Abstract
This study focused on children as well as adolescents and young adults (AYAs) and aimed to examine trends in survival of leukemia over time using population‐based cancer registry data from Osaka, Japan. The study subjects comprised 2254 children (0‐14 years) and 2,905 AYAs (15‐39 years) who were diagnosed with leukemia during 1975‐2011. Leukemia was divided into four types: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and other leukemias. We analyzed 5‐year overall survival probability (5y‐OS), using the Kaplan‐Meier method and expressed time trends using the joinpoint regression model. For recently diagnosed (2006‐2011) patients, a Cox proportional hazards model was applied to determine predictors of 5y‐OS, using age group, gender, and treatment hospital as covariates. Over the 37‐year period, 5y‐OS greatly improved among both children and AYAs, for each leukemia type. Among AYAs, 5y‐OS of ALL improved, especially after 2000 (65% in 2006‐2011), when the pediatric regimen was introduced but was still lower than that among children (87% in 2006‐2011, P < .001). Survival improvement was most remarkable in CML, and its 5y‐OS was over 90% among both children and AYAs after the introduction of molecularly targeted therapy with tyrosine kinase inhibitors. Among patients with recently diagnosed AML, the risk of death was significantly higher for patients treated at nondesignated hospitals than those treated at designated cancer care hospitals. The changes in survival improvement coincided with the introduction of treatment regimens or molecularly targeted therapies. Patient centralization might be one option which would improve survival.
Keywords: adolescents and young adults, children, epidemiology, leukemia, survival
Survival of each type of leukemia in children, adolescents, and young adults has improved dramatically over the past 30 years.

Abbreviations
- 5y‐OS
5‐year overall survival probability
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- AYAs
adolescents and young adults
- CI
confidence interval
- CML
chronic myeloid leukemia
- HR
hazard ratio
- HSCT
hematopoietic stem cell transplantation
- ICD‐O‐3
International Classification of Diseases for Oncology, third edition
- JALSG
Japan Adult Leukemia Study Group
- MHLW
Ministry of Health, Labor, and Welfare
- OCR
Osaka Cancer Registry
- TKIs
tyrosine kinase inhibitors
1. INTRODUCTION
According to national cancer registry data that began in 2016, approximately 2000 children (age 0‐14 years) and 20 000 adolescents and young adults (AYAs, age 15‐39 years) are newly diagnosed with cancer each year in Japan. 1 Improvements in treatment and care for children and AYAs with cancer are included in the 3rd Basic Plan to Promote Cancer Control Programs in Japan. 2
Previous reports revealed that survival of leukemia among children dramatically improved in Japan, and 5‐year overall survival probability (5y‐OS) was over 80% after the 2000s, 3 while among AYAs (age 15‐29 years), 5y‐OS of leukemia was lower (56.9%) than that among children. 4 It was noticeably lower among young adults with acute lymphoblastic leukemia (ALL) (age 20‐29 years, 5y‐OS was 29% in 2001‐2005). 5 The reasons for this difference may include differences in cancer biology and chemotherapy pharmacokinetics, together with barriers to access to specialized centers, a lack of specialist care guidelines, treatment regimens, and clinical trials relevant to AYAs. 5 , 6 , 7 , 8 , 9 Regarding the treatment regimens, several clinical studies, including a Japanese trial, have confirmed the superiority of pediatric regimens in AYAs with ALL over adult regimens, 10 , 11 , 12 , 13 and survival in AYAs after the introduction of pediatric regimens is expected to improve. In Europe and the United States, long‐term trends in survival of ALL among children (age 0‐14 years), adolescents (age 15‐19 years), and young adults (age 20‐39 years) since the 1970s were examined and showed that the 5y‐OS has improved in all generations, but survival in AYAs has still lagged behind that in children. 14 Regarding acute myeloid leukemia (AML), several studies have shown improved procedures for hematopoietic stem cell transplantation (HSCT) and clinical outcomes among both children and AYAs since the 2000s. 15 , 16 Regarding chronic myeloid leukemia (CML), the introduction of molecularly targeted therapy with tyrosine kinase inhibitors (TKIs), which inhibits BCR/ABL tyrosine kinase activity, has revolutionized treatment and prognosis among children and adults in recent decades. 17 However, in Japan, the long‐term trends in survival of each type of leukemia among children and AYAs has never been reported.
The National Cancer Control Act in Japan was established in 2006, initially focusing on major adult cancers. 18 Based on the first Basic Plan to Promote Cancer Control Programs, a total of nearly 400 hospitals that meet the national criteria for the number of cancer patients, physician expertise, and availability of support programs for cancer patients have been designated as specialized cancer care hospitals across the country by the Ministry of Health, Labor, and Welfare (MHLW). 18 , 19 In 2012, the second Basic Plan to Promote Cancer Control Programs raised, for the first time, the issue of care for children, and fifteen hospitals were designated as childhood cancer care hospitals by the MHLW. 18 In the United States, the impact of care at specialized cancer centers on survival was assessed, and AYAs with ALL treated at nonspecialized centers had lower survival than those treated at specialized centers 9 ; however, no such investigation has been reported in Japan.
This study focused on children (age 0‐14 years) and AYAs (age 15‐39 years) and aimed to examine the survival trends of each type of leukemia over time and clarify whether there is still a “survival gap” between children and AYAs, using population‐based cancer registry data from Osaka, Japan. We also examined the impact of age, gender, and type of treatment hospital on prognosis among recently diagnosed patients.
2. MATERIALS AND METHODS
2.1. Data
We identified 5221 children and AYAs diagnosed with leukemia (International Classification of Disease 10th edition: C91‐95) at the age of 0‐39 years during 1975‐2011 from the database of the Osaka Cancer Registry (OCR). The OCR is a population‐based cancer registry which has been operating since 1962 and covers all residents in Osaka prefecture which has a population of 8.8 million (2015 census). 20 Patient data from the OCR include sex, age, date of cancer diagnosis, tumor sequence (ie, the numerical order of occurrence of the neoplasm), site, morphology, behavior, summary of treatment, vital status, treatment hospital, and date of death or the last follow‐up for vital status. If more than one hospital submits the same cancer record, one hospital is selected as the "treatment hospital," and priority is given according to the order in which treatment was performed; surgery, radiation, chemotherapy, or other treatment (including HSCT). If the same treatment is performed at more than one hospital, the hospital with the earliest date of diagnosis is defined as the treatment hospital. Follow‐up for vital status is routinely performed using death certificates. In addition, patients diagnosed with cancer were followed up using official resident registries to verify vital status by 3, 5, 10‐years after diagnosis. 21 Of these, patients who were registered by death certificate only (34 patients, 0.7%) or registered as multiple primary malignancies (28 patients, 0.5%) were excluded; the remaining 5159 patients were analyzed. Extracted leukemia cases were divided into four leukemia types using the International Classification of Diseases for Oncology, third edition (ICD‐O‐3) morphology codes: ALL (ICD‐O‐3 morphology codes: 9835, 9836, 9837), AML (ICD‐O‐3 morphology codes: 9840, 9861, 9866, 9867, 9870‐9874, 9891, 9895‐9897, 9910, 9920, 9931, 9988), CML (ICD‐O‐3 morphology codes: 9863, 9875, 9876), and other leukemias (the rest of the leukemia cases). Based on the designation of 18 hospitals, including two childhood cancer care hospitals, as specialized cancer care hospitals by the MLHW as of March 2019 in Osaka, we divided cases into two categories: those treated at designated cancer care hospitals and those treated at nondesignated hospitals, using information on treatment hospital.
2.2. Statistical analysis
We used the chi‐square test to compare the distribution of categorical variables between children (age 0‐14 years) and AYAs (age 15‐39 years).
Evaluation of survival trends was conducted in three ways. (1) 5y‐OS was estimated for leukemia (all types) and by each leukemia type among children (age 0‐14 years) and AYAs (age 15‐39 years) with 95% confidence interval (CI), using the Kaplan‐Meier method, and compared between children and AYAs using the log‐rank test by each 6‐ or 7‐year period (1975‐1981, 1982‐1987, 1988‐1993, 1994‐1999, 2000‐2005, and 2006‐2011). (2) For the analyses of survival trends, the Joinpoint Regression Program, Version 4.7.0.0, February 2019, was used to obtain joinpoint regressions and inflection points via the logarithmic function and final selection models provided by the program. 14 , 22 We input the 5y‐OS of leukemia (all types) in children (age 0‐14 years) and AYAs (age 15‐39 years) for each year calculated using the Kaplan‐Meier method. Regarding each leukemia type, due to the small number of cases, we input the 5y‐OS over a 3‐year period for ALL and AML and over a 6‐year period for CML. (3) We examined the Kaplan‐Meier curves of each leukemia type over the three decades (1982‐1991, 1992‐2001, 2002‐2011) by three age groups (age 0‐14 years, age 15‐24 years, age 25‐39 years) to assess survival before and after the introduction of pediatric regimens for ALL in 15‐24‐year‐olds in 2002, 12 or before and after the introduction of TKIs for CML in 2001. 23
Focusing on recently diagnosed (2006‐2011) ALL and AML patients, we applied the Cox proportional hazards regression model using age group, gender, and type of treatment hospital as covariates to determine the predictors of 5y‐OS.
For all analyses, a two‐sided P < .05 was considered statistically significant. All analyses except for trend analysis were carried out using Stata 14. This study was approved by the institutional review board of Osaka International Cancer Institute (approval number: 19143).
3. RESULTS
3.1. Characteristics of study subjects
We identified 5192 leukemia cases in the period 1975‐2011. Table 1 shows the differences in patient characteristics between children (age 0‐14 years) and AYAs (age 15‐39 years). Male patients represented a slightly larger proportion of cases than female among both children (54.7%) and AYAs (59.2%). ALL was the most common leukemia in children (63.4%), while AML was the most common in AYAs (46.0%). The number and proportion of CML patients were smaller in children (n = 77) than AYAs (n = 611). Most patients (children: 60.7%, AYAs: 56.0%) were treated in the designated cancer care hospitals. We checked the number of cases by treatment hospital by each 6‐ or 7‐year period (Figure 1). During the most recent period (2006‐2011), over 90% (274 out of 292) of children with leukemia were treated in designated cancer care hospitals compared with 56.3% (245 out of 435) of AYAs with leukemia.
TABLE 1.
Characteristics of study subjects
| Children (age 0‐14 years) | AYAs (age 15‐39 years) | P‐value a | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Total number of patients | 2,254 | 100.0 | 2,905 | 100.0 | |
| Sex | |||||
| Male | 1,232 | 54.7 | 1,719 | 59.2 | .001 |
| Female | 1,022 | 45.3 | 1,186 | 40.8 | |
| Histological type | |||||
| Acute lymphoblastic leukemia | 1,429 | 63.4 | 648 | 22.3 | <.001 |
| Acute myeloid leukemia | 557 | 24.7 | 1,337 | 46.0 | |
| Chronic myeloid leukemia | 77 | 3.4 | 611 | 21.0 | |
| Other leukemias | 191 | 8.5 | 309 | 10.6 | |
| Period at diagnosis | |||||
| 1975‐1981 | 540 | 24.0 | 638 | 22.0 | .023 |
| 1982‐1987 | 434 | 19.3 | 484 | 16.7 | |
| 1988‐1993 | 349 | 15.5 | 480 | 16.5 | |
| 1994‐1999 | 319 | 14.2 | 450 | 15.5 | |
| 2000‐2005 | 320 | 14.2 | 418 | 14.4 | |
| 2006‐2011 | 292 | 13.0 | 435 | 15.0 | |
| Treatment hospital | |||||
| Designated cancer care hospitals | 1,369 | 60.7 | 1,626 | 56.0 | <.001 |
| Nondesignated hospitals | 885 | 39.3 | 1,279 | 44.0 | |
Abbreviation: AYAs, adolescents and young adults.
Comparisons between children and AYAs using chi‐square test.
FIGURE 1.
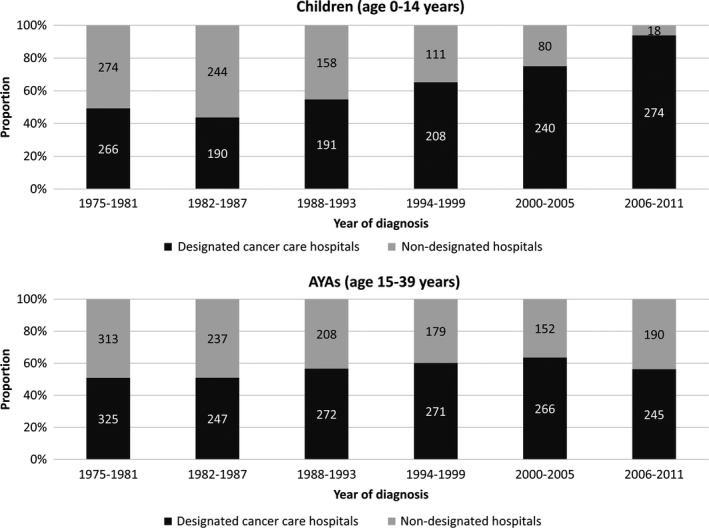
Trends in type of treatment hospital among children (age 0‐14 y) and adolescents and young adults (AYAs; age 15‐39 y)
3.2. Time trends in 5‐y‐OS for each leukemia type
We included 4323 (84%) patients in the survival analysis, excluding 836 patients (16%) without follow‐up information. Table 2 shows 5y‐OS and results of the log‐rank test for children (age 0‐14 years) and AYAs (age 15‐39 years) by each leukemia type, by 6‐ or 7‐year period. Overall, 5y‐OS of leukemia (all types) improved in both children (from 22.0% [95% CI = 18.4‐25.9] to 83.7% [95% CI = 78.9‐87.5]) and AYAs (from 7.2% [95% CI = 4.9‐9.9] to 71.8% [95% CI = 67.1‐75.9]) between 1975 and 2011. Five‐year OS of ALL improved from 30.2% (95% CI = 24.9‐35.5) to 86.7% (95% CI = 80.7‐91.0) in children and from 4.2% (95% CI = 0.8‐12.0) to 64.5% (95% CI = 53.8‐73.3) in AYAs between 1975 and 2011. Among AYAs, 5y‐OS of ALL improved recently (64.5% [95% CI = 53.8‐73.3] in 2006‐2011), but this was still significantly lower than that in children (86.7% [95% CI = 80.7‐91.0], P < .01). Regarding AML, 5y‐OS improved from 7.0% (95% CI = 3.5‐12.3) to 77.0% (95% CI = 66.6‐84.5) in children and from 5.2% (95% CI = 2.8‐8.8) to 66.5% (95% CI = 59.1‐72.9) in AYAs between 1975 and 2011. The difference in 5y‐OS of AML between children and AYAs became nonsignificant in the most recent period (2006‐2011) (P = .09). Regarding CML, 5y‐OS improved from 6.3% (95% CI = 0.4‐24.7) in children and 14.1% (95% CI = 8.0‐22.0) in AYAs during 1975‐1981 to over 90% in both children and AYAs during 2006‐2011, and there has been no survival difference between children and AYAs since 1988.
TABLE 2.
Five‐year overall survival probability among children as well as adolescents and young adults by period at diagnosis and leukemia type, 1975‐2011
| Period at diagnosis | Children (age 0‐14 y) | AYAs (age 15‐39 y) | P‐value a | ||||
|---|---|---|---|---|---|---|---|
| N at risk | 5‐y OS(%) | 95%CI | N at risk | 5‐y OS(%) | 95%CI | ||
| Leukemia (all types) b | 2059 | 2264 | |||||
| 1975‐1981 | 477 | 22.0 | (18.4‐25.9) | 404 | 7.2 | (4.9‐9.9) | <.01 |
| 1982‐1987 | 387 | 45.2 | (40.1‐50.0) | 373 | 16.1 | (12.6‐20.0) | <.01 |
| 1988‐1993 | 301 | 56.8 | (51.0‐62.2) | 360 | 29.2 | (24.6‐34.0) | <.01 |
| 1994‐1999 | 305 | 75.4 | (70.2‐79.9) | 368 | 46.9 | (41.7‐51.9) | <.01 |
| 2000‐2005 | 306 | 81.6 | (76.8‐85.5) | 350 | 52.5 | (47.1‐57.6) | <.01 |
| 2006‐2011 | 283 | 83.7 | (78.9‐87.5) | 409 | 71.8 | (67.1‐75.9) | <.01 |
| Acute lymphoblastic leukemia | 1318 | 515 | |||||
| 1975‐1981 | 289 | 30.2 | (24.9‐35.5) | 60 | 4.2 | (0.8‐12.0) | <.01 |
| 1982‐1987 | 263 | 53.9 | (47.7‐59.7) | 83 | 13.3 | (7.0‐21.5) | <.01 |
| 1988‐1993 | 202 | 58.4 | (51.3‐64.9) | 101 | 25.7 | (17.7‐34.5) | <.01 |
| 1994‐1999 | 190 | 83.2 | (77.0‐87.8) | 89 | 38.7 | (28.5‐48.6) | <.01 |
| 2000‐2005 | 200 | 84.9 | (79.2‐89.2) | 89 | 41.6 | (31.3‐51.5) | <.01 |
| 2006‐2011 | 174 | 86.7 | (80.7‐91.0) | 93 | 64.5 | (53.8‐73.3) | <.01 |
| Acute myeloid leukemia | 511 | 1059 | |||||
| 1975‐1981 | 128 | 7.0 | (3.5‐12.3) | 210 | 5.2 | (2.8‐8.8) | .03 |
| 1982‐1987 | 76 | 19.7 | (11.7‐29.3) | 180 | 15.6 | (10.7‐21.2) | .16 |
| 1988‐1993 | 61 | 50.8 | (37.7‐62.5) | 163 | 25.2 | (18.8‐32.0) | <.01 |
| 1994‐1999 | 76 | 59.1 | (47.2‐69.2) | 168 | 41.7 | (34.2‐49.0) | .01 |
| 2000‐2005 | 83 | 74.5 | (63.6‐82.6) | 153 | 49.6 | (41.4‐57.2) | <.01 |
| 2006‐2011 | 87 | 77.0 | (66.6‐84.5) | 185 | 66.5 | (59.1‐72.9) | .09 |
| Chronic myeloid leukemia | 69 | 502 | |||||
| 1975‐1981 | 16 | 6.3 | (0.4‐24.7) | 92 | 14.1 | (8.0‐22.0) | .03 |
| 1982‐1987 | 17 | 5.9 | (0.4‐23.5) | 89 | 20.2 | (12.6‐29.1) | .01 |
| 1988‐1993 | 12 | 50.0 | (20.8‐73.6) | 77 | 40.7 | (29.7‐51.5) | .50 |
| 1994‐1999 | 13 | 69.2 | (37.3‐87.2) | 79 | 64.6 | (53.0‐74.0) | .73 |
| 2000‐2005 | 5 | 80.0 | (20.4‐96.9) | 73 | 76.7 | (65.2‐84.8) | .80 |
| 2006‐2011 | 6 | 100.0 | ‐ | 92 | 93.3 | (85.8‐97.0) | .52 |
Abbreviations: AYAs, adolescents and young adults; CI, confidence interval; OS, overall survival probability.
Comparisons of survival functions between children and AYAs using the log‐rank test.
Leukemia represents all types of leukemia, including acute lymphoblastic leukemia, acute myeloid leukemia, chronic myeloid leukemia, and other leukemias.
Using the joinpoint model, we examined trends in survival of each leukemia type among children (age 0‐14 years) and AYAs (age 15‐39 years) during 1975‐2011 (Figure 2A‐D). Visually, improvement in 5y‐OS was remarkable among both children and AYAs for all leukemia types. For ALL, 5y‐OS among children improved rapidly during 1975‐1994 and after that it plateaued. Among AYAs, 5y‐OS improved rapidly during 1975‐1991, plateaued, and then improved after 2000. For AML, during 1975‐1978, the 5y‐OS had been under 10% in both children and AYAs but improved rapidly until the 1990s and has been gradually improving recently. Survival improvement in CML was remarkable during the total study period, and 5y‐OS reached 90% after 2006 in both children and AYAs.
FIGURE 2.
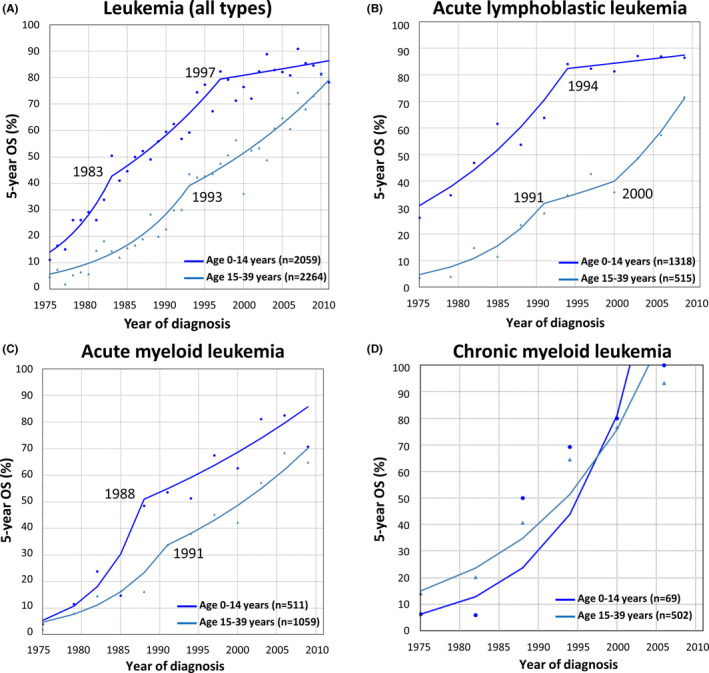
Trends in five‐year overall survival (5y‐OS) of leukemia and each leukemia type among children (age 0‐14 y) and AYAs (age 15‐39 y), 1975‐2011. Leukemia represents all types of leukemia, including acute lymphoblastic leukemia, acute myeloid leukemia, chronic myeloid leukemia, and other leukemias
Figure 3 shows the Kaplan‐Meier survival curves of each leukemia type by three age groups (0‐14 years, 15‐24 years, 25‐39 years) over three decades. For ALL, Kaplan‐Meier survival curves among AYAs aged both 15‐24 years and 25‐39 years improved similarly in the period after the introduction of the pediatric regimen (2002‐2011) (5y‐OS during 1992‐2001: age 0‐14 years: 77.1%, age 15‐24 years: 37.1%, age 25‐39 years: 33.4%; 5y‐OS during 2002‐2011: age 0‐14 years: 87.6%, age 15‐24 years: 59.4%, age 25‐39 years: 57.0%). For AML, Kaplan‐Meier survival curves in all three age groups improved between the period 1982‐1991 and 1992‐2001 by over 20%, and 5y‐OS reached over 60% in the period 2002‐2011. For CML, survival curves in all age groups improved between the period 1982‐1991 and 1992‐2001 by over 30%, and 5y‐OS reached over 80% in the period of 2002‐2011.
FIGURE 3.
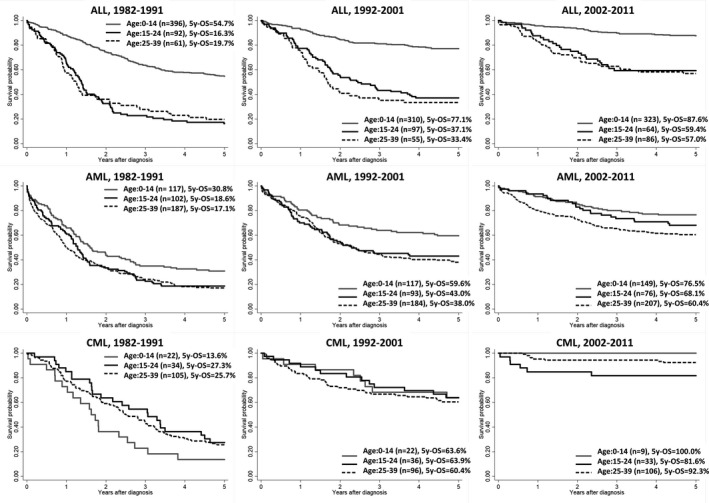
Kaplan‐Meier estimates of survival probability of each leukemia type, by age group (0‐14 y, 15‐24 y, and 25‐39 y), 1982‐1991, 1992‐2001, and 2002‐2011. For acute lymphoblastic leukemia, a pediatric regimen was introduced for adolescents and young adults aged 15‐24 y in 2002 in Japan, by the Japan Adult Leukemia Study Group (JALSG). Tyrosine kinase inhibitor (imatinib mesylate) was introduced as a molecularly targeted drug for chronic myeloid leukemia in all ages in Japan in 2001. 5y‐OS; 5‐year overall survival probability, ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia
3.3. Predictors of survival in recent cases
Focusing on recently diagnosed cases with ALL or AML during 2006‐2011, we applied the Cox proportional hazards regression model using age group, gender, and type of treatment hospital as covariates (Table 3). In the multivariate analysis, for ALL, AYAs aged 15‐39 years were associated with an increased risk of death compared with children (hazard ratio, HR = 3.0 [95% CI = 1.7‐5.4], P < .001). For AML, the risk of death was not significantly different by age group (HR = 1.2 [95% CI = 0.7‐2.1], P = .55) but was significantly higher among patients treated in nondesignated hospitals than in the designated cancer care hospitals (HR = 1.9 [95% CI = 1.2‐3.0], P = .01).
TABLE 3.
Predictors of 5‐year overall survival by Cox proportional hazards regression, among recently diagnosed patients (2006‐2011)
| N at risk | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | P‐value | HR | 95%CI | P‐value | ||
| Acute lymphoblastic leukemia | |||||||
| Age group | |||||||
| Children (age 0‐14 y) | 174 | Ref | Ref | ||||
| AYAs (age 15‐39 y) | 93 | 3.2 | (1.9‐5.4) | <.001 | 3.0 | (1.7‐5.4) | <.001 |
| Treatment hospital | |||||||
| Designated cancer care hospitals | 224 | Ref | Ref | ||||
| Nondesignated hospitals | 43 | 2.0 | (1.1‐3.6) | .03 | 1.1 | (0.6‐2.1) | .76 |
| Sex | |||||||
| Male | 153 | Ref | Ref | ||||
| Female | 114 | 0.8 | (0.5‐1.4) | .53 | 0.9 | (0.5‐1.5) | .71 |
| Acute myeloid leukemia | |||||||
| Age group | |||||||
| Children (age 0‐14 y) | 87 | Ref | Ref | ||||
| AYAs (age 15‐39 y) | 185 | 1.5 | (0.9‐2.6) | .09 | 1.2 | (0.7‐2.1) | .55 |
| Treatment hospital | |||||||
| Designated cancer care hospitals | 180 | Ref | Ref | ||||
| Nondesignated hospitals | 92 | 2.0 | (1.3‐3.1) | <.001 | 1.9 | (1.2‐3.0) | .01 |
| Sex | |||||||
| Male | 143 | Ref | Ref | ||||
| Female | 129 | 0.9 | (0.6‐1.4) | .6 | 0.9 | (0.6‐1.4) | .67 |
Abbreviations: AYAs, adolescents and young adults; CI, confidence interval; HR, hazard ratio.
Bold signifies statistical significance.
4. DISCUSSION
In this study, we overviewed the trends in survival of each type of leukemia among children and AYAs over time and evaluated prognostic factors for recent cases.
4.1. Acute lymphoblastic leukemia
We found that 5y‐OS of ALL improved and after 1994 reached over 80% in children (Table 2 and Figure 2). Treatment for childhood ALL started in 1948; almost all key drugs were approved before the 1980s, and the development of multidrug combination regimens and risk stratification was revised to maximize the antileukemia effect and to minimize toxicity within clinical trials. 24 In Japan, clinical study groups for leukemia in children were developed and followed these treatment modifications. 25 , 26 , 27 , 28 , 29 Our data may indicate these efforts contributed to an improvement in the survival of children with ALL at population level. Among AYAs, 5y‐OS of ALL was especially improved after 2000 (65% in 2006‐2011) when the pediatric regimen was introduced, but was still lower than that in children (87% in 2006‐2011, P < .001) (Tables 2 and 3, Figure 2). The poor prognosis of ALL in AYAs compared with children was reported to be associated with several factors, including differences in biological characteristics; barriers to access to specialized centers; a lack of specialist care guidelines, treatment regimens, and clinical trials relevant to AYAs; and a lower proportion of participation in clinical trials. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 30 , 31 Regarding biological differences, AYAs with ALL have been reported to have a higher proportion of poor prognostic features, including, hypodiploidy, T‐cell immunophenotype, iAMP21, deletion of IKZF1, and Philadelphia chromosome–like ALL, and they are less likely to have favorable hyperdiploidy or t(12;21) translocation, than children with ALL. 6 As for the difference in treatment regimens, most pediatric regimens deliver higher doses of corticosteroids, vincristine, and asparaginase; more doses of intrathecal methotrexate; and lower doses of daunorubicin, cytarabine, and etoposide than conventional adult treatment regimens, 13 and have been confirmed to be superior to adult regimens in AYAs, as shown in clinical trials in several countries. 10 , 11 , 12 , 13 In Japan, a pediatric regimen was introduced for AYAs aged 15‐24 years in 2002 by the Japan Adult Leukemia Study Group (JALSG). 12 In our data, the inflection point of improvement of survival of ALL among AYAs coincided with the introduction of the pediatric regimen, and 5y‐OS among patients aged 15‐24 years, which is the target population of the pediatric regimen in the JALSG, improved after 2002 (Table 2, Figures 2 and 3). Surprisingly, the survival of older young adults aged 25‐39 years has also improved recently (Figure 3); these young adults were not of eligible age for the clinical trials of the JALSG. To elucidate the reasons for this improvement, further investigation such as biology, treatment regimen, or information on HSCT will be needed. With respect to the type of treatment hospital, in the United States, AYAs with ALL treated at nonspecialized centers have been reported to have lower survival rates than those treated at specialized centers. 9 In our data, however, there was no significant difference in the risk of death between patients with ALL treated at the designated cancer care hospitals or nondesignated hospitals. To confirm this, further investigation to provide more information, such as the treatment regimen used in each hospital, is needed.
4.2. Acute myeloid leukemia
In our study, 5y‐OS of AML has improved from under 10% to over 60% in both children and AYAs, and there has been no survival difference between children and AYAs recently (Tables 2 and 3, Figures 2 and 3). In contrast to the reports comparing outcomes for children and AYAs with ALL, only a few reports and no prospective studies compared outcomes for children and AYAs with AML. 16 , 32 , 33 , 34 Canner et al reported that AYA patients were more likely to have high‐risk disease of AML when risk was determined by both molecular and cytogenetic testing results. 32 Several reports demonstrated similarities in terms of event‐free survival between children and AYAs but clearly showed higher rates of treatment‐related mortality among AYA patients. 32 , 34 , 35 Treatments administered by pediatric and adult oncologists for AML are broadly similar; cytarabine and anthracyclines are the mainstay of treatment for AML although pediatric regimens are more intensive and routinely include intensive central nervous system–oriented therapies. 16 Moreover, the indications for HSCT overlap, although the variety of indications in adults is relatively broader. 16 Tomizawa et al have reported recent improvements in HSCT outcomes in Japan among both children and AYAs, 16 which may be partly responsible for the recent improvements in survival in both children and AYAs seen in our data. Focusing on recent cases, patients treated in the designated cancer care hospitals had a lower risk of death than those treated in nondesignated hospitals with a non‐negligibly high HR in the multivariate analysis (Table 3). An association of transplant center size or physician factors with survival in adult patients after HSCT has been reported in the United States, 36 , 37 while another report has shown survival for AYAs with AML did not differ between NCI‐designated and non–NCI‐designated hospitals. 9 A fair assessment of a patient's mortality risk must take account of information such as patient's physical condition at diagnosis, participation in clinical trials, distance between patient's home and treatment hospital, patient's socioeconomic status, 9 and hospital's ability to perform HSCT, as confounding factors. Although our data do not include this information, as more complex treatments such as HSCT are required for AML than for ALL, 16 patient centralization to the designated cancer care hospitals might be one option to improve the survival of young patients with AML.
4.3. Chronic myeloid leukemia
Survival of CML improved dramatically in both children and AYAs during the last three decades (Table 2, Figures 2, and 3). During the 1990s, HSCT became an important treatment modality for the complete cure of CML. 38 After the introduction of TKI, imatinib mesylate, as approved by the US FDA, in Japan in 2001, survival reached over 90% in both children and AYAs. TKIs are considered the most successful targeted anticancer agents with a high cumulative incidence of complete cytogenetic responses in patients with CML. 39 The improvement in survival seen in our data is a striking example of how effective treatments have dramatically changed patient survival.
4.4. Cancer strategy for children and AYAs with cancer in Japan
In our data, children with leukemia had been already centralized to the designated cancer care hospitals in 2006‐2011, while AYAs were not centralized in this way in Osaka (Figure 1). Compared with children with cancer, there are relatively large numbers of AYA patients with more diverse life stages, more variety of needs, and differing psychosocial difficulties, 40 making it harder to determine the ideal cancer care system for them. However, superior survival in AYAs with AML treated in the designated cancer care hospitals in this study indicates that patient centralization to specialized cancer care hospitals might be an option to improve patient survival. On the other hand, since 1974, the Japanese government has subsidized medical expenses for children and adolescents (under 18 years of age) with cancer, 5 and a public long‐term care insurance system is available for Japanese residents aged over 40 years from 2000. Financial issues for AYAs have been reported to be a barrier to care at specialized hospitals and consequently contribute to poor outcomes. 9 , 41 Although the data in our study do not include financial details, considering that financial support for patients aged 19 to 39 years is inadequate compared with other age groups, governmental financial support might be important not only for children but also AYAs.
4.5. Strengths and limitations
The foremost strength of our study is the long‐term nature of the data allowing detection of trends in survival patterns by each leukemia type. In Japan, the national cancer registry started in 2016, founded by the Cancer Registration Promotion Act. 42 The OCR is one of the oldest prefectural cancer registries from which survival analysis is possible. 43 , 44 , 45 Cancer incidence in Osaka has been reported in “Cancer Incidence in Five Continents” volumes III to XI, and cancer survival data in Osaka has been reported in the “CONCORD study”. 46 , 47 , 48 The quality of this data, therefore, can be assumed to have met the standards set in international studies during the last three decades.
On the other hand, a limitation of our study is the relatively small number of cases due to the rarity of this disease in this age group, despite the fact that Osaka is a large prefecture with a population of 8.8 million. Another limitation of this study is the limited variables in the data. To investigate the reasons for the survival gap, clinical details such as patient's physical condition at diagnosis, gene abnormality, chemotherapy regimen, participation in clinical trials, information on the use of HSCT, patient's socioeconomic status, distance between patient's home and treatment hospital, and information on relapse are essential; however, our data do not include these details. A nationwide, population‐based study that allowed links to clinical information would help to identify factors that account for the survival gap.
Our results confirmed a large improvement in survival of each type of leukemia among children and AYAs in Osaka, Japan over a 37‐year period. The survival gap between children and AYAs is narrowing but persists among patients with ALL. Five‐year OS of CML was over 90% among both children and AYAs after the introduction of the molecularly targeted therapy. Risk of death was significantly higher among patients treated outside of the designated cancer care hospitals for patients with AML. Patient centralization might be an option to improve survival.
DISCLOSURE
The authors have no conflict of interest.
ACKNOWLEDGMENTS
We would like to thank all the patients and their families, pediatric oncologists, adult hematologists, and medical staff who cooperate with the Osaka Cancer Registry. This work was supported by a grant from the Children's Cancer Association in Japan in 2019 (to KN) and a Grant‐in‐Aid for Early‐Career Scientists from the Japan Society for the Promotion of Science KAKENHI [JP20K18952] (to KN); Health, Labor and Welfare Sciences Research Grants from the Ministry of Health, Labor, and Welfare of Japan (Grant Number H30‐Gantaisaku‐ippan‐009 [to KN, SO, TM, AS, IM], 20EA1026 [to KN, IM]). We would like to thank Dr Julia Mortimer for helping us with the English language.
Nakata K, Okawa S, Fuji S, et al. Trends in survival of leukemia among children, adolescents, and young adults: A population‐based study in Osaka, Japan. Cancer Sci. 2021;112:1150–1160. 10.1111/cas.14808
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cancer and Disease Control Divesion, Ministry of Health, Labour and Welfare . Cancer Incidence of Japan 2017. Tokyo: Cancer and Disease Control Divesion, Ministry of Health, Labour and Welfare.; 2020. https://www.e‐stat.go.jp/stat‐search/files?page=1&layout=normal&toukei=00450173&tstat=000001133323. Accessed September 14, 2020.
- 2. Foundation for Promotion of Cancer Research (FPCR) . The editorial board of the cancer statistics in Japan. Cancer statistics in Japan 2019. Tokyo: Foundation for Promotion of Cancer Research (FPCR);. 2020. https://ganjoho.jp/data/reg_stat/statistics/brochure/2019/cancer_statistics_2019_app_J.pdf. Accessed September 17, 2020.
- 3. Nakata K, Ito Y, Magadi W, et al. Childhood cancer incidence and survival in Japan and England: A population‐based study (1993–2010). Cancer Sci. 2018;109:422‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ito Y, Miyashiro I, Ito H, et al. Long‐term survival and conditional survival of cancer patients in Japan using population‐based cancer registry data. Cancer Sci. 2014;105:1480‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakata‐Yamada K, Inoue M, Ioka A, et al. Comparison of survival of adolescents and young adults with hematologic malignancies in Osaka, Japan. Leuk Lymphoma. 2016;57:1342‐1348. [DOI] [PubMed] [Google Scholar]
- 6. Harrison CJ. Cytogenetics of paediatric and adolescent acute lymphoblastic leukaemia. Br J Haematol. 2009;144:147‐156. [DOI] [PubMed] [Google Scholar]
- 7. Chiaretti S, Vitale A, Cazzaniga G, et al. Clinico‐biological features of 5202 patients with acute lymphoblastic leukemia enrolled in the Italian AIEOP and GIMEMA protocols and stratified in age cohorts. Haematologica. 2013;98:1702‐1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graubert TA. A call to action for acute lymphoblastic leukemia. N Engl J Med. 2014;371:1064‐1066. [DOI] [PubMed] [Google Scholar]
- 9. Wolfson J, Sun CL, Wyatt L, Stock W, Bhatia S. Adolescents and young adults with acute lymphoblastic leukemia and acute myeloid leukemia: impact of care at specialized cancer centers on survival outcome. Cancer Epidemiol Biomarkers Prev. 2017;26:312‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boissel N, Auclerc M‐F, Lhéritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE‐93 and LALA‐94 trials. J Clin Oncol. 2003;21:774‐780. [DOI] [PubMed] [Google Scholar]
- 11. Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayakawa F, Sakura T, Yujiri T, et al. Markedly improved outcomes and acceptable toxicity in adolescents and young adults with acute lymphoblastic leukemia following treatment with a pediatric protocol: a phase II study by the Japan Adult Leukemia Study Group. Blood Cancer J. 2014;4:e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ram R, Wolach O, Vidal L, Gafter‐Gvili A, Shpilberg O, Raanani P. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric‐inspired regimens: systematic review and meta‐analysis. Am J Hematol. 2012;87:472‐478. [DOI] [PubMed] [Google Scholar]
- 14. Trama A, Bernasconi A, McCabe MG, et al. Is the cancer survival improvement in European and American adolescent and young adults still lagging behind that in children? Pediatr Blood Cancer. 2019;66:e27407. [DOI] [PubMed] [Google Scholar]
- 15. Pinkerton R, Wills RA, Coory MD, Fraser CJ. Survival from haematological malignancy in childhood, adolescence and young adulthood in Australia: is the age‐related gap narrowing? Med J Aust. 2010;193:217‐221. [DOI] [PubMed] [Google Scholar]
- 16. Tomizawa D, Tanaka S, Kondo T, et al. Allogeneic hematopoietic stem cell transplantation for adolescents and young adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2017;23:1515‐1522. [DOI] [PubMed] [Google Scholar]
- 17. Drozdov D, Bonaventure A, Nakata K, Suttorp M, Belot A. Temporal trends in the proportion of "cure" in children, adolescents, and young adults diagnosed with chronic myeloid leukemia in England: a population‐based study. Pediatr Blood Cancer. 2018;65:e27422. [DOI] [PubMed] [Google Scholar]
- 18. Monden M. The basic plan to promote cancer control in Japan. Gan To Kagaku Ryoho. 2013;40:559‐564. [PubMed] [Google Scholar]
- 19. Kakizoe T. Ten Years after Implementation of Cancer Control Act. Gan To Kagaku Ryoho. 2016;43:1023‐1026. [PubMed] [Google Scholar]
- 20. Osaka Prefectural Government . Census 2015. Osaka Prefectural Government. Osaka. 2016. http://www.pref.osaka.lg.jp/attach/1891/00210094/27jinkoutoukihon.pdf.Accessed August 28, 2020.
- 21. Department of Public Health and Medical Affairs, Osaka Prefectural Government, and Cancer Control Center, Osaka International Cancer Institute. Annual Report of Osaka Cancer Registry .Cancer Incidence and Treatment in 2016 and Cancer Survival in 2011 in Osaka. (in Japanese); 2020.
- 22. Surveillance E, and End Results (SEER) Program . Joinpoint Trend Analysis Software; 2019. https://surveillance.cancer.gov/joinpoint/. Accessed May 28, 2019.
- 23. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR‐ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031‐1037. [DOI] [PubMed] [Google Scholar]
- 24. Pui CH, Evans WE. A 50‐year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. 2013;50:185‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takahashi H, Kajiwara R, Kato M, et al. Treatment outcome of children with acute lymphoblastic leukemia: the Tokyo Children's Cancer Study Group (TCCSG) Study L04–16. Int J Hematol. 2018;108:98‐108. [DOI] [PubMed] [Google Scholar]
- 26. Horibe K, Yumura‐Yagi K, Kudoh T, et al. Long‐term results of the risk‐adapted treatment for childhood B‐cell acute lymphoblastic leukemia: report from the japan association of childhood leukemia study all‐97 trial. J Pediatr Hematol Oncol. 2017;39:81‐89. [DOI] [PubMed] [Google Scholar]
- 27. Goto H, Inukai T, Inoue H, et al. Acute lymphoblastic leukemia and Down syndrome: the collaborative study of the Tokyo Children's Cancer Study Group and the Kyushu Yamaguchi Children's Cancer Study Group. Int J Hematol. 2011;93:192‐198. [DOI] [PubMed] [Google Scholar]
- 28. Tsurusawa M, Shimomura Y, Asami K, et al. Long‐term results of the Japanese Childhood Cancer and Leukemia Study Group studies 811, 841, 874 and 911 on childhood acute lymphoblastic leukemia. Leukemia. 2010;24:335‐344. [DOI] [PubMed] [Google Scholar]
- 29. Koh K, Kato M, Saito AM, et al. Phase II/III study in children and adolescents with newly diagnosed B‐cell precursor acute lymphoblastic leukemia: protocol for a nationwide multicenter trial in Japan. Jpn J Clin Oncol. 2018;48:684‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wood WA, Lee SJ. Malignant hematologic diseases in adolescents and young adults. Blood. 2011;117:5803‐5815. [DOI] [PubMed] [Google Scholar]
- 31. Fern LA, Lewandowski JA, Coxon KM, Whelan J. Available, accessible, aware, appropriate, and acceptable: a strategy to improve participation of teenagers and young adults in cancer trials. Lancet Oncol. 2014;15:e341‐e350. [DOI] [PubMed] [Google Scholar]
- 32. Canner J, Alonzo TA, Franklin J, et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients: a report from the Children's Oncology Group. Cancer. 2013;119:4162‐4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woods WG, Franklin AR, Alonzo TA, et al. Outcome of adolescents and young adults with acute myeloid leukemia treated on COG trials compared to CALGB and SWOG trials. Cancer. 2013;119:4170‐4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tomizawa D, Watanabe T, Hanada R, et al. Outcome of adolescent patients with acute myeloid leukemia treated with pediatric protocols. Int J Hematol. 2015;102:318‐326. [DOI] [PubMed] [Google Scholar]
- 35. Rubnitz JE, Pounds S, Cao X, et al. Treatment outcome in older patients with childhood acute myeloid leukemia. Cancer. 2012;118:6253‐6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Majhail NS, Mau LW, Chitphakdithai P, et al. Transplant center characteristics and survival after allogeneic hematopoietic cell transplantation in adults. Bone Marrow Transplant. 2020;55:906‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loberiza FR Jr, Zhang MJ, Lee SJ, et al. Association of transplant center and physician factors on mortality after hematopoietic stem cell transplantation in the United States. Blood. 2005;105:2979‐2987. [DOI] [PubMed] [Google Scholar]
- 38. Masaoka T. Chronic myelogenous leukemia and blood stem cell transplantation. Gan To Kagaku Ryoho. 1999;26:1396‐1400. [PubMed] [Google Scholar]
- 39. Russo D, Garcia‐Gutierrez JV, Soverini S, Baccarani M. Chronic myeloid leukemia prognosis and therapy: criticisms and perspectives. J Clin Med. 2020;9(6):1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bibby H, White V, Thompson K, Anazodo A. What are the unmet needs and care experiences of adolescents and young adults with cancer? A systematic review. J Adolesc Young Adult Oncol. 2017;6:6‐30. [DOI] [PubMed] [Google Scholar]
- 41. Meeneghan MR, Wood WA. Challenges for cancer care delivery to adolescents and young adults: present and future. Acta Haematol. 2014;132:414‐422. [DOI] [PubMed] [Google Scholar]
- 42. Tanaka H, Matsuda T. Arrival of a new era in Japan with the establishment of the Cancer Registration Promotion Act. Eur J Cancer Prev. 2015;24:542‐543. [DOI] [PubMed] [Google Scholar]
- 43. Kinoshita FL, Ito Y, Morishima T, Miyashiro I, Nakayama T. Sex differences in lung cancer survival: long‐term trends using population‐based cancer registry data in Osaka. Japan. Jpn J Clin Oncol. 2017;47:863‐869. [DOI] [PubMed] [Google Scholar]
- 44. Okawa S, Tabuchi T, Morishima T, Koyama S, Taniyama Y, Miyashiro I. Hospital volume and postoperative 5‐year survival for five different cancer sites: a population‐based study in Japan. Cancer Sci. 2020;111:985‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morishima T, Sato A, Nakata K, Miyashiro I. Geriatric assessment domains to predict overall survival in older cancer patients: An analysis of functional status, comorbidities, and nutritional status as prognostic factors. Cancer Med. 2020;9:5839‐5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009:analysis of individual data for 25, 676, 887 patients from 279 population‐based registries in 67 countries (CONCORD‐2). Lancet. 2015;385:977‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet. 2018;391:1023‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bonaventure A, Harewood R, Stiller CA, et al. Worldwide comparison of survival from childhood leukaemia for 1995–2009, by subtype, age, and sex (CONCORD‐2): a population‐based study of individual data for 89828 children from 198 registries in 53 countries. Lancet Haematol. 2017;4:e202‐e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request.


