Abstract
Near‐infrared photoimmunotherapy (NIR‐PIT) is a new type of cancer treatment, which was recently approved in Japan for patients with inoperable head and neck cancer. NIR‐PIT utilizes antibody‐IRDye700DX (IR700) conjugates and NIR light at a wavelength of 690 nm. NIR light exposure leads to physicochemical changes in the antibody‐IR700 conjugate cell receptor complex, inducing rapid necrotic cell death. Just as fluorescence guided surgery is useful for surgeons to resect tumors completely, real‐time information of tumor locations would help clinicians irradiate NIR light more precisely. IR700 is a fluorescence dye that emits at 702 nm; however, there is no clinically available device optimized for detecting this fluorescence. On the other hand, many indocyanine green (ICG) fluorescence imaging devices have been approved for clinical use. Therefore, we investigated whether LIGHTVISION, one of the clinically available ICG cameras, could be employed for tumor detection. We hypothesized that irradiation with even low‐power 690‐nm laser light, attenuated by 99% with a neutral‐density filter, could be detected with LIGHTVISION without fluorescence decay or therapeutic effect because of the long emission tail of IR700 beyond 800 nm (within the detection range of LIGHTVISION). We demonstrated that the LIGHTVISION camera, originally designed for ICG detection, can detect the tail of IR700 fluorescence in real time, thus enabling the visualization of target tumors.
Keywords: near‐infrared, photoimmunotherapy, optical imaging, IR700, ICG camera
A diagnostic imaging method is developed for accurately localizing tumor and IR700 accumulation based on IR700 fluorescence, using weakened therapeutic laser light at a nontherapeutic dose and a commercially available clinical fluorescence camera for indocyanine green (LIGHTVISION).
1. INTRODUCTION
Near‐infrared photoimmunotherapy (NIR‐PIT) is a new type of cancer therapy that employs an antibody labeled with a photosensitizer, IRDye700DX (IR700). An antibody‐photosensitizer conjugate (APC) specifically binds to antigen‐expressing cells, and subsequent exposure to NIR light (approximately 690 nm) selectively kills targeted cells. 1 , 2 , 3 This concept has been successfully demonstrated in a variety of antibodies and tumor types. 4 , 5 , 6 , 7 A phase 1/2 clinical trial using EGFR‐targeted cetuximab‐IR700 (ASP‐1929) in patients with inoperable head and neck cancer has finished with a remarkable response rate (https://clinicaltrials.gov/ct2/show/NCT02422979) in early 2018. The first EGFR‐targeting NIR‐PIT drug received qualified approval from the Japanese Ministry of Health, Labor and Welfare in September 2020, and fast‐track designation from the US Food and Drug Administration (FDA). Currently, a global phase 3 clinical trial is ongoing (https://clinicaltrials.gov/ct2/show/NCT03769506).
As NIR‐PIT requires that light be delivered to the entire tumor, it is significant to determine the accurate spread of lesions prior to NIR light irradiation. Imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) provide detailed information about tumor location prior to treatment, but during treatment options are limited. One promising method is to take advantage of the fluorescence of IR700, which is excited at about 690 nm and emits at a peak of 702 nm but has a long tail of fluorescence extending beyond 800 nm. Among the dyes approved for clinical use, indocyanine green (ICG) is the most successful because of its low toxicity and deeper tissue penetration. 8 , 9 , 10 , 11 As a result, several commercially available imaging systems for ICG have been developed.
The purpose of this study was to demonstrate the feasibility of intratreatment tumor detection using a commercially available imaging hardware (LIGHTVISION; Shimadzu Corporation, Kyoto, Japan) originally designed for ICG imaging. We demonstrated that Trastuzumab‐IR700–bound tumor could be visualized by the irradiation of low‐output‐power NIR light at a wavelength of around 690 nm without therapeutic effect or fluorescence decay.
2. MATERIALS AND METHODS
Conjugation of dye with monoclonal antibody was performed as previously described. 12
Detailed materials and methods are described in Data S1.
3. RESULTS
3.1. Tumor visualization with low‐output‐power therapeutic laser
The wavelength of the therapeutic laser used in NIR‐PIT is between 685 nm and 695 nm, and the tail of emission spectrum of trastuzumab conjugated with IR700 (Tra‐IR700) extends to over 800 nm (Figure 1A). Therefore, we examined whether the LIGHTVISION camera could detect tumors when they were irradiated with low‐output‐power laser at 685‐695 nm. This is the same laser light used in NIR‐PIT but at a much lower power setting. The experimental setup is shown in Figure 1B. Tumor was irradiated with the therapeutic laser whose output power was attenuated with an neutral‐density (ND) filter, and emission light from tumors was detected by LIGHTVISION. The skin contralateral to the tumor was also irradiated to measure background fluorescence. Subcutaneous tumors could be visualized by the irradiation of 690 ± 5 nm laser at a photon density of at least 0.5 mW/cm2 (Figure 1C). 1.0‐mW/cm2‐output‐power lasers were used in subsequent experiments. When using LIGHTVISION, Target‐to‐Background ratios (TBRs) of 55.1 were produced, which were higher than TBRs of 6.0 when using Pearl Imager (Figure 1D and E).
FIGURE 1.
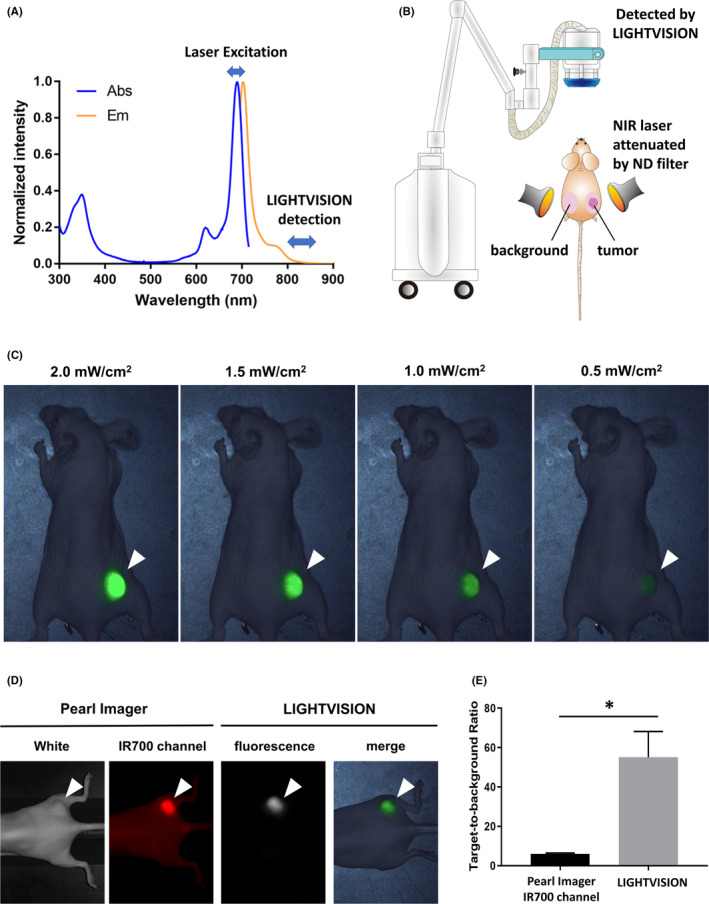
A, Excitation and emission spectrum of Tra‐IR700. B, Schema of the experimental setup. C, LIGHTVISION images of N87‐GFP/luc tumor‐bearing mouse excited by weak‐output‐power near‐infrared (NIR) laser 1 day after Tra‐IR700 injection. A white arrowhead indicates the location of the subcutaneous tumor. D, Fluorescence images of N87‐GFP/luc tumor‐bearing mouse obtained by Pearl Imager and LIGHTVISION. A laser at 1.0 mW/cm2 output power was used for LIGHTVISION imaging. A white arrowhead indicates the location of the subcutaneous tumor. E, Target‐to‐background ratio in each imaging device. Data are shown as mean ± SEM (n = 7, *P < .05)
3.2. Weak laser irradiation shows no therapeutic effect and fluorescence decay
We examined whether 1.0‐mW/cm2‐output‐power laser showed a therapeutic effect in vitro. Up to a light dose of 0.1 J/cm2 irradiation, no increase of cell death was observed (Figure 2A). This was also observed with the 2.0‐mW/cm2‐output‐power laser which showed no therapeutic effect up to the light dose of 0.1 J/cm2 irradiation (Figure S1). Next, we examined whether fluorescence decay was observed during weak‐output‐power laser irradiation. Fluorescence decay indicates photobleaching of the dye with possible implications for therapeutic effect. Absence of fluorescence decay implies absence of photobleaching. Tumor fluorescence intensity was monitored during irradiation with the laser at 1.0 mW/cm2 output power. Compared with the fluorescence intensity at 0 minutes, no statistically significant fluorescence decay was observed after 5, 10, and 15 minutes of irradiation (Figure 2B and C). Histologically, only the specimens of 1.0 J/cm2 irradiation showed necrotic cell death (Figure 2D).
FIGURE 2.
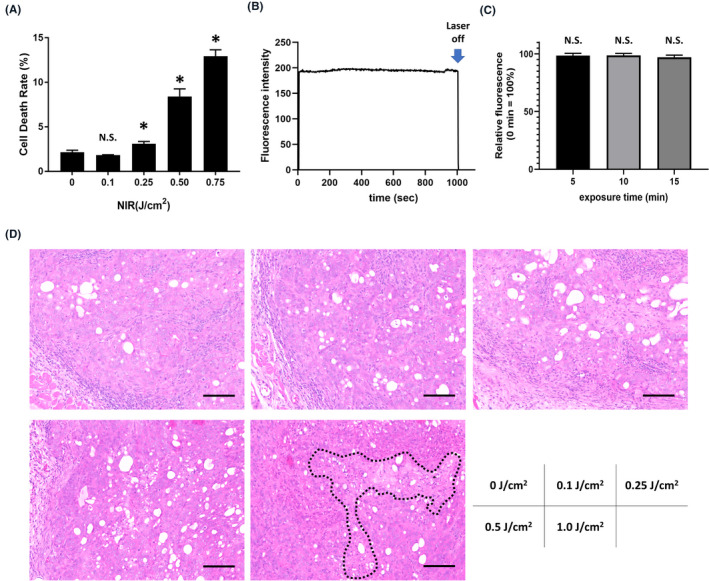
Therapeutic effect and fluorescence decay by weak near‐infrared (NIR) laser. A, Cell viability after weak NIR laser irradiation (1 mW/cm2). Cell viability was measured by propidium iodide (PI) staining. Data are presented as mean ± SEM (n = 4, *P < .05 vs no light exposure group). B, Time course of tumor fluorescence intensity during weak NIR laser irradiation (1 mW/cm2). C, Relative change in fluorescence intensity when irradiated for 5, 10, and 15 minutes. The fluorescence intensity at 0 minutes was calculated as 100%. Data are presented as mean ± SEM (n = 6). D, H&E staining of N87 tumors irradiated by 1‐mW/cm2‐output‐power laser (scale bar = 100 µm). Necrotic cell death area was surrounded by black dot line
4. DISCUSSION
Optical imaging is a powerful tool to detect tumors in real time. In NIR‐PIT, in order to set the light irradiation field accurately, it is considered beneficial to provide real‐time feedback of tumor locations.
IR700 is a phthalocyanine‐based dye, which has a large extinction coefficient and high fluorescence quantum yield. Therefore, although the emission peak of IR700 is about 100 nm shorter than that of ICG, we speculated LIGHTVISION had the potential to detect the IR700 fluorescence. A laser with 150 mW/cm2 output power was used for treatment in the ongoing clinical trials, but a laser with weaker output power was preferable for the purpose of diagnostic imaging. We tried lasers with 0.5 mW/cm2, 1.0 mW/cm2, 1.5 mW/cm2, and 2.0 mW/cm2 output power, and it was possible to detect tumors even with laser irradiation with an output of only 1.0 mW (Figure 1C). Interestingly, up to 0.1 J/cm2 irradiation using low‐output‐power laser, neither photobleaching nor cell damage was observed (Figure 2). Thus, for the first 100 seconds of NIR irradiation, the tumors can be imaged without fear of causing cell damage. This method of imaging is relatively low‐cost, portable, and provides real‐time information. In addition, imaging over 800 nm wavelength that was ~100 nm off the emission peak, where there is no conflict with the excitation light, can achieve lower background signal and higher TBR due to less autofluorescence and scattering and deeper penetration than imaging at 700 nm.
This study has several limitations. The first limitation is the use of a single xenograft tumor model, which does not reflect the full repertoire of cells in a tumor microenvironment unlike orthotopic models. 13 Therefore, in order to confirm the feasibility of this method, further clinical studies will be needed. Additionally, in this study, we used LIGHTVISION as an imaging modality, but many similar devices are commercially available now. 14 , 15 Each device has its own light source to excite ICG, optics for ICG fluorescence collection, and charge‐coupled device detector. Different device properties cause differences in imaging performance. Therefore, although LIGHTVISION worked very effectively in this study, it is not guaranteed that other devices will work equally.
In conclusion, LIGHTVISION, a currently available ICG‐imaging camera, was suitable to visualize NIR‐PIT target tumors by the irradiation of IR700‐conjugated antibodies with very‐low‐output‐power therapeutic laser. This method demonstrates that a camera designed for ICG can also provide real‐time feedback of tumor locations prior to NIR‐PIT based on IR700 fluorescence.
DISCLOSURE
The authors have no conflict of interest.
Supporting information
Supinfo
ACKNOWLEDGMENTS
All authors were supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (ZIABC011513, Funder Id: http://dx.doi.org/10.13039/100000054). FFI was also supported with a grant from the National Center for Global Health and Medicine Research Institute, Tokyo, Japan.
Inagaki FF, Fujimura D, Furusawa A, et al. Diagnostic imaging in near‐infrared photoimmunotherapy using a commercially available camera for indocyanine green. Cancer Sci. 2021;112:1326–1330. 10.1111/cas.14809
REFERENCES
- 1. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell‐selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kobayashi H, Choyke PL. Near‐infrared photoimmunotherapy of cancer. Acc Chem Res. 2019;52:2332–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inagaki FF, Furusawa A, Choyke PL, Kobayashi H. Enhanced nanodrug delivery in tumors after near‐infrared photoimmunotherapy. Nanophotonics. 2019;8:1673–1688. [Google Scholar]
- 4. Lum YL, Luk JM, Staunton DE, Ng DKP, Fong WP. Cadherin‐17 Targeted near‐infrared photoimmunotherapy for treatment of gastrointestinal cancer. Mol Pharm. 2020;17:3941–3951. [DOI] [PubMed] [Google Scholar]
- 5. Kiss B, van den Berg NS, Ertsey R, et al. CD47‐targeted near‐infrared photoimmunotherapy for human bladder cancer. Clin Cancer Res. 2019;25:3561–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagaya T, Nakamura Y, Okuyama S, et al. Near‐infrared photoimmunotherapy targeting prostate cancer with prostate‐specific membrane antigen (PSMA) antibody. Mol Cancer Res. 2017;15:1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maawy AA, Hiroshima Y, Zhang Y, et al. Near infra‐red photoimmunotherapy with anti‐CEA‐IR700 results in extensive tumor lysis and a significant decrease in tumor burden in orthotopic mouse models of pancreatic cancer. PloS One. 2015;10:e0121989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image‐guided cancer surgery using near‐infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg. 2010;97:1369–1377. [DOI] [PubMed] [Google Scholar]
- 10. Ishizawa T, Fukushima N, Shibahara J, et al. Real‐time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115:2491–2504. [DOI] [PubMed] [Google Scholar]
- 11. Kitai T, Inomoto T, Miwa M, Shikayama T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12:211–215. [DOI] [PubMed] [Google Scholar]
- 12. Fujimura D, Inagaki F, Okada R, et al. Conjugation ratio, light dose, and ph affect the stability of panitumumab‐IR700 for near‐infrared photoimmunotherapy. ACS Med Chem Lett. 2020;11:1598–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tashiro Y, Hollandsworth HM, Nishino H, et al. Indocyanine green labels an orthotopic nude‐mouse model of very‐early colon‐cancer liver metastases. Vivo. 2020;34:2277–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012:940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu B, Sevick‐Muraca EM. A review of performance of near‐infrared fluorescence imaging devices used in clinical studies. Br J Radiol. 2015;88:20140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supinfo


