Abstract
Objective:
Varicella zoster virus (VZV) vasculopathy and cerebral amyloid angiopathy (CAA) have similar clinical presentations: both affect cerebrovasculature in the elderly, produce hemorrhage, and can have a protracted course of cognitive decline and other neurological deficits. The cause of CAA is unknown, but amyloid-beta (Aβ) is found within arterial walls. Recent studies show that VZV induces Aβ and amylin expression and an amyloid-promoting environment. Thus, we determined if VZV was present in CAA-affected arteries.
Methods:
Two subjects with pathologically-verified CAA were identified postmortem and frontal lobes analyzed by immunohistochemistry for arteries containing VZV, Aβ, and amylin and H&E for pathological changes. VZV antigen detection was confirmed by PCR for VZV DNA in the same region.
Results:
In both CAA cases, sections with cerebral arteries containing VZV antigen with corresponding VZV DNA were identified; VZV antigen co-localized with Aβ in media of arteries with histological changes characteristic of CAA. Amylin was also seen in the intima of a VZV-positive artery in the diabetic subject. Not all Aβ-containing arteries had VZV, but all VZV-positive arteries contained Aβ.
Conclusions:
VZV antigen co-localized with Aβ in some affected arteries from two CAA cases, suggesting a possible association between VZV infection and CAA.
Keywords: Varicella zoster virus, Cerebral amyloid angiopathy, Amyloid, Amyloid-β, Amylin, Vasculopathy
1. Introduction
Varicella zoster virus (VZV) is an alphaherpesvirus that latently infects >90% of the world’s population and reactivates with immunosuppression or aging. Upon reactivation from dorsal root ganglia, VZV travels along neurites to skin, causing herpes zoster (shingles) in the corresponding dermatomal distribution. VZV can also reactivate from right and/or left trigeminal or upper cervical ganglia that innervates cerebral arteries, infect ipsilateral arteries, and produce transient ischemic attacks (TIAs), ischemic and hemorrhagic stroke, and aneurysms (VZV vasculopathy; also referred to as VZV vasculitis or VZV angiitis) with or without associated zoster rash [reviewed in ref. 1,2]. VZV vasculopathy patients can present with headache, cognitive changes, and focal neurological deficits. Importantly, VZV vasculopathy can have a protracted course as demonstrated by cases of : (1) a 73-year-old man with mental status changes and progressive neurological deificts attributed to a waxing and waning vasculitis of 314 days duration [3]; diagnosis was confirmed by the presence of VZV DNA, antigen, and herpesvirus particles in affected arteries; (2) a 69-year-old man with 4 strokes manifesting as a multi-infarct dementia of 2 years duration; diagnosis was confirmed by detection of intrathecal anti-VZV antibody synthesis [4]; and (3) a 63-year-old man with zoster; 3 months later, he developed cognitive difficulties that progressed over a 6-year period; diagnosis was confirmed by detection of VZV antigen in a brain biopsy [5].
VZV-infected arteries exhibit varying degrees of inflammation depending on disease duration, ranging from rare neutrophil infiltration to widespread vasculitis with a predominance of T cells and macrophages [6]. Additional histopathological changes include fragmented internal elastic lamina, a thickened intima comprised of myofibroblasts, and a disorganized media with a paucity of smooth muscle cells [7]. Aside from VZV vasculopathy, VZV can cause encephalitis and both diseases have been associated with cognitive decline and long-term cognitive impairment that often resolves following antiviral treatment [4,5,8].
Cerebral amyloid angiopathy (CAA) is characterized by the aggregation of amyloid-beta (Aβ) peptides into amyloid that deposits within adventitia and media of cerebral arteries [9]. CAA can manifest as rapid cognitive decline and is frequently associated with Alzheimer’s disease [10,11]. CAA is often diagnosed following intracerebral hemorrhage in the elderly [11]. The cause of CAA is not known, yet it has been speculated to involve increased production of Aβ or abnormal clearance, overall leading to arterial amyloid deposition and vascular damage.
VZV vasculopathy and CAA have many similarities: both (1) are diseases of the elderly, (2) directly affect cerebral arteries (TIAs, ischemia, and hemorrhage), and (3) can have a protracted course presenting as progressive cognitive decline and other neurological deficits. A recent case study raised the intriguing possibility that these two diseases are associated. Takeshita and colleagues [12] described an elderly man with dementia who presented with a left parietal intracerebral hemorrhage and cerebellar infarct. Microbleeds attributed to CAA in the subcortical areas and multiple vascular stenoses were also seen by magnetic resonance imaging (MRI) and magnetic resonance angiopathy. Six days later, the patient developed herpes zoster and VZV meningitis, confirmed by the presence of VZV DNA in cerebrospinal fluid (CSF). Additional intracerebral hemorrhages developed and he died 18 days later. Postmortem analysis confirmed the diagnosis of CAA.
Three recent studies further support a potential link between VZV infection and CAA because of VZV’s ability to induce amyloid deposition. Specifically, VZV-infected spinal astrocytes in vitro induced intracellular Aβ and amylin expression and amyloid formation that was absent in uninfected cells; furthermore, the extracellular environment of VZV-infected cells was amyloidogenic, likely due in part through the generation of self-aggregating and catalyzing VZV glycoprotein B peptides [13]. Amylin is a peptide hormone, co-secreted with insulin from pancreatic β cells in response to food intake, to slow gastric emptying and promote satiety. Similar to Aβ42, amylin can aggregate to form amyloid fibrils independently or mixed with Aβ42 in plaques of Alzheimer’s brains [14], of which up to 90% have CAA pathology. A second study showed that, compared to non-herpes zoster control plasma, plasma from acute herpes zoster subjects had elevated levels of amyloid that positively correlated with Aβ42 and amylin levels [15]. A third study showed that compared to stroke controls, CSF from VZV vasculopathy patients had significantly increased amylin and amyloid that correlated with anti-VZV antibody titers and contained factors that induced amyloid formation [16]. Furthermore, VZV vasculopathy CSF had reduced Aβ40 which is also decreased in Dutch-type hereditary CAA [17].
Given the ability of VZV to induce amyloid formation in vitro and in vivo and VZV’s ability to infect cerebral arteries and cause features similar to CAA, as well as the recent report of a case of CAA associated with herpes zoster and VZV meningitis [12], we determined if VZV antigen and DNA were present in cerebral arteries of two pathologically-verified cases of CAA.
2. Material and methods
De-identified, formalin-fixed, paraffin-embedded (FFPE) brain samples from 2 pathologically-verified CAA cases were obtained from the University of Colorado Department of Pathology. Clinical data was only available from the accompanying autopsy reports. These samples were used to investigate a potential link between VZV infection of cerebral arteries and CAA.
2.1. Case presentations
2.1.1. Case 1
An 84-year-old man presented with sudden onset left arm and leg weakness. His history was remarkable for dementia, diabetes mellitus, cardiovascular disease, and chronic renal failure. Three years prior, he suffered a stroke with residual speech deficits and an MRI showed low T1 signal in the cerebellar hemispheres, suggestive of bilateral vascular malformations. On admission, exam was remarkable for a left hemiparesis; he was unable to ambulate but could perform transfers. A brain MRI identified lesions in the posterolateral aspect of the posterior limb of the right internal capsule, the anterolateral margin of the right atrium, and right parietal subcortical white matter. Two weeks later, the patient became febrile and unresponsive. MRI and an electrocardiogram revealed a large left frontal-parietal intra-parenchymal hemorrhage (8 × 6 cm) and a non-ST-elevation myocardial infarction, respectively. He died three weeks later and a full autopsy was performed. Postmortem macroscopic analysis of brain revealed multifocal, small cerebral hemisphere hemorrhages (acute to subacute) that were consistent with CAA. CAA was diagnosed microscopically via Congo Red and Aβ stains and characterized as diffuse and moderate to severe. These stains corresponding to areas of cerebral hemorrhage lead to the determination of cause of death as hemorrhage due to chronic, moderate to severe CAA that was complicated by hypertension.
2.1.2. Case 2
A 72-year-old woman with a 30-year history of TIAs and on clopidogrel presented with sudden unresponsiveness; MRI revealed a large intraparenchymal hemorrhage of the left frontal lobe (7.8 × 4.7cm), including an intraventricular hemorrhage in the left lateral ventricle, without aneurysm or other vascular malformation. The patient remained comatose (Glasgow coma score = 7) and was intubated. Due to her poor prognosis, the family withdrew care and the patient expired within 1 week of presentation. A full autopsy was performed and several neurological findings were noted. Macroscopically, the intraparenchymal hemorrhage obliterated the local left superior parasagittal cortex, extending into the sub-adjacent subcortical white matter, and dissecting to the anterior left lateral frontal ventricular horn. Microscopic analysis revealed necrosis due to the large hemorrhage and several subcortical satellite hemorrhages adjacent to the large hemorrhage. Many arteries and arterioles in the bilateral frontal, left temporal, left parietal, and right occipital lobes contained significant amounts of Aβ. There was also a left parietal lobe infarct due to previous embolic thrombosis. Cause of death was determined to be complications of a large frontal lobe hemorrhage due to diffuse, moderate to severe CAA.
2.2. Immunohistochemical analysis
Formalin-fixed, paraffin-embedded brain blocks were obtained from the two cases of CAA. Specifically, two blocks (frontal lobe and hippocampus) from case 1 and three blocks of bilateral frontal lobe from case 2 were studied. One-hundred 5-μm sections from each of the five blocks were cut and 50 alternating sections were immunostained for the presence of VZV glycoprotein E (gE, cat. no. Sc-56995; Santa Cruz Biotechnology, Dallas, TX, USA) antigen as described [18]. Once slides containing VZV antigen in arteries were identified, the adjacent slide was stained by hematoxylin and eosin (H&E) to determine if there were associated histopahtological changes. A second adjacent slide was dual-immunostained for the presence of amylin and Aβ using a rabbit anti-amylin antibody (1:250, cat. no. ab55411, Abcam, Cambridge, MA, USA) with antigen retrieval in citrate buffer prior to blocking and a mouse anti-β-amyloid antibody that detects amyloid precursor protein and all Aβ isoforms (1:500, cat. no. 803001, San Diego, CA, USA) using Vector Laboratories’ (Burlingame, CA, USA) ImmPACT NovaRED Peroxidase and Vector Blue Alkaline Phosphatase substrates as described [19]. Positive controls for Aβ and amylin consisted of pathologically confirmed Alzheimer’s disease brain and human pancreas, respectively. Negative controls consisted of replacing primary rabbit and mouse antibodies with the same dilutions of normal rabbit serum (NRS) and mouse IgG1 isotype control antibody (mIgG1; Agilent, Santa Clara, CA, USA), respectively.
2.3. DNA extraction and quantitative PCR analysis
VZV gE-positive areas from each block were identified via light microscopy, scraped, pooled, DNA extracted, and analyzed by quantitative PCR (qPCR) in triplicate for the presence of VZV and glyceraldehyde 3-phosphate dehydrogenase (GAPdH) DNA as described [20]. Cycling conditions consisted of a 95°C holding stage for 10 minutes followed by 45 cycles of 95°C for 30 seconds and 60°C for 1 minute.
3. Results
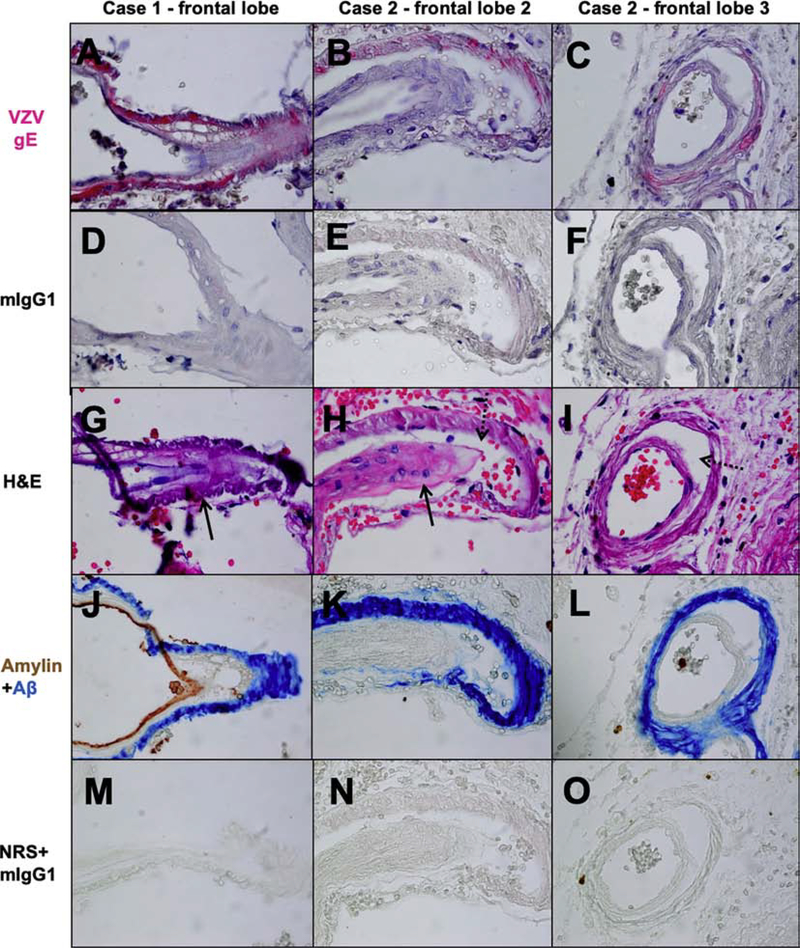
VZV antigen was detected in 3 different cerebral arteries across multiple sections and non-contiguous skip lesions of 3 blocks (Fig. 1A-C, pink color): case 1 frontal lobe contained 2 VZV-positive arteries and 2 skip lesions (Fig. 1A); case 2 frontal lobe block 2 contained 3 VZV-positive arteries and 2 skip lesions (Fig. 1B); and case 2 frontal lobe block 3 contained 3 VZV-positive arteries and 3 skip lesions (Fig. 1C). No staining was seen with the negative control, mIgG1 (Fig. 1D-F). The hippocampus from case 1 and the frontal lobe block 1 from case 2 contained VZV antigen in brain parenchyma but not arteries (data not shown). VZV is not latent in brain, so the viral antigen is not due to reactivation in brain. Most likely, the VZV antigen that we observed in brain parenchyma is due to virus spread from trigeminal and autonomic nerve fibers to arteries, where the fibers terminate, to surrounding tissue.
Fig. 1.
Varicella zoster virus (VZV) antigen co-localizes with amyloid-beta (Aβ) and amylin in some arteries from cases of cerebral amyloid angiopathy (CAA). Formalin-fixed, paraffin-embedded frontal lobes from 2 cases of CAA were analyzed. Immunohistochemical analysis of slide sections using a mouse anti-VZV glycoprotein E (gE) antibody revealed cerebral arteries containing VZV antigen (pink color) in case 1 (A) and in two separate frontal lobe blocks of case 2 (B-C); specifically, VZV antigen was present within the medial smooth muscle cell layer. No staining was seen in nearby sections when primary antibody was replaced with mouse anti-IgG1 isotype control (mIgG1, D-F). Hematoxylin and eosin (H&E) stained adjacent slide sections showed that the VZV antigen-positive arteries had foamy medial layers (G-H, arrows) and/or a vessel-in-vessel appearance characteristic of CAA (H-I, dotted arrows). Immunohistochemical analysis of another slide adjacent to the VZV antigen-positive slides using a mouse anti-Aβ amino acids 1–16 antibody and a rabbit anti-amylin antibody revealed Aβ (blue color) in the same artery and medial smooth muscle cell layer where VZV antigen was found in case 1 and 2 (J-L); in addition, amylin (brown color) was seen in the intimal endothelial cell layer of case 1 (J). No staining was seen when primary antibodies were replaced with normal rabbit serum (NRS) and mIgG1 (M-O) control antibodies. Magnification 600X.
Slides adjacent to VZV gE-positive arteries evaluated by H&E revealed amorphous material in the media of blood vessels strongly suspicious for Aβ in cerebral arteries (Fig. 1G-H, arrows) with focal vessel-in-vessel appearance (Fig. 1H-I, dotted arrows) characteristic of CAA. As a result, another adjacent slide was dual-immunostained for amylin and Aβ. Among all arteries stained, amylin was detected in only one VZV gE-positive artery in the frontal lobe of case 1 (Fig. 1J, brown color), while Aβ was detected in VZV gE-positive arteries in the frontal lobe of both cases (Fig. 1J-L, blue color). No staining was seen with NRS and mIgG1 negative controls (Fig. 1M-O).
While many intracerebral arteries contained Aβ, and some of these Aβ-positive arteries also contained amylin and VZV, several arteries did not contain any VZV, amylin, or Aβ (Fig. 2A-C and J-L) and, histologically, showed only variable degrees of arteriolosclerosis with no evidence of Aβ deposition by H&E (Fig. 2G-I). No staining was seen with mIgG1 (Fig. 2D-F) or NRS and mIgG1 (Fig. 2M-O).
Fig. 2.
Varicella zoster virus (VZV), amyloid-beta (Aβ) and amylin are not present in all cerebral arteries in cases of cerebral amyloid angiopathy (CAA). Immunohistochemical analysis using a mouse anti-VZV glycoprotein E (gE) antibody revealed that not all arteries in CAA cases 1 and 2 contained VZV antigen (A-C, no pink color). No staining was seen when primary antibody was replaced with mouse anti-IgG1 isotype control (mIgG1, D-F). Hematoxylin and eosin (H&E) staining of adjacent slide sections showed that VZV antigen-negative arteries showed only arteriolosclerotic thickening of the media without evidence of Aβ deposition (G-I). Immunohistochemical analysis of adjacent slides using a mouse anti-Aβ amino acids 1–16 antibody and a rabbit anti-amylin antibody revealed that these arteries were negative for Aβ and amylin (J-L, no blue or brown color, respectively). No staining was seen when primary antibodies were replaced with normal rabbit serum (NRS) and mIgG1 (M-O) control antibodies. Magnification 600X.
Quantitative PCR analysis confirmed viral immunohistochemical findings in both cases. All VZV gE-positive areas from each of the 5 blocks analyzed contained cellular GAPdH DNA (average threshold cycle [Ct] = 30.63). The three blocks containing VZV antigen in cerebral arteries (case 1 frontal lobe and case 2 frontal lobe 2–3) also contained VZV DNA: case 1 frontal lobe = 5.9 VZV copies/50μL DNA, ΔCt = 8.8; case 2 frontal lobe 2 = 57.7 VZV copies/50μL DNA, ΔCt = 8.5; and case 2 frontal lobe 3 = 8.7 VZV copies/50μL DNA, ΔCt = 10.3. The remaining two blocks that contained VZV antigen in brain tissue, but not in cerebral arteries (case 1 hippocampus and case 2 frontal lobe 1) did not amplify VZV DNA.
4. Discussion
Herein, we describe two cases of CAA that contained VZV antigen within cerebral arteries affected by CAA; the specificity of VZV antigen staining was confirmed by scraping the VZV antigen-positive regions and amplifying VZV DNA by qPCR. This is the first demonstration of an association between VZV antigen/DNA and amyloid deposition in cases of CAA. While one previous case report described a patient with intracerebral hemorrhage and cerebellar infarction and a dual diagnosis of CAA and VZV central nervous system disease [12], VZV antigen/DNA has never been shown in CAA-diseased vasculature.
In the CAA samples, both normal arteries and disrupted arteries containing Aβ were seen throughout the brain. In every instance when VZV antigen and DNA were found in an artery, that same artery contained Aβ and histological features consistent with CAA. However, not every CAA artery contained VZV. While this report only examined two cases of CAA, we can speculate on potential reasons for the presence of VZV in affected arteries. One possibility is that the presence of VZV in CAA arteries is an incidental finding. However, VZV has not been incidentally found in normal cerebral arteries [21]. Another possibility is that VZV infection of cerebral arteries may contribute to the pathogenesis of CAA, specifically, amyloid deposition. There is growing evidence in vitro and in vivo that herpesvirus infections can induce amyloid deposition, which most likely functions to sequester virus and prevent CNS spread [22]. A recent report found that VZV-infected primary human spinal astrocytes contain intracellular Aβ, amylin, and amyloid that is absent in uninfected cells, showing a direct effect of virus on amyloid formation [13]; furthermore, the conditioned supernatant of VZV-infected cells induced rapid aggregation of amyloidogenic peptides (Aβ42 and amylin), in part through self-aggregating and catalytic viral peptides, suggesting that VZV-infected cells secrete factors that produce an amyloidogenic extracellular environment. Additional support is provided by a study showing that plasma from individuals with acute zoster contained higher levels of amyloid and induced aggregation of amyloidogenic peptides compared to non-zoster control plasma [15]. Finally, CSF from VZV-vasculopathy patients contained elevated levels of amylin and amyloid that positively correlated with VZV antibody titers [16]. While these studies show that VZV induces amylin expression, it is still unclear why only one of 8 VZV-containing arteries contained amylin. The VZV and amylin-containing artery was seen in a subject with diabetes, and diabetics frequently have elevated serum amylin levels that can cross the blood-brain-barrier to deposit as amyloid in brain; thus this may explain the presence of amylin in a single artery [14]. However, amylin has been found in CAA arteries in non-diabetic patients, therefore further research into amylin’s contribution is warranted [14].
The absence of VZV in all affected CAA arteries may be due to effective viral clearance (through innate and adaptive immune mechanisms), yet the “amyloid footprint” still persists. Specifically, our in vitro studies show that by 3 days post-infection, VZV-infected cells have intracellular Aβ and amyloid [13]. The release of amyloid into the extracellular space upon virus-induced cell death/immune clearance and the release of viral peptides that can self-aggregate to form amyloid would form a nidus for subsequent amyloid deposition longitudinally along the artery, without requiring the presence of the precipitating virus. This process could occur from months to years until clinical symptoms appear, depending on host and environmental factors that facilitate amyloid degradation or formation. Therefore, VZV deposition into cerebral arteries following reactivation from trigeminal or autonomic ganglia may produce focal amyloid deposition, initiating or accelerating pre-existing CAA. Interestingly, CAA may be noninflammatory and, in rare cases, inflammatory [23]. Because arteries from patients with VZV vasculopathy typically contain immune infiltrates [6], the interplay between VZV vasculopathy and Aβ-related angiitis and CAA-related inflammation is an intriguing field to explore.
5. Conclusions
The presence of VZV antigen and DNA in arteries containing Aβ in two cases of CAA further supports an in vivo link between VZV infection and Aβ diseases. Based upon the similar clinical features seen in CAA and VZV central nervous system infection (e.g. cognitive changes, cerebrovascular disease, predominance in the elderly) and given the detection of VZV antigen and DNA in CAA-affected cerebral arteries, there may be an association between the two diseases that warrants further investigation. If these additional studies demonstrate a significant link between VZV infection and CAA, clinical management would potentially change with the evaluation for VZV infection in suspected CAA cases and treatment with antiviral agents.
HIGHLIGHTS.
VZV vasculopathy and CAA have similar clinical presentations
VZV vasculopathy and CAA cause cerebrovascular disease (hemorrhage)
VZV antigen and DNA co-localized with some CAA-affected arteries in two cases of CAA
VZV vasculopathy may contribute to CAA pathogenesis through induction of amyloid
Acknowledgements
The authors wish to thank Bette K. Kleinschmidt-DeMasters, M.D., Department of Pathology, University of Colorado School of Medicine, Aurora, CO, for acquisition of tissue samples used in this study; and Cathy Allen for manuscript preparation.
Funding
This work was supported by the National Institutes of Health (P01 AG032958 to M.A.N; and R01 NS093716 to A.N.B. and M.A.N.).
Abbreviations:
- Aβ
amyloid-beta
- CAA
cerebral amyloid angiopathy
- Ct
threshold cycle
- CVA
cerebrovascular accident
- GAPdH
glyceraldehyde 3-phosphate dehydrogenase
- gE
glycoprotein E
- mIgG1
mouse IgG1 isotype control
- MRI
magnetic resonance imaging
- NRS
normal rabbit serum
- qPCR
quantitative PCR
- TIA
transient ischemic attacks
- VZV
varicella zoster virus
Footnotes
Competing interests/declaration of interest: none
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Liberman AL, Nagel MA, Hurley MC, Caprio FZ, Bernstein RA, Gilden D, Rapid development of 9 cerebral aneurysms in varicella zoster virus vasculopathy, Neurology 82 (2014) 2139–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nagel MA, Bubak AN, Varicella zoster virus vasculopathy, J. Infect. Dis. 218 (2018) S107–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 5–1995, A 73-year-old man with focal brain lesions and peripheral-nerve disease, N. Engl. J. Med. 332 (1995) 452–459. Erratum in N. Engl. J. Med. 332 (1995) 1527. [DOI] [PubMed] [Google Scholar]
- [4].Silver B, Nagel MA, Mahalingam R, Cohrs R, Schmid DS, Gilden D, Varicella zoster virus vasculopathy: a treatable form of rapidly progressive multi-infarct dementia after 2 years’ duration, J. Neurol. Sci. 323 (2012) 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gilden D, Grose C, White T, Nagae L, Hendricks RL, Cohrs RJ, Nagel MA, Successful antiviral treatment after 6 years of chronic progressive neurological disease attributed to VZV brain infection, J. Neurol. Sci. 368 (2016) 240–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nagel MA, Traktinskiy I, Stenmark KR, Frid MG, Choe A, Gilden D, Varicella-zoster virus vasculopathy: immune characteristics of virus-infected arteries, Neurology 80 (2013) 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nagel MA, Traktinskiy I, Azarkh Y, Kleinschmidt-DeMasters B, Hedley-Whyte T, Russman A, VanEgmond EM, Stenmark K, Frid M, Mahalingam R, Wellish M, Choe A, Cordery-Cotter R, Cohrs RJ, Gilden D, Varicella zoster virus vasculopathy: Analysis of virus-infected arteries, Neurology 77 (2011) 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grahn A, Nilsson S, Nordlund A, Lindén T, Studhal M, Cognitive impairment 3 years after neurological Varicella-zoster virus infection: a long-term case control study, J. Neurol. 260 (2013) 2761–2769. [DOI] [PubMed] [Google Scholar]
- [9].Weber SA, Patel RK, Lutsep KL, Cerebral amyloid angiopathy: diagnosis and potential therapies, Expert Rev. Neurother. 18 (2018) 503–513. [DOI] [PubMed] [Google Scholar]
- [10].Planton M, Raposo N, Albucher JF, Pariente J, Cerebral amyloid angiopath-yrelated cognitive impairment: The search for a specific neuropsychological pattern, Rev. Neurol. (Paris) 173 (2017) 562–565. [DOI] [PubMed] [Google Scholar]
- [11].Yanagawa T, Takao M, Yasuda M, Kamide T, Sato H, Suzuki K, Ikeda T, Kikkawa Y, Kurita H, Clinical and neuropathologic analysis of intracerebral hemorrhage in patients with cerebral amyloid angiopathy, Clin. Neurol. Neurosurg. 176 (2019) 110–115. [DOI] [PubMed] [Google Scholar]
- [12].Takeshita J, Nomura E, Himeno T, Shimoe Y, Kuriyama M, Rapidly deteriorated lobar intracerebral hemorrhages: possible association of varicella zoster virus-vasculopathy, Clin. Neurol. 58 (2018) 245–248. [DOI] [PubMed] [Google Scholar]
- [13].Bubak AN, Como CN, Coughlan CM, Johnson NR, Hassell JE Jr., Mescher T, Niemeyer CS, Mahalingam R, Cohrs RJ, Boyd TD, Potter H, Russ HA, Nagel MA, Varicella-zoster virus infection of primary human spinal astrocytes produces intracellular amylin, amyloid-β, and an amyloidogenic extracellular environment, J. Infect. Dis. 221 (2020)1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jackson K, Barisone GA, Diaz E, Jin L-W, DeCarli C, Despa F, Amylin deposition in the brain: a second amyloid in Alzheimer’s disease? Ann. Neurol. 74 (2013) 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bubak AN, Beseler C, Como CN, Tyring SK, Haley C, Mescher T, Hassell JE Jr., Cohrs RJ, Potter H, Nagel MA, Acute zoster plasma contains elevated amyloid, correlating with Aβ42 and amylin levels, and is amyloidogenic, J. Neurovirol. 26 (2020) 422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bubak AN, Beseler C, Como CN, Coughlan CM, Johnson NR, Hassell JE, Burnet AM, Mescher T, Schmid DS, Coleman C, Mahalingam R, Cohrs RJ, Boyd TD, Potter H, Shilleh AH, Russ HA, Nagel MA, Amylin, Aβ42, and amyloid in VZV vasculopathy cerebrospinal fluid and infected vascular cells, J. Infect. Dis. (2020) [Published online ahead of print] Aug 18, 2020. Available from https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiaa513/5893839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schultz AP, Kloet RW, Sohrabi HR, van der Weerd L, van Rooden S, Wermer MJH, Moursel LG, Yaqub M, van Berckel BNM, Chaterjee P, Gardener SL, Taddei K, Fagan AM, Benzinger TL, Morris JC, Sperling R, Johnson K, Bateman RJ, Dominantly Inherited Alzheimer Network, Gurol ME, van Buchem MA, Martins R, Chhatwal JP, Greenberg SM, Amyloid imaging of dutch-type hereditary cerebral amyloid angiopathy carriers, Ann. Neurol. 86 (2019) 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gilden D, White T, Khmeleva N, Heintzman A, Choe A, Boyer PJ, Grose C, Carpenter JE, Rempel A, Bos N, Kandasamy B, Lear-Kaul K, Holmes DB, L Bennett J, Cohrs RJ, Mahalingam R, Mandava N, Eberhart CG, Bockelman B, Poppiti RJ, Tamhankar MA, Fogt F, Amato M, Wood E, Durairaj V, Rasmussen S, Petursdottir V, Pollak L, Mendlovic S, Chatelain D, Keyvani K, Brueck W, Nagel MA, Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis, Neurology 84 (2015) 1948–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mahalingam R, Kaufer BB, Ouwendijk WJD, Verjans GM, Coleman C, Hunter M, Das A, Palmer BE, Clambey E, Nagel MA, Traina-Dorge V, Attenuation of simian varicella virus infection by enhanced green fluorescent protein in rhesus macaques, J. Virol. 92 (2018) e02253–e022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Blackmon AM, Como CN, Bubak AN, Mescher T, Jones D, Nagel MA, Varicella zoster virus alters expression of cell adhesion proteins in hu///manperineurial cells via interleukin 6, J. Infect. Dis. 220 (2019) 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nagel MA, Choe A, Khmeleva N, Overton L, Rempel A, Wyborny A, Traktinskiy I, Gilden D, Search for varicella zoster virus and herpes simplex virus-1 in normal human cerebral arteries, J. NeuroVirol. 19 (2013) 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eimer WA, Kumar DKV, Shanmugam NKN, Rodriguez AS, Mitchell T, Washicosky KJ, Gyӧrgy B, Breakefield XO, Tanzi RE, Moir RD, Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection, Neuron 99 (2018) 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Salvarani C, Morris JM, Giannini C, Brown RD Jr., Christianson T, Hunder GG, Imaging findings of cerebral amyloid angiopathy, Aβ-related angiitis (ABRA), and cerebral amyloid angiopathy-related inflammation: A single-institution 25-year experience, Medicine (Baltimore) 95 (2016) e3613. [DOI] [PMC free article] [PubMed] [Google Scholar]