863–871. doi: 10.1097/EDE.0b013e3181ba333c [DOI] [PubMed] [Google Scholar]
- 24.VanderWeele TJ, Robins JM. Four types of effect modification: a classification based on directed acyclic graphs. Epidemiol Camb Mass. 2007;18(5):561–568. doi: 10.1097/EDE.0b013e318127181b [DOI] [PubMed] [Google Scholar]
- 25.Zullo AR, Howe CJ, Galárraga O. Estimating the Effect of Health Insurance on Personal Prescription Drug Importation. Med Care Res Rev MCRR. 2017;74(2):178–207. doi: 10.1177/1077558716629039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 27.Scott SC, Pennell NA. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2018;13(11):1771–1775. doi: 10.1016/j.jtho.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 28.Fucà G, Galli G, Poggi M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open. 2019;4(1):e000457. doi: 10.1136/esmoopen-2018-000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalemkerian GP, Akerley W, Bogner P, et al. Small Cell Lung Cancer. J Natl Compr Cancer Netw JNCCN. 2013;11(1):78–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.