Abstract
Microsatellite instability‐high (MSI‐H) is an important biomarker for predicting the effect of immune checkpoint inhibitors (ICIs) on advanced solid tumors. Microsatellite instability‐high is detected in various cancers, but its frequency varies by cancer type and stage. Therefore, precise frequency is required to plan ICI therapy. In this study, the results of MSI tests actually carried out in clinical practice were investigated. In total, 26 469 samples of various cancers were examined between December 2018 and November 2019 to determine whether programmed cell death‐1 blockade was indicated. The results of MSI tests were obtained for 26 237 (99.1%) of these samples. The male : female ratio was 51:49 and mean age was 64.3 years. In all samples, the overall frequency of MSI‐H was 3.72%. By gender, the frequency of MSI‐H was higher in female patients (4.75%) than in male patients (2.62%; P < .001). A comparison by age revealed that the frequency of MSI‐H was significantly higher in patients younger than 40 years of age (6.12%) and 80 years or older (5.77%) than in patients aged between 60 and 79 years (3.09%; P < .001). Microsatellite instability‐high was detected in 30 cancer types. Common cancer types were: endometrial cancer, 16.85%; small intestinal cancer, 8.63%; gastric cancer, 6.74%; duodenal cancer, 5.60%; and colorectal cancer, 3.78%. Microsatellite instability‐high was detected in cancer derived from a wide variety of organs. The frequency of MSI‐H varied by cancer type and onset age. These data should prove especially useful when considering ICI treatment.
Keywords: advanced solid tumor, immune checkpoint inhibitor, microsatellite instability, mismatch repair, PD‐1 blockade
In this study, we presented real‐world data on microsatellite instability status in various unresectable or metastatic solid tumors to determine if pembrolizumab treatment was indicated.
Abbreviations
- dMMR
deficiency in mismatch repair function
- ICI
immune checkpoint inhibitor
- MSI
microsatellite instability
- MSI‐H
microsatellite instability‐high
- NGS
next‐generation sequencing
- PD‐1
programmed cell death‐1
- PD‐L1
programmed cell death‐ligand 1
- QMVR
quasimonomorphic variation range
1. INTRODUCTION
Cancer drug therapy with ICIs has made remarkable progress but is not effective for all cancers. Therefore, it is necessary to narrow down those patients expected to respond to ICIs. Currently, biomarkers to predict therapeutic response, such as PD‐L1 expression, 1 MSI‐H, or loss of mismatch repair protein expression 2 , 3 , 4 and tumor mutation burden‐high 5 are used. Microsatellite instability‐high results from dMMR. Furthermore, dMMR causes hypermutation and results in the production of many neoantigens in tumor cells; thus, treatment with anti‐PD‐1/‐L1 Abs appears to be an effective option. That is, dMMR tumor is one of the real targets for ICI treatment.
To identify dMMR tumors, simple tests such as MSI testing 6 , 7 and immunohistochemistry testing for MMR proteins 8 , 9 are frequently applied. Microsatellite instability testing is a method to assess changes in microsatellites, which are simple repetitive DNA sequences in genomes, using DNA from both normal and tumor tissue. In recent years, however, an MSI testing method that allows assessment only with tumor tissue by utilizing specific microsatellite markers (BAT25, BAT26, NR21, NR24, and MONO27), which have almost no individual differences, has been developed. 7 In Japan, this method was approved in December 2018 as a companion diagnostic technique to determine whether pembrolizumab is indicated for unresectable or metastatic solid tumors. Consequently, this test has been widely adopted in Japan.
Several recent large‐scale investigations predicted the MSI‐H frequency in each tumor type. However, as these results are based on unique MSI assessment methods (pipeline and algorithm) using NGS, 11 , 12 , 13 , 14 , 15 it is unclear whether they are equivalent to conventional MSI testing. In addition, the cohorts examined in those studies were different from the actual target patients for whom MSI testing is used to determine ICI therapy.
To plan ICI therapy for unresectable or metastatic solid tumors, the precise frequency of MSI‐H in each tumor type needs to be determined.
In this study, the results of MSI testing undertaken as a companion diagnostic technique was used to elucidate the frequency of MSI‐H in each cancer type. These results were obtained from the actual target patients and actually used in clinical practice; that is, these are real‐world data on MSI status in each cancer type.
2. MATERIALS AND METHODS
2.1. Samples
The samples had been submitted by nationwide medical institutes to SRL Inc. (CLIA‐certificated CAP‐accredited central laboratory) for MSI testing as a companion diagnostic technique to determine whether pembrolizumab was indicated for unresectable or metastatic solid tumors. Analyses were undertaken between December 2018 and November 2019. All results were anonymized by SRL, and the aggregated results of MSI were analyzed in this study. The use of anonymized results was approved by the ethics review board of SRL (No. 20‐69).
2.2. DNA extraction
Thin slices of formalin‐fixed paraffin‐embedded tumor tissue specimens were used for the test after confirmation by pathologists. 10 DNA was isolated using QIAsymphony DNA Mini kit (Qiagen) according to the manufacturer’s recommendations.
2.3. Microsatellite instability test
As previously reported, MSI testing with an assessment method using the QMVR was generally carried out. 7 The MSI status was classified as MSI‐H with the presence of two or more unstable markers, as MSI‐low with only one unstable marker, and as microsatellite stable with no unstable marker. 6
2.4. Statistical analyses
Patient characteristics were compared using t tests for continuous variables and χ2 tests or Fisher’s exact tests by SPSS for categorical variables.
3. RESULTS
3.1. Cohort of this study
Of the 26 469 samples, 232 samples were excluded due to poor or insufficient sample conditions. Microsatellite instability testing results were obtained from the remaining 26 237 samples and used for this study (Figure 1). The testing success rate was as high as 99.1%. Among cancer types tested, colorectal cancer was the most common, found in 10 226 samples and accounting for 39% of all samples, followed by pancreatic cancer in 2775 samples (10.6%), gastric cancer in 1929 samples (7.4%), and endometrial cancer in 1389 samples (5.3%) (Figure 2, Table S1).
FIGURE 1.
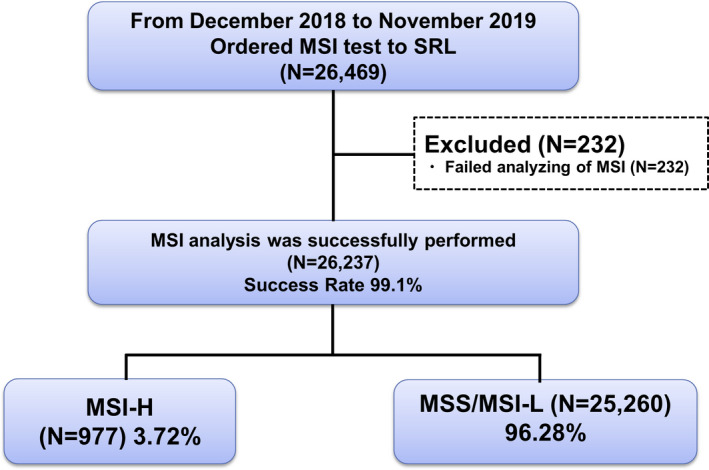
CONSORT flow diagram of this study. Of the 26 469 samples, 232 were excluded due to poor or insufficient sample conditions. Microsatellite instability (MSI) testing results were obtained from the remaining 26 237 samples. Success rate of tumor‐agnostic MSI testing was 99.1% and 3.72% of all tumor showed MSI‐high (MSI‐H) status. MSI‐L, microsatellite instability‐low; MSS, microsatellite stable.
FIGURE 2.
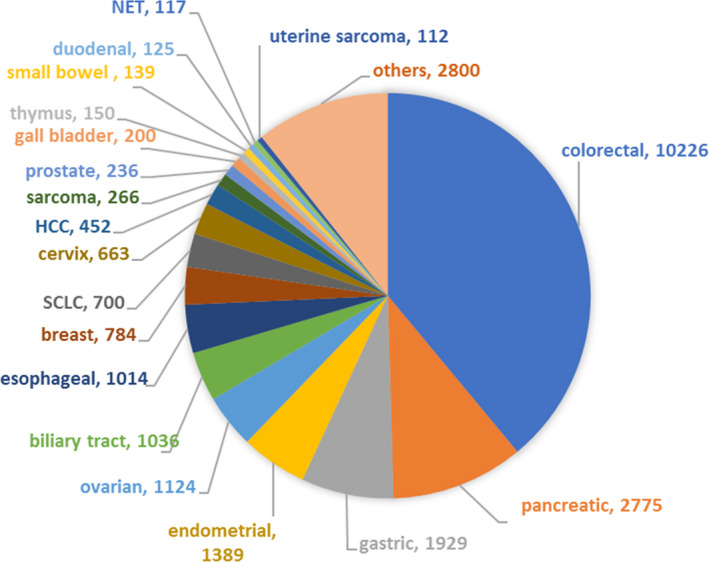
Number and proportion for each tumor sample from patients with unresectable or metastatic solid tumors examined with the microsatellite instability test. HCC, hepatocellular carcinoma; NET, neuroendocrine tumor; SCLC, small‐cell lung carcinoma
3.2. Patient characteristics and MSI‐H frequency
The ratio of male to female patients who could be analyzed was 51:49. The mean age was 64.3 years. The frequency of MSI‐H was higher in female patients (4.7%) than in male patients (2.6%, P < .001). The frequency of MSI‐H in all the samples was 3.72% (Table 1).
TABLE 1.
Characteristics of patients with unresectable or metastatic solid tumors and frequency of microsatellite instability‐high (MSI‐H) tumors
|
All cases N = 26 237 |
MSI‐L/MSS N = 25 260 |
MSI‐H N = 977 |
Frequency of MSI‐H 3.72* |
|
|---|---|---|---|---|
| Gender | ||||
| Male | 12 803 | 12 468 | 335 | 2.62 |
| Female | 12 277 | 11 694 | 583 | 4.75 |
| Unknown | 1157 | 1098 | 59 | 5.1 |
| Mean age (y) | ||||
| All | 64.3 (±12.0) | 64.3 (±11.99) | 63.4 (±13.98) | NA |
| range, 2‐96 | range, 2‐96 | range, 13‐93 | ||
| Male | 66.0 (±11.26) | 66.1 (±11.11) | 63.5 (±15.42) | NA |
| range, 5‐96 | range, 5‐96 | range, 13‐91 | ||
| Female | 62.5 (±12.61) | 62.4 (±12.59) | 63.2 (±13.06) | NA |
| range, 2‐96 | range, 2‐96 | range, 18‐93 | ||
| Stage | ||||
| I‐III | 5464 | 5137 | 327 | 5.98 |
| IV | 17 970 | 17 427 | 543 | 3.02 |
| Unknown | 2803 | 2696 | 107 | 3.82 |
MSI‐L, microsatellite instability‐low; MSS, microsatellite stable; NA, not applicable.
*The frequency of MSI‐H in all the samples.
By age, the frequency of MSI‐H was significantly higher in patients aged less than 40 years (6.1%) and 80 years or older (5.8%) than in patients aged 50‐59 years (3.8%, P = .002, P = .001), 60‐69 years (3.1%, P < .0001, P < .0001), and 70‐79 years (3.1%, P < .0001, P < .0001) (Table 2, Figure 3).
TABLE 2.
Age of disease onset and microsatellite stability status in patients with unresectable or metastatic solid tumors
| Age (y) |
All cases N = 23 379 |
MSI‐L/MSS N = 22 530 |
MSI‐H N = 849 |
Frequency of MSI‐H 3.72* |
|---|---|---|---|---|
| <10 (n = 13) | 13 | 13 | 0 | 0 |
| 10s (n = 44) | 44 | 41 | 3 | 6.82 |
| 20s (n = 131) | 131 | 121 | 10 | 7.63 |
| 30s (n = 695) | 695 | 654 | 41 | 5.90 |
| 40s (n = 2098) | 2098 | 2004 | 94 | 4.48 |
| 50s (n = 4038) | 4038 | 3885 | 153 | 3.79 |
| 60s (n = 7374) | 7374 | 7146 | 228 | 3.09 |
| 70s (n = 7407) | 7408 | 7179 | 229 | 3.09 |
| 80s (n = 1526) | 1526 | 1442 | 84 | 5.51 |
| 90s (n = 52) | 52 | 45 | 7 | 13.46 |
MSI‐H, microsatellite instability‐high; MSI‐L, microsatellite instability‐low; MSS, microsatellite stable.
*The frequency of MSI‐H in all the samples.
FIGURE 3.
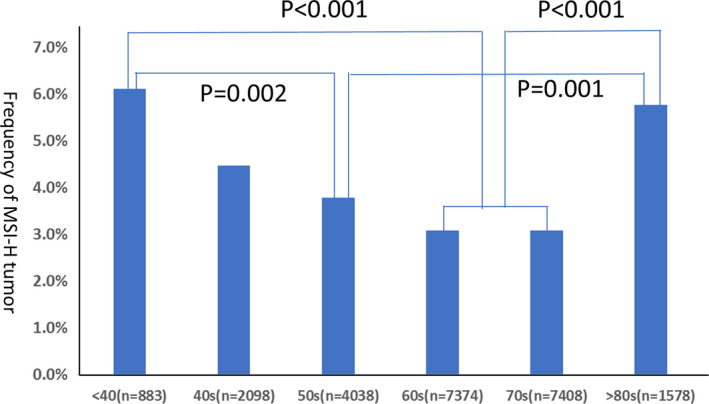
Frequency of microsatellite instability‐high (MSI‐H) tumor in each generation of patients with unresectable or metastatic solid tumors. The frequency of MSI‐H was significantly higher in patients aged <40 y (6%) and 80 y or older (5.77%) compared with patients aged between 60 and 79 y (3.09%; P < .001)
3.3. Frequency of MSI‐H in each cancer type
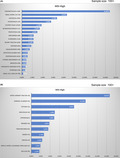
In this analysis, MSI‐H was detected in 30 cancer types (Table S1). Among cancer types for which the number of analyzable samples was 100 or more, MSI‐H was frequently detected in the following: endometrial cancer, 16.9%; small intestinal cancer, 8.6%; gastric cancer, 6.7%; duodenal cancer, 5.6%; and colorectal cancer, 3. 8% (Figure 4A, Table 3). Among cancer types for which the number of analyzable samples was <100, MSI‐H was frequently detected in the following: upper urinary tract cancer, 16.7% (3/18); adrenal cancer, 11.5% (3/26); and testicular cancer, 9.1% (2/22; Figure 4B, Table 4).
FIGURE 4.
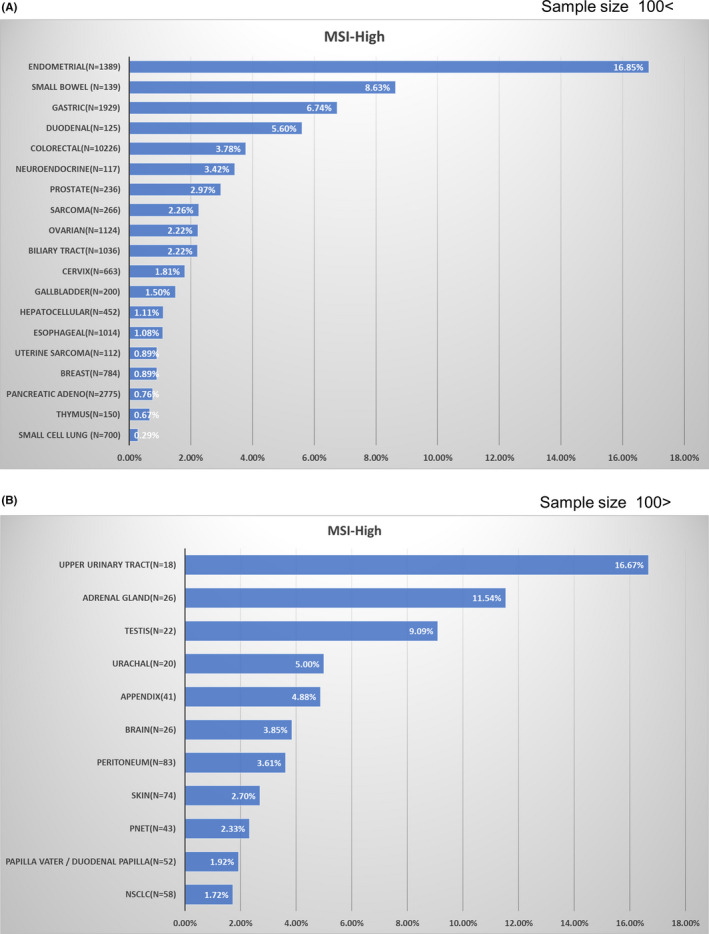
Frequency of microsatellite instability‐high (MSI‐H) in each tumor type among patients with unresectable or metastatic solid tumors. A, Tumors for which the number of analyzable samples was 100 or more. B, Tumors for which the number of analyzable samples was less than 100. adeno, adenocarcinoma; NSCLC, non‐small‐cell lung carcinoma; PNET, pancreatic neuroendocrine tumor
TABLE 3.
Microsatellite stability status in each tumor type classified by anatomical site or histology
| Tumor type |
All cases N = 26 237 |
MSI‐L/MSS N = 25 260 |
MSI‐H N = 977 |
Frequency of MSI‐H 3.72* |
|---|---|---|---|---|
| Endometrial | 1389 | 1155 | 234 | 16.85 |
| Small bowel | 139 | 127 | 12 | 8.63 |
| Gastric | 1929 | 1799 | 130 | 6.74 |
| Duodenal | 125 | 118 | 7 | 5.60 |
| Colorectal | 10 226 | 9839 | 387 | 3.78 |
| Neuroendocrine carcinoma a | 117 | 113 | 4 | 3.42 |
| Prostate | 236 | 229 | 7 | 2.97 |
| Biliary tract | 1036 | 1013 | 23 | 2.22 |
| Ovarian | 1124 | 1099 | 25 | 2.22 |
| Sarcoma | 266 | 260 | 6 | 2.26 |
| Cervix | 663 | 651 | 12 | 1.81 |
| Gallbladder | 200 | 197 | 3 | 1.50 |
| Hepatocellular | 452 | 447 | 5 | 1.11 |
| Esophageal | 1014 | 1003 | 11 | 1.08 |
| Breast | 784 | 777 | 7 | 0.89 |
| Uterine sarcoma | 112 | 111 | 1 | 0.89 |
| Pancreatic adenocarcinoma | 2775 | 2754 | 21 | 0.76 |
| Thymus | 150 | 149 | 1 | 0.67 |
| Small cell lung | 700 | 698 | 2 | 0.29 |
MSI‐H, microsatellite instability‐high; MSI‐L, microsatellite instability‐low; MSS, microsatellite stable.
Excluding pancreatic neuroendocrine tumor.
*The frequency of MSI‐H in all the samples.
TABLE 4.
Microsatellite stability status in each tumor type classified by anatomical site or histology
| Tumor type |
All cases N = 26 237 |
MSI‐L/MSS N = 25 260 |
MSI‐H N = 977 |
Frequency of MSI‐H 3.72* |
|---|---|---|---|---|
| Upper urinary tract | 18 | 15 | 3 | 16.67 |
| Adrenal gland | 26 | 23 | 3 | 11.54 |
| Testis | 22 | 20 | 2 | 9.09 |
| Urachus | 20 | 19 | 1 | 5.00 |
| Appendix | 41 | 39 | 2 | 4.88 |
| Brain | 26 | 25 | 1 | 3.85 |
| Peritoneum | 83 | 80 | 3 | 3.61 |
| Skin | 74 | 72 | 2 | 2.70 |
| Pancreatic neuroendocrine tumor | 43 | 42 | 1 | 2.33 |
| Papilla vater/duodenal papilla | 52 | 51 | 1 | 1.92 |
| Non‐small‐cell lung cancer | 58 | 57 | 1 | 1.72 |
MSI‐H, microsatellite instability‐high; MSI‐L, microsatellite instability‐low; MSS, microsatellite stable.
*The frequency of MSI‐H in all the samples.
4. DISCUSSION
In this study, we presented real‐world data on MSI status based on data from MSI testing undertaken on patients with unresectable or metastatic solid tumors to determine whether pembrolizumab was indicated. To the best of our knowledge, this was the first time this has been done using the largest MSI dataset in Asia. The data and results obtained from this nationwide large‐scale investigation should prove very useful when ICI treatment is being considered.
In our study, the frequency of MSI‐H in all the cases was 3.72%. In previous studies, Latham et al 11 reported that MSI‐H was detected in 2.1% of all samples based on NGS analysis data from 15 045 samples of various cancer types. Le et al 12 carried out an NGS analysis using 12 019 samples consisting of 32 cancer subtypes and reported that the frequency of MMR‐deficient cancer in stage IV patients was 4%. In these cohorts, cancers at different stages were included, and they did not confine themselves to collecting only those patients who might be candidates for treatment with ICIs. The frequency of MSI‐H varies by cancer type 11 , 12 , 13 , 14 , 15 and stage 12 , 16 ; thus, in actual clinical practice, when considering whether ICIs are indicated, the frequency by tumor type is more useful than the frequency for all samples. In our study, MSI‐H was most frequently detected in endometrial cancer (16.9% of all cases) followed by, in descending order of frequency, cancer of the small intestine (8.6%), gastric cancer (6.7%), duodenal cancer (5.6%) and colorectal cancer (3.8%). This order is generally consistent with the results reported by Le et al 12 and Trabucco et al. 15
Among cancer types for which the number of analyzable samples was less than 100, and for which the frequency could not be determined with a high degree of accuracy, MSI‐H was frequently detected in the following cancer types: upper urinary tract cancer, 16.7% (3/18); adrenal cancer, 11.5% (3/26); and testicular cancer, 9.1% (2/22).
In this study, MSI‐H was detected in 30 of 43 cancer types. For the 13 cancer subtypes in which MSI‐H was not detected, the number of cases tested was as few as 97 or less. If the number of cases could be increased, it might be possible to identify MSI‐H cases for these cancer types as well. More cases are needed to obtain an accurate estimation of frequency for these cancer types.
In addition, the classification of cancer type could also affect the frequency of MSI‐H. For example, MSI‐H was not detected among 97 thyroid carcinoma cases in this study, whereas Le reported that 2% of thyroid carcinoma showed MSI‐H. 12 A recent study reported that MSI‐H was observed in 2.5% of follicular thyroid carcinoma, but was either entirely absent or rare in other histology subtypes of thyroid carcinoma. 17 Thus, the classification of cancer type might also affect the frequency of MSI‐H.
The frequency of MSI‐H by gender was approximately 1.8‐fold higher for female patients (4.75%) than male patients (2.62%). This could be attributable to the effect of the high MSI‐H frequency in endometrial cancer, unique to female patients. Actually, when frequency was calculated excluding endometrial cancer, it was 3.2%, similar to the frequency seen in male patients.
The frequency of MSI‐H by age was higher in the young and elderly patients than in other age groups. In younger patients, MSI‐H was likely attributable to a genetic background that makes these patients susceptible to the development of cancer. It was assumed that Lynch syndrome 18 , 19 and constitutional mismatch repair deficiency syndrome 20 , 21 were included among these predisposing conditions.
The reason why the frequency was high in more elderly patients might be because their MSI‐H status occurs through MLH1 promoter methylation. 22 In fact, MSI‐H was frequently detected in elderly female patients with colorectal cancer 23 and endometrial cancer. 24
The MSI test kit (FALCO) used in this study is an MSI assessment method using the QMVR and only requires tumor tissue. 7 This testing method was developed based mainly on the use of colorectal cancer. Consequently, sufficient data had not been obtained from cancer types other than colorectal cancer. In this study, however, MSI testing with various solid tumors was successfully carried out and the success rate for this method was as high as 99.1%. In addition, MSI‐H was detected in various cancer types. This indicated that the five microsatellite markers (BAT25, BAT26, NR21, NR24, and MONO27) showed their utility in a tumor‐agnostic manner.
Although MSI testing is used for the purpose of cancer treatment, it is anticipated that patients with Lynch syndrome will be found among MSI‐H patients at a certain level of frequency 11 ; a medical management system that includes genetic counseling, genetic diagnosis, and surveillance in patients and their blood relatives needs to be organized.
In this study, we presented real‐world data using the results of MSI testing undertaken for determining whether pembrolizumab treatment was indicated for patients with unresectable or metastatic solid tumors. The data and results were obtained from nationwide large‐scale investigations. Such real‐world data on MSI status in unresectable or metastatic solid tumors have not been reported previously and should therefore prove highly useful when devising strategies for cancer treatment with ICIs.
DISCLOSURE
K. Akagi reports research funding received from Ono and Falco Biosystems and lecture fees received from MSD. E. Oki reports lecture fees received from Bayer, Chugai, Eli Lilly, Merck, Ono, Taiho, Takeda, and Yakult Honsha. H. Taniguchi reports honoraria received from Bayer, Bristol‐Myers Squibb, Chugai, Daiichi Sankyo, Eli Lilly, MBL, Merck Serono, Mitsubishi Tanabe Pharma, MSD, Nippon Kayaku, Novartis, Sanofi, Taiho, Takeda, and Yakult Honsha and research funding received from Array Bio Pharma, Daiichi Sankyo, Dainippon Sumitomo Pharma, MSD, Novartis, Ono, Sysmex, and Takeda. D. Aoki reports research funding received from Chugai and Takada and honoraria received from Astra Zeneka, Chugai, and MSD. T. Kuwata reports research funding from Ono and Daiichi Sankyo and honoraria from MSD, Astra Zeneca, and Taiho. T. Yoshino reports research funding received from Chugai, Daiichi Sankyo, Dainippon Sumitomo Pharma, GSK, MSD, Novartis, Ono, Parexel, and Sanofi. The other authors have no conflicts of interest.
Supporting information
Table S1
ACKNOWLEDGMENT
We thank SRL Inc. for providing anonymized MSI data. This research was supported by the Japan Agency for Medical Research and Development (AMED) under grant JP18kk0205004, JSPS KAKENHI Grant Number JP18K07339, and National Cancer Center Research and Development Fund Grant Number 31‐A‐2.
Akagi K, Oki E, Taniguchi H, et al. Real‐world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci. 2021;112:1105–1113. 10.1111/cas.14798
Funding information
Japan Agency for Medical Research and Development (AMED), Grant/Award Number: JP18kk0205004; JSPS KAKENHI, Grant/Award Number: JP18K07339; National Cancer Center Research and Development Fund, Grant/Award Number: 31‐A‐2.
Correction added on 16 Jan 2021, after initial online publication. A duplicate of this article was published under the DOI 10.1111/cas.14804. This duplicate has now been deleted and its DOI redirected to this version of the article.
REFERENCES
- 1. Patel SP, Kurzrock R. PD‐L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847‐856. [DOI] [PubMed] [Google Scholar]
- 2. Le DT, Uram JN, Wang H, et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med. 2015;372:2509‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD‐1 blockade. Clin Cancer Res. 2016;22:813‐820. [DOI] [PubMed] [Google Scholar]
- 4. Mishima S, Taniguchi H, Akagi K, et al. Japan Society of Clinical Oncology provisional clinical opinion for the diagnosis and use of immunotherapy in patients with deficient DNA mismatch repair tumors, cooperated by Japanese Society of Medical Oncology, First Edition. Int J Clin Oncol. 2020;25:217‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD‐1 inhibition. N Engl J Med. 2017;377:2500‐2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boland CR, Thibodeau SN, Stanley R, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Can Res. 1998;58:5248‐5257. [PubMed] [Google Scholar]
- 7. Bando H, Okamoto W, Fukui T, Yamanaka T, Akagi K, Yoshino T. Utility of the quasi‐monomorphic variation range in unresectable metastatic colorectal cancer patients. Cancer Sci. 2018;109:3411‐3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10:293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stelloo E, Jansen AML, Osse EM, et al. Practical guidance for mismatch repair‐deficiency testing in endometrial cancer. Ann Oncol. 2017;28:96‐102. [DOI] [PubMed] [Google Scholar]
- 10. Fujii S, Yoshino T, Yamazaki K, et al. Histopathological factors affecting the extraction of high quality genomic DNA from tissue sections for next‐generation sequencing. Biomed Rep. 2019;11:171‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of lynch syndrome pan‐cancer. J Clin Oncol. 2019;37:286‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science. 2017;357:409‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujimoto A, Fujita M, Hasegawa T, et al. Comprehensive analysis of indels in whole‐genome microsatellite regions and microsatellite instability across 21 cancer types. Genome Res. 2020;30:315‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22:1342‐1350. [DOI] [PubMed] [Google Scholar]
- 15. Trabucco SE, Gowen K, Maund SL, et al. A novel next‐generation sequencing approach to detecting microsatellite instability and pan‐tumor characterization of 1000 microsatellite instability‐high cases in 67,000 patient samples. J Mol Diagn. 2019;21:1053‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujiyoshi K, Yamamoto G, Takenoya T, et al. Metastatic pattern of stage IV colorectal cancer with high‐frequency microsatellite instability as a prognostic factor. Anticancer Res. 2017;37:239‐247. [DOI] [PubMed] [Google Scholar]
- 17. Genutis LK, Tomsic J, Bundschuh RA, et al. Microsatellite instability occurs in a subset of follicular thyroid cancers. Thyroid. 2019;29:523‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Biller LH, Syngal S, Yurgelun MB. Recent advances in Lynch syndrome. Fam Cancer. 2019;18:211‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tabori U, Hansford JR, Achatz MI, et al. Clinical Management and Tumor Surveillance Recommendations of Inherited Mismatch Repair Deficiency in Childhood. Clin Cancer Res. 2017;23:e32‐e37. [DOI] [PubMed] [Google Scholar]
- 21. Abedalthagafi M. Constitutional mismatch repair‐deficiency: current problems and emerging therapeutic strategies. Oncotarget. 2018;9:35458‐35469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLHJ Promoter Correlates with Lack of Expression of hMLH1 in Sporadic Colon Tumors and Mismatch Repair‐defective Human Tumor Cell Lines. Can Res. 1997;57:808‐881. [PubMed] [Google Scholar]
- 23. Fujiyoshi K, Yamaguchi T, Kakuta M, et al. Predictive model for high‐frequency microsatellite instability in colorectal cancer patients over 50 years of age. Cancer Med. 2017;6:1255‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pasanen A, Loukovaara M, Bützow R. Clinicopathological significance of deficient DNA mismatch repair and MLH1 promoter methylation in endometrioid endometrial carcinoma. Mod Pathol. 2020;33:1443‐1452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


