Abstract
The purposes of this study were to re‐confirm the usefulness of PET/CT in the differentiation of benignity/malignancy of neurogenic tumors in NF1 patients, and to analyze the natural course of plexiform neurofibroma (pNF) and clarify whether PET/CT is also useful for detecting tumors other than neurogenic tumors. PET/CT was prospectively imaged in 36 NF1 patients. There were 14 malignant peripheral nerve sheath tumors (MPNSTs) in 14 patients, and 54 pNFs in 30 patients. Nine patients had both MPNST and pNF. Maximal standardized uptake value (SUVmax) was significantly higher in MPNST (median 7.6: range 4.1‐10.4) (P < .001) compared with that of pNF (median 3.7: range 1.6‐9.3). The cut‐off value of 5.8 resulted in a sensitivity of 78.6% and specificity of 88.9%. Median age was 29 y, and median maximum tumor diameter was 82 mm in 14 MPNST patients. The 5‐y overall survival rate was 46.8%. Three patients with low‐grade MPNST were alive without disease at the time of this report. In 9 patients in which pNF and MPNST co‐existed, 2 showed a higher SUVmax of pNF than that of MPNST. Natural history analysis of pNF (n = 43) revealed that no factors significantly correlated with increased tumor size. Nine lesions other than neurogenic tumors were detected by PET/CT including 5 thyroid lesions and 3 malignant neoplasms. This study revealed the usefulness and limitation of PET/CT for NF1 patients. In the future, it will be necessary to study how to detect over time the malignant transformation of pNF to MPNST, via an intermediate tumor.
Keywords: MPNST, natural history, PET, plexiform neurofibroma, SUVmax
Analyzing 68 neurogenic tumors in NF1 patients with PET/CT, SUVmax was significantly higher in MPNST compared with that of pNF, which re‐confirmed results of previous reports from other countries. In NF1 patients, plexiform neurofibroma and MPNST co‐existed, differentiation of malignancy/benignity with SUVmax of PET/CT should be carefully evaluated. PET/CT is useful to detect lesions other than neurogenic tumors such as thyroid diseases in NF1 patients.
![]()
Abbreviations
- ANF
atypical neurofibroma
- AUC
area under the curve
- AYA
adolescent and young adult
- CDF
continuous disease free
- CI
confidence interval
- CT
computed tomography
- DNL
distinct nodular lesion
- DOD
dead of disease
- DWI
diffusion‐weighted imaging
- FDG
18F‐fluorodeoxyglucose
- FNCLCC
Federation Nationale des Centres de Lutte le Cancer
- GIST
gastrointestinal stromal tumor
- HR
Hazard ratio
- MPNST
malignant peripheral nerve sheath tumor
- MRI
magnetic resonance imaging
- NED
no evidence of disease
- NF1
neurofibromatosis type 1
- PET
positron emission tomography
- pNF
plexiform neurofibroma
- RECIST
Response Evaluation Criteria in Solid Tumors
- ROC
receiver operating characteristic
- SUVmax
maximal standardized uptake value
- VOI
volume of interest
1. INTRODUCTION
MPNST is a rare, aggressive soft tissue sarcoma (STS) that accounts for 2%‐4% of all STSs, has a high risk of recurrence, and has a poor prognosis. 1 , 2 , 3 , 4 Half of MPNST occurs in patients with neurofibromatosis type 1 (NF1), which is an autosomal abnormality with an incidence of 1:3000. The lifetime incidence of MPNST in NF1 is 8%‐15.8%, and MPNST is the leading cause of death in NF1. 1 , 5 , 6 Unlike sporadic MPNST, NF1‐related MPNST is considered to develop when the precursor lesion, atypical neurofibroma (ANF), becomes malignant. 7 ANF can also originate within isolated nodular or plexiform neurofibroma (pNF). Once transformed to high‐grade MPNST, resection with a wide operative margin is the only treatment modality available for curative intent. Even with extensive resection for localized MPNST, survival remains poor. 8 , 9 NF1‐related MPNST is often diagnosed when it is larger than sporadic MPNST, 9 and so early diagnosis and treatment are important, and may improve the prognosis, especially in NF1‐related MPNST.
Several imaging modalities have been used to accurately distinguish MPNST from benign lesions, including standard MRI with or without contrast enhancement, 10 , 11 , 12 functional MRI with diffusion‐weighted imaging (DWI), 13 and 18F‐fluorodeoxyglucose (FDG) consumption on PET. 14 , 15 There have been multiple reports on PET‐CT to differentiate benign and MPNST, showing the effective cut‐off value of the maximal standardized uptake value (SUVmax) in the tumor, and its high sensitivity/specificity.
From 2007 to 2014, we prospectively conducted FDG‐PET/CT (PET/CT) to differentiate MPNST from pNF in NF1 patients who were referred to our hospital with some tumor‐related symptoms. Therefore, PET/CT was not performed for screening purposes for asymptomatic NF1 patients. Since 2014, whole body MRI has been performed to screen for deep‐seated pNF even in asymptomatic NF1 patients, and prospective PET/CT imaging was discontinued due to insurance coverage issues.
One purpose of this study was to confirm the usefulness of prospective PET/CT in benign/malignant discrimination and to compare it with past studies. Few studies have reported the course of pNF detected by PET/CT or have described the usefulness of PET/CT to detect lesions other than nervous system tumors. Secondary purposes of this study were to analyze the natural course of pNF and to clarify whether PET/CT is useful for detecting tumors other than neurogenic tumors, in addition to analyzing the survival of patients diagnosed with MPNST that were detected by PET/CT.
2. MATERIALS AND METHODS
2.1. Patients
Among 93 NF1 patients who were referred to our outpatient clinic between 1985 and 2014, there were 50 NF1 patients between March 2007 and July 2014. PET/CT was prospectively imaged consecutively for all the patients during this period from whom informed consent was obtained. Patients who were referred had some complaints of deep‐seated tumors or evidence of tumor presence by MRI or CT imaging in the pre‐referral hospital/clinic. Since August 2014, all NF1 patients with informed consent were prospectively imaged with whole body MRI, even in the absence of any evidence or complaint of the existence of deep‐seated tumors in the outpatient clinic of our institution.
2.2. 18F‐Fluorodeoxyglucose (FDG)‐PET/CT
18F‐Fluorodeoxyglucose (FDG)‐PET/CT was performed in a total of 36 NF1 patients excluding those without informed consent. Diagnosis of NF1 was made using the National Institutes of Health (NIH) criteria. 16 NF1 was diagnosed when 2 or more of the 7 criteria described by NIH were satisfied. PET/CT examinations were performed using a Biograph 16 scanner (Siemens Medical Solutions). The patient had to fast for at least 6 h before imaging. PET/CT was not measured when the blood glucose level of a patient was higher than 150 mg/dL. The FDG dose was determined by body weight using either 3.7 MBq/kg for patients weighing <60 kg or 4.07 MBq/kg for patients weighing 60 kg or more. At 50 min after intravenous injection of FDG, emission scans were acquired for the area between the proximal femur and the base of the skull. Fusion PET/CT images were reconstructed on a workstation, a VOI was placed around the neoplastic lesion, and the maximum SUV at a particular VOI was defined as SUVmax.
Tumors with a maximum diameter of 15 mm or more and SUVmax value of 1.5 or more were defined as significant ones to be investigated or followed up carefully. Sixty‐eight lesions were detected as significant tumors in 36 patients. For patients with these tumors, needle or incisional biopsies were performed in all lesions with a SUVmax ≥ 5. In lesions with SUVmax of 3 or 4, if the patient complained of pain or tumor growth, or if there were findings on MRI suggesting the possibility of malignancy according to our previous report, 12 a biopsy was actively considered. All MPNSTs were pathologically diagnosed, and grade 2 and 3 malignant tumors were defined as high grade, and grade 1 as low grade according to the FNCLCC classification. 17 Differentiation between pNF and MPNST, particularly low‐grade MPNST is occasionally worrisome. Based on the histological findings of cytological atypia, loss of neurofibroma architecture, cellularity, and mitotic index, pathological diagnosis was determined. 18 Benign neurofibroma was diagnosed by experienced pathologists by biopsy in some lesions, and by the clinical course by attending physicians without biopsy in others.
2.3. Longitudinal study for pNFs
For the longitudinal study for pNFs, pNFs detected by PET/CT were followed up by MRI once every 3 mo to 1 y at the discretion of the attending physicians. During this course, if the pNF was evaluated as progressive disease according to RECIST criteria, 19 it was defined as “size up.” No patients received MEK inhibitor treatment in this period because this drug class had not yet been approved for use in Japan, and could not be applied to these patients. When pNF and MPNST were present in the same patient, their sizes and SUVmax values were compared.
For patients with MPNST diagnosis, surgical treatment aiming at R0 resection was performed if possible. Chemotherapy and radiotherapy were administered according to the circumstances of the individual patients. For these 14 patients with MPNST, various clinical factors related to prognosis were analyzed.
In NF1 patients, PET/CT is expected to detect lesions other than neurogenic tumors. When other diseases were suspected, the pathological or clinical diagnosis was determined by biopsy and/or other imaging modality.
This study was approved by the institutional ethical review board (approval number: 2012‐0195), and all patients provided informed consent. The study was conducted according to the principles set out in the Declaration of Helsinki.
2.4. Statistical analyses
Comparisons of various factors between groups were performed using the chi‐square test or Fisher's exact test for categorical variables, and the Mann‐Whitney test for comparison of medians of non‐parametric continuous variables between 2 groups. For each PET measurement, a receiver operating characteristic (ROC) curve was created and the AUC was calculated with cut points for diagnosing malignancies with optimized sensitivity and specificity. Overall survival was estimated using the Kaplan‐Meier method, and statistically analyzed by Log rank test. The effects of potential risk factors on overall survival were analyzed using multivariate Cox proportional hazards regression models. All variables were tested, with simultaneous forced entry, in multivariate models. P values below .05 were considered statistically significant. All analyses were undertaken using SPSS Statistics version 17.0 (IBM).
3. RESULTS
3.1. Patients for analyses
There were 77 high and clinically meaningful FDG uptake lesions analyzed by PET/CT in 36 patients. Nine of these lesions in 8 patients were diagnosed as non‐neurogenic lesions by biopsy. Another 68 neurogenic tumorous lesions were diagnosed as pNF or MPNST by histological diagnosis or clinical features/course. Of the 36 patients, which were referred as suspected neurogenic tumors, 1 patient was diagnosed as high‐grade liposarcoma by biopsy, with no pNF present. Therefore, the analysis of whether PET is useful for distinguishing pNF from MPNST was performed on 35 patients. There were 14 MPNSTs in 14 patients, and 54 pNFs in 30 patients. Nine patients had both MPNST and pNF. Excluding pNFs that were not evaluated by MRI during the course (6 pNFs) or received surgical treatment (5 pNFs), 43 pNFs in 22 patients were subjected to longitudinal study (natural course) (Figure 1).
FIGURE 1.
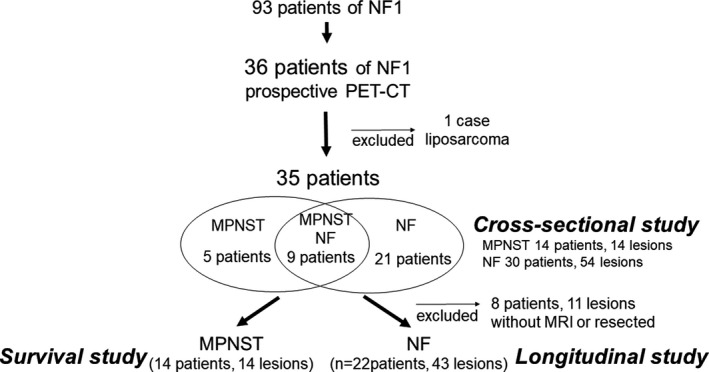
Flowchart of the present study. Flowchart shows the inclusion of patients for cross‐sectional, longitudinal, survival cohort in the present study
3.2. Cross‐sectional study
In the comparison of various factors between MPNST and pNF, there was no significant difference in gender (P = .71), age (P = .45), or site of occurrence (P = .67). Tumor size tended to be larger in MPNST (P = .055), and SUVmax was significantly higher in MPNST (median 7.6: range 4.1‐10.4) (P < .001) compared with that of pNF (median 3.7: range 1.6‐9.3) (Table 1). Among 54 patients with pNFs, 11 lesions were subjected to biopsy or resection to confirm the pathological diagnosis because of the relatively high SUVmax (median 4.8: range 4.1‐9.3). As shown in the box plot analyses (Figure 2), several points were determined to be “outliers,” indicating a large variation in both tumor size and SUVmax for pNF. The ROC curves for SUVmax and tumor size revealed that AUC for SUVmax and tumor size were 0.864 and 0.667, respectively (Figure 3). Optimal cut points were selected to maximize sensitivity and specificity based on the patient population. The cut‐off value of 5.8 resulted in sensitivity of 78.6% and specificity of 88.9%.
TABLE 1.
Comparison of various factors between MPNST (n = 14) and neurofibroma (n = 30)
| MPNST (14 lesions in 14 patients) | Neurofibroma (54 lesions in 30 patients) | P value | |
|---|---|---|---|
| Gender (male) | 7 | 24 | .71 |
| Age a (median, range) | 29 (13‐58) | 33.5 (10‐74) | .45 |
| Location | |||
| Head and neck | 5 | 12 | .67 |
| Trunk | 4 | 25 | |
| Extremity b | 5 | 17 | |
| Tumor size a (median, range) | 82 (20‐148) | 50.5 (20‐425) | .055 |
| SUVmax a (median, range) | 7.6 (4.1‐10.4) | 3.7 (1.6‐9.3) | <.001 |
Statistical analysis: Mann‐Whitney U test.
Extremity: shoulder and pelvic girdle included.
FIGURE 2.
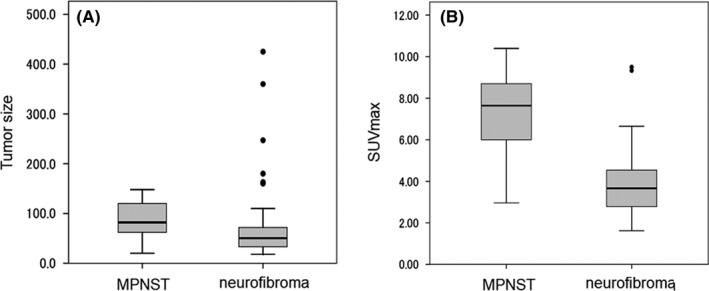
Box plots for tumor size and SUVmax value in 35 patients. Box plots show the distribution of quantitative data that facilitated comparisons between plexiform neurofibroma and MPNST. A, Tumor size, B, SUVmax
FIGURE 3.
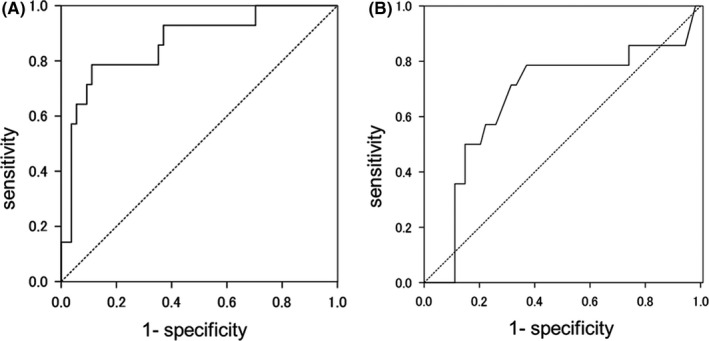
Receiver operating characteristic (ROC) curves. ROC curves for differentiation of MPNST from neurofibroma by SUVmax (A) and tumor size (B). AUC, area under the curve
When there are multiple neurogenic tumors in the same NF1 patient, it may be occasionally difficult to distinguish between benign and malignant tumors. There were 9 patients in which pNF and MPNST co‐existed in the same patients. If patients have multiple pNFs, the pNF that showed the maximum value of SUVmax was selected for comparison with that of MPNST. Comparing MPNST and pNF in the 9 patients, neither tumor size (P = .16) nor SUVmax (P = .063) reached a significant difference (Figure 4). In 2 of 9 patients, pNF showed a higher SUVmax value than that of MPNST (Table 2). Of the 9 patients, all 3 patients of low‐grade MPNST showed CDF, while 5 patients of 6 high‐grade MPNST showed DOD, and 1 patient showed NED after resection of local recurrence. Regarding 2 cases in which SUVmax was reversed, 1 patient was a 13‐y‐old female with both MPNST (SUVmax: 2.96) and pNF (SUVmax: 4.05) located in neck region. Maximum diameters of MPNST and pNF were 62 mm and 49 mm, respectively. MRI findings showed distinct nodular lesion (DNL) on MPNST that was gradually increasing in size. Another patient was a 22‐y‐old male with MPNST (SUVmax: 4.2) in the mediastinum and pNF (SUVmax: 6.5) in the upper arm. Maximum diameters of MPNST and pNF were 86 and 36 mm, respectively. MPNST was gradually increasing in size, whereas pNF was not. MRI findings indicated cystic change in MPNST, and DNL in pNF. MIB‐1 indices of biopsy specimens for MPNST and pNF were 30%‐40% and 1%‐2%, respectively. Summarizing these, it seemed difficult to distinguish between MPNST and pNF that occurred in the same NF1 patient, so evaluation with image follow‐up at short intervals or active biopsy will be required.
FIGURE 4.
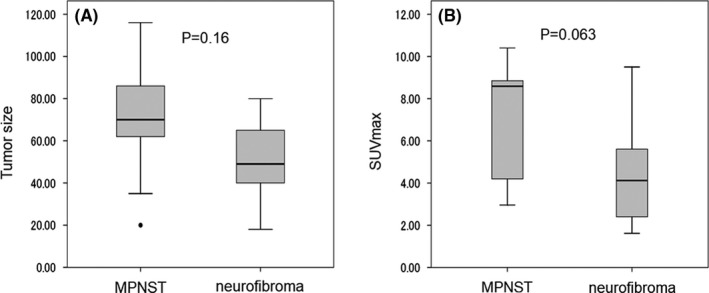
Box plots for tumor size and SUVmax value in 9 patients in which pNF and MPNST co‐existed. Box plots show the distribution of quantitative data that facilitated comparisons between plexiform neurofibroma and MPNST co‐existent in the same patient (n = 9). A, Tumor size, B, SUVmax
TABLE 2.
Patients with both MPNST and neurofibroma evaluated with PET‐CT
| Age | Gender | MPNST | Neurofibroma a | |||
|---|---|---|---|---|---|---|
| Tumor size | SUVmax | prognosis | Tumor size | SUVmax | ||
| 44 | F | 70 | 4.1 b | CDF | 18 | 1.6 |
| 16 | F | 93 | 8.7 | DOD | 51 | 2.4 |
| 17 | M | 116 | 8.6 b | CDF | 65 | 4.1 |
| 28 | M | 70 | 9.8 b | CDF | 43 | 5.6 |
| 13 | F | 62 | 3.0 | DOD | 49 | 4.1 |
| 22 | M | 86 | 4.2 | DOD | 36 | 6.5 |
| 35 | M | 20 | 6.6 | DOD | 80 | 2.0 |
| 21 | F | 35 | 10.4 | DOD | 40 | 9.5 |
| 42 | F | 78 | 8.9 | NED | 78 | 5.0 |
Abbreviations: CDF, continuous disease free; DOD, dead of disease; NED, no evidence of disease.
Neurofibroma with maximum SUVmax value was selected in patients with multiple NFs.
Low‐grade MPNST.
3.3. Survival study for MPNST patients
Survival analysis was performed on 14 MPNSTs, although the number of patients was small. Seven patients were males, median age was 29 y (range; 13‐58), median maximum tumor diameter was 82 mm (range; 20‐148), high‐grade tumors were found in 11 patients, and low grade in 3 patients. At the time of referral, only 1 patient had distant metastasis (lung) and the other patients had only localized disease. There were 5 patients with tumors of limbs/limb girdle, 5 patients with tumors of head and neck, and 4 patients with trunk or abdomen involvement. Median follow‐up time was 30.5 mo (range; 8‐135 mo) after PET. Surgery was performed in 9 patients, chemotherapy in 7 patients, and radiation therapy in 3 patients. There were 5 patients who were CDF, 1 patient alive with disease (AWD), 1 patient who had no evidence of disease (NED), and 7 patients died of disease (DOD). The 5‐y overall survival rate was 46.8%, and the median and mean estimated survival times were 40 mo (confidence interval (CI): not available) and 73 mo (CI: 42‐105), respectively (Figure S1). Univariate analysis showed a tendency for poor prognosis at a young age (<29 y) (P = .053). However, there were no significant differences in any factors including gender, tumor size, site, and SUVmax (Figure S2). There were no significant factors in the multivariate analysis and patients of younger ages tended to have a poorer prognosis (P = .09).
3.4. Longitudinal study
Longitudinal analysis of pNF tumor growth was performed in 43 pNFs. Among them, only 4 pNFs showed progressive disease according to RECIST criteria. Median duration of follow‐up was 56 mo in the size up (+) group, and 68 mo in the size up (−) group (P = .92). Median age was higher and tumor size was smaller in the size up (−) group; however no factors were of significant predictive value for size up of tumors including the SUVmax value (P = .67) (Table 3). Logistic regression analysis found no factors associated with the increased size of pNF (Table S1).
TABLE 3.
Changes in size of neurofibroma and various factors
| Size up (+) (4 lesions) | Size up (−) (39 lesions) | P value | |
|---|---|---|---|
| Gender a (male), lesions | 3 | 15 | 0.29 |
| Age b (median, range) | 26 (13‐35) | 31 (16‐68) | 0.18 |
| Tumor size b (mm, median, range) | 59 (49‐80) | 43 (18‐180) | 0.11 |
| SUVmax b (median, range) | 4.1 (2‐6.33) | 3.6 (1.62‐9.5) | 0.67 |
| Duration b (f/u, median, range) | 56 (17‐81) | 68 (3‐145) | 0.92 |
Abbreviation: f/u, follow‐up (mo).
Fisher exact test.
Mann‐Whitney U test.
18F‐Fluorodeoxyglucose uptake in positive lesions other than neurogenic tumors included thyroid (n = 5), neck (n = 1), back (n = 1), abdomen (n = 2), and pelvis (n = 1). Four lesions were diagnosed as adenomatous goiter, 2 gastrointestinal stromal tumor (GIST), and 1 liposarcoma, teratoma, and chronic thyroiditis each (Table 4). Median SUVmax values in malignant and benign lesions were 7.6 and 6.5, respectively, without any statistical difference (P = .41).
TABLE 4.
Other diseases found by FDG accumulation with PET‐CT
| Gender | Age a | Location | Diagnosis | SUVmax |
|---|---|---|---|---|
| F | 37 b | Neck | Adenomatous goiter | 7.5 |
| F | 37 b | Trunk | Liposarcoma (high grade) | 5.9 |
| F | 41 | Pelvis | Uterine fibroids and teratomas | 14.7 |
| F | 37 | Abdomen | GIST | 9.5 |
| F | 64 | Abdomen | GIST | 7.6 |
| F | 30 | Neck | Chronic thyroiditis | 15.4 |
| F | 41 | Neck | Adenomatous goiter | 4.0 |
| F | 46 | Neck | Adenomatous goiter | 5.4 |
| M | 68 | Neck | Adenomatous goiter | 2.7 |
Abbreviation: GIST, gastrointestinal stromal tumor.
Age at PET‐CT.
Same patient.
4. DISCUSSION
Various studies have reported the usefulness of FDG‐PET/CT for differentiation between benign tumors and MPNST in NF1 patients. 20 , 21 , 22 , 23 , 24 , 25 , 26 Recently, a paper reviewing these studies was published. Tovmassian et al reviewed 13 papers on 796 tumors, and indicated that the mean SUVmax values of benign and malignant lesions were 1.93 vs 7.48, respectively, while sensitivity ranged from 89% to 100% and specificity from 72% to 94% with various SUVmax cut‐off values ranging from 3.1 to 6.1. 15 The present study revealed that median SUVmax of benign and malignant lesions were 3.7 vs 7.6, respectively, while the sensitivity and specificity were 78.6% and 88.9%, respectively with a cut‐off value of 5.8, which was slightly lower than those in previous reports. However, with the cut‐off value set as 4.1 in the present study, the sensitivity increased to 92.9%, which was comparable with those in previous reports. In addition to analyzing SUVmax, there have been reports of other analysis methods using PET/CT. A semi‐quantitative index, the tumor/liver ratio with a cut‐off 1.5 had a negative predictive value of 98.8% and a positive predictive value of 61.5%, values that were better than the standard evaluation with SUVmax analyzing 113 NF1 patients and 145 tumors. 27 In the comparative analysis of early and delayed imaging with FDG‐PET/CT, the results of the 2 imaging modalities showed a similar accuracy. 28 However, most of these studies were cross‐sectional and it is doubtful that they truly contributed to the early detection or treatment of MPNST, or improved the prognosis of NF1 patients including those in the present study.
For early detection and treatment of MPNST, it is important to accurately evaluate and detect intermediate tumors, ATF, and low‐grade MPNST that have been subjected to less‐invasive surgical treatment. 29 , 30 Higham et al 31 reported the results of an analysis including clinical symptoms and FDG‐PET/CT for 76 atypical NFs in 69 patients, and observed increased accumulation in most ANFs. Azizi et al conducted FDG‐PET/CT on 41 children and adolescent NF1 patients with extensive pNF, and reported that asymptomatic malignant lesions were detected with a sensitivity of 100%, a negative predictive value of 100% and a specificity of 45.1%. All patients with asymptomatic malignant lesions were alive at the time of this report, demonstrating the usefulness of early detection. 32
Most past studies analyzed MPNST and pNF in different patients. However, in the medical care of NF1 patients, the distinction between MPNST and pNF in the same patient is also a problem for both physicians and patients. In this study, MPNST and pNF co‐existed in 9 NF1 patients. For the results of these 9 patients, there was no significant difference in SUVmax (P = .063) or tumor size (P = .16) between MPNST and pNF. In 2 patients, the SUVmax value for pNF was higher than that for MPNST. In the overall cohort (n = 35), SUVmax was significantly different (P < .001) and tumor size tended to be different (P = .055), suggesting that differentiation in patients who had both MPNST and pNF, was especially difficult.
The present study revealed that the 5‐y overall survival rate was 46.8%, which was unfavorable compared with previous reports including both NF1‐related and sporadic MPNST. 8 , 9 Several studies have indicated that patients with NF1‐related MPNST tended to have a worse prognosis than those with sporadic disease. 1 , 33 , 34 , 35 The present study analyzed only patients with NF‐1‐related MPNST, with this possibly accounting for their relatively poor prognosis. PET/CT was performed prospectively in the present study, although most patients had some symptoms of deep‐seated tumors at the time of referral to our hospital. PET/CT was not performed as a screening for asymptomatic patients. This background highlights the importance of screening for deep‐seated asymptomatic pNF in NF1 patients for the early detection of MPNST. In addition, all 3 patients with low‐grade MPNSTs among the 14 patients survived disease free in the present study, suggesting a better prognosis with low‐grade MPNST if surgical treatment is performed before their conversion from low grade to high grade.
A few reports have concluded that PET/CT screening of NF1 patients would also be useful in detecting lesions other than nervous system tumors. In the analysis of FDG‐PET/CT performed on 69 NF1 patients focusing on thyroid, 4 NF1 patients were diagnosed with multinodular goiter, 2 with benign chronic lymphocytic thyroiditis, 1 with metastasis to the thyroid, and 1 with medullary thyroid cancer; 36 9 lesions other than neurogenic tumors were also found in the present study and 5 were thyroid diseases. It is also noteworthy that 3 of 9 lesions were malignant (1 liposarcoma and 2 GISTs) and required immediate surgical treatment, confirming that NF1 is a tumor predisposing syndrome. The median age of MPNST patients in this study was 29 y, which is much younger than that of other STSs, making NF‐1‐related MPNST an important malignant tumor in the AYA population. This was also due to NF1 being a tumor predisposing syndrome. Screening such as PET/CT or whole body MRI may be required.
Examining the natural history of pNF is important because pNF itself causes morbidity in NF1 patients, and pNF occasionally undergoes malignant conversion to MPNST via intermediate tumors. Seventy‐five pNFs in 28 patients with NFI were analyzed in a previous report, and the growth rate of pNF correlated significantly positively with SUVmax (P = .003). 37 Akshintala et al 38 reported the growth rate of pNF to show an inverse correlation with age and a moderate inverse correlation with baseline tumor volume. Growth of DNL has been reported to be a sign of conversion to ANF. 31 Therefore, it is also important to observe the growth of DNL along with pNF. The growth rate of DNL showed a weak inverse correlation with age. 38 Association of pNF volume change and development of clinical morbidities with analysis of 57 pNFs demonstrated that 27 patients with pNF needed increased doses of pain suppressants. pNFs in patients that needed such drug adjustments had a faster growth rate. 39 In the present study, only 4 of 43 pNFs increased in size. Neither age, tumor size, nor SUVmax value showed a significant association with increased size. As the natural history analysis is based on fewer than 100 patients, including some from several previous reports, it will be necessary to analyze many more patients through collaborative research in the future.
There are several limitations in the present study. First, although the imaging equipment for PET/CT was the same throughout the analysis period (2007‐2014) in our institution, the imaging conditions may not have been exactly the same. However, the sensitivity and specificity for differentiation in the present study were not so different from those in past reports, indicating no significant effect due to any differences in imaging conditions. Second, this study was not designed to analyze the usefulness of PET/CT in delineating the process of conversion from pNF to ANF and MPNST. Research that contributes to early detection will be necessary to improve the prognosis of patients. Third, no consideration was given to DNL or ANF in pNF. Currently, deep‐seated pNF is prospectively evaluated by whole body MRI, and DNL and ANF are being examined in greater detail in our institution.
In conclusion, the present study has re‐confirmed the usefulness of PET/CT in differentiation between malignant and benign PNST. However, the SUVmax value occasionally varied, and care should be taken in this evaluation, particularly when pNF and MPNST may co‐exist in the same NF1 patient. As the prognosis of low‐grade MPNST patients was good and that of high‐grade MPNST was quite poor in the present study and previous reports, it will be necessary to study how quickly and most appropriately the process of malignant transformation from pNF to MPNST can be detected.
DISCLOSURE
Yoshihiro Nishida received grants from Zimmer‐Biomet, personal fees from Eisai Co., Ltd., personal fees from Eli Lilly Japan KK, personal fees from Kaken Pharmaceutical Co. Ltd., personal fees from Hisamitsu Pharmaceutical Co. Inc, personal fees from Kyowa Hakko Kirin Co., Ltd., personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Daiichi Sankyo Company Ltd, personal fees from Novartis Pharma K. K., and personal fees from Asahi Kasei Pharma Corporation, outside the submitted work; and is a consultant for Seikagaku Corp. and Ono Pharmaceutical Co. Ltd. Shiro Imagama has received grant support from AbbVie Inc, Asahi Kasei Pharma Corporation, Zimmer‐Biomet Dental GK, Mitsubishi Tanabe Pharma Corporation, Eisai Co., Ltd., Takeda Pharmaceutical Company Limited, Stryker Japan KK, Teijin Pharma Ltd., Daiichi Sankyo Company, Ltd, Hisamitsu Pharm Co., Inc, Mochida Pharm Co., Ltd., Nippon Kayaku Co., Ltd., and has received personal fee from Daiichi Sankyo Company, Ltd, outside the submitted work. Other authors have nothing to declare.
Supporting information
Fig S1
Fig S2
Table S1
ACKNOWLEDGMENTS
This work was supported in part by the National Cancer Center Research and Development Fund (29‐A‐3). We thank the patients who participated in our study. We thank Y. Kawai, T. Naganuma, and M. Yoshino for secretarial assistance.
Nishida Y, Ikuta K, Ito S, et al. Limitations and benefits of FDG‐PET/CT in NF1 patients with nerve sheath tumors: A cross‐sectional/longitudinal study. Cancer Sci. 2021;112:1114–1122. 10.1111/cas.14802
DATA AVAILABILITY STATEMENT
The research data are available from a data base repository at our institution, and can be available upon reasonable request.
REFERENCES
- 1. Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39(5):311‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farid M, Demicco EG, Garcia R, et al. Malignant peripheral nerve sheath tumors. Oncologist. 2014;19(2):193‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62(5):1573‐1577. [PubMed] [Google Scholar]
- 4. Ng VY, Scharschmidt TJ, Mayerson JL, Fisher JL. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res. 2013;33(6):2597‐2604. [PubMed] [Google Scholar]
- 5. Spurlock G, Knight SJ, Thomas N, Kiehl TR, Guha A, Upadhyaya M. Molecular evolution of a neurofibroma to malignant peripheral nerve sheath tumor (MPNST) in an NF1 patient: correlation between histopathological, clinical and molecular findings. J Cancer Res Clin Oncol. 2010;136(12):1869‐1880. [DOI] [PubMed] [Google Scholar]
- 6. Uusitalo E, Rantanen M, Kallionpää RA, et al. Distinctive Cancer Associations in Patients With Neurofibromatosis Type 1. J Clin Oncol. 2016;34(17):1978‐1986. [DOI] [PubMed] [Google Scholar]
- 7. Beert E, Brems H, Daniëls B, et al. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer. 2011;50(12):1021‐1032. [DOI] [PubMed] [Google Scholar]
- 8. Stucky CC, Johnson KN, Gray RJ, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012;19(3):878‐885. [DOI] [PubMed] [Google Scholar]
- 9. Valentin T, Le Cesne A, Ray‐Coquard I, et al. Management and prognosis of malignant peripheral nerve sheath tumors: the experience of the French Sarcoma Group (GSF‐GETO). Eur J Cancer. 1990;2016(56):77‐84. [DOI] [PubMed] [Google Scholar]
- 10. Demehri S, Belzberg A, Blakeley J, Fayad LM. Conventional and functional MR imaging of peripheral nerve sheath tumors: initial experience. AJNR Am J Neuroradiol. 2014;35(8):1615‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsumine A, Kusuzaki K, Nakamura T, et al. Differentiation between neurofibromas and malignant peripheral nerve sheath tumors in neurofibromatosis 1 evaluated by MRI. J Cancer Res Clin Oncol. 2009;135(7):891‐900. [DOI] [PubMed] [Google Scholar]
- 12. Wasa J, Nishida Y, Tsukushi S, et al. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol. 2010;194(6):1568‐1574. [DOI] [PubMed] [Google Scholar]
- 13. Yun JS, Lee MH, Lee SM, et al. Peripheral nerve sheath tumor: differentiation of malignant from benign tumors with conventional and diffusion‐weighted MRI. Eur Radiol. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Brinkman M, Jentjens S, Boone K, et al. Evaluation of the most commonly used (semi‐)quantitative parameters of 18F‐FDG PET/CT to detect malignant transformation of neurofibromas in neurofibromatosis type 1. Nucl Med Commun. 2018;39(11):961‐968. [DOI] [PubMed] [Google Scholar]
- 15. Tovmassian D, Abdul Razak M, London K. The role of [(18)F]FDG‐PET/CT in predicting malignant transformation of plexiform neurofibromas in neurofibromatosis‐1. Int J Surg Oncol. 2016;2016:6162182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neurofibromatosis . Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45(5):575‐578. [PubMed] [Google Scholar]
- 17. Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. 2006;130(10):1448‐1453. [DOI] [PubMed] [Google Scholar]
- 18. Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1‐a consensus overview. Hum Pathol. 2017;67:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228‐247. [DOI] [PubMed] [Google Scholar]
- 20. Benz MR, Czernin J, Dry SM, et al. Quantitative F18‐fluorodeoxyglucose positron emission tomography accurately characterizes peripheral nerve sheath tumors as malignant or benign. Cancer. 2010;116(2):451‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bredella MA, Torriani M, Hornicek F, et al. Value of PET in the assessment of patients with neurofibromatosis type 1. AJR Am J Roentgenol. 2007;189(4):928‐935. [DOI] [PubMed] [Google Scholar]
- 22. Brenner W, Friedrich RE, Gawad KA, et al. Prognostic relevance of FDG PET in patients with neurofibromatosis type‐1 and malignant peripheral nerve sheath tumours. Eur J Nucl Med Mol Imaging. 2006;33(4):428‐432. [DOI] [PubMed] [Google Scholar]
- 23. Cardona S, Schwarzbach M, Hinz U, et al. Evaluation of F18‐deoxyglucose positron emission tomography (FDG‐PET) to assess the nature of neurogenic tumours. Eur J Surg Oncol. 2003;29(6):536‐541. [DOI] [PubMed] [Google Scholar]
- 24. Cook GJR, Lovat E, Siddique M, Goh V, Ferner R, Warbey VS. Characterisation of malignant peripheral nerve sheath tumours in neurofibromatosis‐1 using heterogeneity analysis of (18)F‐FDG PET. Eur J Nucl Med Mol Imaging. 2017;44(11):1845‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferner RE, Golding JF, Smith M, et al. [18F]2‐fluoro‐2‐deoxy‐D‐glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated malignant peripheral nerve sheath tumours (MPNSTs): a long‐term clinical study. Ann Oncol. 2008;19(2):390‐394. [DOI] [PubMed] [Google Scholar]
- 26. Tsai LL, Drubach L, Fahey F, Irons M, Voss S, Ullrich NJ. [18F]‐Fluorodeoxyglucose positron emission tomography in children with neurofibromatosis type 1 and plexiform neurofibromas: correlation with malignant transformation. J Neurooncol. 2012;108(3):469‐475. [DOI] [PubMed] [Google Scholar]
- 27. Combemale P, Valeyrie‐Allanore L, Giammarile F, et al. Utility of 18F‐FDG PET with a semi‐quantitative index in the detection of sarcomatous transformation in patients with neurofibromatosis type 1. PLoS One. 2014;9(2):e85954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chirindel A, Chaudhry M, Blakeley JO, Wahl R. 18F‐FDG PET/CT qualitative and quantitative evaluation in neurofibromatosis type 1 patients for detection of malignant transformation: comparison of early to delayed imaging with and without liver activity normalization. J Nucl Med. 2015;56(3):379‐385. [DOI] [PubMed] [Google Scholar]
- 29. Bernthal NM, Putnam A, Jones KB, Viskochil D, Randall RL. The effect of surgical margins on outcomes for low grade MPNSTs and atypical neurofibroma. J Surg Oncol. 2014;110(7):813‐816. [DOI] [PubMed] [Google Scholar]
- 30. Nelson CN, Dombi E, Rosenblum JS, et al. Safe marginal resection of atypical neurofibromas in neurofibromatosis type 1. J Neurosurg. 2020;133(5):1516‐1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higham CS, Dombi E, Rogiers A, et al. The characteristics of 76 atypical neurofibromas as precursors to neurofibromatosis 1 associated malignant peripheral nerve sheath tumors. Neuro Oncol. 2018;20(6):818‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azizi AA Slavc I, Theisen BE, et al. Monitoring of plexiform neurofibroma in children and adolescents with neurofibromatosis type 1 by [(18) F]FDG‐PET imaging. Is it of value in asymptomatic patients? Pediatr Blood Cancer. 2018;65(1):e26733. [DOI] [PubMed] [Google Scholar]
- 33. Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005;23(33):8422‐8430. [DOI] [PubMed] [Google Scholar]
- 34. Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57(10):2006‐2021. [DOI] [PubMed] [Google Scholar]
- 35. Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys. 1998;42(2):351‐360. [DOI] [PubMed] [Google Scholar]
- 36. van Lierop Z, Jentjens S, Anten M, et al. Thyroid gland (18)F‐FDG uptake in neurofibromatosis type 1. Eur Thyroid J. 2018;7(3):155‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reinert CP, Schuhmann MU, Bender B, et al. Comprehensive anatomical and functional imaging in patients with type I neurofibromatosis using simultaneous FDG‐PET/MRI. Eur J Nucl Med Mol Imaging. 2019;46(3):776‐787. [DOI] [PubMed] [Google Scholar]
- 38. Akshintala S, Baldwin A, Liewehr DJ, et al. Longitudinal evaluation of peripheral nerve sheath tumors in neurofibromatosis type 1: growth analysis of plexiform neurofibromas and distinct nodular lesions. Neuro Oncol. 2020;22:1368‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gross AM, Singh G, Akshintala S, et al. Association of plexiform neurofibroma volume changes and development of clinical morbidities in neurofibromatosis 1. Neuro Oncol. 2018;20(12):1643‐1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Data Availability Statement
The research data are available from a data base repository at our institution, and can be available upon reasonable request.


