Abstract
Background
In the global FLAURA study, first-line osimertinib, a third-generation irreversible tyrosine kinase inhibitor (TKI) of epidermal growth factor receptor (EGFR), significantly improved progression-free survival (PFS) and overall survival (OS) versus comparator EGFR TKIs in patients with EGFR mutation-positive (EGFRm) advanced non-small-cell lung cancer (NSCLC).
Objective
The FLAURA China study assessed first-line osimertinib in Chinese patients with EGFRm advanced NSCLC (NCT02296125).
Methods
FLAURA China was a double-blind, randomized, phase III study. Adults from mainland China with previously untreated EGFRm (Exon 19 deletion or L858R) advanced NSCLC were enrolled in the global study or a China-only study under the same protocol; 136 patients were randomized to osimertinib (80 mg once daily [od]; n = 71) or comparator EGFR TKI (gefitinib or erlotinib; all sites selected gefitinib 250 mg od; n = 65). Patients were randomized and allocated to treatment groups by a central computer system. Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent. The primary endpoint was investigator-assessed PFS; OS was a secondary endpoint.
Results
All 136 randomized patients were analyzed. Osimertinib extended median PFS by 8.0 months versus comparator EGFR TKI (17.8 vs. 9.8 months; hazard ratio [HR] 0.56; 95% confidence interval [CI] 0.37–0.85). Median OS was 33.1 months in the osimertinib group versus 25.7 months in the comparator group (HR 0.85; 95% CI 0.56–1.29). At 3 years, 20% of patients on osimertinib and 8% on the comparator remained on randomized treatment. Grade 3 or higher adverse events (AEs) were reported in 54 and 28% of patients in the osimertinib and comparator groups, respectively, driven by increased local reporting of laboratory- and disease-related AEs. No new safety signals were identified.
Conclusions
First-line osimertinib treatment resulted in a clinically meaningful PFS and OS benefit versus comparator EGFR TKI in Chinese patients with EGFRm advanced NSCLC. Safety data were consistent with the known safety profile of osimertinib.
Clinical Trial Registration
ClinicalTrials.gov NCT02296125, registered 20 November 2014
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-021-00794-6.
Key Points
| FLAURA assessed first-line osimertinib in patients with previously untreated epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer. |
| The FLAURA China study enrolled 136 patients from mainland China, including 19 patients from the global FLAURA study; all were enrolled using the same FLAURA study protocol. |
| A clinically meaningful benefit in progression-free survival and overall survival was observed with osimertinib versus comparator EGFR tyrosine kinase inhibitor. No new safety signals were reported in the FLAURA China study. |
Introduction
In China, the prevalence of epidermal growth factor receptor (EGFR) mutations in patients with non-small-cell lung cancer (NSCLC) is high, ranging from 36 to 48% [1–4]. For Chinese patients with advanced NSCLC harboring an EGFR tyrosine kinase inhibitor (EGFR TKI)-sensitizing mutation (EGFRm), EGFR TKIs are the recommended first-line standard of care [5].
First-line therapy with first- and second-generation EGFR TKIs has been shown to improve progression-free survival (PFS) compared with chemotherapy in previously untreated Chinese patients with EGFRm advanced NSCLC; however, the PFS benefits have not translated to benefits in overall survival (OS) [6–9].
Osimertinib is a third-generation irreversible oral EGFR TKI that potently and selectively inhibits both EGFRm and EGFR T790M resistance mutations and has demonstrated efficacy in NSCLC central nervous system (CNS) metastases [10–14]. In the global phase III FLAURA study (NCT02296125), osimertinib demonstrated significantly longer PFS than comparator EGFR TKIs (erlotinib or gefitinib) in the first-line treatment of patients with EGFRm advanced NSCLC (hazard ratio [HR] 0.46; 95% confidence interval [CI] 0.37–0.57; P < 0.001), with median PFS of 18.9 versus 10.2 months [14]. A final OS analysis in FLAURA also demonstrated significantly longer OS with osimertinib than with comparator EGFR TKI (median duration 38.6 vs. 31.8 months; HR 0.80; 95.05% CI 0.64–1.00; P = 0.046) [15].
The FLAURA China study assessed the efficacy and safety of first-line osimertinib in Chinese patients with advanced EGFRm NSCLC who were either enrolled in the global FLAURA study or a China-only study under the same protocol. Here, we report efficacy and safety data from the FLAURA China study.
Materials and Methods
Full methodological details for the FLAURA study have been previously published [14, 15]. The FLAURA China study in patients from mainland China was conducted under the same protocol, and brief details are given in the following section.
Study Design and Patients
In this double-blind, randomized, phase III study, eligible patients were aged ≥ 18 years, had locally advanced or metastatic NSCLC with local or central confirmation of Exon 19 deletion (Ex19del) or L858R mutations by biopsy tissue testing, had received no previous treatment for advanced disease, and had a World Health Organization (WHO) performance status of 0–1. Neurologically stable patients with CNS metastases were eligible if definitive treatment or corticosteroids were completed ≥ 2 weeks before enrollment.
Patients were randomized 1:1 to oral osimertinib 80 mg once daily or comparator EGFR TKI (erlotinib or gefitinib; all Chinese sites selected oral gefitinib 250 mg once daily as erlotinib did not have marketing authorization in China until after initiation of enrollment) and were stratified by mutation status (Ex19del/L858R) and race (Asian/non-Asian; however, all patients were in the stratum of Asian race). Treatment continued until disease progression as defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, unacceptable toxicity, or withdrawal of consent. Treatment after disease progression was allowed if patients continued to show clinical benefit, as judged by the investigator. Patients receiving comparator EGFR TKI were eligible to cross over to open-label osimertinib after confirmation of disease progression by blinded independent central review (BICR), or by investigator assessment if disease progression occurred after the primary PFS data cutoff (DCO), and post-progression tumor T790M-positive status by local or central testing.
The study was approved by the institutional review board or independent ethics committee of each study center. The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines (as defined by the International Conference on Harmonization), applicable regulatory requirements, and the policy on bioethics and human biologic samples of the study sponsor, AstraZeneca. All patients provided written informed consent prior to treatment.
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Study Endpoints
The primary endpoint was investigator-assessed PFS according to RECIST v1.1. A sensitivity analysis of PFS was conducted based on BICR of imaging data. Secondary endpoints included OS and safety; after the primary endpoint PFS analysis, central collection of progression events by RECIST v1.1 ceased. Exploratory endpoints also included time to first or second subsequent therapy (TFST/TSST).
Study Assessments
For PFS, tumor assessments were performed at baseline, every 6 weeks for 18 months, then every 12 weeks until disease progression. For OS, assessments for survival were performed every 6 weeks after objective disease progression up to the final OS analysis cutoff. PFS was defined as the time from randomization to objective disease progression or death from any cause in the absence of progression, irrespective of withdrawal from the study or treatment with another anticancer therapy before progression. OS was defined as the time from randomization until death from any cause. TFST/TSST was defined as the time from the date of randomization to the earliest start date of first/second subsequent anticancer therapy following study drug discontinuation, or death.
Adverse events (AEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.
Statistical Analyses
All prespecified statistical analyses for the FLAURA China study are considered exploratory, and the reported P values are nominal. The full analysis set consisted of all randomized patients in the FLAURA China study. The safety analysis set consisted of all patients in the full analysis set who received at least one dose of study treatment.
Kaplan–Meier methodology with a log-rank test, stratified according to mutational status (Ex19del/L858R), was used to compare PFS and OS in the two treatment groups, and the Breslow approach was used to handle tied events. Data for patients who had not had a progression event or had not died at the time of the analysis were censored at the time of the last RECIST assessment that could be evaluated for PFS or at the last recorded date that the patient was known to be alive for OS. PFS and OS were also assessed in predefined subgroups using a Cox proportional hazards model.
DCOs for the primary PFS analysis and final OS analysis were 10 January 2018 and 25 June 2019, respectively.
Results
Patient Demographics and Characteristics
Overall, 136 Chinese patients were randomized, including 19 Chinese patients who were part of the global study and an additional 117 Chinese patients enrolled under the same protocol after the global recruitment; 71 patients received osimertinib and 65 patients received comparator EGFR TKI (gefitinib) (Fig. 1 in the electronic supplementary material [ESM]). All patients received at least one dose of study drug. Patient demographics and clinical characteristics at baseline were generally well balanced between treatment groups (Table 1) with the exception of a higher proportion of female patients in the comparator EGFR TKI group (71%) than in the osimertinib group (61%), more patients with CNS metastases (32 vs. 24%), and more patients with extrathoracic visceral metastases (46 vs. 35%). At baseline, 90 and 80% of patients receiving osimertinib and comparator EGFR TKI had a WHO performance status of 1, respectively.
Table 1.
Patient baseline demographics and clinical characteristics (full analysis set)
| Characteristics | Osimertinib (n = 71) |
Comparator EGFR TKI (n = 65) |
|---|---|---|
| Age (years) | 60 (29–80) | 61 (32–82) |
| Female | 43 (61) | 46 (71) |
| Smoking | ||
| Never | 53 (75) | 50 (77) |
| Current | 3 (4) | 4 (6) |
| Former | 15 (21) | 11 (17) |
| WHO performance status | ||
| 0 | 7 (10) | 13 (20) |
| 1 | 64 (90) | 52 (80) |
| Overall disease classification | ||
| Metastatica | 69 (97) | 65 (100) |
| Locally advancedb | 2 (3) | 0 |
| Metastases | ||
| Extrathoracic visceral metastasesc | 25 (35) | 30 (46) |
| CNS metastasesd | 17 (24) | 21 (32) |
| EGFR mutation typee | ||
| L858R | 35 (49) | 32 (49) |
| Exon 19 deletion | 36 (51) | 33 (51) |
| Tumor lesion size (mm)f | 52.0 (14‒171) | 44.0 (14‒163) |
Data are presented as median (range) or n (%) unless otherwise indicated
CNS central nervous system, EGFR epidermal growth factor receptor, EGFR TKI EGFR tyrosine kinase inhibitor, WHO World Health Organization
aPatient had any metastatic site of disease
bPatient had only locally advanced sites of disease
cVisceral metastases were determined programmatically from baseline data for which the disease site was described as adrenal, ascites, brain or CNS, gastrointestinal, genitourinary, hepatic (including gallbladder), liver, other CNS, pancreas, peritoneum, or spleen. Also included were other metastatic sites, such as the eye and thyroid, as identified as extrathoracic visceral sites by AstraZeneca physicians
dCNS metastases were determined programmatically from baseline data for the CNS lesion site, medical history, surgery, or radiotherapy
eEGFR based on local or central test used for randomization strata
fLongest diameter
Treatment
At the DCO for the final OS analysis, the median (range) duration of treatment exposure was 20.0 (0.3–39.7) months in the osimertinib group and 13.6 (1.1–39.1) months in the comparator EGFR TKI group. At 12, 24, and 36 months, respectively, 52 (73%), 27 (38%), and 14 (20%) patients remained on study treatment in the osimertinib group versus 37 (57%), 9 (14%), and five (8%) patients in the comparator EGFR TKI group.
In total, 15 patients in the osimertinib group (21%) and three patients in the comparator EGFR TKI group (5%) were ongoing with their randomized treatment (Fig. 1 in the ESM).
Progression-Free Survival
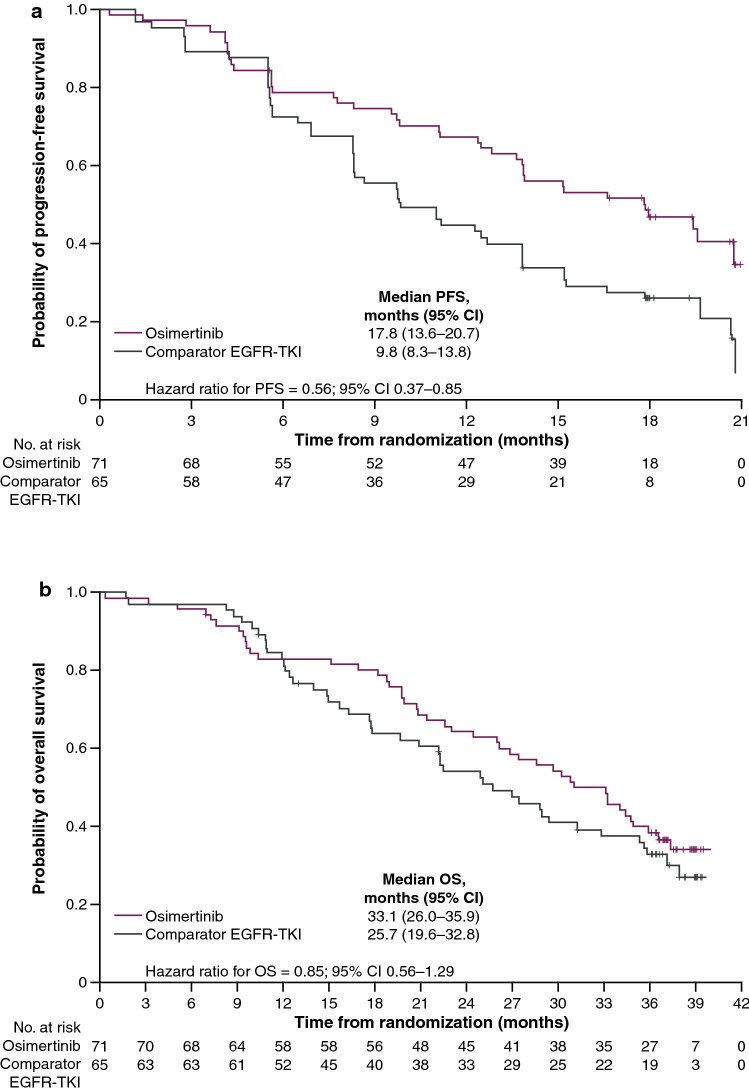
The median follow-up for PFS was 16.7 months in the osimertinib group and 9.8 months in the comparator EGFR TKI group. At the DCO for the primary endpoint PFS (10 January 2018), RECIST-defined disease progression or death had occurred in 40 (56%) and 51 (78%) patients in the osimertinib and comparator EGFR TKI groups, respectively, resulting in an overall 67% PFS maturity (Table 2). Investigator-assessed PFS was longer in the osimertinib group than in the comparator EGFR TKI group (HR 0.56; 95% CI 0.37–0.85; P = 0.007), with median PFS extended by 8 months: 17.8 (95% CI 13.6–20.7) versus 9.8 months (95% CI 8.3–13.8) (Table 2). There was separation of the Kaplan–Meier curves in favor of osimertinib over comparator EGFR TKI from 6 months onward (Fig. 1a). At 18 months, 47% of patients in the osimertinib group and 26% of patients in the comparator EGFR TKI group were alive and progression free. A sensitivity analysis based on BICR-assessed PFS was consistent with the investigator-based assessment (see the supplementary information and Fig. 2 in the ESM).
Table 2.
Progression-free survival and overall survival (full analysis set)
| Osimertinib (n = 71) |
Comparator EGFR TKI (n = 65) |
|
|---|---|---|
| PFS | ||
| Patients with PFS events | ||
| RECIST progression | 35 (49) | 49 (75) |
| Death | 5 (7) | 2 (3) |
| PFS (months) | 17.8 (13.6–20.7) | 9.8 (8.3–13.8) |
| HRa | 0.56 (0.37–0.85); P = 0.007 | |
| Proportion of patients progression-free | ||
| At 6 months | 78.8 (67.3–86.6) | 72.3 (59.7–81.6) |
| At 12 months | 67.3 (55.0–76.9) | 44.6 (32.3–56.2) |
| At 18 months | 46.9 (34.8–58.1) | 25.8 (15.9–36.9) |
| OS | ||
| Deaths | 45 (63) | 44 (68) |
| OS, months | 33.1 (26.0–35.9) | 25.7 (19.6–32.8) |
| HRa | 0.85 (0.56–1.29); P = 0.442 | |
| Survival | ||
| At 12 months | 82.9 (71.9–89.9) | 81.4 (69.6–89.0) |
| At 24 months | 64.3 (52.0–74.3) | 54.2 (41.2–65.5) |
| At 36 months | 38.6 (27.3–49.8) | 32.6 (21.3–44.3) |
Data are presented as n (%) or median (95% CI) unless otherwise indicated. Proportion of patients progression-free and survival are presented as % (95% CI)
CI confidence interval, EGFR TKI epidermal growth factor receptor tyrosine kinase inhibitor, HR hazard ratio, OS overall survival, PFS progression-free survival, RECIST Response Evaluation Criteria in Solid Tumors
aP value is nominal
Fig. 1.
Kaplan–Meier plots of a investigator-assessed progression-free survival and b overall survival (full analysis set). Censored data are indicated by tick marks. Data from patients who had not died at the time of the analysis were censored on the basis of the last recorded date on which the patient was known to be alive. CI confidence interval, EGFR TKI epidermal growth factor receptor tyrosine kinase inhibitor, OS overall survival, PFS progression-free survival
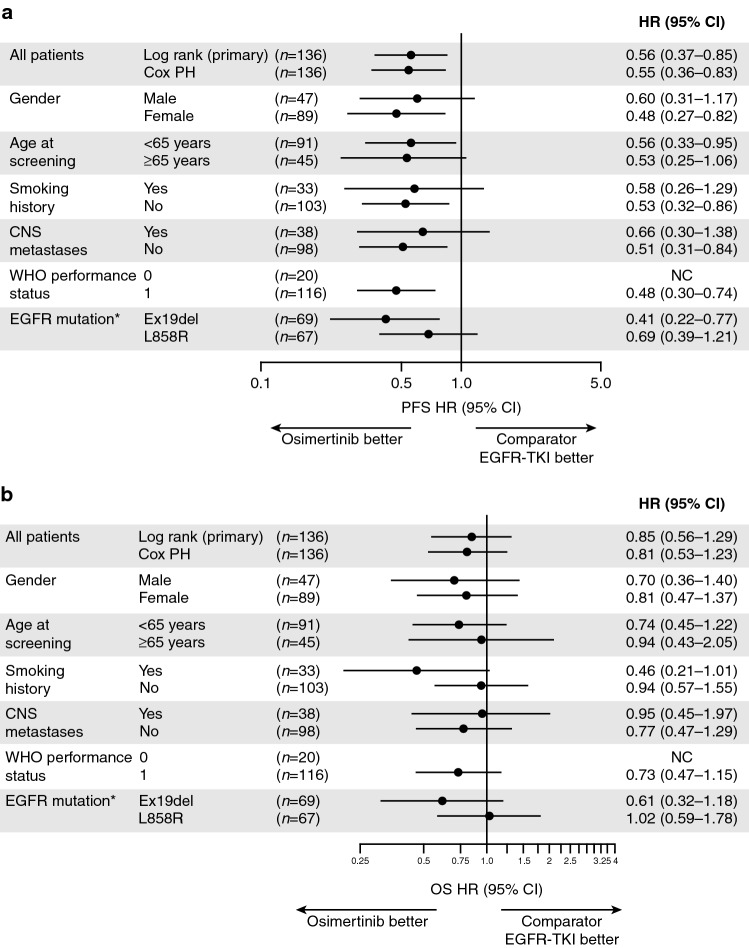
PFS benefit with osimertinib was consistent across all predefined subgroups (Fig. 2a). Regardless of the status of known or treated CNS metastases at study entry, CNS progression was observed in two patients (3%) in the osimertinib group and 13 patients (20%) in the comparator EGFR TKI group.
Fig. 2.
Subgroup analyses of a investigator-assessed progression-free survival and b overall survival (full analysis set). This analysis was performed using a Cox proportional hazards model, including treatment, subgroup, and a treatment-by-subgroup interaction term. Subgroup categories with < 20 events were excluded from the analysis. A hazard ratio of <1.00 indicates a lower risk of death with osimertinib than with the comparator EGFR TKI. CI confidence interval, CNS central nervous system, EGFR epidermal growth factor receptor, HR hazard ratio, NS not calculable, OS overall survival, PFS progression-free survival, PH proportional hazards, TKI tyrosine kinase inhibitor, WHO World Health Organization. *EGFR mutation is by method used at randomization
Overall Survival
All patients had the opportunity to have ≥ 36 months of follow-up. The median follow-up for OS was 31.0 months in the osimertinib group and 24.9 months in the comparator EGFR TKI group. At DCO for the final OS analysis (25 June 2019), 45 (63%) and 44 (68%) patients had died in the osimertinib and comparator EGFR TKI groups, respectively, resulting in an overall 65% OS maturity. The HR for OS was 0.85 (95% CI 0.56–1.29; nominal P=0.442), with median OS extended by 7.4 months in the osimertinib group compared with comparator EGFR TKI group: 33.1 months (95% CI 26.0–35.9) versus 25.7 months (95% CI 19.6–32.8) (Table 2). There was clear separation of the Kaplan–Meier curves from 12 months onward in favor of osimertinib compared with comparator EGFR TKI (Fig. 1b). Survival rates were similar between the treatment groups at 12 months and higher in the osimertinib group than in the comparator EGFR TKI group at 24 and 36 months (Table 2). OS benefit with osimertinib compared with comparator EGFR TKI was observed across most predefined subgroups, with some variation in the magnitude of benefit (Fig. 2b).
Subsequent Anticancer Therapies
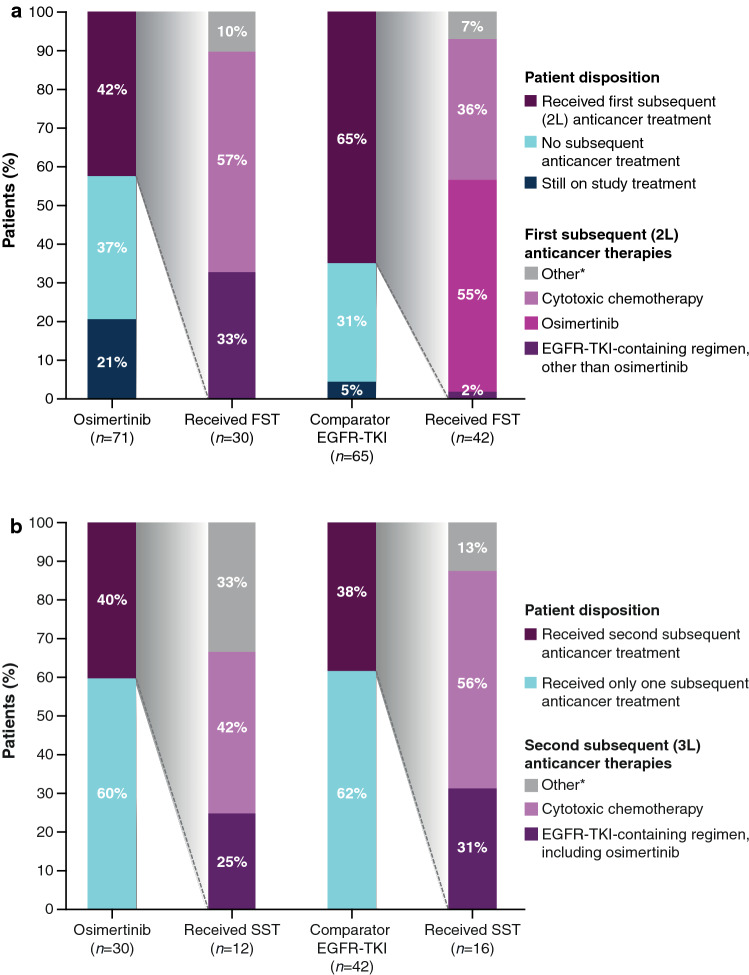
Following discontinuation of randomized treatment, 30 patients (42%) in the osimertinib group and 42 patients (65%) in the comparator EGFR TKI group started a first subsequent anticancer therapy (FST) (Fig. 3a). The majority of patients (n = 17; 57%) in the osimertinib group received cytotoxic chemotherapy agents as FST, one-third (n = 10; 33%) received EGFR TKIs (excluding osimertinib), and the remainder (n = 3; 10%) received other treatments (i.e., not chemotherapy or an EGFR TKI). Most patients in the comparator EGFR TKI group (n=23; 55%) received osimertinib as FST, approximately one-third received cytotoxic chemotherapy (n=15; 36%), whereas n=3 (7%) received other treatments, and n=1 (2%) received EGFR TKIs excluding osimertinib (Table 1 in the ESM). The median (95% CI) TFST was 21.4 months (18.8–27.4) and 15.8 months (11.9–20.0) in the osimertinib and comparator EGFR TKI groups, respectively (HR 0.62; 95% CI 0.42–0.92) (Fig. 3a in the ESM).
Fig. 3.
a First and b second subsequent anticancer therapies received (full analysis set). The first/second subsequent anticancer therapy is the first/second treatment started on or after the last dose date of randomized study treatment. 2L second line, 3L third line, EGFR epidermal growth factor receptor, FST first subsequent therapy, SST second subsequent therapy, TKI tyrosine kinase inhibitor. *Other therapy refers to patients who did not receive either chemotherapy or an EGFR TKI
A second subsequent anticancer therapy was started in 12 patients (17%) in the osimertinib group and 16 patients (25%) in the comparator EGFR TKI group (Fig. 4b; Table 2 in the ESM). The median (95% CI) TSST was 29.6 (23.1–33.9) and 22.1 (17.4–28.8) months in the osimertinib and comparator EGFR TKI groups, respectively (HR 0.79; 95% CI 0.52–1.18) (Fig. 3b in the ESM).
Safety
At the time of the DCO for OS (25 June 2019), the most commonly reported AEs in the osimertinib group and the comparator EGFR TKI group, respectively, were decreased white blood cell count (41 and 9%), anemia (38 and 17%), decreased platelet count (28 and 2%), diarrhea (24 and 29%), decreased neutrophil count (24 and 5%), and decreased weight (24 and 12%) (Table 3). AEs considered by the investigator to be possibly causally related to study drug are reported in Table 2 in the ESM.
Table 3.
Adverse events reported in ≥10% of the patients in either study group (safety analysis set)
| Adverse events | Osimertinib (n = 71) |
Comparator EGFR TKI (n = 65) |
|---|---|---|
| AE, any cause [n (%)] | ||
| Any AE | 70 (99) | 64 (99) |
| Any AE grade 3 or higher | 38 (54) | 18 (28) |
| Any fatal AE | 7 (10) | 3 (5)a |
| Any serious AE | 25 (35) | 12 (19) |
| Any AE leading to discontinuation of treatment | 9 (13) | 4 (6) |
| AE, possibly causally related to treatment [n (%)]b | ||
| Any AE | 66 (93) | 56 (86) |
| Any AE grade ≥ 3 | 18 (25) | 10 (15) |
| Any fatal AE | 3 (4) | 1 (2) |
| Any serious AE | 9 (13) | 4 (6) |
| Any AE leading to discontinuation of treatment | 6 (9) | 2 (3) |
| Most common AEs in ≥10% of the patients in either study group [n (%)] | ||
| White blood count decreased | 29 (41) | 6 (9) |
| Anemia | 27 (38) | 11 (17) |
| Rash or acnec | 26 (37) | 25 (39) |
| Platelet count decreased | 20 (28) | 1 (2) |
| Diarrhea | 17 (24) | 19 (29) |
| Neutrophil count decreased | 17 (24) | 3 (5) |
| Weight decreased | 17 (24) | 8 (12) |
| Cough | 14 (20) | 11 (17) |
| Hypoalbuminemia | 12 (17) | 6 (9) |
| Hypokalemia | 12 (17) | 8 (12) |
| Leucopenia | 12 (17) | 2 (3) |
| Mouth ulceration | 12 (17) | 7 (11) |
| Neutropenia | 12 (17) | 2 (3) |
| AST increased | 11 (16) | 28 (43) |
| Lymphocyte count decreased | 11 (16) | 3 (5) |
| Decreased appetite | 10 (14) | 8 (12) |
| Dyspnea | 10 (14) | 5 (8) |
| Nail effectsc | 10 (14) | 2 (3) |
| Nausea | 10 (14) | 7 (11) |
| Vomiting | 10 (14) | 5 (8) |
| Hyponatremia | 9 (13) | 0 |
| Proteinuria | 9 (13) | 7 (11) |
| Upper respiratory tract infection | 9 (13) | 3 (5) |
| Hematuria | 8 (11) | 5 (8) |
| Non-cardiac chest pain | 8 (11) | 5 (8) |
| Urinary tract infection | 8 (11) | 8 (12) |
| Dry skinc | 7 (10) | 10 (15) |
| Hypocalcemia | 7 (10) | 7 (11) |
| ALT increased | 6 (9) | 29 (45) |
| Chest discomfort | 6 (9) | 8 (12) |
| GGT increased | 6 (9) | 7 (11) |
AE adverse event, ALT alanine aminotransferase, AST aspartate aminotransferase, EGFR TKI epidermal growth factor receptor tyrosine kinase inhibitor, GGT gamma-glutamyl transferase
aAfter the 28-day follow-up period, one additional fatal AE occurred in the comparator EGFR TKI group
bAs assessed by investigator
cThis category is a grouped term
AEs of grade 3 or higher were reported in 54% of patients in the osimertinib group and 28% of patients in the comparator EGFR TKI group, with the most frequent being decreased neutrophil count, decreased lymphocyte count, increased aspartate aminotransferase, and decreased white blood cell count (Table 3 in the ESM). The difference between treatment arms was largely driven by the increased reporting of laboratory- and disease-related symptoms as AEs in the osimertinib arm, due to specific local AE-reporting habits and requirements at some sites. However, no new safety concerns were identified for laboratory-related AEs, and shifts in grade of severity did not result in any clinically significant sequelae.
Cardiac effects (QT) were reported in more patients in the osimertinib group (n=7; 10%) than in the comparator EGFR TKI group (n=5; 8%). AEs of QT prolongation in the osimertinib group and the comparator EGFR TKI group, respectively, were of grade 1 (2 patients [3%] and 2 patients [3%]), grade 2 (2 patient [3%] and 1 patient [2%]) or grade 3 intensity (2 patients [3%] and 0 patients). No cases of fatal QT prolongation occurred in either group. Analysis of the QT interval as measured by electrocardiography showed that median QT interval corrected for heart rate by Fridericia’s formula (QTcF) at baseline was 408.3 msec in the osimertinib group and 410.7 ms in the comparator EGFR TKI group, which increased from baseline by 17.7 and 7.8 ms, respectively, within a few weeks before becoming stable in both groups. Patients with changes in prespecified QTcF thresholds are shown in Table 4 in the ESM.
AEs of interstitial lung disease (ILD) and pneumonitis (grouped term) were reported in two patients (3%) in each group. These events comprised one serious AE (SAE) of ILD and one AE of pneumonitis, both grade 3, in the osimertinib group, and one SAE of ILD, which was fatal, and one AE of pneumonitis (grade unknown) in the comparator EGFR TKI group.
SAEs were reported in 25 patients (35%) in the osimertinib group and 12 patients (19%) in the comparator EGFR TKI group (Table 5 in the ESM). The majority of SAEs were reported in one patient only, except for SAEs of pleural effusion in three patients and pneumonia in three patients in the osimertinib group.
Fatal AEs occurred in seven patients (10%) in the osimertinib group (cardiac arrest, cardiac tamponade, “death” [no further specifics supplied], depression, poisoning, respiratory failure, and upper gastrointestinal hemorrhage) and three patients (5%) in the comparator EGFR TKI group (blood disorder, ILD, and “death” [no further specifics supplied]). In addition, in the comparator EGFR TKI group, one death occurred after the 28-day follow-up period (lung infection). Three fatal AEs were due to the disease under investigation and an AE: cardiac tamponade and upper gastrointestinal hemorrhage in the osimertinib group and lung infection in the comparator EGFR TKI group. Four fatal AEs were considered by the investigator to be possibly causally related to study drug: cardiac tamponade, “death,” and upper gastrointestinal hemorrhage in the osimertinib group and ILD in the comparator EGFR TKI group. On review of the data, all of the fatal AEs in the osimertinib arm were confounded or indicative of disease progression.
The proportion of AEs leading to discontinuation was higher in the osimertinib group (n = 9; 13%) than in the comparator EGFR TKI group (n = 4; 6%) (Table 6 in the ESM), largely driven by greater reporting of disease-related fatal events that were also reported as AEs leading to discontinuation (n = 5) in the osimertinib arm. The number of remaining AEs leading to discontinuation in the osimertinib group were comparable.
Discussion
In the FLAURA China study, patients with EGFRm NSCLC treated with first-line osimertinib had a longer PFS and OS than those who received a comparator EGFR TKI. The median PFS of 17.8 months observed at the first DCO translated to a median OS of 33.1 months at the final DCO. There was an extension in the median OS of 7.4 months, together with a 15% reduction in the risk of death. After 36 months, 20% of patients in the osimertinib group and 8% of patients in the comparator EGFR TKI group remained on randomized study treatment. The safety profile of osimertinib in FLAURA China was generally consistent with the global FLAURA population, and no new safety signals were reported.
The magnitude of clinical benefit observed with osimertinib in the FLAURA China study was consistent with results from the global FLAURA study [14, 15]. In the FLAURA China and global FLAURA populations, osimertinib extended PFS by 8.0 and 8.7 months and OS by 7.4 and 6.8 months, respectively [14, 15]. Median PFS and OS values for both treatment groups were lower in the FLAURA China study than in the global FLAURA study. For the osimertinib and comparator EGFR TKI groups, respectively, median PFS was 17.8 versus 9.8 months in the FLAURA China study and 18.9 versus 10.2 months in the global FLAURA study. The corresponding results for median OS were 33.1 versus 25.7 months in FLAURA China and 38.6 versus 31.8 months in FLAURA [14, 15]. This difference may have been due, in part, to the higher disease burden of patients enrolled in the FLAURA China study than the global population, as indicated by a higher proportion of patients with WHO performance status 1 in FLAURA China (85%) than in FLAURA (59%) and a higher proportion of patients with extrathoracic visceral metastases (40 vs. 35%) [14]. More patients also had known or treated CNS metastases at study entry in FLAURA China than in the global population (28 vs. 21%, respectively) [14].
The PFS benefit was consistent across all predefined subgroups, including patients with or without known or treated CNS metastases at baseline. Regardless of CNS metastases status at study entry, patients receiving osimertinib had lower progression in CNS than those receiving comparator EGFR TKI (n = 2 [3%] vs. n = 13 [20%]). This finding is consistent with CNS efficacy in the global FLAURA study [12, 14]. The OS benefit also favored osimertinib across most subgroups, with variations in the magnitude of the benefit; the EGFR L858R mutation subgroup had an HR of 1.02, with CIs that overlapped those of the EGFR Ex19del subgroup (HR 0.61). The subgroup analyses for the FLAURA China study should be interpreted with caution given the patient numbers and exploratory nature of the analysis.
All patients in the comparator EGFR TKI group received gefitinib as this was the only approved first-line treatment in China during enrollment. The median PFS and OS for the comparator EGFR TKI group was consistent with those in previous reports for Asian or Chinese patients with EGFRm NSCLC receiving first-line first- or second-generation EGFR TKIs (PFS 9.2‒16.6 months; OS 20.7‒34.2 months) [6, 7, 9, 16–21], particularly when accounting for the exclusion of patients with CNS metastases from many first- and second-generation EGFR TKI studies. Most of these trials, which compared EGFR TKI with chemotherapy or head to head with another EGFR TKI, have not demonstrated statistically significant differences in OS [7, 9, 16, 17, 21].
The clinically meaningful longer TFST (HR 0.62; 95% CI 0.42–0.92) was maintained through to TSST (HR 0.79; 95% CI 0.52–1.18). The subsequent therapies received by patients after discontinuing randomized treatment were most frequently chemotherapy in the osimertinib group (in accordance with treatment guidelines) [5], followed by other EGFR TKIs, similar to the pattern observed in the global FLAURA study. In the comparator EGFR TKI group, the most frequently received FST was another EGFR TKI; of the 42 patients who discontinued comparator EGFR TKI and received an FST, 23 (55%) received osimertinib, for which they were eligible due to T790M positivity. This is in line with expectations because most patients will develop resistance, despite initial responses to first-generation EGFR TKIs, with one of the most common mechanisms being the EGFR T790M mutation, occurring in 36–56% of Chinese patients [22–25]. Emergence of the T790M resistance mutation and progressive disease in the comparator group was accommodated by the FLAURA China study design, where patients had the opportunity to cross over to receive osimertinib. Osimertinib was shown to be more effective than other earlier-generation EGFR TKIs in pretreated patients who developed the T790M resistance mutation [11, 26, 27]. Despite a crossover rate of 34% (n = 22) from the comparator group after disease progression and developing the T790M mutation, to receive osimertinib, a survival benefit was seen with osimertinib.
Overall, safety data in FLAURA China were similar to those from the global FLAURA population [15], although the proportion of reported grade 3 or higher AEs and SAEs was higher in the osimertinib group than in the comparator EGFR TKI group. These were largely driven by the local practice of increased reporting of laboratory- and disease-related symptoms as AEs. Per protocol, events due to progression were not to be reported as AEs or SAEs, and abnormal laboratory results were not to be reported as AEs unless they were SAEs, the reason for discontinuation of osimertinib, or clinically significant. However, despite this increased reporting, no new safety signals were identified, including QTcF prolongation and ILD, compared with previous studies [14, 15]. Discontinuations due to AEs were comparable with those in the global FLAURA study [15], although local reporting habits and requirements to report disease-related fatal AEs as discontinuations led to a higher proportion of discontinuations being reported for osimertinib than for the comparator EGFR TKI group. Overall, osimertinib demonstrated acceptable tolerability in this population. It should be noted that treatment duration was 6.4 months longer in the osimertinib group than in the comparator EGFR TKI group, and—even with this increased exposure—the incidence of AEs was similar in both groups.
Conclusions
A clinically meaningful PFS benefit was observed with osimertinib compared with comparator EGFR TKI in Chinese patients with EGFRm advanced NSCLC, which translated into clinically meaningful increases in TFST, TSST, and OS. The safety profile of osimertinib in this patient population was generally consistent with that in the global trial, and no new safety signals were reported.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank all the patients and their families. The authors would like to acknowledge Sally Cotterill, PhD, CMPP, of Ashfield Healthcare Communications, Macclesfield, UK, part of UDG Healthcare plc, for medical writing support that was funded by AstraZeneca, Cambridge, UK, in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Declarations
Funding
The study (NCT02296125) was funded by AstraZeneca, Cambridge, UK, the manufacturer of osimertinib. AstraZeneca was involved in the study design; the collection, analysis, and interpretation of data; the writing of this article; and its approval for submission.
Conflicts of Interest
YC, WL, HZ, and CL have no conflicts of interest that are directly relevant to the content of this article. YH has received personal fees from AstraZeneca, Eli Lilly, Pfizer, and Roche for speaker bureau/expert testimony. QZ has received personal honoraria from AstraZeneca and Roche. BW has received personal fees from AstraZeneca, Boehringer Ingelheim, and Roche for advisory/consultancy and speaker bureau/expert testimony and from Eli Lilly for speaker bureau/expert testimony. AW, MS, and XH are employees and shareholders/stockholders of and hold stock options for AstraZeneca. MF received personal fees from AstraZeneca during the conduct of the study and is an employee of AstraZeneca. JW is an employee of AstraZeneca. SSR has received grants and personal fees from Amgen, BMS, Merck, Tesaro, and Takeda; grants from Advaxis and Genmab; grants, personal fees, and non-financial support from AstraZeneca; and personal fees from Genentech and Glaxo Smith Kline, outside the submitted work.
Ethics approval
The study was approved by the institutional review board or independent ethics committee of each study center. The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines (as defined by the International Conference on Harmonization), applicable regulatory requirements, and the policy on bioethics and human biologic samples of the study sponsor, AstraZeneca.
Consent to participate
All patients provided written informed consent prior to treatment.
Consent for publication
Not applicable.
Availability of data and material
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Code availability
Not applicable.
Author contributions
YC, WL, AW, and SSR contributed to the conception and design of the study. YH, WL, HZ, QZ, BW, CL, AW, and JW contributed to collection and assembly of data. YH, WL, QZ, CL, AW, MS, XH, MF, JW, and SSR contributed to data analysis and interpretation. All authors critically reviewed and drafted the manuscript, provided final approval, and agreed to be accountable for all aspects of the work.
References
- 1.Wen S, Dai L, Wang L, Wang W, Wu D, Wang K, et al. Genomic signature of driver genes identified by target next-generation sequencing in Chinese non-small cell lung cancer. Oncologist. 2019;24(11):e1070–e1081. doi: 10.1634/theoncologist.2018-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48):78985–78993. doi: 10.18632/oncotarget.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang H, Song X, Zhang Y, Zhang S, Li F, Fang J, et al. Real-world data on EGFR/ALK gene status and first-line targeted therapy rate in newly diagnosed advanced non-small cell lung cancer patients in Northern China: a prospective observational study. Thorac Cancer. 2019;10(7):1521–1532. doi: 10.1111/1759-7714.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S, Li L, Zhu Y, Huang C, Qin Y, Liu H, et al. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer. 2014;110(11):2812–2820. doi: 10.1038/bjc.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS. SSO and TOS. Ann Oncol. 2019;30(2):171–210. doi: 10.1093/annonc/mdy554. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 7.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 8.Wu YL, Xu CR, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus gemcitabine/cisplatin for first-line treatment of Chinese patients with advanced non-small-cell lung cancer harboring EGFR mutations: subgroup analysis of the LUX-Lung 6 trial. Onco Targets Ther. 2018;11:8575–8587. doi: 10.2147/ott.S160358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802) Ann Oncol. 2015;26(9):1877–1883. doi: 10.1093/annonc/mdv276. [DOI] [PubMed] [Google Scholar]
- 10.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non–small-cell lung cancer. J Clin Oncol. 2018;36(33):3290–3297. doi: 10.1200/jco.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 13.Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3) J Clin Oncol. 2018;36(26):2702–2709. doi: 10.1200/JCO.2018.77.9363. [DOI] [PubMed] [Google Scholar]
- 14.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 15.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 16.Yang JJ, Zhou Q, Yan HH, Zhang XC, Chen HJ, Tu HY, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer. 2017;116(5):568–574. doi: 10.1038/bjc.2016.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443–2450. doi: 10.1093/annonc/mdx359. [DOI] [PubMed] [Google Scholar]
- 18.Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244–2250. doi: 10.1200/JCO.2018.78.7994. [DOI] [PubMed] [Google Scholar]
- 19.Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466. doi: 10.1016/s1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 20.Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. doi: 10.1016/s1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 21.Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 22.Jin Y, Shao Y, Shi X, Lou G, Zhang Y, Wu X, et al. Mutational profiling of non-small-cell lung cancer patients resistant to first-generation EGFR tyrosine kinase inhibitors using next generation sequencing. Oncotarget. 2016;7(38):61755–61763. doi: 10.18632/oncotarget.11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y, Ma Y, Shi H, Du Y, Huang Y. Epidermal growth factor receptor T790M mutations in non-small cell lung cancer (NSCLC) of Yunnan in southwestern China. Sci Rep. 2018;8(1):15426. doi: 10.1038/s41598-018-33816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng Q, Xie B, Wu L, Ji X, Li C, Feng L, et al. Competitive evolution of NSCLC tumor clones and the drug resistance mechanism of first-generation EGFR-TKIs in Chinese NSCLC patients. Heliyon. 2018;4(12):e01031. doi: 10.1016/j.heliyon.2018.e01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Zhang L, Si X, Zhang X, Wang M. Re-biopsy status among Chinese non-small-cell lung cancer patients who progressed after icotinib therapy. Onco Targets Ther. 2018;11:7513–7519. doi: 10.2147/ott.S174075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis PM, Shepherd FA, Millward M, Perrone F, Seymour L, Liu G, et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR2.6): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2014;15(12):1379–1388. doi: 10.1016/S1470-2045(14)70472-3. [DOI] [PubMed] [Google Scholar]
- 27.Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13(5):528–538. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.