Abstract
The synaptonemal complex (SC) is a proteinaceous structure that is transiently formed during meiosis to promote homologous recombination between maternal and paternal chromosomes. As this structure is required only for meiotic recombination, the proteins constituting the complex are almost undetectable in normal somatic cells, but they can be expressed under the conditions in which the transcriptional machinery is deregulated. Accumulating evidence indicates that they are epigenetically expressed in cancers of various origin. Not surprisingly, in contrast to their meiotic roles, the somatic roles of the SC proteins remain to be investigated. However, it has recently been reported that SYCP3 and SYCE2 control DNA double‐strand break repair negatively and positively, respectively, suggesting that the ectopic expression of the SC proteins in somatic cells could be associated with the maintenance of genomic instability. Thus, it is highly likely that the investigation of the somatic roles of the SC proteins would improve our understanding of the mechanisms underlying tumor development.
Keywords: cancer therapy, DNA damage response, homologous recombination, nuclear microenvironment, synaptonemal complex protein
The synaptonemal complex (SC) is a proteinaceous structure that is transiently formed during meiosis to promote homologous recombination. Although the proteins constituting the complex are highly expressed in meiotic cells and almost undetectable in normal somatic cells, accumulating evidence indicates that they are epigenetically expressed in cancers of various origin. Recent studies report that the SC proteins regulate the endogenous cellular machinery that maintains genome integrity in somatic cells. In this review, we focus on the clinical significance of the SC proteins and the possible mechanism underlying their ectopic expression in human cancers.

1. INTRODUCTION
The identification of molecules that are selectively expressed in cancer is a prerequisite for the development of cancer‐specific treatments with fewer adverse effects than currently available molecularly targeted drugs. To realize this approach to cancer, an understanding of the somatic roles of molecules that are thought to be expressed exclusively during meiosis could provide important clues. Among these molecules, the proteins constituting the synaptonemal complex (SC), a proteinaceous structure that holds together homologous chromosomes, are worth investigating, because evidence that they are expressed in cancer but not in normal somatic cells is accumulating. The SC is a meiosis‐specific structure that is formed between homologous sister chromatid pairs and essential for cross‐over formation. 1
The SC has a unique ladder‐like structure, which is never observed during mitosis in somatic cells, indicating that meiosis‐specific proteins are assembled during meiotic prophase I. 2 Because cell culture systems to investigate the role of the SC proteins in meiosis are not available, mouse genetic approaches have been used and have revealed the biological significance of the proteins in this higher‐ordered structure. 3 , 4 , 5 , 6 Additionally, an alternative approach for investigating the structural properties of these proteins is analysis of the exogenous expression of genes encoding the SC proteins in cultured somatic cells in the absence of other SC proteins. 7 , 8 Under these experimental conditions, some SC proteins can self‐assemble to form higher‐ordered structures. Although these conditions do not reflect the endogenous expression levels of these proteins in cancer, these observations suggest that they could interfere with the cellular function in somatic cells by forming abnormal structures with endogenous proteins.
In this review, we will focus on the clinical significance of the SC proteins and the possible mechanism underlying their ectopic expression in human cancers to shed light on their biological importance in the maintenance of somatic cellular functions.
2. MEIOTIC ROLES OF SC PROTEINS
The structure of the SC is conserved during evolution. 9 The SC has a tripartite structure consisting of two lateral elements (LEs) and a central region (CR) that contains the central element (CE) and the transverse filaments (TFs) (Figure 1). The SC components progressively assemble to form the synaptic chromosome. The LEs, which are termed axial elements (AEs) prior to the loading of CR proteins, assemble with cohesin and form the chromosome axis during the leptotene stage. During the zygotene stage, the LEs are connected by the CR. The SC is fully assembled in the pachytene and disassembled in the diplotene stage.
FIGURE 1.
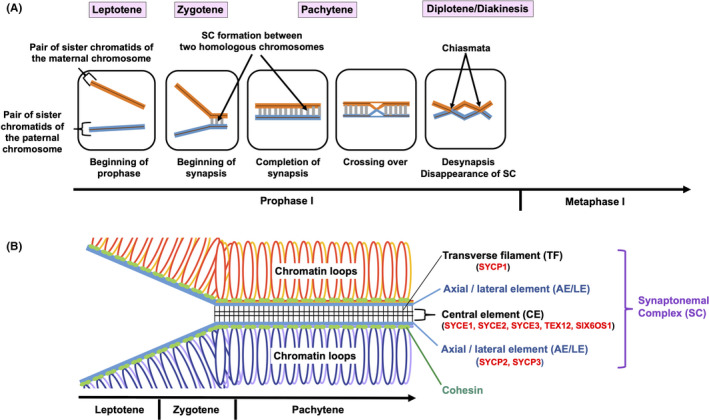
Chromosomal events in each stage of meiotic prophase I. A, Interactions between one pair of homologous chromosomes (red and blue) within a nucleus in each stage of prophase I are schematically represented. In the leptotene stage, the chromosomes begin to condense. In the following zygotene stage, synapsis begins with the formation of the synaptonemal complex (SC) (gray) between homologous chromosomes. In the pachytene stage, synapsis is completed and the SC is fully assembled. Crossing over of genetic materials also occurs in the pachytene stage. In the diplotene and diakinesis stages, the SC is disassembled and chiasmata resulting from interhomolog recombination become visible and serve to connect the homologs. B, Structures of the SC in each stage of prophase I are indicated. SC components progressively assemble to form the synaptic chromosome. In the leptotene stage, the axial elements (AEs), which will be termed lateral elements (LEs) in the next stage, assemble with cohesin and form the chromosome axis. In the zygotene stage, the LEs are formed from AEs, connected by the central region (CR) to form the SC. In the pachytene stage, the SC is fully assembled. The SC is a ladder‐like proteinaceous structure that is formed between two homologous chromosomes, and consists of two LEs (comprised of SYCP2 and SYCP3) and a CR that contains the central element (CE) (comprised of SYCE1, SYCE2, SYCE3, TEX12, and SIX6OS1) and the transverse filaments (TFs) (comprised of SYCP1). The SC is important for stabilizing homologous pairs and facilitating the completion of meiotic recombination
Eight SC proteins have been identified in mammals so far. 2 SYCP1 is a component of the TFs. It has N and C terminal globular domains and central coiled‐coil domains. SYCP2 and SYCP3 are components of the LEs with coiled‐coil domains and potential DNA binding motifs. SYCP2 and SYCP3 directly interact with each other. 10 The CE is composed of SYCE1, SYCE2, SYCE3, TEX12, and SIX6OS1. With the exception of TEX12, all of the CE components contain coiled‐coil domains. 6 , 11 SYCE2 tetramers directly interact with TEX12 dimers through the coiled‐coil domain of SYCE2. 12 SYCE1 interacts with SIX6OS1 multivalently. 13 The multiple hydrophobic interactions among SC proteins through the coiled‐coil domains contribute to the stabilization of the SC structure.
Meiotic roles of the SC proteins have been extensively studied in mice. SYCP1, SYCE1, SYCE2, SYCE3, TEX12, and SIX6OS1 deficient mice are infertile due to synaptic failure and massive meiocyte death. 3 , 6 , 11 SYCP2 and SYCP3 deficient mice show sexual dimorphisms. 3 , 11 The males are sterile whereas the litter sizes of the females are reduced. In humans, mutations in SYCP3 and SYCE1 have been identified as causes of idiopathic infertility. 14
3. ECTOPIC EXPRESSION OF SC PROTEINS IN HUMAN CANCERS
Although the SC proteins were first considered to be expressed only in meiotic cells, accumulating evidence shows that the SC proteins are ectopically and aberrantly expressed in various human cancers, as summarized in Table 1. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 This evidence suggests that the SC proteins are so‐called cancer/testis antigens, whose expression is normally limited to the germ cells but can be activated in cancer by a demethylation‐dependent process. 43
TABLE 1.
Ectopic expression of synaptonemal complex (SC) proteins in human cancers and its potential clinical significance
| Molecule | Localization in SC | Type(s) of cancer | Frequency (%) | Potential clinical significance | Reference(s) |
|---|---|---|---|---|---|
| SYCP1 | TF | Melanoma | 14.3 | 15 | |
| Breast cancer | 27.3‐34.1 | 15, 18 | |||
| Glioma | 40.0 | 15 | |||
| Stomach cancer | 7.1‐23.5 | 15, 18 | |||
| Non‐small‐cell lung cancer | 7.1 | 15 | |||
| Renal cell carcinoma | 8.3 | 15 | |||
| Acute myelogenous leukemia | 5.8 | 16 | |||
| Chronic myelogenous leukemia | 23.3 | 16 | |||
| Multiple myeloma | 10.0 | 16 | |||
| Meningioma | 18.4 | 17 | |||
| Astrocytoma | 38.5 | 17 | |||
| Oligoastrocytoma | 75.0 | 17 | |||
| Hepatocellular carcinoma | 29.0 | 19 | |||
| B‐cell lymphoma | 19.2 | 20 | |||
| T‐cell lymphoma | 60.0 | 20 | |||
| Acute lymphatic leukemia | 11.8 | 21 | |||
| Pancreatic adenocarcinoma | 47.5 | 22 | |||
| Testicular germ‐cell tumor | 42.9 | 23 | |||
| Head and neck squamous cell carcinoma | 11.8 | 24 | |||
| Medulloblastoma | 44.0 | 25 | |||
| Cutaneous T‐cell lymphoma | ~36.7 | 26, 27 | |||
| SYCP2 | AE/LE | Head and neck cancer | 30.6‐42.9 | Associated with human papillomavirus infection | 28, 29, 30, 32 |
| Cervical cancer | ~90.0 | Associated with human papillomavirus infection | 29, 31 | ||
| SYCP3 | AE/LE | Acute lymphoblastic leukemia | 47.1 | 21 | |
| Ovarian tumor | ~25.0 | 34, 36 | |||
| Brain tumor | 16.7 | 34 | |||
| Cervical cancer | ~61.3 | Poor prognosis, associated with increased phosphorylation of AKT | 35, 37 | ||
| Adrenal tumor, bladder tumor, breast tumor, colon tumor, gall bladder tumor, kidney tumor, liver tumor, lung tumor, rectal tumor, small intestine tumor, soft tissue tumor, stomach tumor, thyroid tumor | Variable | 36 | |||
| Cervical intraepithelial neoplasia | 14.2 | 37 | |||
| Cutaneous T‐cell lymphoma | 23.3 | 26 | |||
| Non‐small‐cell lung cancer | 19.4‐27.0 | Poor outcome, prognosis, associated with lymphangiogenesis | 38, 39 | ||
| SYCE1 | CE | Lung adenocarcinoma | 10.8 | 40 | |
| Prostate cancer | Variable | Associated with cases with Gleason score of 7 or greater | 41 | ||
| SYCE2 | CE | Cervix, ovary, thyroid, uterus, kidney, and stomach tumors, lymphoma | Variable | 42 | |
| SYCE3 | CE | N/R | |||
| TEX12 | CE | N/R | |||
| SIX6OS1 | CE | N/R |
Abbreviations: AE, axial element; CE, central element; LE, lateral element; TF, transverse filament; N/R, not reported.
Aberrant expression of SYCP1 was first observed in melanoma, breast cancer, glioma, stomach cancer, non‐small‐cell lung cancer, and renal cell carcinoma. 15 Subsequently, elevated SYCP1 expression was also reported in other types of tumors, including acute myelogenous leukemia, chronic myelogenous leukemia, multiple myeloma, meningioma, astrocytoma, oligoastrocytoma, hepatocellular carcinoma, B‐cell lymphoma, T‐cell lymphoma, acute lymphatic leukemia, pancreatic adenocarcinoma, testicular germ‐cell tumor, head and neck squamous cell carcinoma, medulloblastoma, and cutaneous T‐cell lymphoma. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 SYCP1 is thus specifically expressed in a broad variety of tumors, but there are currently no reports describing the mechanism or biological effects of ectopic expression of SYCP1 in somatic cancer cells.
SYCP2 expression has been reported only in cervical and head and neck cancers, but its potential clinical significance has been investigated thoroughly. 28 , 29 , 30 , 31 , 32 Notably, SYCP2 expression has been associated with human papillomavirus (HPV) infection in almost all of the investigations on SYCP2 and cancer. SYCP2 expression is not detected in the human keratinocyte line NIKS, which lacks an extrachromosomal HPV16 genome, but it is detectable in the HPV16‐positive line NIKS‐16. 29 At the mRNA level, SYCP2 expression was more than 15‐fold higher in NIKS‐16 cells compared to NIKS cells. 29 This induction of SYCP2 expression was dependent on the viral oncogenes E6 and E7, which contribute to the oncogenic potential of HPV. Although the mechanism still remains to be clarified, these findings suggest a potential link between SYCP2 expression and the risk of viral infection. With respect to the regulatory mechanism for SYCP2 expression, a genome‐wide DNA methylation analysis recently identified SYCP2 as one of the three hypomethylated CpG island‐associated genes in immunodeficiency, centromeric instability, and facial anomalies (ICF) syndrome. 33 A rare autosomal recessive disease, ICF syndrome, is caused by mutations in the DNA methyltransferase gene DNMT3B. As SYCP2 is likely to be the direct target of DNMT3B, the hypomethylation in the SYCP2 gene might induce the overexpression of SYCP2 observed in ICF syndrome. This finding supports the notion that a demethylation‐dependent process plays a major role in inducing the ectopic expression of SC proteins in somatic cells, which is in agreement with the previous reports on cancer/testis antigens. 43
SYCP3 expression has been documented in various cancers, including acute lymphoblastic leukemia, ovarian tumor, brain tumor, cervical cancer, adrenal tumor, bladder tumor, breast tumor, colon tumor, gall bladder tumor, kidney tumor, liver tumor, lung tumor, rectal tumor, small intestine tumor, soft tissue tumor, stomach tumor, thyroid tumor, cervical intraepithelial neoplasia, cutaneous T‐cell lymphoma, and non‐small‐cell lung cancer. 21 , 34 , 35 , 36 , 37 , 38 , 39 It was reported that SYCP3 expression can be induced in the colorectal adenocarcinoma cell line DLD1 by treatment with the demethylating agent 5‐azacytidine, 36 indicating that ectopic expression of SYCP3 in somatic cells is also regulated by a demethylation‐dependent process, like ectopic expression of SYCP2. The clinical significance of SYCP3 expression has been described in cervical cancer and non‐small‐cell lung cancer. Cho et al examined SYCP3 expression in tumor specimens from cervical cancer patients by immunohistochemistry and analyzed the correlation between SYCP3 expression and clinicopathologic factors or survival. 37 High expression of SYCP3 was significantly associated with the tumor stage and tumor grade. They also showed that overexpression of SYCP3 in NIH3T3 cells increased the expression of phosphorylated AKT, which agreed with a previous report showing increased phosphorylation of AKT in SYCP3‐expressing cervical cell lines. 35 In primary tumor samples, the survival times for patients with cervical cancer overexpressing both SYCP3 and phosphorylated AKT were actually significantly shorter than those for patients with low expression of either SYCP3 or phosphorylated AKT. 37 In non‐small‐cell lung cancer, there are two studies describing the clinical significance of SYCP3 expression. 38 , 39 One was an immunohistochemical and tissue microarray analysis in early stage non‐small‐cell lung cancer patients, and it showed that SYCP3 upregulation was correlated with a higher risk of disease progression and poor survival. 38 The other study assessed the expression of SYCP3 and the members of the vascular endothelial growth factor (VEGF) family, which are major mediators of tumor angiogenesis and lymphangiogenesis, in tumor tissues from 89 non‐small‐cell lung cancer patients with lymph node metastasis by a combination of immunohistochemistry and quantitative digital image analysis. 39 The results showed that SYCP3 was positively correlated with VEGF‐C and VEGF‐D expression, suggesting that SYCP3 might also be associated with lymphangiogenesis. Moreover, SYCP3 expression was correlated with worse overall survival. Taken together, these results indicated that SYCP3 can be used as a prognostic predictor for overall survival in cervical cancer as well as in both early and metastatic stages of non‐small‐cell lung cancer.
SYCE1 expression has been reported in lung adenocarcinoma and prostate cancer. 40 , 41 Although SYCE1 expression was not associated with the clinical characteristics of lung adenocarcinoma patients, 40 it was associated with a Gleason score of 7 or greater in prostate cancer, which is known to confer a higher risk for disease progression compared with a Gleason score of 6 or less. 41
SYCE2 expression has been reported in tumors of cervix, ovary, thyroid, uterus, kidney, and stomach, and in lymphomas. 42 Induction of SYCE2 expression was observed in DLD1 cells and the fibrosarcoma cell line HT1080 after treatment with the demethylating agent 5‐azacytidine, 42 indicating that SYCE2 expression is also regulated by a demethylation‐dependent process, as has been described for other cancer‐testis antigens. 43
As for the SC proteins SYCE3, TEX12, and SIX6OS1, which were identified relatively recently, there have been no reports describing their expression in primary tumors.
4. LINK BETWEEN SC PROTEINS AND HOMOLOGOUS RECOMBINATION MACHINERY
As shown above, accumulating evidence suggests the clinical importance of the SC proteins in cancer. This raises the question: what exactly are the roles of the SC proteins in somatic cancer cells? To answer this question, it could be worth reviewing the common features and differences between meiotic recombination and mitotic recombination in somatic cells. In contrast to mitotic recombination, which repairs accidental double‐strand breaks (DSBs) by using undamaged sister chromatids as templates, meiotic recombination is a programmed physiological event that is initiated by active induction of DSBs and takes place between two homologous chromosomes, one of paternal origin and the other of maternal origin. 44 As meiotic recombination includes an interchange of the genetic material between homologous chromosomes, it could resemble the process of genomic instability in cancer, where genetic alterations are accumulated.
As the SC is necessary for meiotic recombination and cross‐over formation, it is highly likely that the SC proteins interact with proteins involved in homologous recombination. Although the processes of mitotic recombination in somatic cells appear to differ from those of meiotic recombination, they share some of the same steps. 45 An excellent example is seen in the early stage of both types of recombination, when RAD51 plays a role in strand invasion as a key recombinase, although DMC1, the meiosis‐specific RAD51 paralog, is also a critical player in meiotic recombination. Thus, the identification of an interaction between the SC proteins and the recombination components that play both meiotic and mitotic roles would provide novel insights into the somatic roles for the SC proteins.
During meiosis prophase I before synapsis is initiated, RAD51 and DMC1 are associated with the AEs and mark sites of DNA DSBs that are generated by Spo11 in a programmed manner. 1 , 11 During the leptotene and zygotene stages, RPA, BLM, MSH4, and MSH5 localize to the sites between the AEs and chromosomes. During the pachytene stage, MLH1 is recruited to the CE and marks sites of chiasmata on the chromosome. Two of these proteins, RAD51 and RPA, also play roles at the early stage of homologous recombination in somatic cells.
The physical link between the SC proteins and the recombination machinery has been investigated in mice. Mouse RAD51 was shown to interact with SYCP1 or SYCP3 by analysis using a yeast two‐hybrid system and a pull‐down assay. 46 RAD51 was also shown to form a complex with SYCE2 in the mouse testis by immunoprecipitation. 4 Although these studies provided new clues regarding the link between the SC proteins and the homologous recombination machinery, it is unclear whether the interactions are preserved in somatic cells.
In addition to studies using mouse genetic models, studies using cultured somatic cells also have the potential to provide novel insights into the link between the SC proteins and the homologous recombination machinery. Experiments using a combination of exogenous expression of SYCP3 at low levels comparable to the endogenous levels in normal human epithelial cells and knockdown of SYCP3 in cancer cells in which it is epigenetically expressed have revealed that SYCP3 inhibits the RAD51‐dependent homologous recombination machinery by interacting with BRCA2, a mediator of RAD51 and a tumor suppressor whose mutations are responsible for hereditary breast and ovarian cancers. 36 BRCA2 can interact directly with RAD51 and deliver this key recombinase to the site of junction between a double‐stranded DNA and a single‐stranded DNA (ssDNA) that is protected by RPA. In SYCP3‐expressing somatic cells, the BRCA2‐mediated recruitment of RAD51 to the break site is inhibited, resulting in defective sister‐chromatid recombination (Figure 2). The inhibitory effect of SYCP3 on the recombination machinery not only causes genomic instability, which is a driving force for tumor development, but also specifically sensitizes tumor cells to poly(ADP‐ribose) polymerase inhibition, based on the principle of synthetic lethality. 47 Thus, experiments using somatic cells in which the SC proteins are either expressed or repressed are expected to become powerful tools for understanding their somatic roles.
FIGURE 2.
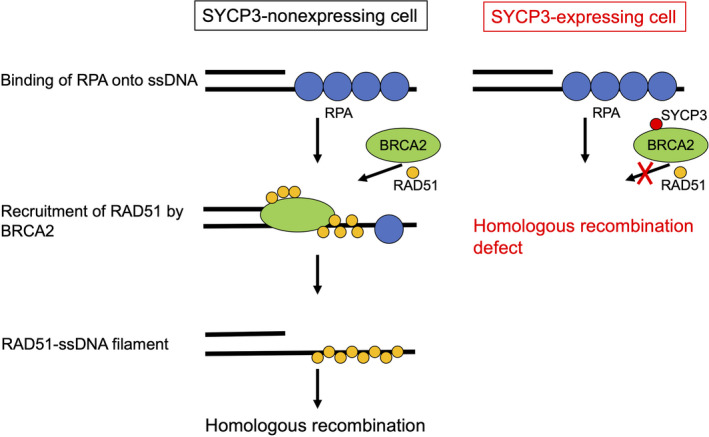
Proposed model for inhibition of RAD51‐dependent homologous recombination by SYCP3 is depicted. In the early stage of homologous recombination in SYCP3‐nonexpressing cells (left), the DNA double‐strand break ends are resected, resulting in generation of 3′ single‐stranded DNA (ssDNA) overhangs on both sides of the break. These overhangs are coated and stabilized by replication protein A (RPA). Next, BRCA2 directly binds RAD51 and recruits it to the double‐stranded DNA‐ssDNA junction, and promotes the loading of RAD51 onto ssDNA. This step is followed by displacement of RPA from ssDNA ends and assembly of the RAD51‐ssDNA filament, leading to strand invasion into an undamaged homologous DNA template. In SYCP3‐expressing cells (right), SYCP3 forms a complex with BRCA2 and impairs the recruitment of RAD51 to resected DNA double‐strand breaks, resulting in defective homologous recombination
The somatic role of SYCP3 raises a new question: does SYCP3 also inhibit the function of BRCA2 in meiotic recombination in germ cells? In germ cells, BRCA2 binds to the meiosis‐specific proteins MEILB2 and BRME1, resulting in the formation of the stable BRCA2‐MEILB2‐BRME1 ternary complex, which is essential for meiotic DSB repair, homolog synapsis, and cross‐over formation. 48 Interestingly, MEILB2‐BRME1 is ectopically expressed in many human cancers, and somatically expressed MEILB2‐BRME1 impairs mitotic BRCA2 functions, just like somatic SYCP3. However, the functional interaction between meiotic SYCP3 and meiotic BRCA2 has not been reported yet. As meiotic SYCP3 exists as one of the components of a highly ordered structure of the SC, the mobility of meiotic SYCP3 in germ cells might be quite limited compared to that of somatic SYCP3 ectopically expressed as a single protein in cancer cells with no SC formation. This could make meiotic SYCP3 difficult to bind to meiotic BRCA2 and affect its function in meiotic recombination, even though it has such a binding potential.
5. LINK BETWEEN SC PROTEINS AND DNA DAMAGE RESPONSE MACHINERY
The SC proteins in yeast share little primary sequence homology to those in mammals. 9 , 49 However, the TF proteins in yeast and mammals are similar in structure. Both have the N‐ and C‐terminal globular domains and the central coiled‐coil domains. In yeast, the functional homolog of SYCP1 is ZIP1. 50 In the phosphatase PP4 mutants in Saccharomyces cerevisiae, the persistent phosphorylation of ZIP1 on serine 75 is coupled with defects in homology‐independent centromere pairing. 51 Mec1 kinase, the homolog of the PI3K‐like kinase (PIKK) ATR, phosphorylates ZIP1 on serine 75, and centromere pairing is restored in mec1 mutants. In mice lacking ATM, a member of PIKK involved in the DNA damage response, levels of cross‐over formation are increased on autosomes. 52 These findings indicate that the DNA damage response signaling is implicated in cross‐over control in meiotic recombination. However, it is unclear whether ATM phosphorylates SC proteins in mammals. 2
Conversely, a recent report indicates that SYCE2 activates the ATM‐dependent DNA damage response machinery in human somatic cells, a result determined based on a combination of the exogenous expression of SYCE2 in normal epithelial cells and knockdown in cancer cells. Surprisingly, the effect of SYCE2 on ATM is mediated by its direct interaction with heterochromatin protein 1α (HP1α), a key player in the maintenance of the nuclear microenvironment. 42 Although SYCE2 has a coiled‐coil domain in the central region, its interaction with HP1α is mediated by the N‐terminal hydrophobic sequence, indicating that this region contributes to protein‐protein interactions independently of the coiled‐coil domain. The interaction between SYCE2 and HP1α reduces the direct association of HP1α with the heterochromatin marker trimethylated histone H3 lysine 9 (H3K9me3) without affecting H3K9me3 levels and promotes ATM‐mediated homologous recombination and nonhomologous end joining (Figure 3). Consequently, DNA DSB repair activity is increased in SYCE2‐expressing cells, conferring cellular resistance to radiation and DNA cross‐linking chemotherapeutic agents. In other words, SYCE2 is a potential target for sensitization of cancer cells to DNA‐damaging treatments. Thus, the identification of the somatic role of SYCE2 is likely to lead to the development of a strategy for improving the efficacy of cancer therapy by directly linking the ectopic expression of the SC protein with DNA damage response potentials. Although ATM plays an important role in meiotic recombination, as described above, there is currently no evidence showing that SYCE2 directly affects ATM‐mediated meiotic recombination. Thus, this finding opens a novel research field in which somatic roles of the SC proteins are independent of their meiotic roles, even if there might be partial overlap.
FIGURE 3.
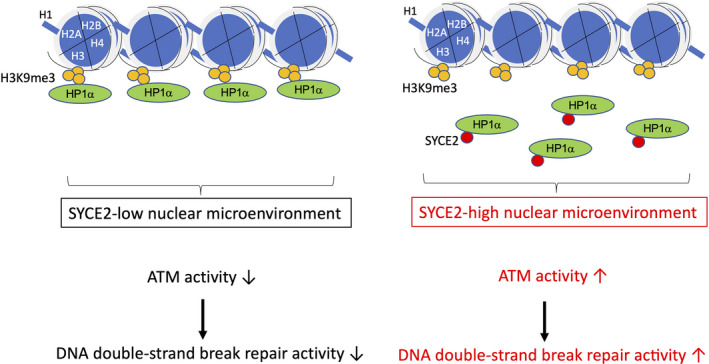
Schematic representation showing that expression levels of SYCE2 in somatic cells define the steady‐state ATM activity by affecting heterochromatin protein 1α (HP1α) localization in the nuclear microenvironment. When the expression level of SYCE2 is low (left), HP1α is bound to the heterochromatin marker trimethylated histone H3 lysine 9 (H3K9me3), and the steady‐state ATM activity and DNA double‐strand break repair activity are kept low. When the expression level of SYCE2 is high (right), SYCE2 directly binds to HP1α and dissociates HP1α from H3K9me3, namely heterochromatin. As a result, HP1α will be distributed in euchromatin as well, which will potentiate the steady‐state ATM activity and increase the levels of total DNA double‐strand break repair activity following the induction of exogenous DNA damage
6. CONCLUSION
The SC proteins are epigenetically expressed in cancers of various origin. The presence of coiled‐coil domains in their structures implies that they play roles as scaffold proteins by protein‐protein interactions during meiosis and in somatic cells. Recent reports have clearly indicated that the SC proteins regulate the endogenous cellular machinery that maintains genome integrity either negatively or positively in somatic cells. Thus, further understanding of their somatic roles will lead to novel cancer therapeutic strategies with extremely high tissue specificity.
DISCLOSURE
The authors have no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI Grant Numbers JP23591836, JP25125705, JP26461881, JP16H01298, JP17K10471, and JP20K08127 to N. Hosoya and by grants from the Takeda Science Foundation and the Naito Foundation to N. Hosoya.
Hosoya N, Miyagawa K. Synaptonemal complex proteins modulate the level of genome integrity in cancers. Cancer Sci. 2021;112:989–996. 10.1111/cas.14791
Funding information
Japan Society for the Promotion of Science KAKENHI, Grant/Award Numbers: JP23591836, JP25125705, JP26461881, JP16H01298, JP17K10471, and JP20K08127; Takeda Science Foundation; Naito Foundation.
REFERENCES
- 1. Zickler D, Kleckner N. Recombination, pairing, and synapsis of homologs during meiosis. Cold Spring Harb Perspect Biol. 2015;7:a016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao J, Colaiácovo MP. Zipping and unzipping: protein modifications regulating synaptonemal complex dynamics. Trends Genet. 2018;34:232‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costa Y, Cooke HJ. Dissecting the mammalian synaptonemal complex using targeted mutations. Chromosome Res. 2007;15:579‐589. [DOI] [PubMed] [Google Scholar]
- 4. Bolcun‐Filas E, Speed R, Taggart M, et al. Mutation of the mouse Syce1 gene disrupts synapsis and suggests a link between synaptonemal complex structural components and DNA repair. PLoS Genet. 2009;5:e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schramm S, Fraune J, Naumann R, et al. A novel mouse synaptonemal complex protein is essential for loading of central element proteins, recombination, and fertility. PLoS Genet. 2011;7:e1002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gómez‐H L, Felipe‐Medina N, Sánchez‐Martín M, et al. C14ORF39/SIX6OS1 is a constituent of the synaptonemal complex and is essential for mouse fertility. Nat Commun. 2016;7:e13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Öllinger R, Alsheimer M, Benavente R. Mammalian protein SCP1 forms synaptonemal complex‐like structures in the absence of meiotic chromosomes. Mol Biol Cell. 2005;16:212‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuan L, Pelttari J, Brundell E, et al. The synaptonemal complex protein SCP3 can form multistranded, cross‐striated fibers in vivo. J Cell Biol. 1998;142:331‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cahoon CK, Hawley RS. Regulating the construction and demolition of the synaptonemal complex. Nat Struct Mol Biol. 2016;23:369‐377. [DOI] [PubMed] [Google Scholar]
- 10. Yang F, De La Fuente R, Leu NA, Baumann C, McLaughlin KJ, Wang PJ. Mouse SYCP2 is required for synaptonemal complex assembly and chromosomal synapsis during male meiosis. J Cell Biol. 2006;173:497‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fraune J, Schramm S, Alsheimer M, Benavente R. The mammalian synaptonemal complex: protein components, assembly and role in meiotic recombination. Exp Cell Res. 2012;318:1340‐1346. [DOI] [PubMed] [Google Scholar]
- 12. Davies OR, Maman JD, Pellegrini L. Structural analysis of the human SYCE2‐TEX12 complex provides molecular insights into synaptonemal complex assembly. Open Biol. 2012;2:e120099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sánchez‐Sáez F, Gómez‐H L, Dunne OM, et al. Meiotic chromosome synapsis depends on multivalent SYCE1‐SIX6OS1 interactions that are disrupted in cases of human infertility. Sci Adv. 2020;6(36):eabb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geisinger A, Benavente R. Mutations in genes coding for synaptonemal complex proteins and their impact on human fertility. Cytogenet Genome Res. 2016;150:77‐85. [DOI] [PubMed] [Google Scholar]
- 15. Türeci Ö, Sahin U, Zwick C, Koslowski M, Seitz G, Pfreundschuh M. Identification of a meiosis‐specific protein as a member of the class of cancer/testis antigens. Proc Natl Acad Sci USA. 1998;95:5211‐5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim SH, Austin S, Owen‐Jones E, Robinson L. Expression of testicular genes in haematological malignancies. Br J Cancer. 1999;81:1162‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sahin U, Koslowski M, Türeci Ö, et al. Expression of cancer testis genes in human brain tumors. Clin Cancer Res. 2000;6:3916‐3922. [PubMed] [Google Scholar]
- 18. Mashino K, Sadanaga N, Tanaka F, et al. Expression of multiple cancer‐testis antigen genes in gastrointestinal and breast carcinomas. Br J Cancer. 2001;85:713‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luo G, Huang S, Xie X, et al. Expression of cancer‐testis genes in human hepatocellular carcinomas. Cancer Immun. 2002;2:11. [PubMed] [Google Scholar]
- 20. Xie X, Wacker HH, Huang S, et al. Differential expression of cancer testis genes in histological subtypes of non‐Hodgkin's lymphomas. Clin Cancer Res. 2003;9:167‐173. [PubMed] [Google Scholar]
- 21. Niemeyer P, Türeci Ö, Eberle T, Graf N, Pfreundschuh M, Sahin U. Expression of serologically identified tumor antigens in acute leukemias. Leuk Res. 2003;27:655‐660. [DOI] [PubMed] [Google Scholar]
- 22. Kubuschok B, Xie X, Jesnowski R, et al. Expression of cancer testis antigens in pancreatic carcinoma cell lines, pancreatic adenocarcinoma and chronic pancreatitis. Int J Cancer. 2004;109:568‐575. [DOI] [PubMed] [Google Scholar]
- 23. Zhang C, Kawakami T, Okada Y, Okamoto K. Distinctive epigenetic phenotype of cancer testis antigen genes among seminomatous and nonseminomatous testicular germ‐cell tumors. Genes Chromosomes Cancer. 2005;43:104‐112. [DOI] [PubMed] [Google Scholar]
- 24. Atanackovic D, Blum I, Cao Y, et al. Expression of cancer‐testis antigens as possible targets for antigen‐specific immunotherapy in head and neck squamous cell carcinoma. Cancer Biol Ther. 2006;5:1218‐1225. [DOI] [PubMed] [Google Scholar]
- 25. Oba‐Shinjo SM, Caballero OL, Jungbluth AA, et al. Cancer‐testis (CT) antigen expression in medulloblastoma. Cancer Immun. 2008;8:7. [PMC free article] [PubMed] [Google Scholar]
- 26. Litvinov IV, Cordeiro B, Huang Y, et al. Ectopic expression of cancer‐testis antigens in cutaneous T‐cell lymphoma patients. Clin Cancer Res. 2014;20:3799‐3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use of transcriptional profiling to improve personalized diagnosis and management of cutaneous T‐cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820‐2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slebos RJC, Yi Y, Ely K, et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:701‐709. [DOI] [PubMed] [Google Scholar]
- 29. Pyeon D, Newton MA, Lambert PF, et al. Fundamental differences in cell cycle deregulation in human papillomavirus‐positive and human papillomavirus‐negative head/neck and cervical cancers. Cancer Res. 2007;67:4605‐4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martinez I, Wang J, Hobson KF, Ferris RL, Khan SA. Identification of differentially expressed genes in HPV‐positive and HPV‐negative oropharyngeal squamous cell carcinomas. Eur J Cancer. 2007;43:415‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Espinosa AM, Alfaro A, Roman‐Basaure E, et al. Mitosis is a source of potential markers for screening and survival and therapeutic targets in cervical cancer. PLoS One. 2013;8:e55975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Masterson L, Sorgeloos F, Winder D, et al. Deregulation of SYCP2 predicts early stage human papillomavirus‐positive oropharyngeal carcinoma: a prospective whole transcriptome analysis. Cancer Sci. 2015;106:1568‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simo‐Riudalbas L, Diaz‐Lagares A, Gatto S, et al. Genome‐wide DNA methylation analysis identifies novel hypomethylated non‐pericentromeric genes with potential clinical implications in ICF syndrome. PLoS One. 2015;10:e0132517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mobasheri MB, Jahanzad I, Mohagheghi MA, Aarabi M, Farzan S, Modarressi MH. Expression of two testis‐specific genes, TSGA10 and SYCP3, in different cancers regarding to their pathological features. Cancer Detect Prev. 2007;31:296‐302. [DOI] [PubMed] [Google Scholar]
- 35. Kang TH, Noh KH, Kim JH, et al. Ectopic expression of X‐linked lymphocyte‐regulated protein pM1 renders tumor cells resistant to antitumor immunity. Cancer Res. 2010;70:3062‐3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hosoya N, Okajima M, Kinomura A, et al. Synaptonemal complex protein SYCP3 impairs mitotic recombination by interfering with BRCA2. EMBO Rep. 2012;13:44‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cho H, Noh KH, Chung JY, et al. Synaptonemal complex protein 3 is a prognostic marker in cervical cancer. PLoS One. 2014;9:e98712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung JY, Kitano H, Takikita M, et al. Synaptonemal complex protein 3 as a novel prognostic marker in early stage non‐small cell lung cancer. Hum Pathol. 2013;44:472‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kitano H, Chung JY, Noh KH, et al. Synaptonemal complex protein 3 is associated with lymphangiogenesis in non‐small cell lung cancer patients with lymph node metastasis. J Transl Med. 2017;15:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taguchi A, Taylor AD, Rodriguez J, et al. A search for novel cancer/testis antigens in lung cancer identifies VCX/Y genes, expanding the repertoire of potential immunotherapeutic targets. Cancer Res. 2014;74:4694‐4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu X, Lv D, Lei M, et al. A 10‐gene signature as a predictor of biochemical recurrence after radical prostatectomy in patients with prostate cancer and a Gleason score ≥7. Oncol Lett. 2020;20:2906‐2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hosoya N, Ono M, Miyagawa K. Somatic role of SYCE2: an insulator that dissociates HP1α from H3K9me3 and potentiates DNA repair. Life Sci Alliance. 2018;1:e201800021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615‐625. [DOI] [PubMed] [Google Scholar]
- 44. Hunter N. Meiotic recombination: the essence of heredity. Cold Spring Harb Perspect Biol. 2015;7:a016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229‐257. [DOI] [PubMed] [Google Scholar]
- 46. Tarsounas M, Morita T, Pearlman RE, Moens PB. RAD51 and DMC1 form mixed complexes associated with mouse meiotic chromosome cores and synaptonemal complexes. J Cell Biol. 1999;147:207‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hosoya N, Miyagawa K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014;105:370‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang J, Gurusaran M, Fujiwara Y, et al. The BRCA2‐MEILB2‐BRME1 complex governs meiotic recombination and impairs the mitotic BRCA2‐RAD51 function in cancer cells. Nat Commun. 2020;11:2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fraune J, Brochier‐Armanet C, Alsheimer M, Volff JN, Schücker K, Benavente R. Evolutionary history of the mammalian synaptonemal complex. Chromosoma. 2016;125:355‐360. [DOI] [PubMed] [Google Scholar]
- 50. Sym M, Engebrecht JA, Roeder GS. ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell. 1993;72:365‐378. [DOI] [PubMed] [Google Scholar]
- 51. Falk JE, Chan AC, Hoffmann E, Hochwagen A. A Mec1‐ and PP4‐dependent checkpoint couples centromere pairing to meiotic recombination. Dev Cell. 2010;19:599‐611. [DOI] [PubMed] [Google Scholar]
- 52. Barchi M, Roig I, Di Giacomo M, de Rooij DG, Keeney S, Jasin M. ATM promotes the obligate XY crossover and both crossover control and chromosome axis integrity on autosomes. PLoS Genet. 2008;4:e1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]


