Abstract
Anticancer immunotherapies have revolutionized cancer management, yet the effect of systemic anti‐programmed cell death protein 1 (PD‐1) treatment is predominantly studied in tumor‐infiltrating lymphocytes (TILs). Its impact on PD‐1 expressing cells in tumor‐draining lymph nodes (TDLNs) is not well understood and yet to be explored. Thus, further research aiming for better understanding of the PD‐1 pathway not only in tumor tissue but also in TDLNs is warranted. In this study, we investigated the expression of PD‐1, CD69, and HLA‐DR on CD4+ and CD8+ T cells by flow cytometry analysis of peripheral blood mononuclear cells (PBMCs), TDLNs, and tumor samples from patients with oral squamous cell carcinoma (OSCC). Our data showed that both helper and cytotoxic T lymphocytes in OSCC tissue were highly activated and expressed high level of PD‐1 (over 70% positivity). Lymphocytes in TDLNs and peripheral blood expressed significantly lower levels of PD‐1 and other activation markers compared to TILs. Moreover, we demonstrated that a significant fraction of PD‐1 negative TILs expressed high levels of human leukocyte antigen – DR isotype and CD69. In contrast, PD‐1 negative cells in TDLNs and PBMCs scarcely expressed the aforementioned activation markers. Furthermore, we proved that patients with a high percentage of CD3+ PD‐1+ cells in tumor‐draining lymph nodes had significantly lower disease‐free and overall survival rates (log‐rank test P = .0272 and P = .0276, respectively). Taken together, we proved that flow cytometry of lymph nodes in OSCC is feasible and may be used to investigate whether PD‐1 levels in TDLNs correspond with survival and potentially with response to anti‐PD‐1 therapy. Such knowledge may ultimately help guide anti‐PD‐1 treatment.
Keywords: head and neck cancer, metastatic head and neck squamous cell carcinoma, PD‐1, sentinel node, tumor‐draining lymph nodes
Short abstract
Flow cytometry is a powerful tool that can be used in head and neck cancers to investigate immunological milieu of tumor‐draining lymph nodes. In this project, we provide a characterization of T cells within head and neck cancer tumors, tumor‐draining lymph nodes, and blood.
Abbreviations
- APC
antigen‐presenting cell
- HLN
neck lymph node derived from noncancer patient
- LN
nonsentinel lymph node derived from cancer patient
- Met LN
lymph node with detected metastasis in pathological examination
- OSCC
oral squamous cell carcinoma
- PBMCs
peripheral blood mononuclear cells
- SN
sentinel node derived from cancer patient; the first lymph node(s) to which cancer cells are likely to spread from a primary tumor
- TDLNs
tumor‐draining lymph nodes
- TILs
tumor‐infiltrating lymphocytes
1. INTRODUCTION
Over the last decade, anticancer immunotherapies have revolutionized cancer management and improved prognosis for patients suffering from a wide range of advanced solid and hematopoietic malignancies. 1 In particular, treatment with programmed cell death protein 1 (PD‐1) pathway inhibitors has emerged as the most efficacious among available cancer immunotherapy agents. Nonetheless, even though some patients show spectacular and long‐lasting remission in response to anti‐PD‐1 treatment, there is still a significant proportion of patients who respond poorly or not at all to immune checkpoint blockade. Furthermore, the effect of systemic anti‐PD‐1 treatment is predominantly studied in TILs, 2 , 3 but its impact on PD‐1 expressing cells in TDLN, an important immunological site, has not yet been explored. Thus, further research aiming for better understanding of the PD‐1 pathway not only in tumor, but also in TDLN is warranted.
In 2016, nivolumab became the first anti‐PD‐1 agent approved for treatment of platinum‐refractory recurrent/metastatic head and neck squamous cell carcinoma (HNSCC). 4 Since then, several other agents, such as pembrolizumab, atezolizumab, durvalumab, and avelumab, have been approved for the treatment of advanced HNSCC. 5 , 6 , 7 , 8 Despite significant advances in the development of new immunotherapies and the introduction of combination schemes with conventional chemo‐ and radiotherapy, the response rate of HNSCC to anti‐PD‐1 agents is still far below that of malignant melanoma. An additional obstacle in improving the efficacy of immunotherapy in HNSCC is the absence of reliable biomarkers, which could help to identify likely responders. Unfortunately, programmed death‐ligand 1 (PD‐L1) immunohistochemistry alone is not sufficient to predict responders in HNSCC and there is a definite need to identify new, more reliable biomarkers for improved patient selection and reduction of therapy costs. 9 , 10 , 11
Recent research has focused on finding predictive biomarkers in the peripheral blood or tumor microenvironment. However, one cannot forget the importance of TDLN as the source of tumor‐specific CD4+ and CD8+ cells and the site of important anticancer immunological events such as antigen presentation, immune cell activation, priming, proliferation, and differentiation. Subsequently, these highly specialized and matured cells egress from lymph nodes and migrate into cancer tissue where they act. Therefore, any disturbances in lymph node immunity are expected to have striking consequences for the whole immune system, including its response to cancer. Thus, we expect that TDLNs contain predictive information enabling identification of immunotherapy responders, and that knowing the PD‐1 expression pattern in TDLNs in HNSCC will guide further research and development of novel immunotherapies.
Considering the immunological importance of TDLNs, we investigated the expression of PD‐1 and two other activation markers—CD69 and human leukocyte antigen – DR isotype (HLA‐DR)—on CD4+ and CD8+ T cells by flow cytometry analysis. We present PD‐1 expression and activation signatures of T cells in TDLNs, lymph nodes with metastasis, nonsentinel lymph nodes from cancer patients, lymph nodes from noncancer patients, PBMCs, and OSCC tumor samples. To extend these findings, by means of a high‐dimensional analysis, we also describe heterogeneous subsets of CD4+ and CD8+ T cells within the studied compartments.
2. MATERIALS AND METHODS
2.1. Patient characteristics
Eligible patients enrolled in this study met the following inclusion criteria: (a) diagnosis of primary or recurrent OSCC, (b) tumor excision with sentinel node‐assisted neck dissection performed at Karolinska University Hospital, Stockholm, Sweden. Sentinel nodes were identified with preoperative single‐photon emission computed tomography and their location was confirmed intraoperatively by gamma probe in combination with fluorescence detection by indocyanine green light; (c) willingness to participate in the study. Exclusion criteria were as follows: (a) systemic autoimmune diseases; (b) second malignancy or history of hemo‐lymphopoietic malignancies; (c) any other acute or chronic condition that could influence the immunological milieu in lymph nodes.
2.2. Sample preparation
The unfixed neck sample and tumor samples after excision were transferred directly to the pathology department, where one of the designated pathologists (PFS) handled samples and separated lymph nodes halves (all sentinel nodes and one or two nonsentinel nodes per patient). After surgical excision, the lymph nodes and tumor samples were kept in prechilled MACS Tissue Storage Solution and used within 1 hour for further analysis. A Tumor Dissociation Kit (Miltenyi Biotec #130‐100‐008) was used to mechanically and enzymatically dissociate surgical specimens. After dissociation, cells were filtered through a 100 µm cell strainer (BD Biosciences #352360). Cells were resuspended in Brilliant Stain Buffer (BD Biosciences #563794) at 40 × 106 cells/mL and used for downstream analysis. Lymph nodes from noncancer patients were obtained during surgeries for benign salivary gland disease (such as submandibular gland removal due to a blocked salivary duct) or neck cyst removal and were handled in the same way as those from cancer patients.
2.3. Flow cytometry
Single‐cell suspensions with purified cells from blood and surgical specimens were first blocked with Fc‐block for 5 minutes at room temperature. Next, samples were stained with an antibody panel (CD3, CD4, CD8, CD69, HLA‐DR, and PD‐1; Table S2) for 20 minutes at room temperature. Staining was followed by two washing steps performed with PBS, 400 g, for 5 minutes. Cells were resuspended in PBS with 1% paraformaldehyde (HistoLab #02178) and analyzed on LSR FORTESSA (BD Biosciences). Analysis of the flow cytometry data was performed with FlowJo version 10.6.2 (LLC).
Cells were first gated based on side scatter (SSC‐A) and forward scatter (FSC‐A) to exclude debris. Cells were then gated manually to delineate the following cell subpopulations: CD3+CD4+, CD3+CD8+, CD3+CD4+PD‐1+/‐, CD3+CD8+PD‐1+/‐. Expression of CD69 and HLA‐DR was then analyzed individually on the aforementioned populations. Fluorescence minus one negative controls were used for CD69, HLA‐DR, and PD‐1 antibodies.
2.4. t‐SNE and phenograph
FACS3.0 files were imported into FlowJo software version 10.6.2 (LLC). Dimensionality reduction was performed using the t‐SNE tool in FlowJo (v.10.6.2). The downsample plugin (v.3.3) and concatenation tool were used to visualize multiparametric data from a comparable number of CD3+ cells from each studied location. HLN, LN, SN and Met LN contributed 100 000 events each, while PBMCs and tumor samples were underrepresented, contributing 54 265 and 16 323 events, respectively. The following parameters were used to create t‐distributed stochastic neighbor embedding (t‐SNE) plots: iterations = 1000, perplexity = 30, learning rate (eat) = 32 941, gradient algorithm Barnes‐Hut. Clusters of phenotypically related cells were then detected by Phenograph (v.2.4). Phenograph was run with K = 80. The following markers were used to delineate the t‐SNE and perform Phenograph clustering: CD4, CD8, CD69, HLA‐DR, PD‐1.
2.5. Statistical analysis
Statistical analyses were performed with GraphPad Prism version 6.01 (GraphPad Software) and IBM SPSS Statistics 27 (IBM Corporation). The Kolmogorov–Smirnov normality test was used to determine if data sets were normally distributed, and ordinary one‐way ANOVA or Kruskal–Wallis tests were performed, depending on the distribution of the data. For the multiple comparisons, Tukey's or Dunn's tests were used, respectively. A paired t test was used to compare paired groups of data, while an unpaired t test with Welch's correction was used to compare unpaired groups of data. Pearson correlation coefficients were calculated to analyze correlation between activation markers. The outcome was presented as the mean ± standard deviation. Survival analysis was performed using the Kaplan–Meier method with log‐rank test and Cox proportional hazard model. For our binary outcomes (recurrence, death), the cut‐off point for CD3+ PD‐1+ high and low subgroups was set at the mean percentage of CD3+PD‐1 in all TDLNs (mean % = 33.2%).
2.6. Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Ethics Committee Approvals: 2015/1650‐31/2 and 2019‐03518.
3. RESULTS
3.1. Patient clinical and pathological characteristics
Fourteen patients were included in this study. For logistical reasons, blood samples from two patients were missed and not included in the analysis. The responsible pathologist declined to take samples from tumor material in seven cases due to the small size of the primary tumor. One patient contributed with two tumor samples, which were extranodal neck tumor masses samples. All patients had pathologically confirmed diagnosis of OSCC. Eleven had cancer in the mobile tongue, two in the floor of the mouth, and one in the inner cheek. Seven patients (50%) had occult metastases in their TDLNs. Table S1 summarizes the clinical characteristics of enrolled patients. Analyzed samples included fresh HNSCC tumor samples (n = 8), head and neck cancer patient peripheral blood mononuclear cells (HNSCC PBMC, n = 12), neck lymph nodes from controls without cancer (HLN, n = 5), neck nonsentinel lymph nodes from HNSCC patients (LN, n = 15), neck sentinel lymph nodes (SN, n = 22), and metastatic lymph nodes (Met LN, n = 9). Eleven patients received postoperative radiotherapy, 10 received brachytherapy and four patients received postoperative chemotherapy with cisplatin.
3.2. TILs are highly activated compared with lymphocytes in TDLNs
In the analyzed material, tumor tissue demonstrated a significantly decreased proportion of CD4+ T cells compared to HLN, LN, and SN (P < .05; 57.9 ± 9.9% vs 85.0 ± 4.5%, 79.7 ± 9.8% and 79.1 ± 12.3%, respectively; Figure 1A). There was no significant difference in distribution of CD8+ cells and CD4/CD8 ratio between tumor and lymph nodes (Figure 1A).
FIGURE 1.
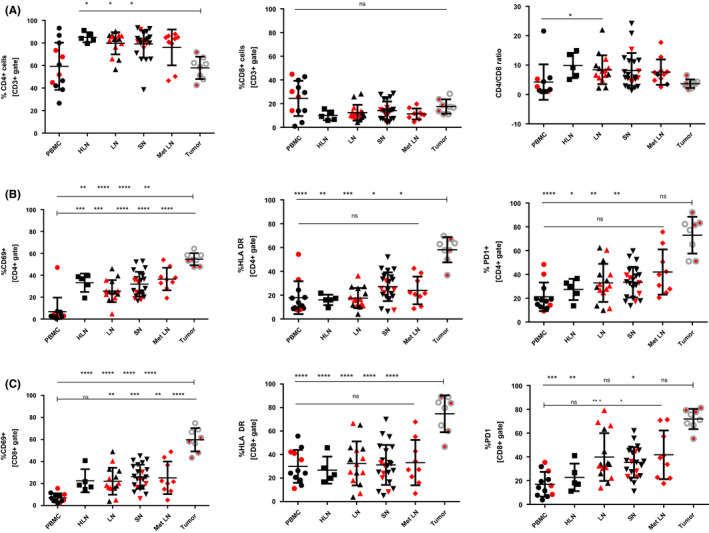
Expression of CD69, HLA‐DR, and PD1 in TDLNs and tumor in head and neck cancer patients. A, Percentage of CD4+ T cells, CD8+ T cells, and CD4/CD8 ratio among analyzed compartments. B, Expression of CD69, HLA‐DR, and PD1 within CD4+ T cells. C, Expression of CD69, HLA‐DR, and PD1 within CD8+ T cells. Red represents samples from patients with nodal metastases. *<.05, **<.01, ***<.001, **** <.0001
Activated T cells, identified by surface expression of CD69, HLA‐DR, and PD‐1, were significantly increased in tumor tissue both in CD4+ and CD8+ compartments compared with PBMC, HLN, LN, SN, and Met LN (Figure 1B,C). On average, CD4+ TILs expressed CD69 at 54.7 ± 5.5%, HLA‐DR at 58.2 ± 10.6%, and PD‐1 at 73.0 ± 15.5%. CD8+ TILs expressed these markers at 59.7 ± 10.6%, 74.7 ± 15.7, and 71.9 ± 8.5%, respectively. Lymph nodes expressed CD69 at 25.5%–36.6% in the CD4+ compartment and at 22.0%–26.1% in the CD8+ compartment, HLA‐DR at 16.1%–27.1% and 26.8%–33.2%, respectively, and PD‐1 at 27.4%–42.1% and 22.8%–41.8%, respectively (Figure 1B,C). Expression of CD69 on PBMC CD4+ and CD8+ and PD‐1 on CD8+ T cells was significantly lower compared with LN, SN, and Met LN (Figure 1B,C), whereas expression of HLA‐DR and PD‐1 was comparable between PBMC and lymph nodes.
3.3. The majority of TILs express PD‐1 on their surface
The analysis of PD‐1 expression in the CD3+ compartment revealed that contrary to T cells in PBMCs and lymph nodes, most TILs express PD‐1 on their surface (Figure 2A–H). The frequency of PD‐1 positive CD3+ cells in PBMCs and lymph nodes did not prove to be significantly different between compartments and ranged from 23.5 ± 8.4% in PBMCs to 38.0 ± 14.8% in Met LN. As mentioned, the frequency of PD‐1 positive CD3+ cells in tumor samples was significantly increased compared with remaining tissues and equaled 63.1 ± 10.4% (data not shown).
FIGURE 2.
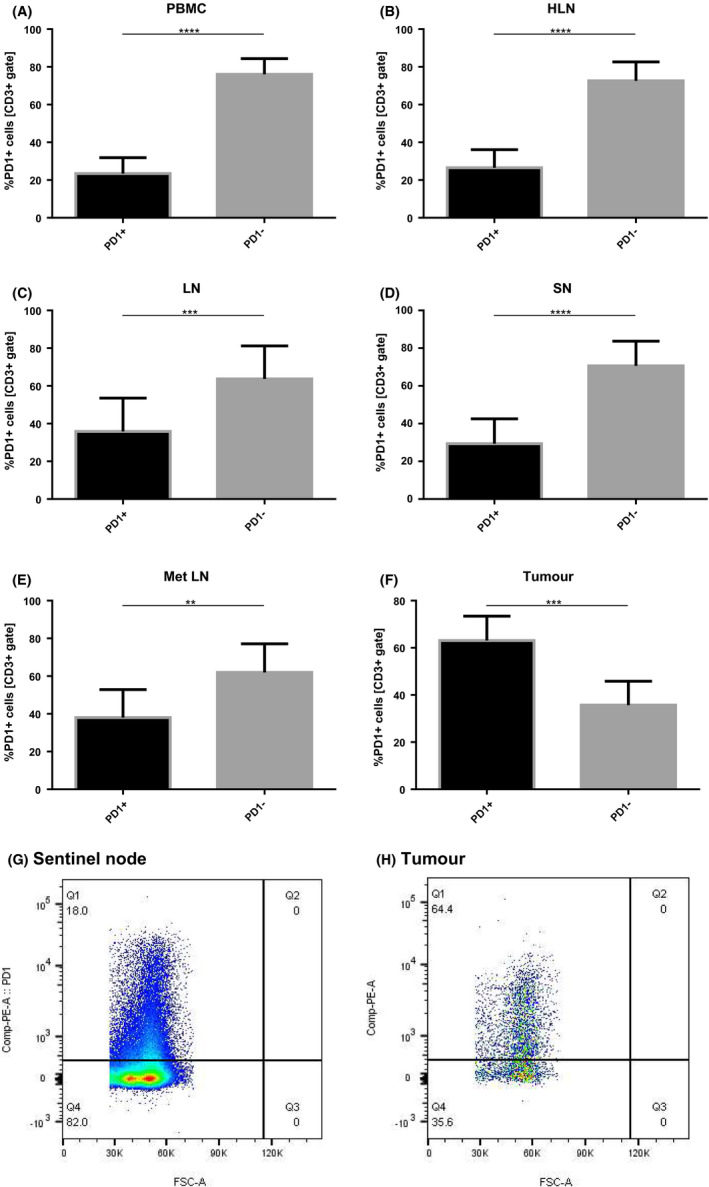
Percentage of PD1 positive CD3+ cells among PBMC, healthy LN, TDLNs and tumor samples. A–F, Percentage of PD1neg vs PD1pos cells within CD3+ compartment among analyzed locations. G, H, Scatter plots showing PD1pos populations in sentinel node and tumor, respectively. *<.05, **<.01, ***<.001, ****<0.0001
3.4. PD‐1 negative TILs are characterized by high expression of CD69 and HLA‐DR
In contrast to TILs, the PD‐1 negative fraction in lymph nodes showed low expression of both CD69 and HLA‐DR. In particular, a remarkable difference was seen in expression of CD69 between the PD1 negative vs positive fractions. The PD‐1 negative CD4+ fraction expressed CD69 at 15.7%–21.5% in TDLNs and the PD‐1 positive fraction at 59.6%–62.9% (Figure 3A). The same trend was observed regarding HLA‐DR expression; however, the difference was not as pronounced (Figure 3C,D). In PBMCs, the PD‐1 negative and positive fractions both expressed low levels of CD69. The expression of HLA‐DR by these cells was higher than that of CD69, but still lower than in lymph nodes and tumor.
FIGURE 3.
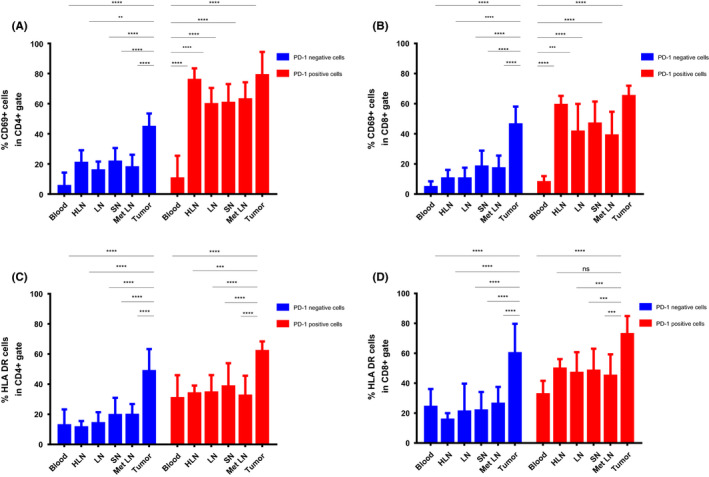
Comparison of expression of CD69 and HLA‐DR within PD1pos and PD1neg fractions of CD4+ and CD8+ T cells. A, B. Comparison of CD69 expression on PD1pos and PD1neg CD4+ and CD8+ T cells among compartments. C, D, Comparison of HLA‐DR expression on PD1pos and PD1neg CD4+ and CD8+ T cells among compartments
3.5. Patients with high expression of PD‐1 on CD3+ cells in TDLNs have significantly lower short‐term disease‐free and overall survival rates
Patients with nodal metastasis in at least one of the lymph nodes had a significantly higher percentage of CD3+ cells expressing PD‐1 compared with patients who did not have metastatic lymph nodes (36.7% vs 26.0%, respectively; P = .0317; Figure 4A). There was no significant difference in expression of PD‐1 between different T stages of the primary tumor (P > .05; Figure 4B).
FIGURE 4.
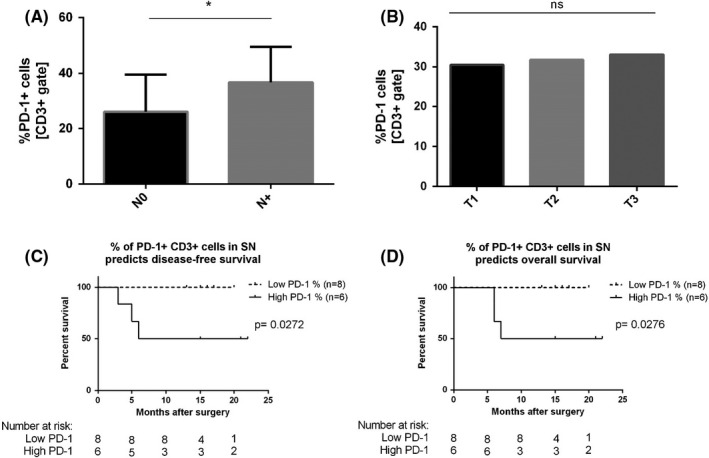
A, Expression of PD‐1 on CD3+ cells in relation to N‐status. B, Expression of PD‐1 on CD3+ cells in relation to T‐status. C, D, Kaplan–Meier analysis of DFS and OS according to percentage of PD‐1 expression CD3+ cells in sentinel nodes. The P value for the difference between the two curves was determined by the log‐rank test
During the study's follow‐up period, three patients (21.4%) developed recurrence. To investigate the influence of PD‐1 expression in TDLNs on survival in the studied cohort, we stratified patients into two groups: High PD‐1 and Low PD‐1. The cut‐off point for the Low and High subgroups was set at the mean percentage of CD3+PD‐1 in all TDLNs (mean % = 33.2%). Patients with a high percentage of CD3+ PD‐1+ cells in TDLNs proved to have significantly lower disease‐free and overall survival rates (log‐rank test P = .0272 and P = .0276, respectively; Figure 4C,D). For those with high PD‐1 expression in sentinel nodes 12‐month disease‐free and overall survival equaled 50% compared with 100% for those with low PD‐1 level in sentinel nodes. A worse disease‐free and overall survival was also observed for patients with nodal metastases compared with N0 patients (log‐rank test P = .0272 and P = .0276, respectively; Table S3). Cox regression analysis failed to confirm that level of PD‐1 or nodal involvement are independent factors influencing disease‐free survival (DFS) or overall surviva (OS) (Table S3).
3.6. PD‐1 expression positively correlates with expression of CD69 and HLA‐DR in metastatic lymph nodes and sentinel nodes
To better understand the co‐expression of the studied activation markers, a linear regression model with goodness‐of‐fit test was performed (Figure S1A–H). The expression of PD‐1 positively correlated with expression of CD69 in sentinel nodes and metastatic lymph nodes on both CD4+ and CD8+ cells (R 2 = 0.3154, P = .0065; R 2 = 0.5412, P = .0239; R 2 = 0.2669, P = .0138; R 2 = 0.7000, P = .0049, respectively). The same trend was observed for correlation of PD‐1 and HLA‐DR in CD4+ cells in metastatic lymph nodes and CD8+ cells in both metastatic lymph nodes and sentinel nodes (R 2 = 0.7622, P = .0021; R 2 = 0.8308, P = .0006 and R 2 = 0.4637, P = .0005, respectively). The remaining immunological compartments did not show a significant correlation between percentage of CD69pos and HLA‐DRpos with PD‐1pos cells. (data not shown).
3.7. High‐dimensional analysis reveals heterogeneous subsets of CD4+and CD8+ T cells within PBMC, TDLNs, and tumour tissue
To extend these findings, we concatenated data as described in the Methods section and mapped the populations on t‐SNE composite plots, which revealed a clear localization of most populations. Phenograph analysis revealed 26 unique clusters in the t‐SNE space. Figure 5A summarizes the distribution and localization of plotted populations. Figure 5B presents a heat map of surface markers among 26 clusters. Twenty‐one clusters were delineated within CD4+ cells (Figure 5C,D) and five clusters within CD8+ cells (Figure 5E,F). Of those clusters, six contained PD1high cells, as shown in Figure 5G,H.
FIGURE 5.
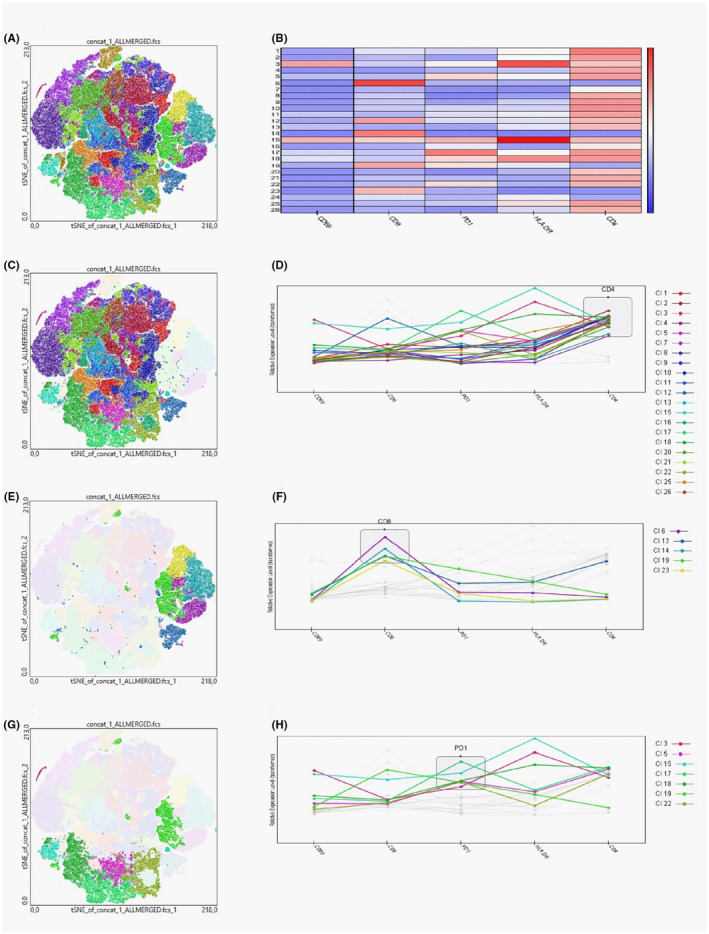
t‐SNE plots generated after data concatenation with hierarchical clustering of expression intensity (z score) for each of the indicated markers in each cluster derived using Phenograph. A, Overview of all 26 clusters delineated within concatenated data. B, Heat map showing markers intensity within 26 clusters identified by Phenograph. C, D, Clusters containing CD4+ T cells. E, F, Clusters containing CD8+ T cells. G, H Clusters containing PD1high T cells
In further analysis, we compared cluster distribution within six immunological compartments: PBMCs, healthy lymph nodes (HLNs), nonsentinel lymph nodes, sentinel nodes, lymph nodes with metastases, and tumor samples. We identified unique CD4+ and CD8+ subpopulation patterns for every location (Figure 6A). The frequencies of the clusters in each location are presented in Figure 6B,C.
FIGURE 6.
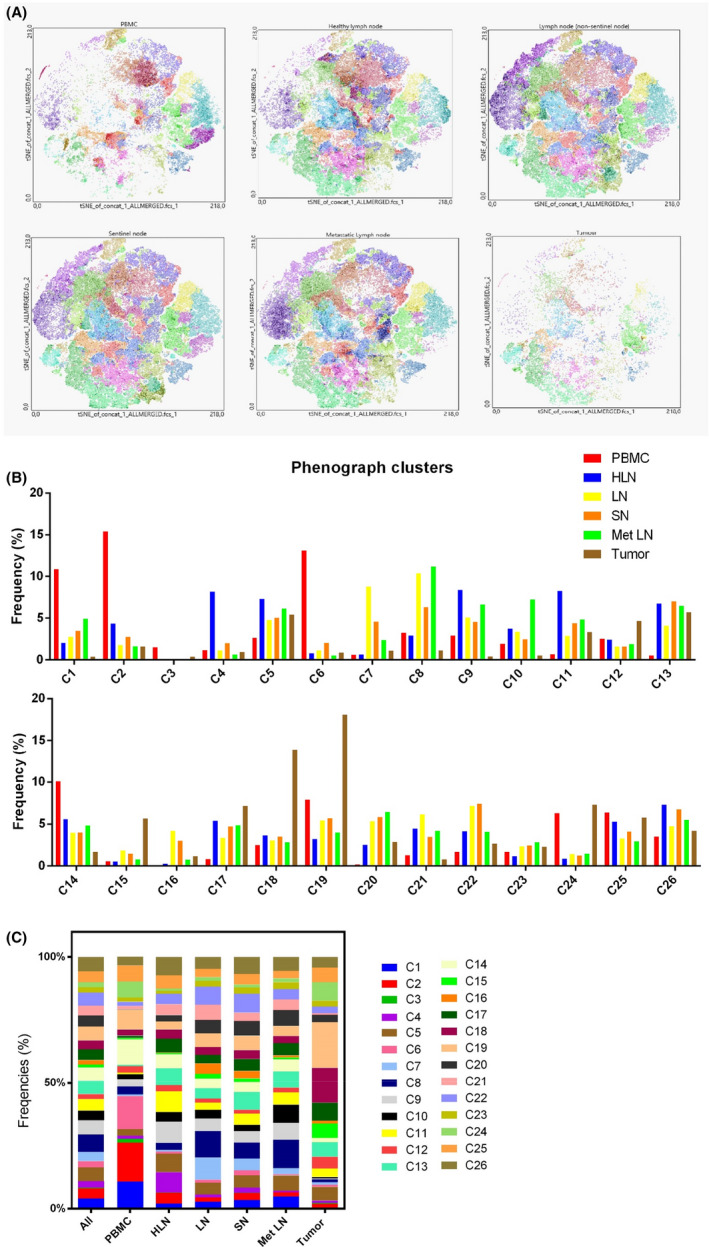
Phenograph‐derived clusters pattern in different locations A, t‐SNE plots showing expression pattern for the indicated markers among PBMC, HLN, LN, SN, Met LN, and tumor samples. B, C, Distribution of clusters in different locations shown in a bar graph (B) and a stacked bar graph (C)
PBMCs were rich in cluster 1 (CD4+ CD69neg HLA‐DRdim PD‐1dim), cluster 2 (CD4+ CD69neg HLA‐DRdim PD‐1neg), cluster 6 (CD8+ CD69neg HLA‐DRdim PD‐1dim), and cluster 14 (CD8+ CD69neg HLA‐DRlow PD‐1low). HLN did not show a predominant subpopulations, but compared with other locations cluster 4 and cluster 9 constituted significant percentages in HLN, representing naïve CD4+ T cells (CD69neg HLA‐DR dim and PD‐1neg/dim). HLN also contained a significant percentage of clusters 11 and 13, representing activated CD4+ T cells (CD69pos HLA‐DRhigh PD‐1dim). Nonsentinel nodes were characterized by a high proportion of cells within clusters 7 and 8, representing CD4+ cells negative for all activation markers. Cluster 22 (CD4+ CD69neg HLA‐DRdim PD‐1high) was also remarkably present within nonsentinel lymph nodes in cancer patients. Sentinel nodes presented a similar subpopulations pattern to nonsentinel nodes with the exception of clusters 7 and 8, which constituted a significantly lower proportion in SN. Lymph nodes with metastases were characterized by a higher presence of cluster 10 (CD4+ CD69neg HLA‐DRdim PD‐1dim) and cluster 5 (CD4+ CD69pos HLA‐DRhigh PD‐1high). Phenograph analysis also revealed a unique subpopulations pattern within tumor samples, which differed noticeably from analyzed lymph node samples and PBMCs. The dominant clusters were highly positive for PD‐1 (cluster 17 and 18,representing CD4+ CD69pos HLA‐DRdim/high PD‐1high cells and cluster 18 representing CD8+ CD69pos HLA‐DRhigh PD‐1high cells). Interestingly, cluster 15, representing CD8+ cells negative for all activation markers, was remarkably overrepresented in tumor compared with other locations.
4. DISCUSSION
In this study, we described a PD‐1, CD69, and HLA‐DR expression pattern on CD4+ and CD8+ T cells across three major immunological compartments: PBMC, TDLNs, and tumor tissue in OSCCs. We showed differences in expression of activation markers between these locations and identified diversity in expression of PD‐1 and other T cell activation markers between nonsentinel nodes, sentinel nodes, lymph nodes with metastases, and tumor tissue in OSCC. Moreover, we proved that the presence of metastases in TDLNs is associated with higher PD‐1 expression in sentinel nodes and that patients with a high percentage of PD‐1 expressing CD3+ cells in sentinel nodes have significantly worse short‐term disease‐free and overall survival. To our knowledge, this is the first study in OSCC investigating PD‐1 expression on T cells in TDLNs and one of few looking into PD‐1 expression on TILs by flow cytometry. Previous studies relied predominantly on immunohistochemical analyses, which have certain limitations compared with flow cytometry, such as the subjective and random manner of quantification. 12 , 13
Our results demonstrate that TILs are highly activated compared with lymphocytes in TDLNs and blood. It is known that a high density of TILs in HNSCC is associated with better prognosis. 14 , 15 It is, however, unclear how expression of T cell activation markers and PD‐1 influences survival and response to anticancer treatment. In particular, PD‐1 expression is important to investigate, as modulation of this molecule via anti‐PD‐1 therapy has the potential to reinvigorate tumor‐specific T cells, which were suppressed by binding to PD‐L1/PD‐L2. It is also worth noting that PD‐1 has been shown by different groups to have an ambiguous role in effective immune response to cancer. It can be considered as both a marker of dysfunctional/exhausted T cells and a marker of T cell activation and tumor specificity. 16 , 17 , 18
Here, the flow cytometry analysis found that both CD4+ and CD8+ tumor infiltrating T cells were characterized by significantly higher frequencies of CD69, HLA‐DR, and PD‐1 positivity compared with other locations. Over 70% of TILs expressed PD‐1 on CD4+ cells and both HLA‐DR and PD‐1 on CD8+ cells. The high expression of PD‐1 on TILs may indicate a state of exhaustion and susceptibility of these cells to PD‐L1/2 inhibition. Overall, these findings are in accordance with findings reported by Lechner et al 19 In their study, high PD‐1 expression (55.7%) was observed on CD3+ TILs in HNSCC tissue. In our cohort, 63.1% CD3+ TILs expressed PD‐1 on their surface. However, when comparing our results to those of Lechner et al, it must be pointed out that there were significant differences in the primary tumor localization of enrolled patients between the two studies. In the study performed by Lechner et al only 14.7% of patients had OSCC, while a plurality (47.1%) suffered from oropharyngeal cancer. In our present study, all included patients had OSCCs. From this standpoint, our observation could indicate a higher T cell exhaustion and/or dysfunctionality in OSCC, as this type of cancer seems to be characterized by higher PD‐1 expression on TILs compared with other types of HNSCC. A high expression of PD‐1 may be a contributing factor to lower survival, higher metastasis rate, and worse prognosis for this group of patients.
Another interesting finding was that the PD‐1 negative fraction of TILs (both CD4+ and CD8+) expressed high levels of HLA‐DR and CD69, which was rather unexpected. Since only the PD‐1 positive fraction is known to contain T lymphocytes specific for tumor neoantigens, one would expect low expression of other activation molecules on PD‐1 negative (naïve) lymphocytes. 17 , 18 , 20 A high CD69 expression can be explained by the fact that CD69, besides being an activation marker, is also a typical marker of peripheral tissue‐resident memory T cells and has been shown to be abundantly expressed on T cells in the periphery. 21 , 22 , 23 Nevertheless, high expression of HLA‐DR on PD‐1 negative cells is unexpected, as both HLA‐DR and PD‐1 are recognized as markers of T cell activation/antigen recognition and should be co‐expressed on activated cells. 24 This assumption was later confirmed in our correlation analysis, where we showed that in both sentinel node and metastatic lymph nodes the percentages of HLA‐DR and PD‐1 correlated closely with each other, which indicates that priming and activation with tumor antigens stimulates both markers. Therefore, we speculate that the PD‐1 negative activated fraction contains tumor‐specific T cells, which had their PD‐1 molecules blocked with PD‐L1/2 ligands present in the tumor microenvironment and hence did not bind anti‐PD‐1 antibody in flow cytometry. Alternatively, these activated cells could downregulate the expression of PD‐1 on their surface to escape an immunosuppressive tumor microenvironment. However, further research is warranted to confirm those hypotheses and fully elucidate the role of this TIL subset in HNSCC.
The role and expression of immune checkpoint molecules and activation markers on T cells in HNSCC has been studied previously by different groups, but to our knowledge, no studies have investigated PD‐1 expression in TDLNs in OSCC using flow cytometry. Improved understanding of the immunological milieu is important in OSCC since this type of HNSCC is characterized by the highest rate of occult nodal neck involvement among HNSCC cancers, which has a direct impact on prognosis and survival for these patients. 25 In this study, we went beyond immunohistochemistry and analyzed those molecules that can represent activated and/or exhausted T cell state in flow cytometry. Our data showed that T cells in TDLNs compared with PBMCs express comparable levels of HLA‐DR and higher levels of PD‐1. The expression of CD69 in TDLNs was higher than in blood but lower than in cancer tissue, which is in accordance with findings of Bankovich et al and Labiano et al. 21 , 22 In contrast to the finding that metastatic lymph nodes express high levels of HLA‐DR presented by Saraiva et al, 26 we did not find any significant differences in the expression level of the aforementioned marker among analyzed groups of lymph nodes. When it comes to PD‐1 expression, it was also expressed at comparable levels on CD4+ and CD8+ cells, with the highest PD‐1 level observed in metastatic lymph nodes, though the observed difference was not statistically significant. Following our expectation, the PD‐1 negative fraction in blood and TDLNs scarcely expressed other activation markers, while the PD‐1 positive fraction in TDLNs expressed CD69 and HLA‐DR abundantly. Overall, these findings are in accordance with findings reported by van de Ven et al, who reported comparable PD‐1 expression on PBMC and TDLN in nonsmall cell lung cancer (NSCLC). 27 Another study investigating PD‐1 expression in TDLN in NSCLC published by Ma et al 28 showed that metastatic lymph nodes express significantly higher levels of PD‐1. Again, even though there was a notable difference and higher expression in metastatic lymph nodes in OSCC, these differences did not reach statistical significance. However, when we compared PD‐1 expression on CD3+ cells between patients diagnosed by pathologist with N+ stage and N0 stage, we saw a significant upregulation of PD‐1 expression in all sentinel nodes of patients who had metastasis detected in at least one of their TDLNs. This proves that PD‐1 is upregulated not only in a metastatic lymph node, but tends to be higher in all TDLNs if metastasis is present in at least one of them.
To further extend our findings, we performed a high‐dimensional analysis with the use of Phenograph to investigate differences in T cell subpopulations between studied immunological compartments. These results go beyond previous reports, showing subtle differences in T cell subpopulations between TDLNs and tumor. The analysis revealed that sentinel nodes have a lower proportion of naïve T cells (negative for HLA‐DR and PD‐1) compared with nonsentinel nodes in HNSCC. This finding may reflect the fact that the sentinel node has earlier and more extensive contact with cancer neoantigens carried by antigen‐presenting cells. Our data also showed that tumor tissue, besides having numerous clusters of PD‐1high cells, contained a notable fraction of CD8+ cells negative for all studied activation markers (cluster 15), which may represent naïve cytotoxic T cells.
The limitations of the present study include a small sample size and lack of prior similar research in HNSCC. Further research is warranted to elucidate the functional aspects of T cell immunity in TDLN in HNSCC. In conclusion, we confirm that TILs in OSCC are highly activated and express high levels of PD‐1. In this study, we described also a fraction of highly activated PD‐1 negative T cells in tumor tissue that needs to be further investigated. Moreover, we show that patients with a high percentage of PD‐1 expressing CD3+ cells in sentinel node have significantly worse short‐term disease‐free and overall survival. Taken together, we demonstrated that flow cytometry of lymph nodes in HNSCC is feasible and may be used in the future to investigate whether PD‐1 levels in TDLNs correspond with response to anti‐PD‐1 therapy. Such knowledge may ultimately help guide anti‐PD‐1 treatment.
CONFLICTS OF INTEREST
Authors have no conflicts to declare.
Supporting information
Figure S1
Tables S1–S3
ACKNOWLEDGMENTS
We would like to thank Dr Christopher Illies (Karolinska University Hospital, Stockholm, Sweden) for pathological evaluation of lymph node samples in some of the enrolled patients.
Piersiala K, Farrajota Neves da Silva P, Hjalmarsson E, et al. CD4+ and CD8+ T cells in sentinel nodes exhibit distinct pattern of PD‐1, CD69, and HLA‐DR expression compared to tumor tissue in oral squamous cell carcinoma. Cancer Sci. 2021;112:1048–1059. 10.1111/cas.14816
Funding information
This work was funded by grants from the Swedish Cancer Foundation (No. 190287) and The Cancer Research Funds of Radiumhemmet (No. 194062).
REFERENCES
- 1. Chen L, Han X. Anti‐PD‐1/PD‐L1 therapy of human cancer: past, present, and future. J Clin Investig. 2015;125(9):3384‐3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism‐driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hurkmans DP, Kuipers ME, Smit J, et al. Tumor mutational load, CD8(+) T cells, expression of PD‐L1 and HLA class I to guide immunotherapy decisions in NSCLC patients. Cancer Immunol Immunother. 2020;69(5):771‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehra R, Seiwert TY, Gupta S, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long‐term follow‐up in KEYNOTE‐012. Br J Cancer. 2018;119(2):153‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colevas AD, Bahleda R, Braiteh F, et al. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol. 2018;29(11):2247‐2253. [DOI] [PubMed] [Google Scholar]
- 7. Segal NH, Ou SI, Balmanoukian A, et al. Safety and efficacy of durvalumab in patients with head and neck squamous cell carcinoma: results from a phase I/II expansion cohort. European Journal of Cancer (Oxford. England. 1990;2019(109):154‐161. [DOI] [PubMed] [Google Scholar]
- 8. Yu Y, Lee NY. JAVELIN Head and Neck 100: a Phase III trial of avelumab and chemoradiation for locally advanced head and neck cancer. Future Oncol. 2019;15(7):687‐694. [DOI] [PubMed] [Google Scholar]
- 9. Pai SI, Cohen EEW, Lin D, et al. SUPREME‐HN: a retrospective biomarker study assessing the prognostic value of PD‐L1 expression in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. J Transl Med. 2019;17(1):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2‐year long‐term survival update of CheckMate 141 with analyses by tumor PD‐L1 expression. Oral Oncol. 2018;81:45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khunger M, Hernandez AV, Pasupuleti V, et al. Programmed cell death 1 (PD‐1) ligand (PD‐L1) expression in solid tumors as a predictive biomarker of benefit from PD‐1/PD‐L1 axis inhibitors: a systematic review and meta‐analysis. JCO Precis Oncol. 2017;1:1‐15. [DOI] [PubMed] [Google Scholar]
- 12. Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T‐cells and CD20+ B‐cells in metastatic lymph nodes are associated with favourable outcome in patients with oro‐ and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Russell S, Angell T, Lechner M, et al. Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head Neck Oncol. 2013;5(3):24. [PMC free article] [PubMed] [Google Scholar]
- 14. Balermpas P, Michel Y, Wagenblast J, et al. Tumour‐infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110(2):501‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ward MJ, Thirdborough SM, Mellows T, et al. Tumour‐infiltrating lymphocytes predict for outcome in HPV‐positive oropharyngeal cancer. Br J Cancer. 2014;110(2):489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Day CL, Kaufmann DE, Kiepiela P, et al. PD‐1 expression on HIV‐specific T cells is associated with T‐cell exhaustion and disease progression. Nature. 2006;443(7109):350‐354. [DOI] [PubMed] [Google Scholar]
- 17. Gros A, Robbins PF, Yao X, et al. PD‐1 identifies the patient‐specific CD8⁺ tumor‐reactive repertoire infiltrating human tumors. J Clin Investig. 2014;124(5):2246‐2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gros A, Parkhurst MR, Tran E, et al. Prospective identification of neoantigen‐specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22(4):433‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lechner A, Schlößer H, Rothschild SI, et al. Characterization of tumor‐associated T‐lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget. 2017;8(27):44418‐44433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inozume T, Hanada K, Wang QJ, et al. Selection of CD8+PD‐1+ lymphocytes in fresh human melanomas enriches for tumor‐reactive T cells. J Immunother. 2010;33(9):956–964. 10.1097/CJI.0b013e3181fad2b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1‐phosophate receptor‐1 (S1P1) function through interaction with membrane helix 4. J Biol Chem. 2010;285(29):22328‐22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Labiano S, Meléndez‐Rodríguez F, Palazón A, et al. CD69 is a direct HIF‐1α target gene in hypoxia as a mechanism enhancing expression on tumor‐infiltrating T lymphocytes. Oncoimmunology. 2017;6(4):e1283468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mackay LK, Kallies A. Transcriptional regulation of tissue‐resident lymphocytes. Trends Immunol. 2017;38(2):94‐103. [DOI] [PubMed] [Google Scholar]
- 24. Viallard JF, Blanco P, André M, et al. CD8+HLA‐DR+ T lymphocytes are increased in common variable immunodeficiency patients with impaired memory B‐cell differentiation. Clin Immunol. 2006;119(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 25. Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26(3–4):645‐662. [DOI] [PubMed] [Google Scholar]
- 26. Saraiva DP, Jacinto A, Borralho P, Braga S, Cabral MG. HLA‐DR in cytotoxic T lymphocytes predicts breast cancer patients' response to neoadjuvant chemotherapy. Front Immunol. 2018;9:2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van de Ven R, Niemeijer A‐LN, Stam AGM, et al. High PD‐1 expression on regulatory and effector T‐cells in lung cancer draining lymph nodes. ERJ Open Res. 2017;3(2):00110‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma H, Mao G, Zhang G, Huang H. The expression and clinical signification of PD‐1 in lymph nodes of patients with non‐small cell lung cancer. Immunol Invest. 2017;46(7):639‐646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Tables S1–S3


