Abstract
Approximately 1 in 2 Japanese people are estimated to be diagnosed with cancer during their lifetime. Cancer still remains the leading cause of death in Japan, therefore the government of Japan has decided to develop a better cancer control policy and launched the Cancer Genomic Medicine (CGM) program. The Ministry of Health, Labour, and Welfare (MHLW) held a consortium at their headquarters with leading academic authorities and the representatives of related organizations to discuss ways to advance CGM in Japan. Based on the report of the consortium, the CGM system under the national health insurance system has gradually been realized. Eleven hospitals were designated in February 2018 as core hospitals for CGM; subsequently, the MHLW built the Center for Cancer Genomics and Advanced Therapeutics (C‐CAT) as an institution to aggregate and manage genomic and clinical information on cancer patients, and support appropriate secondary use of the aggregated information to develop research aimed at medical innovation. As the first step in Japan's CGM in routine practice, in June 2019 the MHLW started reimbursement of 2 types of tumor profiling tests for advanced solid cancer patients using the national insurance system. Japan's CGM has swiftly been spreading nationwide with the collaboration of 167 hospitals and patients. The health and research authorities are expected to embody personalized cancer medicine and promote CGM utilizing state‐of‐the‐art technologies.
Keywords: genes, genomics, Japan, neoplasms, organization and administration
As the first step in Cancer Genomic Medicine (CGM) in routine practice in Japan, since June 2019 the Ministry has started reimbursement of 2 gene panel tumor profiling tests for advanced solid cancer patients using the national insurance system. CGM has swiftly been spreading nationwide in Japan with the collaboration of 167 hospitals and patients. The Ministry are expected to realize further personalized cancer medicine and promote CGM utilizing state‐of‐the‐art technologies.
Abbreviations
- C‐CAT
Center for Cancer Genomics and Advanced Therapeutics
- CGM
Cancer Genomic Medicine
- CKDB
Cancer Knowledge DataBase
- MHLW
Ministry of Health, Labour, and Welfare
- NCC
National Cancer Center
- NGS
next generation sequencing
1. CANCER STATISTICS IN JAPAN
For several years after World War II, approximately 50 000‐60 000 people in Japan died from cancer each year. Since then, the number of cancer deaths has increased steadily and cancer has become the leading cause of death, surpassing strokes in 1981. According to statistics compiled by the MHLW, in 2017 approximately 370 000 people died from cancer, accounting for 1 in every 3 deaths (Figure S1). Heart disease, the second biggest cause of mortality in Japan, accounted for only about half the number of cancer deaths.
The cumulative lifetime risk of cancer incidence in Japan is 62% for males and 46% for females, according to the incidence and mortality data in 2013. In other words, 1 in 2 Japanese people are estimated to be diagnosed with cancer during their lifetime. Conversely, the cancer survival rate has steadily improved over the past few decades, with the latest 5‐y relative survival rate for cancer patients in Japan being 62.1%, as reported by NCC in a survey report. 1
2. BACKGROUND OF CGM IN JAPAN
The National Diet of Japan enacted the Cancer Control Act in 2006 to enforce cancer control further, and formulated the 1st‐term “Basic Plan to Promote Cancer Control Programs” (hereinafter referred to as the “Basic Plan”) to promote cancer control comprehensively and systematically, in 2007. Following the first edition, in 2012 the government formulated the 2nd‐term Basic Plan to expand its focused areas and goals by adding pediatric cancer, rare cancer, and social problems associated with cancer patients including cancer education and patients’ employment. These efforts produced positive expected results, such as a decrease in mortality rates and increase in 5‐y relative survival rates. As mentioned above, the cancer survival rate has steadily improved, however cancer remains the leading cause of death in Japan and a serious problem for the lives and health of Japanese citizens.
As a result, the government decided to progress cancer medicine further, formulating the 3rd‐term Basic plan in 2018. In the 3rd‐term Basic plan, 3 main targets were set: “Enhancement of cancer prevention and cancer screening based on scientific backgrounds,” “Realization of patient‐oriented cancer medicine,” and “Establishment of a society where people with cancer can live with dignity and relief.” The CGM was set at the top of the list to ensure the “Realization of patient‐oriented cancer medicine.”
3. ESTABLISHMENT OF CGM IN JAPAN
In 2017, the MHLW convened an Expert Meeting for the Cancer Genomic Medicine Promotion Consortium. This not only provided Japanese citizens with early access to leading‐edge CGM, but the Expert Meeting called for capitalization of Japan's strengths to develop innovative treatment methods. Furthermore, they called for contributions from other Asian countries by integrating the nation's knowledge and creating a framework to lead CGM on a global scale. In addition, the Expert Meeting clarified the ownership of the constructed framework as an asset of Japanese citizens and set directions of the functions and roles of medical institutions providing CGM and institutions for aggregating, managing, and promoting the use of the CGM data. Following the Expert Meeting for the Cancer Genomic Medicine Promotion Consortium, the MHLW set the Council of Cancer Genomic Medicine Promotion Consortium to discuss and decide the direction of CGM in Japan as ongoing (Figure 1).
FIGURE 1.
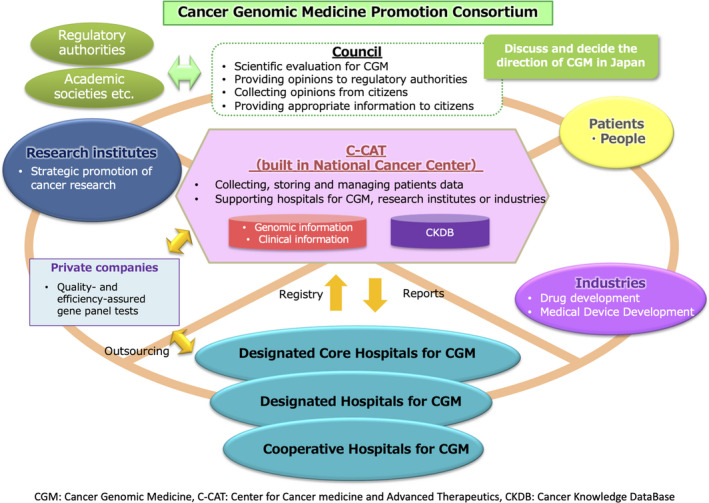
The outline of Cancer Genomic Medicine in Japan. The council plays an important role in discussing and deciding the direction of CGM in Japan. Patients, people, hospitals, academia, industry, etc, all work together to advance CGM in Japan
For providing CGM, under the national insurance system in Japan, the Ministry issued the “Guideline of Establishment of the network of the CGM hospitals. 2 ” This guideline describes the elements required for hospitals that carry out CGM in Japan based on the report of the Expert Meeting for the Cancer Genomic Medicine Promotion Consortium in December 2017. Based on the guideline, in February 2018 the Ministry designated 11 hospitals throughout Japan to serve as core hospitals for CGM (termed designated core hospitals for CGM) (the Ministry has since designated 1 additional hospital in April 2020, bringing the total to 12 hospitals.). The designated core hospitals were required to fulfill the guideline requirements, such as providing treatment for a sufficient number of cancer cases and a patient biobank for quality assurance, among other aspects mentioned in Table 1. The government also publicized 161 hospitals as liaison hospitals (called cooperative hospitals for CGM) to work with the core hospitals in unison by April 2020 (Figure 2). As Expert Panels required to implement CGM in Japan were allowed to be held in only the 11 designated core hospitals, there was concern that the 11 designated core hospitals alone would not be sufficient to respond to the increasing demand for CGM as it spreads throughout Japan. Hence, in September 2019, the Ministry designated 34 hospitals (called designated hospitals for CGM) among the cooperative hospitals in order to increase the number of hospitals that could conduct Expert Panels (in April 2020, 1 of the 34 hospitals was designated as the designated core hospitals, bringing the total number of hospitals to 33). In June 2019, 2 types of cancer tumor profiling tests became available at the 167 hospitals, all of which were affiliated to CGM; Expert Panels were held at 45 hospitals (the total of the designated core hospitals and the designated hospitals for CGM).
TABLE 1.
Requirements of designated core hospitals for Cancer Genomic Medicine in Japan
| 1 | Quality‐assured gene panel tests (outsourcing is acceptable) |
| 2 | Expert panels on cancer gene panel tests (cooperation with external experts in certain areas is acceptable) |
| 3 | Expert genomic counseling |
| 4 | Enough cases of cancers |
| 5 | Certificated system that can preserve genomic and clinical information safely, and which can register required information to C‐CAT |
| 6 | Certificated biobank |
| 7 | Appropriate system that can implement clinical trials such as advanced medical care, investigator‐initiated clinical trial, etc |
| 8 | Service window easily available for cancer patients and their families, and that can efficiently provide clinical trial information to patients |
Based on these requirements, in March 2018, 11 hospitals were designated throughout Japan, and 1 additional hospital was designated in April 2020, bringing the total to 12 hospitals.
Abbreviation: C‐CAT, Center for Cancer Medicine and Advanced Therapeutics.
FIGURE 2.
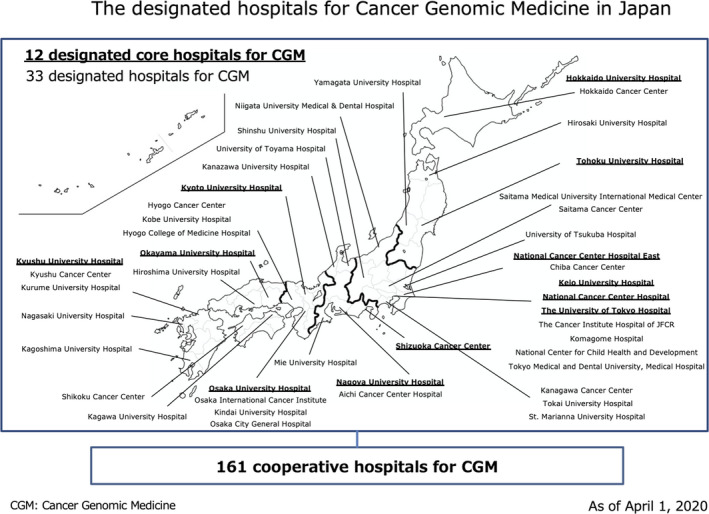
The designated hospitals for Cancer Genomic Medicine in Japan. In total, 206 hospitals as of April 1, 2020; 12 designated core hospitals, 33 designated hospitals, and 161 cooperative hospitals for Cancer Genomic Medicine
Three major Japanese cancer‐related academic societies (the Japanese Society of Medical Oncology, the Japanese Society of Clinical Oncology, and the Japanese Cancer Association) issued a consensus on clinical practice guidance for NGS‐based cancer tests (the Consensus Clinical Practice Guidelines for Next Generation Sequencing in Cancer Diagnosis and Treatment [edition 1.0]) in October 2017. 3 This clinical practice guideline was revised to edition 2.0 in March 2020. 4 Different from “Guideline of Establishment of the network of the CGM hospitals,” this guidance provides a certain direction for concerns when conducting tumor profiling tests for patients with solid cancers who can receive cancer pharmacotherapy under the national health insurance.
After the MHLW, in December 2018 the Japanese regulatory agency, approved 2 types of tumor profiling tests as comprehensive genomic profiling tests for all solid tumors (Table S1). Since June 1st 2019, the 2 types of hospitals for CGM (the designated core hospitals and the cooperative hospitals) became able to provide cancer tumor profiling tests under the national health insurance system; this includes informing patients on tumor profiling tests, submitting their samples, conducting the Expert Panels and feedback to patients by referring to the reports of tumor profiling tests and the Expert Panels. In the course of CGM in Japan, the designated core hospitals and the designated hospitals are required to implement that the Expert Panels liaise with the cooperative hospitals where all cases of tumor profiling tests are performed (Figure 3). CGM is supported by the analyses of genomic information of individual patients’ cancer cells, providing underpinning data to facilitate the optimization of treatments for individual patients. For extracting medically useful information from the source data for a comprehensive genomic analysis, this process calls for deliberations by multidisciplinary specialists such as oncologists, geneticists, pathologists, bioinformaticians, and genetic counselors. This body is called an “Expert Panel.” The Expert Panels are set at all designated core hospitals and the designated hospitals. The reports of the Expert Panels are generated and sent back to the physicians in the hospitals where patients had cancer tumor profiling tests based on the informed consent detailing the purpose and the significance of the tests. Physicians convey the content of the reports to the patients and choose the optimal treatment for patients.
FIGURE 3.
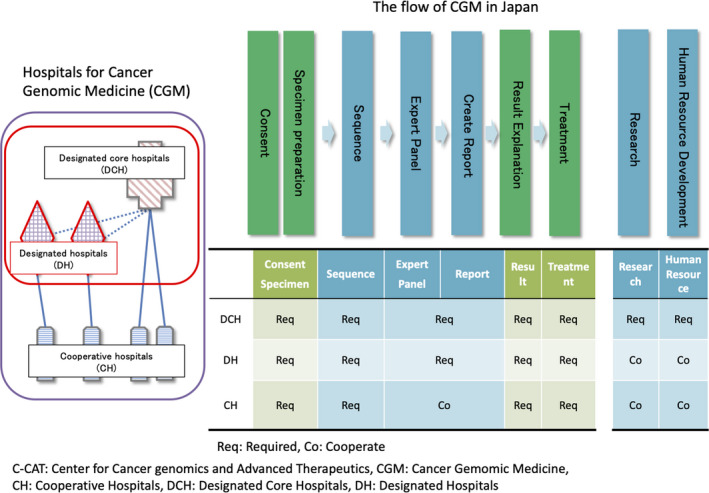
The function of the network of Cancer Genomic Medicine hospitals. Designated core hospitals (DCH) play a central role in CGM, research, and human resource development. DCH and designated hospitals (DH) can hold the Expert Panels, and cooperative hospitals have to take part in the Expert Panels held in DCH or DH in CGM
4. AGGREGATION AND MANAGEMENT OF INFORMATION OF CGM
The NCC established the Center for Cancer Genomics and Advanced Therapeutics (C‐CAT) in June 2018 based on the 3rd Basic Plan 5 with the aim to aggregate and manage genomic and clinical information, and support appropriate secondary use of these information to develop new types of medicine. The center developed and created a cancer genomic information repository, a master database for cancer genomic medicine and research that commenced trial operations in early 2019. The center will construct a cancer knowledge database (CKDB) for CGM. The center will fully utilize this framework and promote CGM in Japan alongside the network of the CGM hospitals.
In addition, C‐CAT will aggregate and manage clinical information pertaining to drug efficacy and adverse events obtained through the templates embedded in the electronic medical records in the hospitals and electronic data capture systems (Figure 4). At the same time, C‐CAT will build a system for quality control with the network with the CGM hospitals, with avenues for correction when required. The center annotates mutations described on the tumor profiling test report of each patient, referencing against the CKDB to create “C‐CAT findings” which are to be provided to the Expert Panels in the designated core hospitals and the designated hospitals for CGM. The center will also serve as a gateway to share part of the clinical and genomic information accumulated, to advance the quality of healthcare covered by the national healthcare insurance, and promote clinical studies at the designated core hospitals. Furthermore, the center will provide datasets for secondary use for medical research and development by academia and the private sector after proper review of applications for secondary use. Additionally, the center aims to establish a system supporting the network of the CGM hospitals as necessary.
FIGURE 4.
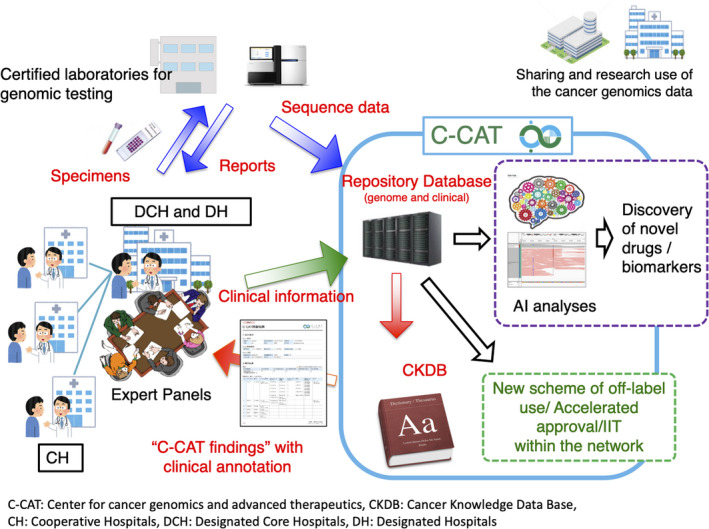
The function of the Center for Cancer medicine and Advanced Therapeutics (C‐CAT). C‐CAT collects and stores sequencing and clinical data of patients who have undergone tumor profiling tests, and supports the secondary use of these data for academia and industries
5. IMPLEMENTATION OF CGM IN JAPAN
As described above, the Ministry has established a system for providing CGM and for aggregating or managing information related to CGM. Some of the tumor profiling tests may be implemented initially as “advanced medical care” with clinical trials in Japan. “Advanced Medical Care” is a governmental scheme introduced as a way to assess the efficacy and toxicity of new treatments or devices on a clinical trial basis, for future inclusion into mainstream healthcare, with health insurance coverage. The first tumor profiling test approved as “advanced medical care” was an “NCC Oncopanel” offered by National Cancer Center Hospital. 6 It was examined by a hospital‐based prospective study (TOP‐GEAR project, 2nd stage) to investigate the feasibility and utility of NGS‐based analysis of 114 cancer‐associated genes, termed the NCC Oncopanel test. 7 It was examined by using it for analyses of 230 patients with advanced solid tumors, all of whom were also analyzed for the matching non‐tumor samples. In addition to this project, some marked research on clinical sequencing for CGM has also been conducted in Japan. 8 , 9 , 10
By referring to advanced medical care and other clinical data results, in December 2019 the MHLW approved 2 genomic profiling tests for solid cancer, NCC Oncopanel and FoundationOne CDx. The 2 tests were approved as a comprehensive genomic profiling test for all solid tumors. The latter was also approved as a broad companion diagnostic test for individuals living with advanced cancer. Both tests have been reimbursed by the national insurance system run by the MHLW since June 1, 2019 after deliberation by the Expert Committee. Under the national health insurance system, patients applicable for cancer tumor profiling tests were those who met the following 2 conditions: first, the attending physician determines that the patient status is sufficient for them to be able to undergo chemotherapy after the tumor profiling tests; second, each physician evaluates that a patient has no standard therapies, or has completed or will soon complete standard therapies for his/her solid cancer with local progression or metastasis. Thus, both tumor profiling tests are allowed to be implemented in the 167 hospitals for CGM.
Comparing the clinical sequence status with other countries, for example, the United States announced the following in March 2018: Centers for Medicare & Medicaid Services finalized a National Coverage Determination covering diagnostic laboratory tests using NGS for patients with advanced cancer. 11 Japan is the same as the United States in the following respects: firstly, these tests are used as a companion diagnostic to identify patients with specific genetic mutations that may benefit others; secondly, when a known cancer mutation cannot be matched to treatment, results from the diagnostic laboratory test using NGS can help to determine a patient's candidacy for cancer clinical trials. Conversely, the difference between Japan and the United States is that these tests are implemented only for stage 3 or 4 cancer patients in the United States, whereas these tests are implemented for cancer patients who have completed standard treatment in Japan. In South Korea, the government started in March 2017 to grant selective health insurance coverage for NGS‐based gene panel tests for the 10 most common cancer types. The scope of reimbursement was expanded to all types of solid cancer from May last year. Japan was 2 y behind South Korea in allowing insurance benefits for NGS testing. However, Japan established a national database for integrating relevant NGS data with clinical data, drug prescriptions, and therapeutic outcomes. The number of patients undergoing these tumor profiling tests is currently limited in Japan; the number of patients is expected to increase in the future as it is covered by universal health insurance.
Japan currently also has a unique system for secondary findings, germline mutations. The NCC Oncopanel searches for germline mutations in the 2 types of tumor profiling tests currently covered by insurance. Before taking this test, the patient may disclose his or her choice of whether or not to undergo disclosure of germline mutation information. When germline mutations are found, and preventive or therapeutic measures exist, the policy informs the patients and their blood relatives of the beneficial results for their health care. The Japan Agency for Medical Research and Development has also made recommendations on the process and specific policies for communicating the results. 12 As germline mutations have to be discussed in the Expert Panels, the Expert Panel members include experts in genetic medicine. This unique system is useful because it leads to the possibility of early detection of cancer in a patient's blood relatives.
In December 2019, the Ministry implemented a questionnaire survey for the network of the CGM hospitals and the response rate to the survey was 80.2% (134 out of 167: 11 of 11 designated core hospitals, 34 of 34 designated hospitals, and 89 of 122 cooperative hospitals). According to the survey, 805 cancer patients received the cancer tumor profiling tests in the 134 hospitals in the 5 mo from June 1, 2019 to October 31, 2019. Of the patients who have undergone tumor profiling tests, 99.3% (799 of 805) of the patients agreed to both data enrollment to C‐CAT and secondary use of their data, and the individualized treatments were identified for 10.9% (88 of 805) of the patients based on the results of the tumor profiling tests. The Ministry plans to continue the annual survey of all CGM hospitals to grasp the actual situation for CGM.
6. FUTURE PERSPECTIVES OF CGM IN JAPAN
Cancer tumor profiling tests had not been implemented in routine oncological practice until 2019 in Japan. However, the system and guidelines for providing CGM have been arranged antecedently and the cancer tumor profiling tests are rapidly spreading, being used for clinical practice after reimbursement by the national insurance system. Meanwhile, there are many issues that require solving in order to continue high‐quality CGM in Japan, and the government and academia are working together to promote CGM. First, the current tumor profiling tests are covered by insurance for patients with solid tumors who are no longer responding to standard treatment or who do not have a standard of care. However, in order to take advantage of their characteristics, it is necessary to be able to perform the tests at an early stage of treatment intervention. Of course, as companion diagnostics are approved by insurance in Japan, tumor profiling tests need to show greater efficacy at an early stage. The second issue is that a low percentage of patients who undergo tumor profiling tests are linked to treatment. It is vital to increase the proportion of patients whose results from tumor profiling tests lead to treatment, including clinical trials and off‐label use with appropriate procedures. In the revised March 2020 clinical practice guideline mentioned above, patients should be considered for inclusion in clinical trials and off‐label use whenever possible, based on the tumor profiling test results. In Japan, “C‐CAT findings” allow patients and their physicians to obtain information on clinical trials that may be enrolled based on tumor profiling tests. Additionally, there is a “Patient‐requested medical care system” for unapproved drugs and off‐label medication in Japan. The “Patient‐requested medical care system” is a system that allows unapproved drugs and other drugs to be used promptly as uninsured combined treatment and can only be implemented in hospitals that meet specific requirements. Finally, an ecosystem must be established to effectively use the genomic data obtained from tumor profiling tests and return it to the public. It is essential to establish a safe and effective system that can combine the genomic data with clinical data and other data.
Technologies for CGM are developing rapidly with novel systems in the process of being developed. For example, the use of liquid biopsies, which are gradually being put into practice mainly in western countries, are being implemented in Japan. Particularly, those involving cell‐free DNA from plasma are rapidly emerging as an important and minimally invasive adjunct to standard tumor biopsies, and, in some cases, even as a potential alternative approach. 13 Considering such circumstances, the Consensus Clinical Practice Guidelines for Next Generation Sequencing in Cancer Diagnosis and Treatment was revised to edition 2.0 by the 3 major Japanese cancer‐related societies.
Overall, in December 2019, the Ministry issued a concrete action plan for comprehensive analysis, including whole genome sequencing, for cancer and rare diseases (edition 1.0). 14 This action plan describes a concrete execution plan including numerical targets of specimens, human resource development, and a system development with reference to the UK, Genomics England, and other foreign projects, while studying issues of current CGM in Japan.
Through this project, the appropriate authorities are expected to realize the need for a further personalized cancer medicine and to promote CGM by utilizing leading‐edge technologies to help in aiding solutions for the issue of growing cancer incidence in Japan.
DISCLOSURE
The authors have no conflicts of interest.
Supporting information
Fig S1
Table S1
ACKNOWLEDGMENTS
This work was not supported by any grant and foundation. We are particularly grateful for the assistance provided by Hiroyuki Mano, Teruhiko Yoshida, and Takashi Kohno.
Mukai Y, Ueno H. Establishment and implementation of Cancer Genomic Medicine in Japan. Cancer Sci. 2021;112:970–977. 10.1111/cas.14754
REFERENCES
- 1. CANCER STATISTICS IN JAPAN 2018. https://ganjoho.jp/data/reg_stat/statistics/brochure/2018/cancer_statistics_2018_fig_E.pdf. Accessed November 17, 2020.
- 2. Guideline of Establishment of Designated Core Hospitals and etc. for Cancer Genomic Medicine <Resolved by the Ministry, December 25, 2019> (Japanese). https://www.mhlw.go.jp/content/000532262.pdf. Accessed November 17, 2020.
- 3. Sunami K, Takahashi H, Tsuchihara K, et al. Clinical practice guidance for next‐generation sequencing in cancer diagnosis and treatment (Edition 1.0). Cancer Sci. 2018;109(9):2980‐2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical practice guidance for next‐generation sequencing in cancer diagnosis and treatment (Edition 2.0). (Japanese). http://www.jca.gr.jp/researcher/topics/2020/files/20200518.pdf. Accessed November 17, 2020.
- 5. Basic Plan to Promote Cancer Control Programs (Phase 3) <Resolved by the Cabinet, March 9, 2018> (Japanese). https://www.mhlw.go.jp/file/06‐Seisakujouhou‐10900000‐Kenkoukyoku/0000196975.pdf. Accessed November 17, 2020.
- 6. National Cancer Center Hospital offers Next Generation Sequencing tests as ‘Advanced Medical Care’. Towards coverage under national health insurance system. https://www.ncc.go.jp/en/information/press_release/20180403_2/index.html. Accessed November 17, 2020.
- 7. Sunami K, Ichikawa H, Kubo T, et al. Feasibility and utility of a panel testing for 114 cancer‐associated genes in a clinical setting: a hospital‐based study. Cancer Sci. 2019;110(4):1480‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kou T, Kanai M, Yamamoto Y, et al. Clinical sequencing using a next‐generation sequencing‐based multiplex gene assay in patients with advanced solid tumors. Cancer Sci. 2017;108(7):1440‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naito Y, Takahashi H, Shitara K, et al. Feasibility study of cancer genome alterations identified by next generation sequencing: ABC study. Jpn J Clin Oncol. 2018;48(6):559‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kohsaka S, Tatsuno K, Ueno T, et al. Comprehensive assay for the molecular profiling of cancer by target enrichment from formalin‐fixed paraffin‐embedded specimens. Cancer Sci. 2019;110(4):1464‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. CMS finalizes coverage of Next Generation Sequencing tests, ensuring enhanced access for cancer patients. <CMS.gov>. https://www.cms.gov/newsroom/press‐releases/cms‐finalizes‐coverage‐next‐generation‐sequencing‐tests‐ensuring‐enhanced‐access‐cancer‐patients. Accessed November 17, 2020.
- 12. Recommendations for the Information Transfer Process in Genomic Medicine. <Japan Agency for Medical Research and Development> (Japanese). https://www.amed.go.jp/news/seika/kenkyu/20200121.html. Accessed November 17, 2020.
- 13. Corcoran RB, Chabner BA. Application of cell‐free DNA analysis to cancer treatment. N Engl J Med. 2018;379(18):1754‐1765. [DOI] [PubMed] [Google Scholar]
- 14. Action plan for the comprehensive analysis including whole genome sequencing for cancer and rare diseases (edition 1.0) (Japanese). https://www.mhlw.go.jp/content/10601000/000579016.pdf. Accessed November 17, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1


