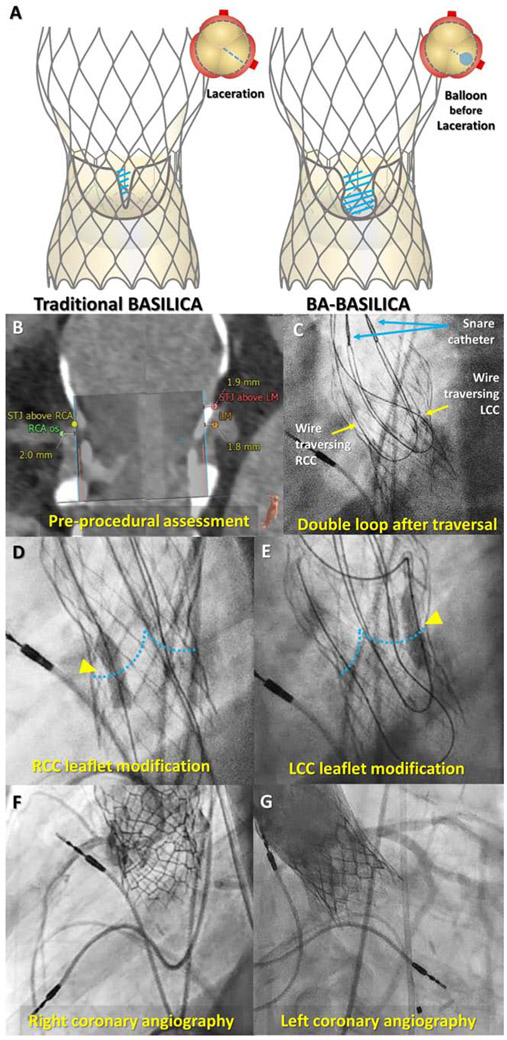
BASILICA can prevent coronary obstruction following TAVR.1 TAV-in-TAV may pose the highest risk for obstruction, because of narrow residual sinuses, tall leaflets, and supra-annular designs. Bench testing suggests that certain TAV devices exhibit inadequate leaflet “splay” following traditional BASILICA.2 In cases risking inadequate splay (heavily calcified leaflets, very small valve-to-coronary distance, TAV-in-TAV), we have performed balloon-assisted (BA)-BASILICA. This expands the traversal point outward by inflating a balloon across the leaflet(s) prior to laceration and increases leaflet splay (Figure 1A). Herein, we describe the first reported case.
Figure.
(A) Concept of balloon-assisted BASILICA; traditional BASILICA (left panel) may not achieve adequate splay due to restriction from the outer frame of a TAV and balloon-assisted BASILICA (right panel) may facilitate a larger splay. (B) Pre-procedural analysis showing threatened coronary arteries. (C) Two wire loops after traversal of both right and left coronary cusp leaflets. (D and E) Right and left leaflet modification with a 5mm balloon prior to laceration. (F and G) Coronary angiography after TAV-in-TAV with no coronary flow limitation.
An 80-year-old of prohibitive surgical risk (STS-PROM: 15.8%) suffered structural deterioration of a 29 mm CoreValve (Medtronic). Computed tomography revealed heavily calcified leaflets extending above both coronary ostia, which would seal at the sinotubular junction (Figure 1B). The procedure was performed under general anesthesia with cerebral protection. In standard fashion, the right and left coronary cusp leaflets were traversed with 0.014” guidewires and ensnared in the left ventricular outflow tract (Figure 1C).3 The right and left leaflet traversal points were then dilatated using 5mm noncompliant balloons (Figure 1D and E). Both leaflets were then lacerated in standard fashion. Leaflet dilation added approximately 15 minutes to the procedure. Neither balloon dilatation nor leaflet laceration changed hemodynamics. Coronary inflow was preserved following implantation of a 26 mm TAV (SAPIEN 3, Edwards) (Figures 1F and G). The cerebral embolic filter captured significant debris. The patient was discharged post-procedure day 5.
BA-BASILICA may be a viable treatment strategy for patients previously thought ineligible for traditional BASILICA. This and other leaflet modification strategies may allow safer TAV-in-TAV for carefully selected patients with threatened coronary obstruction.
Acknowledgments
Funding:
Supported by Emory Structural Heart and Valve program intramural funds, and NIH Z01-HL006040
ABBREVIATIONS AND ACRONYMS
- BASILICA
Bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction
- TAV
Transcatheter aortic valve
- TAVR
Transcatheter aortic valve replacement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
ABG is a proctor for Edwards Lifesciences and Medtronic. He is a consultant with equity in Transmural Systems. His employer has research contracts for investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific.
JPV is a consultant for Edwards Lifesciences
GP is a consultant and proctor for Edwards Lifesciences
KJG is a speaker, proctor, and investigator for Edwards Lifesciences. She is a speaker, proctor, and advisory board member for Boston Scientific. She is a speaker, proctor, investigator, and advisory board member for Medtronic.
RJL is principal investigator in a cooperative research and development agreement (CRADA) between Edwards Lifesciences and NIH on transcatheter modification of the mitral valve.
VCB is a consultant for Edwards Lifesciences. He is a consultant with equity in Transmural Systems. His employer has research contracts for investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific.
All other authors disclosed no financial conflicts.
REFERENCES
- 1.Khan JM, et al. Transcatheter Laceration of Aortic Leaflets to Prevent Coronary Obstruction During Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2018;11(7):677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan JM, et al. TAVR Roulette: Caution Regarding BASILICA Laceration for TAVR-in-TAVR. JACC Cardiovasc Interv. 2020;13(6):787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lederman RJ, et al. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2019;12(13):1197–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]