Abstract
Background
Tazemetostat is a selective and orally available inhibitor of enhancer of zeste homolog 2 (EZH2), a histone methyltransferase and epigenetic regulator of cellular differentiation programs. We carried out a phase I study of tazemetostat in Japanese patients with relapsed or refractory B‐cell non‐Hodgkin‐type lymphoma (B‐NHL) to evaluate its tolerability, safety, pharmacokinetics, and preliminary antitumor activity.
Methods
Tazemetostat was given orally at a single dose of 800 mg on the first day and 800 mg twice daily (BID: total 1600 mg/d) on following days in a 28‐day/cycle manner. Tazemetostat dose‐limiting toxicity (DLT) was evaluated up to the end of the first treatment cycle. Archival tumor tissues were analyzed for hotspot EZH2 mutations.
Results
As of 15 January 2018, seven patients (four follicular lymphoma [FL] and three diffuse large B‐cell lymphoma [DLBCL]) were enrolled. The median age was 73 (range, 59‐85) years, and the median number of prior chemotherapy regimens was three (range, one to five). No DLT was observed (one patient was not evaluable due to early disease progression). The common treatment‐related adverse events (AEs) were thrombocytopenia and dysgeusia (three patients each; 42.9%). No treatment‐related serious AEs were observed. The objective response rate was 57% (4/7 patients), including responses in three of four patients with FL and one of three patients with DLBCL. An EZH2 mutation was detected in one patient with FL responding to treatment.
Conclusions
Tazemetostat at 800 mg BID showed an acceptable safety profile and promising antitumor activity in Japanese patients with relapsed or refractory B‐NHL.
Keywords: diffuse large B‐cell lymphoma, enhancer of zeste homolog 2, follicular lymphoma, phase I study, tazemetostat
Tazemetostat is a selective and orally available inhibitor of enhancer of zeste homolog 2 (EZH2), a histone methyltransferase acting as an epigenetic regulator of cellular differentiation programs. Tazemetostat at 800 mg BID demonstrated an acceptable safety profile and promising antitumor activity in Japanese patients with relapsed or refractory B‐cell non‐Hodgkin‐type lymphoma.

Abbreviations
- AE
adverse event
- AUC
area under the concentration‐time curve
- BOR
best overall response
- CI
confidence interval
- COO
cell‐of‐origin
- CR
complete response
- CT
computed tomography
- CYP3A
cytochrome P450 family 3 subfamily A
- DLBCL
diffuse large B‐cell lymphoma
- DLT
dose‐limiting toxicity
- ECG
electrocardiogram
- EZH2
enhancer of zeste homolog 2
- FL
follicular lymphoma
- GC
germinal center
- GCB‐DLBCL
germinal center B‐cell‐like diffuse large B‐cell lymphoma
- H3K27
lysine 27 on histone 3
- IHC
immunohistochemistry
- NHL
non‐Hodgkin‐type lymphoma
- ORR
objective response rate
- PK
pharmacokinetic
- PR
partial response
- PRC2
polycomb repressive complex 2
- PS
performance status
- R‐CHOP
combination of several chemotherapy drugs (cyclophosphamide, doxorubicin, vincristine, and prednisone) and rituximab (R)
- R/R
relapsed or refractory
- SAE
serious adverse event
- SWI/SNF
switch/sucrose nonfermentable
- t1/2
terminal elimination phase half‐life
- TR‐AE
treatment‐related adverse event
1. INTRODUCTION
The disruption of chromatin modulation has emerged as an important step in oncogenesis, including lymphomagenesis. Mutations in chromatin modifiers, associated with aberrant cell fate decisions, have been reported to frequently occur in a number of tumors. 1 , 2 , 3 Loss‐of‐function mutations in E1A binding protein P300 (EP300), CREB binding protein (CREBBP), or lysine methyltransferase 2D (KMT2D, also known as MLL4) have been shown to occur frequently in B‐NHL. 4 , 5 , 6 Enhancer of zest homolog 2 is a histone methyltransferase known to function as the catalytic subunit of PRC2. Briefly, PRC2 is known to methylate H3K27. 7 , 8 , 9 , 10 , 11 , 12 , 13 Patients with solid tumors characterized by loss of expression of the SWI/SNF subunits of the SWI/SNF‐related matrix‐associated actin‐dependent regulator of chromatin subfamily B member 1 (SMARCB1, also known as INI1, SNF5, and BAF47) protein have an extremely poor prognosis and lack efficacious treatments. More specifically, INI1 is a potent tumor suppressor gene and encodes a core component of the SWI/SNF complex that is known to act in opposition to PRC2, the integrated functions of which have been shown to control diverse cellular processes, such as cell differentiation and proliferation. 14 , 15 Loss of INI1 has been reported to disrupt the function of the SWI/SNF complex, leading to aberrant recruitment of EZH2 to target genes, increased H3K27me3, transcriptional repression of key tumor suppressors, and the upregulation of several oncogenic signaling pathways, including Sonic hedgehog, Wnt/β‐catenin, and myc. 15 , 16 , 17 With regard to B‐NHL, recurrent gain‐of function alterations in EZH2 have been reported to occur in approximately 21.7% of GCB‐DLBCLs and 7%‐27% of FLs. 6 , 18 , 19 Once GC B‐cells complete their affinity maturation, they resume their normal path of plasma cell differentiation. 20 Both GCB‐DLBCL and FL have been reported to arise from this inherently tumorigenic GC B‐cell phenotype. 21 , 22 Accordingly, EZH2 was found to be essential for maintaining the GC phenotype and is thus required for the development of pre‐B cells to acquire a full spectrum of immunoglobulin recombination. 23 Moreover, EZH2 is known to be highly expressed in GC, and conditional deletion of EZH2 in established GC B‐cells results in their failure to form functional GCs. 24 , 25 Tazemetostat (EPZ‐6438, E7438) is an orally administered, highly selective EZH2 inhibitor, and its first‐in‐human study was undertaken in France. 26 In this study, tazemetostat showed a favorable safety profile and antitumor activity in patients with refractory B‐NHL and advanced solid tumors, including epithelioid sarcomas. The recommended dose was set to 800 mg BID. Tazemetostat received accelerated approval by the US FDA in January 2020 for the treatment of adults and adolescents aged 16 years or older with locally advanced or metastatic epithelioid sarcoma not eligible for complete resection, based on the ORR and duration of response observed in the phase II study. 27 With respect to B‐NHL, a separate phase II study reported that the ORR of tazemetostat was 69% (95% CI, 53‐82; 31 of 45 patients) in the EZH2 mutant FL cohort and 3% (95% CI, 23‐49; 19 of 54 patients) in the EZH2 WT FL cohort. 28 Based on this study, tazemetostat also received accelerated approval from the FDA in June 2020 for the treatment of adult patients with R/R FL whose tumors are positive for an EZH2 mutation as detected by an FDA‐approved test and who have received at least two prior systemic therapies, as well as for adult patients with R/R FL who have no satisfactory alternative treatment options. Here, we report a phase I study of tazemetostat in Japanese patients with relapsed or refractory B‐NHL.
2. MATERIALS AND METHODS
2.1. Study design and treatment
This multicenter, single‐arm, phase I study (ClinicalTrials.gov identifier: NCT03009344) in Japanese patients with relapsed or refractory B‐NHL aimed to evaluate the tolerability, safety, PKs, and preliminary antitumor activity of tazemetostat. In addition, the EZH2 mutation status in tumors was explored. For this, 800 mg tazemetostat was given orally in a single dose in cycle 0 (4 days) and in continuous doses of 800 mg BID (1600 mg total daily dose) in cycle 1, and later in 28‐day cycles. Dose reduction and interruption were allowed in case patients experienced toxicity, such as intolerable grade 2 or more toxicity (except for absolute neutrophil counts of 0.75 × 109/L or higher). Dose reductions were in the order of 600 and 400 mg BID (1200 mg and 800 mg total daily dose, respectively) and were not allowed to increase later. Treatment with tazemetostat continued until disease progression, development of unacceptable toxicity, patient request to discontinue, withdrawal of consent, and other activities and were discussed with the sponsor. Follow‐up was carried out until 30 days after the final treatment with tazemetostat.
The selection of initiation dose in this study was based on a phase I/II study of tazemetostat (NCT01897571) undertaken outside of Japan, where the recommended dose of tazemetostat was determined to be 800 mg BID. 26 The tolerability of tazemetostat was determined based on the incidence of DLTs in cycles 0 and 1. If DLTs occurred in two or fewer of six patients, this dosage level was considered tolerable.
2.2. Patient eligibility
Eligible patients were a minimum of 20 years of age with a histological diagnosis of DLBCL or FL (except for transformed lymphoma), for which no standard therapy existed. Patients must have had previous therapy with systemic chemotherapy or Ab therapy, and measurable disease detected by a CT scan. Patients also had to have an ECOG‐PS of 0 or 1 and life expectancy of at least 3 months, as well as adequate renal, liver, bone marrow, and cardiac function. Patients were not eligible if they had allogeneic stem cell transplantation or prior exposure to an EZH2 inhibitor. Patients were also excluded if they were unable to take oral medication, had malabsorption syndrome, or had venous thrombosis or pulmonary embolism within the past 3 months before study drug administration, complications of hepatic cirrhosis, interstitial pneumonia, or pulmonary fibrosis. Other key exclusion criteria included medication comprising potent or moderate inhibitors/inducers of CYP3A, use of H2 blockers or proton‐pump inhibitors, significant cardiovascular impairment, prolongation of QT interval, malignancy other than B‐NHL, and pregnancy or lactation. This study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol and its amendments were approved by the Institutional Review Board, and all patients provided written informed consent.
2.3. Definition of DLT
The following toxicities were regarded as DLTs: (a) grade 4 neutropenia for more than 7 consecutive days or neutropenia requiring hematopoietic growth factors; (b) grade 3 or higher febrile neutropenia; (c) grade 4 thrombocytopenia, grade 3 thrombocytopenia with bleeding, or thrombocytopenia requiring platelet transfusion; (d) grade 4 anemia or anemia requiring erythrocyte transfusion; (e) grade 3 or higher nausea, vomiting, or diarrhea persisting for more than 7 consecutive days despite maximal medical therapy; (f) grade 3 or higher nonhematological laboratory abnormalities with clinical symptoms persisting for more than 7 days; (g) other grade 3 toxicity lasting more than 7 consecutive days or grade 4 nonhematological toxicity of any duration; (h) failure to administer 75% or more of the planned administration number (42 or more of 56 doses) of the study drugs in cycle 1 as a result of treatment‐related toxicity.
2.4. Safety
Safety assessments consisted of monitoring and recording all AEs, including all grading of Common Terminology Criteria for Adverse Events (version 4.03), SAEs, regular laboratory evaluation of hematology, blood chemistry, and urine values, and periodic measurements of vital signs, including 12‐lead ECGs, echocardiograms/multigated acquisition scans to assess left ventricular ejection fraction, ECOG‐PS, and physical examinations.
2.5. Pharmacokinetics
Blood samples for PK analyses were collected as follows: predose, and 0.5, 1, 2, 4, 6, 8, 10, and 12 hours (day 1), 24 hours (day 2), 48 hours (day 3), and 72 hours (day 4) postdose in cycle 0; predose in the first administration on cycle 1 day 3 (C1D3) and cycle 1 day 8 (C1D8); predose and 0.5, 1, 2, 4, 6, 8, 10, and 12 hours postdose in the first administration on cycle 1 day 15 (C1D15); and predose in the first administration on cycle 1 day 22 (C1D22) and cycle 2 day 1 (C2D1). Urine samples for PK analyses of tazemetostat were collected as follows: predose and 0‐72 hours postdose in C0D1; and 0‐12 hours postdose for the first administration in C1D15. Tazemetostat was given in a fasted state in cycle 0 day 1 (C0D1) and at the first administration of cycle 1 day 15 (C1D15) defined as 2 hours or more before and 2 hours or more after a meal (only water was allowed). The plasma and urine concentrations of tazemetostat and the plasma concentrations of its desethyl metabolite (EPZ‐6930) were measured by validated methods using liquid chromatography with tandem mass spectrometry. Pharmacokinetic parameters were calculated using noncompartmental analysis, including C max (maximum plasma concentration), time to C max (t max), and AUC at both first [C0D1] and repeated [C1D15] administrations).
2.6. Antitumor activity
Tumor assessment was carried out according to the Lugano Classification (CT‐based Response). 29 The ORR and BOR were assessed. The CT scans were undertaken within 28 days prior to the initiation of treatment, every 8 weeks (starting at C1D1) during cycle 2‐6, every 12 weeks starting at cycle 7 (C7D1) and beyond, and at discontinuation. Bone marrow aspiration or biopsy was carried out at screening for the evaluation of bone marrow infiltration in the tumor. After studying drug administration, bone marrow aspiration or biopsy was carried out if the result of screening was positive or unconfirmed and when required to confirm CR as the best response or if clinically indicated.
2.7. EZH2 mutation and COO status
Archival, formalin‐fixed tumor tissues from available patients were collected for assessment of the mutational status of the EZH2 (codons Y646, A682, and A692). The COO status of DLBCL patients was collected as patient characteristics. The COO status of all three patients was identified using the Hans IHC‐based algorithm. 30 The frequency of EZH2 mutation status and COO status were calculated.
2.8. Statistical analysis
All subjects who completed treatment cycles 0 and 1 without major protocol deviations with at least 75% treatment compliance in cycle 1 were assessed for DLT, in addition to subjects who experienced DLT during cycles 0 and 1. All subjects who received at least one dose of tazemetostat were analyzed for safety, efficacy, and PKs. The BOR was summarized in total or for each disease (DLBCL and FL). The ORR was presented with corresponding two‐sided Clopper‐Pearson exact 95% CIs. Statistical analyses were performed using SAS Version 9.2 or later and Phoenix WinNonlin software (version 7.0) for PK analysis.
3. RESULTS
3.1. Patient characteristics
This study was carried out between 10 January 2017 and 21 May 2019 at two study sites in Japan. A total of seven patients received at least one dose of the study drug. Two patients were in cycle 29 as of the date of data cut‐off, whereas five patients discontinued the study. Dose‐limiting toxicities were evaluated in six patients, but one patient was not included, due to disease progression with less than 75% treatment compliance in cycle 1. A summary of patient characteristics is presented in Table 1. The median age was 73.0 years (range, 59‐85 years), with four male (57.1%) patients. All patients were Japanese. Baseline ECOG‐PS scores of patients were either 0 (five patients; 71.4%) or 1 (two patients; 28.6%).
TABLE 1.
Demographics and characteristics of Japanese patients with relapsed or refractory B‐cell lymphoma treated with tazemetostat
| Characteristic | Patients (n = 7) |
|---|---|
| Age, years; median (range) | 73.0 (59‐85) |
| Sex, male/female | 4 (57.1)/3 (42.9) |
| ECOG performance status, n (%) | |
| 0/1 | 5 (71.4)/2 (28.6) |
| Histopathologic subtype, n (%) | |
| Follicular lymphoma | 4 (57.1) a |
| Diffuse large B‐cell lymphoma | 3 (42.9) b |
| Number of prior chemotherapy treatments, n (%) | |
| 1 | 3 (42.9) |
| 2 | 0 (0.0) |
| ≥3 | 4 (57.1) |
| Median (range) | 3 (1‐5) |
| Auto‐HSCT, n (%) | 0 (0) |
Abbreviation: Auto‐HSCT, autologous hematopoietic stem cell transplantation.
One patient with EZH2 gene mutation.
One patient was germinal center B‐cell‐like (GCB)‐type, whereas two patients were non‐GCB type, based on Hans criteria‐based diagnosis at the investigator site.
3.2. Treatment
The median number of cycles received was 12 (range, 1‐29), whereas the median duration of exposure was 11.2 months (range, 0.6‐26.4). Of the seven treated patients, one (14.3%) received 100% of their planned starting dosage, five (71.4%) received at least 90% of the dosage, and one (14.3%) patient received at least 70% of the dosage. Tazemetostat treatment was interrupted for three (42.9%) patients. Only one patient (14.3%) received a reduction in the tazemetostat dose, with the time to first dose reduction at 4.9 months.
3.3. Adverse events and DLTs
A summary of treatment‐emergent AEs and TR‐AEs is shown in Table 2. All seven patients experienced at least one AE, with six (85.7%) patients having at least one TR‐AE. Grade 3 or higher AEs occurred in four (57.1%) patients. The common AEs were nasopharyngitis (five patients; 71.4%) and thrombocytopenia, constipation, and dysgeusia (three patients each; 42.9%). Grade 3 AEs reported were thrombocytopenia (two patients; 28.6%) and anemia, leukopenia, neutropenia, lymphopenia, fatigue, increased γ‐glutamyltransferase, hypophosphatemia, and squamous cell carcinoma of the tongue (one patient each; 14.3%). Grade 4 AEs were intestinal perforation and increased levels of blood triglycerides (one patient each; 14.3%). The common TR‐AEs were thrombocytopenia and dysgeusia (three patients each; 42.9%). No DLTs were observed. None of the patients died or had AEs resulting in death. Serious AEs occurred in two (28.6%) patients, consisting of intestinal perforation and squamous cell carcinoma of the tongue, but both assessed as not related to study drug. One patient (14.3%) experienced grade 2 peripheral neuropathy, leading to drug dose reduction. One patient (14.3%) discontinued the treatment due to squamous cell carcinoma of the tongue, which is not a treatment‐related event. One (14.3%) patient experienced an AE leading to dose reduction, whereas four (57.1%) patients experienced AEs leading to dose interruption.
TABLE 2.
D‐limiting toxicity and treatment‐emergent adverse events (TEAEs) (≥2 patients) in Japanese patients with relapsed or refractory B‐cell lymphoma treated with tazemetostat
| TEAEs | Patients (n = 7) | |||
|---|---|---|---|---|
| All TEAEs | Treatment‐related TEAEs | |||
| All grades (%) | Grade ≥3 (%) | All grades (%) | Grade ≥3 (%) | |
| Hematologic toxicity | ||||
| Thrombocytopenia | 3 (42.9) | 2 (28.6) | 3 (42.9) | 2 (28.6) |
| Anemia | 2 (28.6) | 1 (14.3) | 2 (28.6) | 1 (14.3) |
| Leukopenia | 2 (28.6) | 1 (14.3) | 1 (14.3) | 0 (0.0) |
| Neutropenia | 2 (28.6) | 1 (14.3) | 1 (14.3) | 0 (0.0) |
| Nonhematologic toxicity | ||||
| Nasopharyngitis | 5 (71.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Constipation | 3 (42.9) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| Dysgeusia | 3 (42.9) | 0 (0.0) | 3 (42.9) | 0 (0.0) |
| Blood creatinine increased | 2 (28.6) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| Dry eye | 2 (28.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dry skin | 2 (28.6) | 0 (0.0) | 2 (28.6) | 0 (0.0) |
| Fatigue | 2 (28.6) | 1 (14.3) | 2 (28.6) | 1 (14.3) |
| Insomnia | 2 (28.6) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| Muscle spasms | 2 (28.6) | 0 (0.0) | 2 (28.6) | 0 (0.0) |
| Rash | 2 (28.6) | 0 (0.0) | 2 (28.6) | 0 (0.0) |
| Stomatitis | 2 (28.6) | 0 (0.0) | 2 (28.6) | 0 (0.0) |
No AEs of T‐cell lymphoblastic lymphoma/T‐cell acute lymphoblastic leukemia or myeloid malignancy, including myelodysplastic syndrome, were reported during the study. No clinically important changes were observed in the mean or median laboratory values, vital signs, or weight over time. Shift analyses revealed no shifts of clinical concern noted in urinalysis parameters and ECG findings. No abnormal QT interval corrected for heart rate using Fridericia’s formula values was found.
3.4. Antitumor activity and EZH2 mutations
In seven treated patients, one (14.3%) had CR, three (42.9%) had PR, one (14.3%) had stable disease, and two (28.6%) had progressive disease as BOR, based on the investigator assessment (Table 3). The ORR was 57.1% (95% CI: 18.4‐90.1) with response in one patient with DLBCL (n = 3) and three patients with FL (n = 4). Overall, six (85.7%) patients experienced a reduction of tumor burden (Figure 1). One (14.3%) patient had an EZH2 mutation, whereas five (71.4%) patients did not. One patient (14.3%) had an unknown EZH2 mutational status. The COO status of the three patients with DLBCL was GCB type in one patient and non‐GCB type in two patients.
TABLE 3.
Summary of tumor response in Japanese patients with relapsed or refractory B‐cell lymphoma treated with tazemetostat
| Response category | DLBCL (n = 3) | FL (n = 4) | Total (n = 7) |
|---|---|---|---|
| Best overall response, n (%) | |||
| Complete response (CR) | 1 (33.3) | 0 (0.0) | 1 (14.3) |
| Partial response (PR) | 0 (0.0) | 3 (75.0) | 3 (42.9) |
| Stable disease | 0 (0.0) | 1 (25.0) | 1 (14.3) |
| Progressive disease | 2 (66.7) | 0 (0.0) | 2 (28.6) |
| Not evaluable | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Objective response rate (CR + PR), n (%) | 1 (33.3) | 3 (75.0) | 4 (57.1) |
| 95% CI of objective response rate a | (0.8, 90.6) | (19.4, 99.4) | (18.4, 90.1) |
Abbreviations: CI, confidence interval; DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma.
Calculated using Clopper‐Pearson’s exact method.
FIGURE 1.
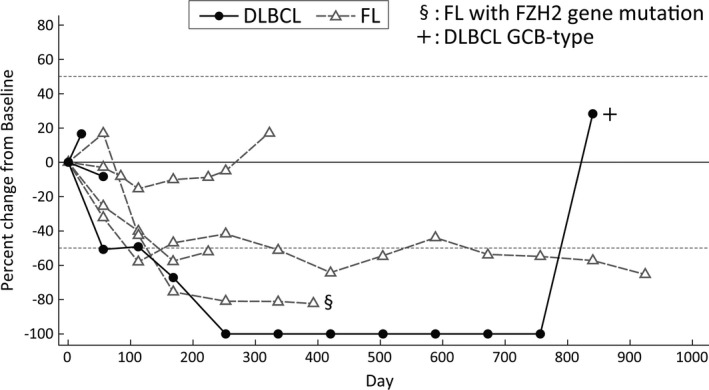
Changes in target tumor burden over time in Japanese patients with relapsed or refractory B‐cell lymphoma treated with tazemetostat. Black circles indicate diffuse large B‐cell lymphoma (DLBCL); gray triangle indicates follicular lymphoma (FL). §Patient with an EZH2 mutation. +Patient with germinal center B‐cell‐like (GCB)‐type DLBCL
3.5. Pharmacokinetics
The plasma concentration profiles and PK parameters of tazemetostat and its desethyl metabolite EPZ‐6930 are shown in Figure 2 and Table 4. After oral administration of 800 mg tazemetostat, tazemetostat was rapidly absorbed. The median t max values for tazemetostat and EPZ‐6930 were approximately 2 hours after the C0D1 dosing and approximately 1 hour after the C1D15 dosing. The mean t 1/2 values of tazemetostat and EPZ‐6930 were 7.59 and 8.83 hours, respectively, after C0D1 dosing and 4.59 and 4.91 hours, respectively after C1D15 dosing. The t 1/2 values were shorter for C1D15 than C0D1, but slopes of decline in mean plasma concentration profiles for tazemetostat and EPZ‐6930 were similar up to 12 hours after dosing between C0D1 and C1D15. In addition, the slope of decline in mean plasma concentration profiles for tazemetostat and EPZ‐6930 were slower after 12 hours after dosing for C0D1 (Unpublished data in Eisai). No remarkable difference in mean PK profiles was observed between the first dose administration for C1D1 and multiple doses for C1D15. The AUC ratios of EPZ‐6930 to tazemetostat were shown to be 141% for C0D1 and 256% for C1D15. Plasma concentrations of tazemetostat reached steady‐state after multiple dosing reached steady‐state by C1D8 in almost all subjects. The mean urinary excretion of tazemetostat was less than 3% both after C0D1 and C1D15 dosing.
FIGURE 2.
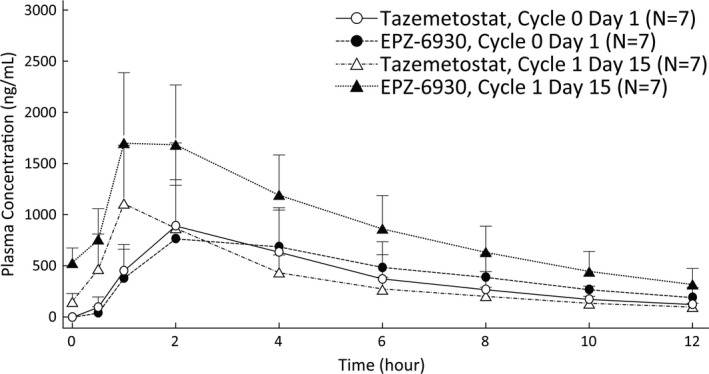
Plasma concentration profiles of tazemetostat and its metabolite, EPZ‐6930, in Japanese patients with relapsed or refractory B‐cell lymphoma. Plasma concentration shown as the mean + SD after single and multiple oral administrations of 800 mg tazemetostat
TABLE 4.
Summary of plasma pharmacokinetic parameters of tazemetostat and EPZ‐6930 after single and multiple doses in Japanese patients with relapsed or refractory B‐cell lymphoma treated with tazemetostat
| Pharmacokinetic parameter |
After single dose Cycle 0 day 1 |
After multiple doses Cycle 1 day 15 |
||
|---|---|---|---|---|
| Tazemetostat | EPZ‐6930 | Tazemetostat | EPZ‐6930 | |
| C max (ng/mL) | 1150 (787) | 948 (556) | 1290 (582) | 1950 (773) |
| C ss,min (ng/mL) | — | — | 95.1 (37.7) | 322 (170) |
| t max (h) | 1.97 (0.95, 4.08) | 1.97 (1.12, 4.08) | 1.05 (0.88, 2.03) | 1.07 (0.88, 2.03) |
| AUC(0‐12 h) (ng·h/mL) | 4700 (2810) | 5280 (2890) | 4500 (1570) | 10 800 (3600) |
| AUC(0‐ t ) (ng·h/mL) | 5990 (3460)a | 7700 (4090)a | 4490 (1560)b | 10 800 (3560)b |
| AUC(0‐inf) (ng·h/mL) | 6030 (3470) | 7730 (4090) | — | — |
| t 1/2 (h) | 7.59 (1.24) | 8.83 (1.43) | 4.59 (1.93) | 4.91 (2.32) |
| CL/F (L/h) | 175 (90.4) | NA | 205 (98.6) | NA |
| V z/F (L) | 1910 (1050) | NA | 1580 (1540) | NA |
| R ac (C max) | — | — | 1.32 (0.604) | 2.59 (1.36) |
| R ac (AUC) | — | — | 1.09 (0.358) | 2.58 (1.44) |
| R ss | — | — | 0.849 (0.277) | 1.76 (1.07) |
Data are shown as the mean (SD) except for t max; for t max, median (minimum, maximum) is shown. The last sampling time point was 72 h after single administration (a) and 12 h after multiple administrations (b). Pharmacokinetic analysis set: N = 7. R ac and R ss values were calculated using these formulas: and
Abbreviations: AUC(0‐12 h), area under the concentration‐time curve from zero time to 12 h; AUC(0‐inf) , area under the concentration‐time curve from zero to infinite; AUC(0‐ t ) , area under the concentration‐time curve from zero time to time of last quantifiable concentration; AUC(0‐ t ) ratio, metabolite to parent area under the concentration‐time curve ratio adjusted in molecular weight; CL/F, apparent total clearance following oral administration; C max, maximum plasma concentration; C ss,min, minimum observed concentration at steady state; NA, not applicable; R ac, accumulation index; R ss, time and concentration dependent accumulation ratio; t 1/2, terminal elimination phase half‐life; t max, time at which the highest drug concentration occurs; V z/F, apparent volume of distribution at the terminal phase.
4. DISCUSSION
In this multicenter, single‐arm phase I study of tazemetostat in Japanese patients with relapsed or refractory B‐NHL, tazemetostat was given orally at a dose of 800 mg BID and was found to be well tolerated. We did not observe any DLT in six DLTs evaluable patients. In a total of seven patients, the common AEs observed included thrombocytopenia, dysgeusia (43%), anemia, dry skin, fatigue, rash, and stomatitis (29%). No treatment‐related SAEs were observed. In the global phase II study, among all 99 patients, common treatment‐related grade 3 or higher AEs were thrombocytopenia (3%), neutropenia (3%), and anemia (2%). Treatment‐related SAEs were reported in four (4%) of 99 patients. Importantly, there were no treatment‐related deaths. 28 There was some difference between safety profiles in the global phase II study and in this phase I study. However, as the number of patients in our present study was limited, the safety profile of tazemetostat in Japanese patients will be confirmed in the next phase II study.
The ORR was 57% (4/7 patients), including response in three of four patients with FL and one of three patients with DLBCL. We observed a gain‐of‐function mutation in EZH2 in one patient with FL showing a partial response with a −82.3% maximum change in the sum of the product of the diameters from baseline in the target lesion. In a preclinical study, tazemetostat showed antiproliferative effects, inducing apoptosis, in EZH2‐mutant and WT cells. 31 It was also reported that treatment of EZH2‐mutant NHL xenograft‐bearing mice with tazemetostat caused dose‐dependent tumor growth inhibition, including complete and sustained tumor regression with a correlative decrease in the levels of H3K27Me3 in tumors and selected normal tissues. Another in vitro study showed that treatment with EZH2 inhibitors reduced viability in both EZH2‐WT and EZH2‐mutated lymphoma cell lines; however, viability was much more reduced in the EZH2‐mutated cells. 25 In a global (ex‐Japan) phase II study, it was reported that tazemetostat showed a greater ORR (69% [95% CI 53‐82; 31 of 45 patients]) in EZH2‐mutated patients compared to that in WT patients (35% [23‐49; 19 of 54 patients]). In our study, one patient, classified as GCB‐DLBCL, showed complete response. The other two patients with DLBCL were identified as non‐GCB type by the Hans IHC‐based algorithm. Generally, EZH2 is known to be expressed in GC, playing a crucial role in its proliferation and differentiation. Hence, the cellular origin of lymphoma might be important in predicting the efficacy of tazemetostat. Moreover, a BCL2 fusion was observed in most patients with FL and GCB‐DLBCL. 32 Interestingly, BCL‐2‐transduced EZH2‐mutant mice were shown to exhibit much faster lymphomagenesis than BCL‐2 transduced WT mice. 33 The other two patients with FL showed PR, despite the WT EZH2 status of their lymphomas. In preclinical animal models, tazemetostat showed potent antitumor effects in EZH2‐mutant NHL xenograft‐bearing mice in a dose‐dependent manner. However, tazemetostat was also found to induce antitumor effects in EZH2‐wt lymphoma xenograft models. 31 Immunodeficient SCID mice lacking mature B and T lymphocytes but showing residual immunity, such as natural killer cells, were used in these xenograft models. Recently, it was reported that an EZH2 mutation was strongly enriched in both MHC‐I and MHC‐II negative lymphomas, with EZH2 inhibitors significantly restoring the expression of MHC in DLBCL cell lines. It was also reported that EZH2 regulates the expression of CD58, which is involved in tumor evasion in lymphoid malignancies. 34 , 35 These results suggested that EZH2 might regulate the immune system by modulating the effects of these molecules, and we thus speculated that tazemetostat might show efficacy through this immune regulation in both EZH2‐mutant and WT patients.
Tazemetostat has been reported to be mainly metabolized by CYP3A4, and was shown to induce and inhibit the activity of CYP3A4 in vitro (Unpublished data in Eisai). The PK profiles of tazemetostat in Japanese patients were comparable to those of non‐Japanese patients previously reported. 26 The mean value of the time‐ and concentration‐dependent accumulation ratio (R ss) was shown to be 0.849, slightly smaller than 1, suggesting that there was no accumulation of tazemetostat and a possible small effect of autoinduction of CYP3A4. We further observed apparent differences in the t 1/2 values of tazemetostat and EPZ‐6930, its demethylated metabolite, between C0D1 and C1D15. We speculated that this was due to the difference in the last blood sampling time points at 72 and 12 hours after dosing for C0D1 and C1D15, respectively. As EPZ‐6930 showed weaker inhibitory activity (1/11‐1/31) against EZH2 than tazemetostat in preclinical studies and its exposure was larger than that of tazemetostat, we assumed that EPZ‐6930 might partially contribute to the observed antitumor activity.
In conclusion, the present phase I study showed that 800 mg BID of tazemetostat showed an acceptable safety profile and promising antitumor activity in Japanese patients with relapsed or refractory B‐NHL. However, most patients in this study carried WT EZH2. Subsequent studies to evaluate the efficacy and safety of tazemetostat in Japanese patients with B‐NHL, especially in patients with EZH2 mutations, are warranted.
DISCLOSURE
The authors declare the following potential conflicts. KT: HUYA Bioscience, consultancy, honoraria; Bristol‐Myers Squibb, honoraria; Verastem, honoraria; Takeda Pharmaceutical, consultancy, honoraria, research funding; Eisai, honoraria, research funding; Kyowa Kirin, honoraria, research funding; Celgene, consultancy, honoraria, research funding; Zenyaku Kogyo, consultancy, honoraria; AbbVie, research funding; Yakult, honoraria; Janssen Pharmaceutical, honoraria, research funding; Mundi Pharma, consultancy, honoraria, research funding; Solasia, honoraria; Meiji Seika, honoraria; Daiichi Sankyo, consultancy, honoraria; Ono Pharmaceutical, consultancy, honoraria, research funding; Chugai Pharmaceutical, honoraria, research funding. SM: personal fees (BMS/Celgene, Chugai, Daiichi‐Sankyo, Eisai, Novartis, Symbio, Takeda). DM: personal fees and grant (Ono Pharmaceuticals, Celgene, Takeda Pharmaceutical, Janssen Pharmaceutical, Chugai Pharmaceutical, Bristol‐Myers Squibb), personal fees (Eisai, Kyowa Kirin, Zenyaku Kogyo Company, Synmosa Biopharma, Nippon Sinyaku), grant (Merck, Amgen Astellas BioPharma, Astellas Pharma, Sanofi, Novartis Pharma, Otsuka Pharmaceutical). KI: four honoraria and research funding (Eisai). TN, SS, SH: employees of Eisai Co., Ltd. KA: research funding (Eisai). The other authors have no conflict of interest.
ACKNOWLEDGMENTS
We thank all participating patients and their families, as well as investigators, physicians, nurses, and clinical research coordinators who helped in this study. We would also like to thank Dr Hirokazu Nagai (Nagoya Medical Center) as the independent safety adviser and Dr Akira Tomonari (Eisai Co., Ltd.) as the medical adviser of the sponsor. We also acknowledge Dr Kenzo Muramoto and Dr Michiko Sugawara (Eisai Co., Ltd.) for their help in preparing this manuscript. This study was funded and supported by Eisai Co., Ltd.
Munakata W, Shirasugi Y, Tobinai K, et al. Phase 1 study of tazemetostat in Japanese patients with relapsed or refractory B‐cell lymphoma. Cancer Sci. 2021;112:1123–1131. 10.1111/cas.14822
REFERENCES
- 1. Gonzalez‐Perez A, Jene‐Sanz A, Lopez‐Bigas N. The mutational landscape of chromatin regulatory factors across 4,623 tumor samples. Genome Biol. 2013;14:r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen T, Dent SY. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat Rev Genet. 2014;15:93‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Comet I, Riising EM, Leblanc B, et al. Maintaining cell identity: PRC2‐mediated regulation of transcription and cancer. Nat Rev Cancer. 2016;16:803‐810. [DOI] [PubMed] [Google Scholar]
- 4. Lunning MA, Green MR. Mutation of chromatin modifiers; an emerging hallmark of germinal center B‐cell lymphomas. Blood Cancer J. 2015;5:e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lohr JG, Stojanov P, Lawrence MS, et al. Discovery and prioritization of somatic mutations in diffuse large B‐cell lymphoma (DLBCL) by whole‐exome sequencing. Proc Natl Acad Sci USA. 2012;109:3879‐3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morin RD, Mendez‐Lago M, Mungall AJ, et al. Frequent mutation of histone‐modifying genes in non‐Hodgkin lymphoma. Nature. 2011;476:298‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Velichutina I, Shaknovich R, Geng H, et al. EZH2‐mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood. 2010;116:5247‐5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chase A, Cross NC. Aberrations of EZH2 in cancer. Clin Cancer Res. 2011;17:2613‐2618. [DOI] [PubMed] [Google Scholar]
- 9. Mahmoudi T, Verrijzer CP. Chromatin silencing and activation by Polycomb and trithorax group proteins. Oncogene. 2001;20:3055‐3066. [DOI] [PubMed] [Google Scholar]
- 10. Cao R, Wang L, Wang H, et al. Role of histone H3 lysine 27 methylation in polycomb‐group silencing. Science. 2002;298:1039‐1043. [DOI] [PubMed] [Google Scholar]
- 11. Müller J, Hart CM, Francis NJ, et al. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell. 2002;111:197‐208. [DOI] [PubMed] [Google Scholar]
- 12. Czermin B, Melfi R, McCabe D, et al. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111:185‐196. [DOI] [PubMed] [Google Scholar]
- 13. Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Italiano A. Role of the EZH2 histone methyltransferase as a therapeutic target in cancer. Pharmacol Ther. 2016;165:26‐31. [DOI] [PubMed] [Google Scholar]
- 15. Wilson BG, Wang XI, Shen X, et al. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell. 2010;18:316‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mora‐Blanco EL, Mishina Y, Tillman EJ, et al. Activation of β‐catenin/TCF targets following loss of tumor suppressor SNF5. Oncogene. 2014;33:933‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jagani Z, Mora‐Blanco EL, Sansam CG, et al. Loss of the tumor suppressor Snf5 leads to aberrant activation of the Hedgehog‐Gli pathway. Nat Med. 2010;16:1429‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bödör C, Grossmann V, Popov N, et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood. 2013;122:3165‐3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morin RD, Johnson NA, Severson TM, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B‐cell lymphomas of germinal‐center origin. Nat Genet. 2010;42:181‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hatzi K, Melnick A. Breaking bad in the germinal center: how deregulation of BCL6 contributes to lymphomagenesis. Trends Mol Med. 2014;20:343‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klein U, Dalla‐Favera R. Germinal centres: role in B‐cell physiology and malignancy. Nat Rev Immunol. 2008;8:22‐33. [DOI] [PubMed] [Google Scholar]
- 22. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B‐cell lymphoma identified by gene expression profiling. Nature. 2000;403:503‐511. [DOI] [PubMed] [Google Scholar]
- 23. Su I‐H, Basavaraj A, Krutchinsky AN, et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4:124‐131. [DOI] [PubMed] [Google Scholar]
- 24. Raaphorst FM, van Kemenade FJ, Fieret E, et al. Cutting edge: polycomb gene expression patterns reflect distinct B cell differentiation stages in human germinal centers. J Immunol. 2000;164:1‐4. [DOI] [PubMed] [Google Scholar]
- 25. Béguelin W, Popovic R, Teater M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23:677‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Italiano A, Soria J‐C, Toulmonde M, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B‐cell non‐Hodgkin lymphoma and advanced solid tumours: a first‐in‐human, open‐label, phase 1 study. Lancet Oncol. 2018;19:649‐659. [DOI] [PubMed] [Google Scholar]
- 27. Gounder M, Schöffski P, Jones RL, et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open‐label, phase 2 basket study. Lancet Oncol. 2020;21:1423‐1432. [DOI] [PubMed] [Google Scholar]
- 28. Morschhauser F, Tilly H, Chaidos A, et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open‐label, single‐arm, multicentre, phase 2 trial. Lancet Oncol. 2020;21:1433‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B‐cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275‐282. [DOI] [PubMed] [Google Scholar]
- 31. Knutson SK, Kawano S, Minoshima Y, et al. Selective inhibition of EZH2 by EPZ‐6438 leads to potent antitumor activity in EZH2‐mutant non‐Hodgkin lymphoma. Mol Cancer Ther. 2014;13:842‐854. [DOI] [PubMed] [Google Scholar]
- 32. Maeshima AM, Omatsu M, Nomoto J, et al. Diffuse large B‐cell lymphoma after transformation from low‐grade follicular lymphoma: morphological, immunohistochemical, and FISH analyses. Cancer Sci. 2008;99:1760‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Souroullas GP, Jeck WR, Parker JS, et al. An oncogenic Ezh2 mutation induces tumors through global redistribution of histone 3 lysine 27 trimethylation. Nat Med. 2006;22:632‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ennishi D, Takata K, Béguelin W, et al. Molecular and genetic characterization of MHC deficiency identifies EZH2 as therapeutic target for enhancing immune recognition. Cancer Discov. 2019;9:546‐563. [DOI] [PubMed] [Google Scholar]
- 35. Otsuka Y, Nishikori M, Arima H, et al. EZH2 inhibitors restore epigenetically silenced CD58 expression in B‐cell lymphomas. Mol Immunol. 2020;119:35‐45. [DOI] [PubMed] [Google Scholar]


