Abstract
Angiosarcoma (AS) is a rare disease with a dismal prognosis. The treatment landscape and prognostic factors for advanced AS, including locally advanced, unresectable, and metastatic disease remain elusive. The Asian Sarcoma Consortium is an international collaborative effort to understand the sarcoma treatment landscape in Asia. We undertook a retrospective chart review of AS patients seen in 8 sarcoma academic centers across Asia. Patients with complete clinical, treatment, and follow‐up data were enrolled. Overall, 276 advanced AS patients were included into this study; 84 (30%) of the patients had metachronous metastatic AS. The median age was 67 y; primary sites of AS was cutaneous in 55% and visceral in 45% of patients. In total, 143 (52%) patients received at least 1 line of systemic chemotherapy. The most common first‐line chemotherapy regimen used was paclitaxel (47.6%) followed by liposomal doxorubicin (19.6%). The median overall survival (OS) was 7.8 mo. Significant prognostic factors for OS included age > 65 (hazard ratio (HR) 1.54, P = .006), male gender (HR 1.39, P = .02), and a cutaneous primary AS site (HR 0.63, P = .004). The median progression‐free survival (PFS) for first‐line chemotherapy was 3.4 mo. PFS for single vs combination or paclitaxel vs liposomal doxorubicin chemotherapy regimens were comparable. This study provides an insight into the treatment patterns and prognostic factors of advanced AS patients in Asia. Prognosis of advanced AS remains poor. Data from this study serve as a benchmark for future clinical study design.
Keywords: advanced disease, angiosarcoma, chemotherapy, ethnicity, prognosis
With a total of 276 advanced angiosarcoma patients, we provided the complete clinicopathological demographics, treatment patterns, and prognostic outcomes of advanced angiosarcoma in Asia.

1. INTRODUCTION
Angiosarcoma (AS) is a subtype of soft tissue sarcoma transformed from vascular endothelial cells. 1 It is ubiquitous and commonly occurs on the skin surface but could also develop in the breast, vascular system, or visceral organs. The incidence of AS is extremely low, accounting for less than 5% of all adult soft tissue sarcomas. 2 While the exact mechanism for malignant transformation is uncertain, risk factors associated with AS included previous radiotherapy, chronic lymphedema, or exposure to toxic chemicals. 3 The prognosis of AS is generally poor, and with its infiltrative and highly metastatic potential, the risk of recurrence and metastasis is high. 3 All of these features make AS a tough‐to‐treat disease.
The optimal treatment for patients who have unresectable or recurrent metastatic AS remains uncertain. Although most may agree that chemotherapy is the main option, the selection of front‐line chemotherapy regimens or sequencing of drug(s) is unclear and lacks formal comparisons. Furthermore, cutaneous or visceral AS may have a different clinical behavior or response to different kinds of treatment. For a rare cancer with such a low incidence, a collaborative effort across various institutions is in pressing need to provide a more concrete understanding of the current treatment landscape in Asia.
It is now recognized that different regions or ethnicities have different cancer incidence patterns, attributed to genetic or environmental factors. 4 Previous reports seem to suggest a higher than expected incidence of AS in Asian patients over the West. 5 However, a lack of a regional collaborative research initiative across Asia impedes the full understanding of the nature of AS. Additionally, the diversity of medical practices and reimbursement systems across Asia must also be taken into consideration when trying to understand the treatment paradigm for AS in this region. 6
The Asian Sarcoma Consortium is a research initiative led by dedicated sarcoma oncologists and surgeons from academic institutions across Asia, with aims to understand sarcoma and improve its treatment in this part of the world. The Consortium has been working since 2015. The first collaborative effort is focused on angiosarcoma. This study aims to provide the results of our findings on the landscape of treatment in advanced AS.
2. METHODS
2.1. Patient populations
Patients with advanced including locally advanced, recurrent metastatic, and/or unresectable AS were eligible. Eight high‐volume academic or designated sarcoma centers across Asia were selected for this study. The 8 centers included: Queen Elizabeth Hospital, Prince of Wales Hospital of The Chinese University of Hong Kong, Hong Kong, SAR; National Cancer Center Hospital, Tokyo, Japan; University of Medicine 1, Yangong, Myanmar; National Cancer Center Singapore and National University Hospital, Singapore; National Taiwan University Hospital, Taipei, Taiwan; and King Chulalongkorn Memorial Hospital, Bangkok, Thailand (in alphabetical order of the Country). Each institution has the approval from their Research Ethics Committee for this project.
2.2. The definition of cutaneous and visceral AS
All AS were designated as either cutaneous or visceral AS in this analysis. Cutaneous AS are those with primary tumors arising from cutaneous regions of scalp, head and neck, limbs, trunks, perineum, and genitalia, or other areas. Visceral AS included tumors arising from liver, cardiovascular system, spleen, musculoskeletal, gastrointestinal tract, lung, genitourinary system, breast, and other non‐cutaneous areas.
2.3. Time to event measurements
Duration of overall survival (OS) was measured from the date of diagnosis of advanced disease to date of death or date of last follow‐up for surviving patients. Patients alive at time of analysis were censored at date of last follow‐up.
Duration of PFS was measured from the date of initiation of first‐line chemotherapy treatment to the date of first progression or death (whichever earlier) or date of last follow‐up for surviving patients free of progressive disease. Surviving patients free of progressive disease were censored at date of last follow‐up.
2.4. Statistical analysis
Baseline categorical variables were summarized as frequency and percentage, and continuous variables were summarized using mean, median with inter‐quartile range (IQR) and range. Comparisons of patient demographics, clinical characteristics and treatment by metastasis diagnosis (synchronous vs metachronous) were performed using Fisher exact test or chi‐squared test for categorical variables (when appropriate) and Mann‐Whitney U test for continuous variables.
Survival curves were estimated by Kaplan‐Meier method and median survival time reported with 95% confidence interval (95% CI). The log‐rank test was used to determine if there was a difference in survival curves between different groups of patients.
Univariable and multivariable analyses were performed using the Cox proportional hazards model. Patient demographics and clinical characteristics associated with survival in the univariable Cox regression model with a significance level of P < .2 and known prognostic factors were included for model selection. Variable selection was performed using a backward selection strategy using the likelihood ratio test with P < .05 as the criteria for inclusion in the final multivariable model. Proportionality assumption for using the Cox regression model was assessed using the Schoenfeld residuals test.
A two‐sided P‐value less than .05 was considered statistically significant. All analyses were performed in STATA version 12.1.
3. RESULTS
Two hundred and 76 advanced AS patients with initial diagnosis between January 1990 and February 2016 from 8 sarcoma academic centers across Asia were included in this study, with the exception that National Cancer Center Hospital Tokyo included AS patients from 2002 to 2017. In total, 192 patients (70%) were presented with de novo advanced AS at the time of initial diagnosis while the remaining 84 patients (30%) developed metachronous metastatic disease at a median of 8.8 mo (IQR: 5.2‐23.1 mo) following initial diagnosis of a previously localized AS.
Patient demographics are presented in Table 1. The median age at diagnosis of advanced disease was 67 y (range 15‐95); the male to female ratio was 1.5. Cutaneous origin of the primary tumor was found in 55% and a visceral origin in 45% of patients. The most common primary cutaneous site was scalp/head and neck while the most common primary visceral site was liver. Different primary AS also had different patterns of spread: Among metachronous metastasis patients, 70% were of cutaneous origin and 30% were visceral origin. While similar proportion of patients had cutaneous and visceral as primary sites in de novo advanced AS cohort, 70% of the recurrent advanced AS had cutaneous primary site. Table S1 provided details of the primary anatomical locations of the advanced AS patients.
TABLE 1.
Demographic and clinical data of the advanced angiosarcoma cohort
| Frequency (%) | P‐value | |||
|---|---|---|---|---|
| Total | LA/UR/Mets at diagnosis | Mets at FU | ||
| N = 276 | n = 192 (70%) | n = 84 (30%) | ||
| Age at metastatic disease, years | .1 # | |||
| Median (IQR) | 67 (49, 77) | 63 (46, 77) | 70 (59, 76) | |
| Range | 15‐95 | 15‐95 | 19‐94 | |
| Age at metastatic disease | .03 | |||
| ≤65 y old | 133 (48.2) | 101 (52.6) | 32 (38.1) | |
| >65 y old | 143 (51.8) | 91 (47.4) | 52 (61.9) | |
| Time to metastasis, months | NA | |||
| Median (IQR) | NA | NA | 8.8 (5.2, 23.1) | |
| Range | NA | NA | 0.5‐112.8 | |
| Gender | .02 | |||
| Female | 112 (40.6) | 87 (45.3) | 25 (29.8) | |
| Male | 164 (59.4) | 105 (54.7) | 59 (70.2) | |
| Primary origin of tumor | .001 ^ (.001) | |||
| Cutaneous | 151 (54.7) | 92 (47.9) | 59 (70.2) | |
| Visceral | 124 (44.9) | 99 (51.6) | 25 (29.8) | |
| Unknown | 1 (0.4) | 1 (0.5) | 0 (0.0) | |
| Chemotherapy | .4 | |||
| No | 133 (48.2) | 89 (46.4) | 44 (52.4) | |
| Yes | 143 (51.8) | 103 (53.6) | 40 (47.6) | |
| Subgroup of patient who had chemotherapy (n = 143) | ||||
| ECOG at start of chemo post metastasis | .4 ^ (.5 ^ ) | |||
| 0 | 22 (15.4) | 14 (13.6) | 8 (20.0) | |
| 1 | 41 (28.7) | 34 (33.0) | 7 (17.5) | |
| 2 | 10 (7.0) | 8 (7.8) | 2 (5.0) | |
| 3 | 5 (3.5) | 4 (3.9) | 1 (2.5) | |
| 4 | 1 (0.7) | 1 (1.0) | 0 (0.0) | |
| Unknown | 64 (44.8) | 42 (40.8) | 22 (55.0) | |
P‐value estimated using chi‐squared test unless otherwise stated.
P‐value within parenthesis excludes the category "Unknown."
Abbreviations: FU, follow‐up; LA, locally advanced; Mets, metastasis; NA, non‐applicable; UR, unresectable.
P‐value estimated using Mann‐Whitney U test.
P‐value estimated using Fisher's exact test.
Fifty‐three out of the 276 (19%) patients had data collected regarding prior radiation history. Among these 53 AS patients, 17 (32%) had prior radiation exposure history. Nine and 8 radiation‐associated AS had primary cutaneous and visceral AS, respectively. The proportion of radiation‐associated AS in the cutaneous and visceral AS cohorts was not significantly different (chi‐square P = .11). Interestingly, all 4 breast AS with information of prior radiation history recorded were confirmed to be radiation‐associated AS.
When the gender proportion was reviewed based on different age cohorts at 10‐y intervals, we observed a disproportionally high percentage of female advanced AS in age cohorts 30‐39 (75%) and 40‐49 (67%) (Figure S1). However, the primary locations of AS were different in these 2 age cohorts. In the age 30‐39 cohort, 7 out of 12 (58.3%) of female patients had breast AS, while in age 40‐49 cohort, the majority were cardiovascular (n = 6/24; 25%) and spleen AS (n = 4/24, 16.7%) and only 2 (8.3%) patients had primary breast AS.
3.1. Treatment landscape of advanced AS in Asia
Regarding the treatment modalities, 21 (7.6%), 33 (11.9%), and 16 (5.8%), 63 (22.8%) received surgery only, radiotherapy only, surgery plus radiotherapy, or palliative care only, respectively, and 143 (51.8%) patients received at least 1 line of chemotherapy after the diagnosis of advanced AS. Among patients who did not receive chemotherapy, more than half (52.6%) had received local treatments consisting of surgery, radiotherapy, or both surgery plus radiotherapy. We also examined if the pattern of practice changed during the period of the study. In the past 2 decades, the proportion of patients who received chemotherapy in advanced AS gradually increased: from 25% in the years 1995‐1999 up to 56% in the years 2015‐2016. As shown in Figure 1, liposomal doxorubicin was the predominant choice of drug while the proportion of patients receiving paclitaxel as first‐line agent gradually increased from year 2005 to year 2016.
FIGURE 1.
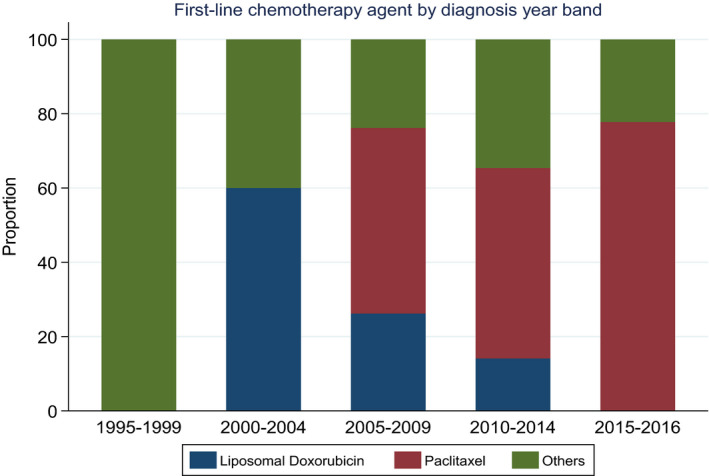
Stacked bar chart of the chemotherapeutic drugs trend changes from year 1995 to year 2016
3.2. Chemotherapy
In total, 143 patients (51.8%) had received at least 1 line of chemotherapy after the diagnosis of advanced AS, and 80 (55.9%), 35 (24.5%) and 28 (19.6%) of these patients received 1, 2, and 3 or more lines of chemotherapy, respectively. Most patients had single agent chemotherapy (79.7%) and the most common first‐line chemotherapy regimens used were paclitaxel (47.6%) and liposomal doxorubicin (19.6%). The most common combination regimen was doxorubicin‐based regimen (8.3%). Only 8 (5.6%) patients received anti‐angiogenic based regimen (either bevacizumab, pazopanib or sunitinib) as first‐line treatment. Ninety (62.9%) patients had less than 6 cycles of chemotherapy for their first‐line treatment. Summary of the first‐line chemotherapy regimens received by this cohort of patients who received chemotherapy post diagnosis of metastatic disease are presented in Table 2, and the details of each regimen was shown in Table S2.
TABLE 2.
The characteristics of the chemotherapy treatment in advanced angiosarcoma cohort
| Frequency (%) | P‐value | |||
|---|---|---|---|---|
| Total | LA/UR/Mets at diagnosis | Mets at FU | ||
| N = 143 | n = 103 (72%) | n = 40 (28%) | ||
| Lines of chemotherapy | .001 # | |||
| Median (IQR) | 1 (1, 2) | 2 (1, 2) | 1 (1, 1) | |
| Range | 1‐5 | 1‐5 | 1‐3 | |
| Lines of chemotherapy | .02 ^ | |||
| 1 | 80 (55.9) | 49 (47.6) | 31 (77.5) | |
| 2 | 35 (24.5) | 30 (29.1) | 5 (12.5) | |
| 3 | 17 (11.9) | 13 (12.6) | 4 (10.0) | |
| 4 | 6 (4.2) | 6 (5.8) | 0 (0.0) | |
| 5 | 5 (3.5) | 5 (4.9) | 0 (0.0) | |
| Lines of chemotherapy | .005 | |||
| 1 | 80 (55.9) | 49 (47.6) | 31 (77.5) | |
| 2 | 35 (24.5) | 30 (29.1) | 5 (12.5) | |
| ≥3 | 28 (19.6) | 24 (23.3) | 4 (10.0) | |
| First‐line chemotherapy agent(s) | .3 | |||
| Single | 114 (79.7) | 80 (77.7) | 34 (85.0) | |
| Combination | 29 (20.3) | 23 (22.3) | 6 (15.0) | |
| First‐line chemotherapy agent(s) | .01 ^ (.007) | |||
| Liposomal doxorubicin | 28 (19.6) | 16 (15.5) | 12 (30.0) | |
| Paclitaxel | 68 (47.6) | 57 (55.3) | 11 (27.5) | |
| Others | 45 (31.5) | 28 (27.2) | 17 (42.5) | |
| Unknown | 2 (1.4) | 2 (1.9) | 0 (0.0) | |
| First‐line chemotherapy agent(s) | <.001 ^ (<.001 ^ ) | |||
| Liposomal doxorubicin | 28 (19.6) | 16 (15.5) | 12 (30.0) | |
| Paclitaxel | 68 (47.6) | 57 (55.3) | 11 (27.5) | |
| Others‐single | 16 (11.2) | 5 (4.9) | 11 (27.5) | |
| Others‐combination | 29 (20.3) | 23 (22.3) | 6 (15.0) | |
| Unknown | 2 (1.4) | 2 (1.9) | 0 (0.0) | |
| First‐line chemo cycles | .5 ^ (.4) | |||
| <6 | 90 (62.9) | 62 (60.2) | 28 (70.0) | |
| ≥6 | 45 (31.5) | 34 (33.0) | 11 (27.5) | |
| Unknown | 8 (5.6) | 7 (6.8) | 1 (2.5) | |
P‐value estimated using chi‐squared test unless otherwise stated.
P‐value within parenthesis excludes the category "Unknown."
P‐value estimated using Mann‐Whitney U test.
P‐value estimated using Fisher's exact test.
3.3. Overall survival
Four patients had no survival follow‐up data and were excluded from the survival analysis. With a median follow‐up of 6.7 mo (range: 0.03‐185.7 mo), 216 deaths (79%) were observed in this cohort of 272 advanced AS patients. Median OS was 7.8 mo (95% confidence interval (CI) 6.41‐9.26 mo, Figure 2A). The median OS for patients who received at least 1 line of chemotherapy was 9.9 mo (95% CI 7.79‐12.32 mo) compared to 4.4 mo (95% CI 3.35‐6.70 mo) for patients who did not receive palliative chemotherapy (P = .007) (Figure 2B).
FIGURE 2.
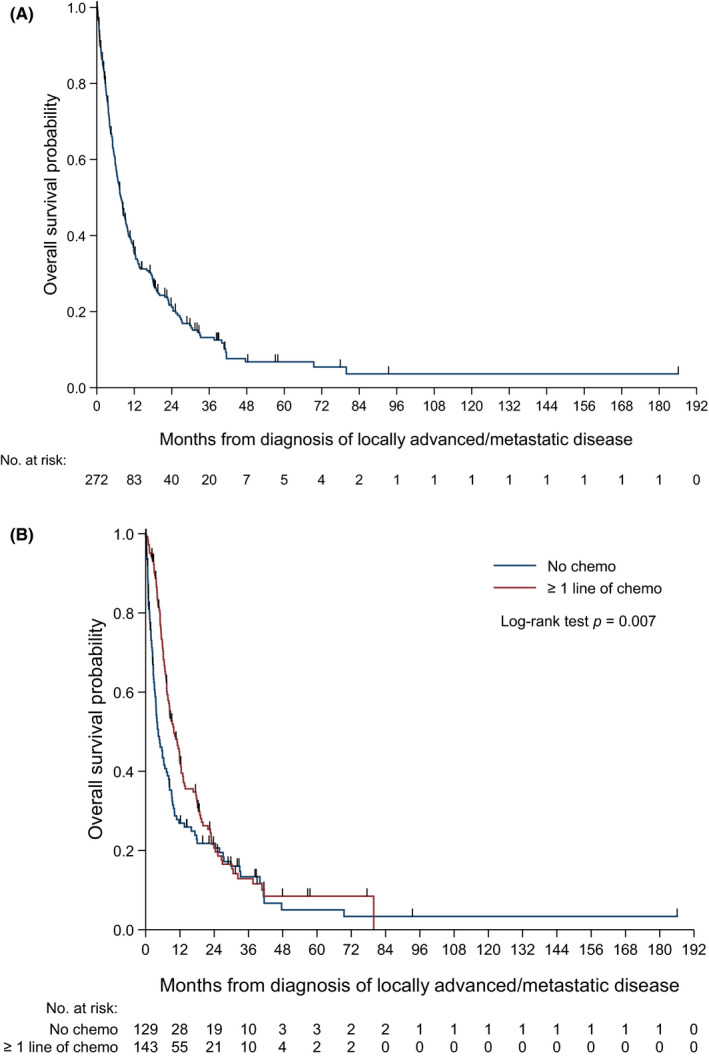
A, Overall survival Kaplan‐Meier curve of the advanced angiosarcoma cohort. B, Overall survival Kaplan‐Meier curve of those who received chemotherapy vs those who had not received chemotherapy in the advanced angiosarcoma cohort
In the univariable analysis, younger age, female gender, and treatment with chemotherapy were associated with longer OS (Table S3). In the multivariable analysis, factors prognostic for OS were age at diagnosis of metastatic disease, gender, and primary origin of AS. After accounting for age at diagnosis of metastatic disease and gender, patients whose tumor developed from a primary cutaneous had better OS than patients whose tumor developed from a primary visceral site (adjusted hazard ratio (HR) = 0.63; 95% CI: 0.46‐0.86; P = .004) (Table 3).
TABLE 3.
Univariate and multivariate models based on age, gender, and primary site of angiosarcoma for overall survival
| OS (Deaths/Pts = 216/272) | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | Adjusted HR (95% CI) | P‐value | |
| Age at diagnosis of metastatic disease | ||||
| ≤65 y old | 1 | 1 | ||
| >65 y old | 1.30 (0.99‐1.70) | .05 | 1.54 (1.13‐2.10) | .006 |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 1.32 (1.00‐1.74) | .05 | 1.39 (1.05‐1.85) | .02 |
| Primary site of angiosarcoma | ||||
| Visceral | 1 | 1 | ||
| Cutaneous | 0.84 (0.64‐1.10) | .2 | 0.63 (0.46‐0.86) | .004 |
| Unknown | 2.20 (0.30‐15.90) | .4 | 1.89 (0.26‐13.87) | .5 |
P‐value calculated using Wald test (from Cox regression model).
Within patients who received at least one line of chemotherapy (n = 143), those who received more than 1 line of chemotherapy and those who had received equal to or more than 6 cycles of systemic treatment in the first‐line chemotherapy had a significant better OS in univariate analysis (Table S3).
3.4. Progression‐free survival (PFS) after chemotherapy
In total, 143 patients had at least 1 line of chemotherapy; 3 patients did not have chemotherapy start date/follow‐up data and were excluded from this analysis. In total, 134 patients (94%) experienced either progressive disease or death. Median PFS was 3.4 mo (95% CI: 2.83‐4.53 mo). The PFS curve is presented in Figure 3.
FIGURE 3.
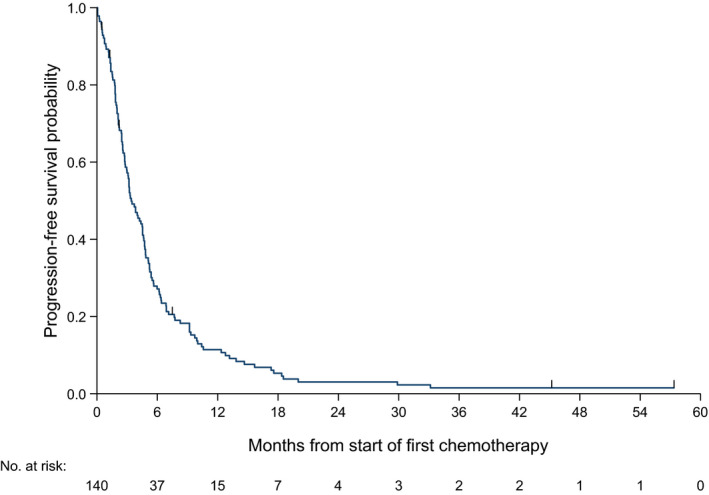
Progression‐free survival Kaplan‐Meier curves for first‐line chemotherapy in advanced angiosarcoma
Notably, median PFS for patients who received single agent vs combination drug chemotherapy was comparable. Patients who received single agent paclitaxel had median PFS of 4.5 mo (95% CI 3.19‐4.86 mo) compared to 2.8 mo (95% CI 2.10‐5.39 mo) for patients who received single agent liposomal doxorubicin. However, the PFS (P = .8) and OS (P = .5) were not significantly different between single agent paclitaxel vs liposomal doxorubicin use (Table 4).
TABLE 4.
Median overall survival (OS) and progression‐free survival (PFS) of the first‐line chemotherapy treatments
| Median OS, months (95% CI) | P‐value 1 | Hazard ratio (95% CI) | P‐value | Median PFS, months (95% CI) | P‐value 1 | Hazard ratio (95% CI) | P‐value | |
|---|---|---|---|---|---|---|---|---|
| First‐line chemo agents | .8 | .6 | ||||||
| Single | 11.0 (7.85‐13.17) | 1 | 3.4 (2.79‐4.73) | 1 | ||||
| Combination | 8.4 (5.49‐11.86) | 0.93 (0.59‐1.47) | .8 | 3.8 (1.91‐5.65) | 0.9 (0.59‐1.37) | .6 | ||
| First‐line chemo agents | .8 | .9 | ||||||
| Liposomal Doxorubicin | 10.6 (6.05‐17.58) | 1 | 2.8 (2.10‐5.39) | 1 | ||||
| Paclitaxel | 11.9 (7.85‐16.66) | 0.85 (0.53‐1.38) | .5 | 4.5 (3.19‐4.86) | 0.94 (0.60‐1.48) | .8 | ||
| Others | 7.4 (6.18‐11.86) | 0.88 (0.52‐1.49) | .6 | 3.0 (2.10‐4.53) | 1.02 (0.63‐1.66) | .9 | ||
| Unknown | 7.4 (NE) | 0.47 (0.06‐3.50) | .5 | NA | NA |
Abbreviations: NA, Not applicable; NE, Not estimable; NR, Not reached.
P‐value calculated using log‐rank test, excluding the category "Unknown."
The only significant factor for better PFS was the disease stage at diagnosis: locally advanced and unresectable patients had a significantly better PFS as compared to patients with metastatic disease (median PFS 5.1 vs 3.0 mo, HR 1.60; 95% CI 1.08‐2.39 mo). Although the duration of chemotherapy may be prone to a lead‐time bias in PFS analysis, other possible confounding clinical factors such as age, gender, primary AS location, ECOG PS at the start of chemotherapy were not significantly associated with PFS in the univariate analysis.
4. DISCUSSION
To the best of our knowledge, this multi‐center study on advanced AS involving 8 academic or designated sarcoma centers from 6 different countries is the largest to be reported in Asia. The real‐world data, taken from a large sample size of patients, treated in expert sarcoma centers, add to the strength of our results.
In this collaborative effort among 8 Asian academic or sarcoma centers, we demonstrated that advanced AS patients in the Asian region have roughly similar median OS (7.8 mo), and similar median PFS (3.4 mo, post first‐line chemotherapy) when compared with AS studies from other populations. Three other large studies that included more than 100 advanced AS patients also reported median OS from 8.5 to 12.1 mo and median PFS from 3.5 to 4.9 mo. 7 , 8 , 9 Despite a higher prevalence of cutaneous AS in our cohort who had a better prognosis, 22.8% of our patients did not receive active oncology treatments for various reasons, which may account for the slightly lower overall median OS. Limitations in access to and diverse variations in availability of advanced medical resources in different Asian health care systems for such patients during the earlier study period could have contributed a negative impact on the outcome. 6
In a study that used data from a national population‐based database, Hung et al reported that the incidence of AS in Taiwan was higher compared with SEER or European data. 10 However, it is uncertain in Asia if cutaneous or visceral AS was more common. In our advanced AS cohort, 55% of patients had a cutaneous primary origin. This was higher than most other reports from the Western world, where the primary cutaneous AS metastatic rate is between 10% and 31%. 7 , 8 , 9 Although our study population involved patients with unresectable but non‐metastatic disease, the number is still high compared with Lahat et al, in which only 45% of all locally recurrent AS were from the cutaneous area. 7 Within our advanced and recurrent cutaneous AS, 84% had primary scalp and head and neck AS. AS from the scalp and head and neck had been reported to have a high rate of recurrence, possibly because negative surgical margins are hard to achieve at time of initial resection. 11 How to better improve the outcomes of advanced cutaneous AS is an important unanswered question for the sarcoma expert community.
Over the course of the study, we noticed interesting changes in the paradigm of care of advanced AS patients in Asia. First, there was a gradual increase in patients receiving chemotherapy and, secondly, a shift toward the use of single agent paclitaxel. This could in part be explained by prospective studies such as the ANGIOTAX informing systemic treatment benefits in patients, recognition of AS as a chemo‐sensitive disease and patients' acceptance of chemotherapy use. Clearly, further development and consolidation of high‐volume sarcoma centers of excellence in Asia, through a consistent network of referrals, conduction of clinical studies and collaboration, will enhance disease understanding and patient outcomes. A centralized treatment for rare diseases such as sarcoma has been advocated in Europe and other parts of the world. 12 An increased volume of patients in dedicated centers along with the establishment of multi‐disciplinary sarcoma teams will further enhance the experience and familiarity of physicians to these rare diseases and provide opportunities for research and clinical trials. 12 , 13
In our study, we found that paclitaxel was the most commonly used first‐line agent in our advanced AS patients associated with a numerically higher PFS (4.5 vs 2.8 mo) than single agent liposomal doxorubicin. However, the median PFS and OS of patients treated by liposomal doxorubicin or paclitaxel did not show statistical difference. Prior studies have suggested that the pharmacokinetic properties of liposomal doxorubicin confer a higher drug concentration in the endothelial venules and therefore efficacy in AS. 14 In the retrospective study by D’Angelo et al, the time to tumor‐progression for liposomal doxorubicin and paclitaxel was similar in the first‐line setting for metastatic AS. 9 In the retrospective analysis from the EORTC group, anthracycline was also not significantly worse in advanced AS in terms of PFS and OS as compared with other soft tissue sarcoma histologies. 15 Although paclitaxel is the preferred first‐line drug of choice in advanced AS in Asia, other systemic treatment options could also be considered based on individual patient selection.
The dismal prognosis of advanced AS patients underscores the fact that newer and more effective treatments are desperately needed. Although rarely used as up‐front treatment for AS in our Asian cohort, anti‐angiogenic agents have been suggested to be efficacious in advanced AS. In a retrospective study, pazopanib, an anti‐angiogenic tyrosine kinase inhibitor, had a response rate of 20% (8/40) in advanced angiosarcoma patients. 16 Vascular endothelial growth factors (VEGF) inhibitor such as bevacizumab have also shown modest activity toward advanced AS. 17 , 18 Endoglin, the latest target in the angiogenesis pathway for vascular tumors, is a molecule that is highly expressed on tumor endothelial cells, suggesting its pivotal role in the resistance of anti‐angiogenesis treatment. 19 Unfortunately, although results of the phase I/II study of the anti‐endoglin antibody TRC105 plus pazopanib in AS were promising, a follow‐up randomized phase III study failed to demonstrate better efficacy with the addition of TRC105. 20
A recent report from the Angiosarcoma Project and other clinical studies found that scalp AS had higher tumor mutation burden and therefore will be more likely to respond to immune checkpoint inhibitors. 21 , 22 This provides a strong scientific rationale for the use of immune checkpoint inhibitor in advanced cutaneous AS. However, AS originating from other non‐cutaneous anatomical locations are genomically diverse with different driver mutations, 22 raising the discussion of routine genetic profiling of AS prior to a tailored or molecularly targeted treatment.
There are several limitations to our study. First, the study was a collaborative effort from 8 sites in 6 Asian countries. We acknowledge that the medical resources available to patients with advanced AS were not uniform across all countries during the study period. Therefore some of the changes in the pattern of chemotherapeutic drug use and care in advanced AS patients may be a reflection of the healthcare systems and/or resources available at hand in each country. Secondly, most of the participating institutions are located in more developed areas in Asia, so the results reported here may not be able to represent the diversity among different Asian countries. Lastly, information on history of prior radiation exposure was only available in 19% of our AS patients, preventing precise evaluation of the prevalence rate of radiation‐associated AS within the AS population. Radiation‐associated AS is associated with a worse prognosis 23 and different molecular features such as c‐myc amplification compared with primary AS patients. 24 Future studies on AS should describe the proportion of radiation‐associated AS to understand if their outcome will differ from those without radiation association.
In conclusion, in this large Asian cohort of 276 locally advanced, unresectable, or metastatic AS patients, the median OS was 7.8 mo and the first‐line PFS for systemic chemotherapy was 3.4 mo. Despite the poor prognosis, we see encouraging trends; more patients are receiving effective systemic chemotherapy after advanced AS diagnosis. We have provided robust clinical outcome data in AS patients and this serves as a basis upon which future studies can be developed. Further investigations into AS are urgently needed to improve the outcome of these patients.
DISCLOSURE
No conflict of interests are reported from the authors.
Supporting information
Fig S1
Table S1‐S3
Chen TW‐W, Pang A, Puhaindran ME, et al. The treatment landscape of advanced angiosarcoma in Asia—A multi‐national collaboration from the Asian Sarcoma Consortium. Cancer Sci. 2021;112:1095–1104. 10.1111/cas.14793
Address of the Institution the study was carried out: National Cancer Center, Singapore. Add: 11 Hospital Crescent, Singapore 169610.
Clinical data were collected from each institution, but the central statistical analysis was carried out at National Cancer Center, Singapore.
Richard Quek was at the National Cancer Center Singapore when the study was carried out.
Contributor Information
Tom Wei‐Wu Chen, Email: tomwchen@ntuh.gov.tw.
Roger K. C. Ngan, Email: rkcngan@hku.hk.
REFERENCES
- 1. Wagner MJ, Ravi V, Menter DG, Sood AK. Endothelial cell malignancies: new insights from the laboratory and clinic. NPJ Precis Oncol. 2017;1(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: an analysis of 26,758 cases. Int J Cancer. 2006;119(12):2922‐2930. [DOI] [PubMed] [Google Scholar]
- 3. Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010;11(10):983‐991. [DOI] [PubMed] [Google Scholar]
- 4. Nahar R, Zhai W, Zhang T, et al. Elucidating the genomic architecture of Asian EGFR‐mutant lung adenocarcinoma through multi‐region exome sequencing. Nat Commun. 2018;9(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin C‐H, Liau J‐Y, Lu Y‐S, et al. Molecular subtypes of breast cancer emerging in young women in Taiwan: evidence for more than just westernization as a reason for the disease in Asia. Cancer Epidemiol Biomarker Prev. 2009;18(6):1807‐1814. [DOI] [PubMed] [Google Scholar]
- 6. Lewin J, Puri A, Quek R, et al. Management of sarcoma in the Asia‐Pacific region: resource‐stratified guidelines. Lancet Oncol. 2013;14(12):e562‐e570. [DOI] [PubMed] [Google Scholar]
- 7. Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16(9):2502‐2509. [DOI] [PubMed] [Google Scholar]
- 8. Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118(13):3330‐3336. [DOI] [PubMed] [Google Scholar]
- 9. D'Angelo SP, Munhoz RR, Kuk D, et al. Outcomes of systemic therapy for patients with metastatic angiosarcoma. Oncology. 2015;89(4):205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hung G‐Y, Horng J‐L, Chen P‐H, et al. Incidence of soft tissue sarcoma in Taiwan: a nationwide population‐based study (2007–2013). Cancer Epidemiol. 2019;60:185‐192. [DOI] [PubMed] [Google Scholar]
- 11. Kuan CH, Yang HW, Huang HF, et al. Prognostic significance of positive surgical margins for scalp angiosarcoma. J Formos Med Assoc. 2021;120(1):217‐225. [DOI] [PubMed] [Google Scholar]
- 12. Casali PG, Abecassis N, Bauer S, et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow‐up†. Ann Oncol. 2018;29(Supplement_4):iv51‐iv67. [DOI] [PubMed] [Google Scholar]
- 13. Bonvalot S, Gaignard E, Stoeckle E, et al. Survival benefit of the surgical management of retroperitoneal sarcoma in a reference center: a nationwide study of the French sarcoma group from the NetSarc database. Ann Surg Oncol. 2019;26(7):2286‐2293. [DOI] [PubMed] [Google Scholar]
- 14. Gabizon AA, Patil Y, La‐Beck NM. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist Updat. 2016;29:90‐106. [DOI] [PubMed] [Google Scholar]
- 15. Young RJ, Natukunda A, Litiere S, Woll PJ, Wardelmann E, van der Graaf WT. First‐line anthracycline‐based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50(18):3178‐3186. [DOI] [PubMed] [Google Scholar]
- 16. Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta oncologica (Stockholm, Sweden). 2017;56(1):88‐92. [DOI] [PubMed] [Google Scholar]
- 17. Bui N, Kamat N, Ravi V, Chawla S, Lohman M, Ganjoo KN. A multicenter phase II study of Q3 week or weekly paclitaxel in combination with bevacizumab for the treatment of metastatic or unresectable angiosarcoma. Rare Tumors. 2018;10:2036361318771771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agulnik M, Yarber JL, Okuno SH, et al. An open‐label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24(1):257‐263. [DOI] [PubMed] [Google Scholar]
- 19. Rosen LS, Gordon MS, Robert F, Matei DE. Endoglin for targeted cancer treatment. Curr Oncol Rep. 2014;16(2):365. [DOI] [PubMed] [Google Scholar]
- 20. Jones RL, Ravi V, Brohl AS, et al. Results of the TAPPAS trial: An adaptive enrichment phase III trial of TRC105 and pazopanib (P) versus pazopanib alone in patients with advanced angiosarcoma (AS). Ann Oncol. 2019;30:v683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Painter CA, Jain E, Tomson BN, et al. The Angiosarcoma Project: enabling genomic and clinical discoveries in a rare cancer through patient‐partnered research. Nat Med. 2020;26(2):181‐187. [DOI] [PubMed] [Google Scholar]
- 23. Merfeld E, Gabani P, Spraker MB, et al. Clinical outcomes and prognostic features of angiosarcoma: significance of prior radiation therapy. Clin Oncol (R Coll Radiol). 2019;31(4):232‐241. [DOI] [PubMed] [Google Scholar]
- 24. Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176(1):34‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S3


