Abstract
Herein, a new magnetic metal–organic frameworks based on Fe3O4 (NMMOFs) with porous and high surface area materials were synthesized. Then, NMMOFs were characterized by FT-IR, XRD, SEM, elemental mapping, energy dispersive X-ray (EDS), TG, DTG, VSM, and N2 adsorption–desorption isotherms (BET). Fe3O4@Co(BDC)-NH2 as a magnetic porous catalyst was applied for synthesis of novel fused pyridines and 1,4-dihydropyridines with pyrazole and pyrimidine moieties as suitable drug candidates under ultrasonic irradiation. The significant advantages of the presented methodology are mild, facile workup, high yields, short reaction times, high thermal stability, and reusability of the described NMMOFs catalyst.
Subject terms: Chemistry, Nanoscience and technology
Introduction
Catalysis under ultrasonic irradiation has been widely applied for the preparation of organic compounds and catalysis1–3. On the other hand, hybrid organic–inorganic catalysts such as metal–organic frameworks (MOFs) as a new class of porous materials have high attention in chemical processes. Porous and magnetic materials have been widely used in biotechnology, magnetic resonance imaging (MRI), catalysis, adsorption, gas separation, and purification, optics, drug delivery, etc.4–6. However, metal–organic frameworks (MOFs) are a widespread strategy for the expansion of new porous materials to reach with the higher surface area. By selecting a suitable plan, reactants and reaction conditions can be correctly controlled by the porosity and structure of desired materials7–13. Magnetic catalysts have been used for the synthesis of a good range of pharmaceutical and chemical compounds, due to their easy removal and convenient separation14–16. The reported catalysts can be easily isolated from the reaction mixture with an external magnetic field17–19. Therefore magnetic metal–organic frameworks (MMOFs) have been used for various purposes due to their exciting properties20–23, such as high thermal stability and application at the hard reaction conditions24–26. The chemistry of magnetic metal–organic frameworks and their corresponding applications comprehensively have been reviewed27–29.
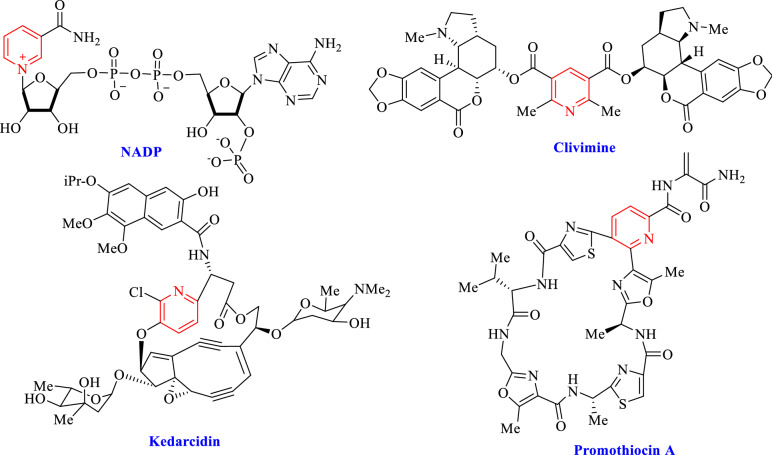
Fused N-heterocycles compounds with pyrazole and pyridines have shown a broad spectrum of biological and agricultural activities such as antitumor, cardiotonic hepatoprotactive, antihypertensive, antibronchitic, and antifungal activity30–33. Therefore, research and develop the new strategy are necessary for the synthesis of pyridines with pyrazole moieties. Pyridine derivatives are the central core of natural products such as NADP, clivimine, kedarcidin, and promothiocin A (Fig. 1)34.
Figure 1.
Structure of pyridine as natural products.
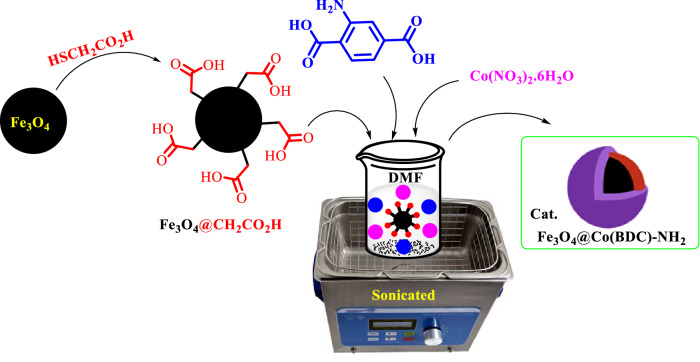
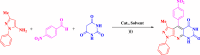
Synthesis of composites of MOF and nano-magnetic Fe3O4 is our great research interest. With this aim, we have decided to synthesize nano-magnetic metal–organic frameworks Fe3O4@Co(BDC)-NH2 as a porous and magnetic catalyst under ultrasonic irradiation condition. This nanomagnetic metal–organic frameworks (NMMOFs) was applied in the synthesis of novel fused pyridines and 1,4-dihydropyridines with pyrazole and pyrimidine moieties by using the corresponding precursors in DMF (5 mL) as solvent under ultrasonic irradiation (Scheme 1).
Scheme 1.
Preparation of novel fused pyridines and 1,4-dihydropyridines with pyrazole and pyrimidine moieties by using Fe3O4@Co(BDC)-NH2 as the catalyst.
Experimental
Materials and methods
All chemicals were purchased from Merck Chemical Company. The known products were identified by comparison of their melting points and spectral data with those reported in the literature. To scrutinize the progress of the reaction silica gel SIL G/UV 254 plates were used. From the model of the BRUKER Ultra shield, NMR spectrometer (δ in ppm) was recorded 1H NMR (400 MHz) and 13C NMR (100 MHz). Recorded on a Büchi B-545 apparatus in open capillary tubes were melting points. The PerkinElmer PE-1600-FTIR device was registered for the infrared spectra of compounds. SEM was performed using a scanning electron microscope for field publishing made by TE-SCAN. Thermal gravimetry (TG), differential thermal gravimetric (DTG) and differential thermal (DTA) were analyzed by a Perkin Elmer (Model: Pyris 1). The analysis 25–1000 °C, temperature increase rate of 10 °C min−1.
General procedure for the synthesis of Fe3O4@CH2CO2H
Fe3O4 was prepared according to the previously reported literature35,36. Then, in a 25 mL round-bottomed flask, a mixture of Fe3O4 (1 g), HSCH2CO2H (10.0 mmol, 1.38 g), and EtOH (30 mL) were added and refluxed for 24 h. After this time, a dark brown precipitate was appeared, which it is isolated by using a magnet. The obtained Fe3O4@CH2CO2H (1.95 g) was dried under vacuum23.
General procedure for the synthesis of Fe3O4@Co(BDC)-NH2
At first, a solution of 45.0 mM of Co(NO3)2·6H2O (2.34 g in 180 mL DMF) (solution I) and 45.0 mM of H2BDC-NH2 (1.46 g in 180 mL DMF) (solution II) were prepared respectively. In a 25 mL glass vials, a mixture of Fe3O4@CH2CO2H (0.5 g) and 10 mL of solution I were sonicated for 20 min. Then, this mixture was separated by a permanent magnet and washed with DMF as step I. Then, a mixture of step I and 10 mL of solution II were sonicated for 45 min. The produced solid was separated by a permanent magnet and washed with EtOH as step II. In continued, two strategies (a mixture of step I and step II) were repeated 18 times, respectively. Finally, Fe3O4@Co(BDC)-NH2 (0.8 g) was dried under vacuum for 2 h (Scheme 2).
Scheme 2.
Synthesis of Fe3O4@Co(BDC)-NH2 as a nanomagnetic metal–organic frameworks.
General procedure for the synthesis of novel fused pyridines and 1,4-dihydropyridines
In a 10 mL round-bottomed, a mixture of aldehyde (1.0 mmol), pyrimidine (1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione or pyrimidine-2,4,6(1H,3H,5H)-trione) and pyrazole-5-amine (3-methyl-1H-pyrazole-5-amine or 3-methyl-1-phenyl-1H-pyrazole-5-amine) derivatives] and Fe3O4@Co(BDC)-NH2 (10 mg) as a catalyst were mixed in DMF (5 mL) as solvent under ultrasonic irradiation. After completion of the reaction (monitor by TLC n-hexane/ethyl acetate; 4:6), the catalyst was separated by an external magnet. Finally, the mixture was poured into H2O and filtered off its precipitate. The obtained residue was washed with warm ethanol and dried at 100 °C (Scheme 1).
Result and discussion
The systematic study of the stereoelectronic effects in target molecules, allows for the design of synthetic strategies based on a nomerically driven stereoselective reactions, or highly biased equilibria among isomeric products. To the best of our knowledge, many biological processes involve the oxidation–reduction of substrates by NAD+/NADH, respectively37–41. The key feature of the oxidation mechanism is hydride transfer from carbon via stereoelectronic interactions. Thus the development of stereoelectronic effects leads to knowledge-based designing of biomimetic reactions. The obtained results from this research will be supporting the idea of rational designs, syntheses, and applications of tasked-specific catalysts and molecules for the development of stereoelectronic effects in the course of organic synthesis. With this aim, a nanomagnetic metal–organic frameworks (NMMOFs) was designed, characterized and applied for the preparation of pyridines fused with pyrazole and pyrimidine under ultrasonic irradiation.
At first, nanomagnetic metal–organic frameworks (NMMOFs) were synthesized (Scheme 2). Its schematic synthesis is showed in Fig. 2. The synthesized Fe3O4@Co(BDC)-NH2 fully characterized by applying FT-IR, XRD, SEM, elemental mapping, energy dispersive X-ray (EDS), TG, DTG, VSM and N2 adsorption–desorption isotherms (BET).
Figure 2.
Stepwise synthesis of the nanomagnetic metal–organic frameworks (NMMOFs) system.
FT-IR spectrum of H2BDC-NH2, Fe3O4, Fe3O4@CH2CO2H, and Fe3O4@Co(BDC)-NH2 are shown in Figure S1 (see supporting information). The absorption bands at 670 cm−1 linked to the stretching vibrational modes of Fe–O groups in Fe3O4. The absorption bands at 1741, 2924, and 3426 cm−1 related to C=O, C–H and, O–H stretching respectively in Fe3O4@CH2CO2H. Also, the absorption bands at 633 cm−1 and 3318–3448 cm−1 are related to Co–O and N–H2 stretching respectively, of Fe3O4@Co(BDC)-NH2. Finally, the differences between H2BC-NH2, Fe3O4, Fe3O4@CH2CO2H, and Fe3O4@Co(BDC)-NH2 in the FT-IR spectrum were confirmed the synthesis of Fe3O4@Co(BDC)-NH2.
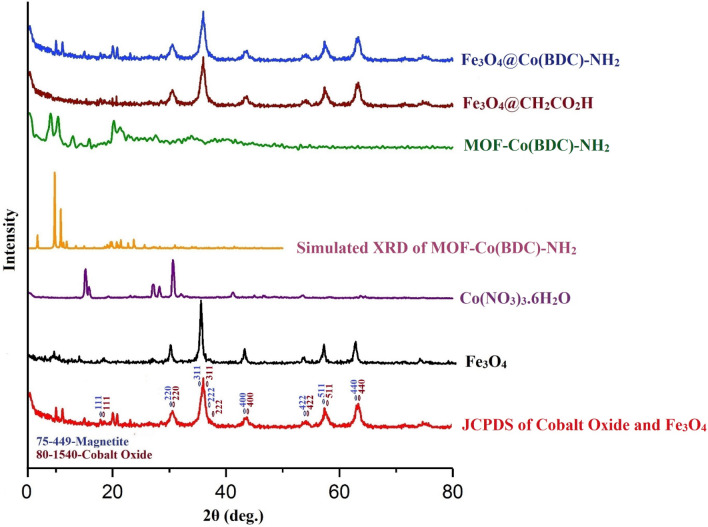
The particle size and shape, as well as the morphology of Fe3O4@CH2CO2H, Fe3O4, MOF-Co(BDC)-NH2, Co(NO3)3·6H2O and Fe3O4@Co(BDC)-NH2 were studied by XRD (Fig. 3), and SEM (Fig. 4). The comparison XRD pattern of JCPDS (red line), Fe3O4 (black line), Co(NO3)3·6H2O (purple line), Simulated XRD (orange line), MOF-Co(BDC)-NH2 (green line), Fe3O4@CH2CO2H (brown line) and Fe3O4@Co(BDC)-NH2 (blue line) is assembled according to the liturture servey at the range of 5°–80° in Fig. 342. The phase of Co oxide and Fe3O4 in Fe3O4@Co(BDC)-NH2 as standard brown line (ICDD Card: 80-1540) of Co and pinks standard line (JCP2: 75-449) of Fe3O4 in the standard references. Also, Peaks of Fe3O4@Co(BDC)-NH2 exhibited 2θ = 18.3°, 30.2°, 35.6°, 43.3°, 53.6°, 57.3°, 62.8° and 74.2° corresponding to diffraction lines (111), (220), (311), (400), (422), (511), (440) and (533). Then, the averaged interlunar distance and sizes of crystal were calculated by the Scherer equation and Bragg equation, which are determined 0.67 nm (single peak at 12.8 and 17.5–37.5 nm range (Table S1 see in supporting information)43,44.
Figure 3.
Comparison XRD pattern of JCPDS (red line), Fe3O4 (black line), Co(NO3)3·6H2O (purple line), Simulated XRD (orange line), MOF-Co(BDC)-NH2 (green line), Fe3O4@CH2CO2H (brown line) and Fe3O4@Co(BDC)-NH2 (blue line).
Figure 4.
Scanning electron microscope (SEM) images of Fe3O4@Co(BDC)-NH2.
For comparison, structure and elementals in the synthesis of step by step Fe3O4@CH2CO2H and Fe3O4@Co(BDC)-NH2 were also studied with energy dispersive X-ray analysis (EDX) analysis (Figure S2 see supporting information). The structures of Fe3O4@Co(BDC)-NH2 and Fe3O4@CH2CO2H were verified with existence of Fe, Co, N, C, O and Fe, C, O, and S atoms respectively 45. Then, elementals dispersed over the surface of the catalyst, and step Fe3O4@Co(BDC)-NH2 was checked out by SEM-elemental mapping (Figure S3 see supporting information). The images in Figure S3 shows that all kinds of elements are well dispersed over the surface of Fe3O4@Co(BDC)-NH2. The difference between EDX analysis and SEM-elemental mapping is confirmed by the structure of Fe3O4@Co(BDC)-NH2.
In another investigation, the particle size and shape, as well as the morphology of Fe3O4@Co(BDC)-NH2 were examined by scanning electron microscope (SEM) (Fig. 4). As shown in Fig. 4, nano-spherical particles of the nanomagnetic metal–organic frameworks (NMMOFs) are in the nanoscale, as the particles are quite overlapped with different crystallite size as observed in SEM Transmission electron microcopy (TEM) images of Fe3O4@Co(BDC)-NH2 catalyst reveal that the particles shape is spherical and the particle size is up to 50 nm (Fig. 5).
Figure 5.
Transmission electron microscopy (TEM) images of Fe3O4@Co(BDC)-NH2.
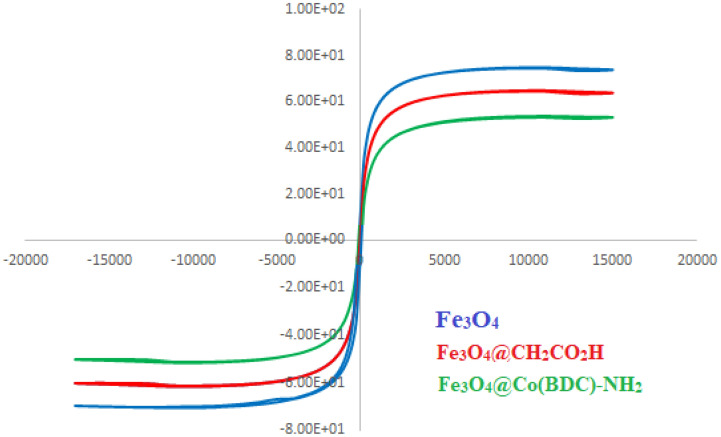
The magnetic measurement of Fe3O4, Fe3O4@CH2CO2H, and Fe3O4@Co(BDC)-NH2 are shown in Fig. 6. Based on Fig. 6, the vibrating sample magnetometer (VSM) of Fe3O4, Fe3O4@CH2CO2H, and Fe3O4@Co(BDC)-NH2 were examined and reduced from 64.4, 60.1 up to 54.3 μg−1 respectively. Therefore, these decreases are the result of coating with its corresponding layers.
Figure 6.
Vibrating sample magnetometer (VSM) of Fe3O4, Fe3O4@CH2CO2H, and Fe3O4@Co(BDC)-NH2.
In another investigation, the structural and thermal stability of Fe3O4@Co(BDC)-NH2 was also determined using the technique of the thermal gravimetric (TG), derivative thermal gravimetric (DTG), as well as the differential thermal analysis (DTA) (Figure S4 see in supporting information). First stage weight loss is about 100 °C, associated with the removal of possible solvents (organic and water), which was used in the course of catalyst preparation. Then, twice a step of weight loss has occurred at about 300 °C, which is the onset of the structural degradation of the catalyst.
For the determination of surface structural parameters, the N2 adsorption/desorption technique was used. The results of N2 adsorption/desorption were plotted in Fig. 7. The obtained surface area based on BET isotherm is 22.35 m2 g−1. The total pore volume of the catalyst is 0.02 cm3 g−1. Also, for studying the textural properties of MOF-Co(BDC)-NH2 the N2 adsorption–desorption isotherms were used (Fig. 7). The adsorption isotherm is type III and the appearance of hysteresis loop shows the presence of mesopores in the sample. The calculated surface areas based on BET equation and total pore volumes are 86 m2 g−1 and 0.36 cm3 g−1 respectively. The pore size distribution of MOF-Co(BDC)-NH2 based on BJH method is shown (Figure S5 see in supporting information). This plot clearly shows presence of micropores (size < 2 nm) and mesopores (2 < size < 50 nm) in the sample, however the micropores are more abundant.
Figure 7.
Nitrogen adsorption–desorption isotherm (BET) of Fe3O4@Co(BDC)-NH2 and MOF-Co(BDC)-NH2.
After the synthesis and characterization of Fe3O4@Co(BDC)-NH2, it was applied for the synthesis of novel mono, bis and tris novel fused pyridines and 1,4-dihydropyridines with pyrazole and pyrimidine moieties by using the corresponding precursors such as 3-methyl-1,4-diphenyl-1,8-dihydro-5H-pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-5,7(6H)-dione and 3-methyl-4-phenyl-1,4,8,9-tetrahydro-5H-pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-5,7(6H)-dione. The above mentioned products were obtained by reaction of 4-nitro benzaldehyde (1.0 mmol, 0.151 g), 3-methyl-1-phenyl-1H-pyrazole-5-amine (1.0 mmol, 0.174 g) and pyrimidine-2,4,6(1H,3H,5H)-trione (1.0 mmol, 0.128 g) as a model for the optimization the reaction conditions. The optimized data is listed in Table 1. As shown in Table 1, the best of choice for the synthesis of 3-methyl-1,4-diphenyl-1,8-dihydro-5H-pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-5,7(6H)-dione was achieved in the presence of 10 mg Fe3O4@Co(BDC)-NH2 in DMF (5 mL) as solvent under ultrasonic irradiation (entry 4, Table 1). The model reaction was also studied by using several solvents such as H2O, CH3CN, n-hexane, CHCl3, MeOH, EtOH, CH2Cl2, EtOAc (5 mL) and solvent-free condition in the presence of 10 mg of Fe3O4@Co(BDC)-NH2. The results of the reaction did not improve (Table 1, entries 6–13). Also, the model reaction was also studied in the magnetic stirrer condition at room temperature under the solvent-free reaction (Table 1, entry 14).
Table 1.
Effect of different amounts of catalysts and solvent (5 mL) in the synthesis of novel fused pyridines and 1,4-dihydropyridines with pyrazole and pyrimidine moieties by using the corresponding precursors under ultrasonic irradiation.
| ||||
|---|---|---|---|---|
| Entry | Solvent | Cat. (mg) | Sonication (min) | Yield (%) |
| 1 | DMF | – | 120 | Trace |
| 2 | DMF | 5 | 75 | 25 |
| 3 | DMF | 7.5 | 60 | 45 |
| 4 | DMF | 10 | 50 | 72 |
| 5 | DMF | 15 | 50 | 72 |
| 6 | H2O | 10 | 60 | 35 |
| 7 | n-Hexane | 10 | 60 | 25 |
| 8 | EtOH | 10 | 60 | Trace |
| 9 | CH2Cl2 | 10 | 60 | Trace |
| 10 | CHCl3 | 10 | 60 | 28 |
| 11 | EtOAc | 10 | 60 | 46 |
| 12 | CH3CN | 10 | 60 | 54 |
| 13 | MeOH | 10 | 60 | Trace |
| 14 | – | 10 | 60 | 15 |
Reaction conditions: 3-methyl-1-phenyl-1H-pyrazole-5-amine (1.0 mmol, 0.174 g), pyrimidine-2,4,6(1H,3H,5H)-trione (1.0 mmol, 0.128 g) and 4-nitrobenzaldehyde (1.0 mmol, 0.151 g).
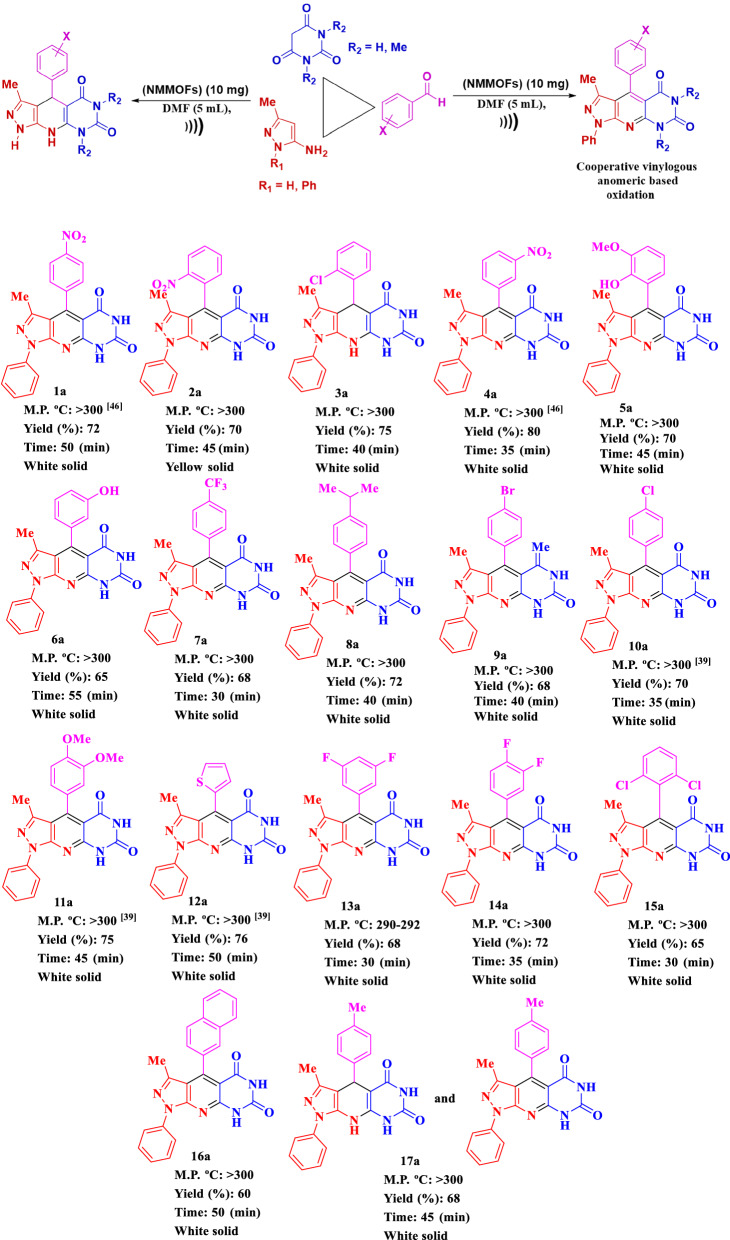
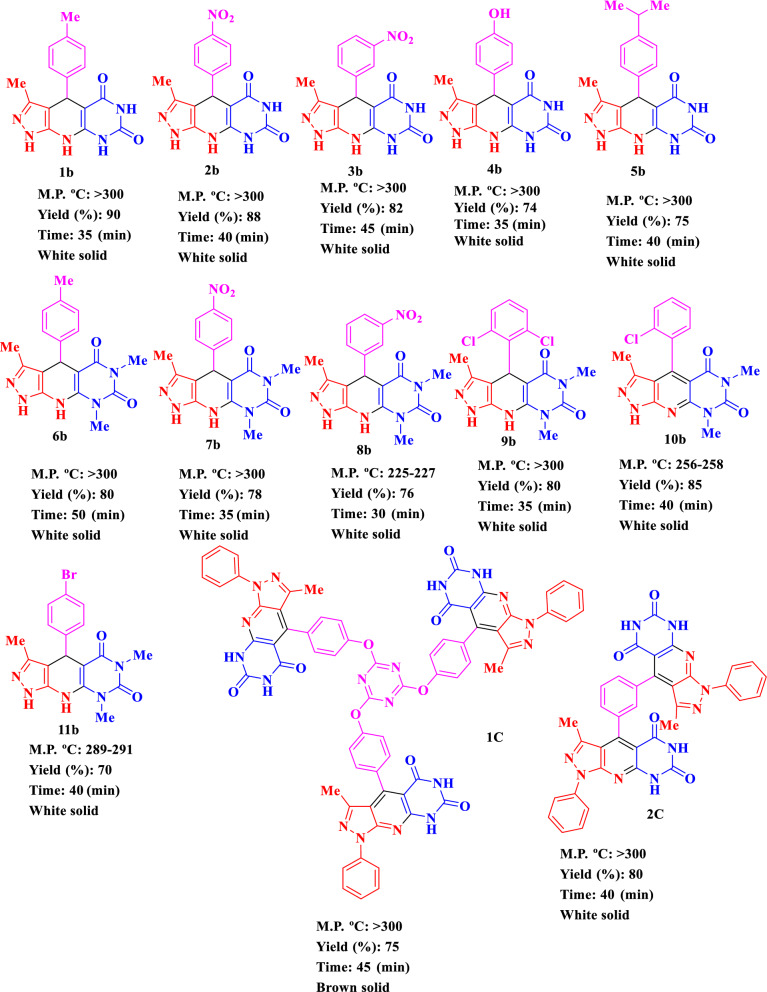
After optimizing the reaction conditions, Fe3O4@Co(BDC)-NH2(10 mg) is applied to synthesis a good range of novel biological and pharmacological candidate compounds using various aromatic aldehydes (trephetaldehyde, iso-trephetaldehyde, tris-aldehyde, bearing electron-donating and electron-withdrawing groups), pyrimidine (1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione, pyrimidine-2,4,6(1H,3H,5H)-trione) and pyrazole-5-amine (3-methyl-1H-pyrazole-5-amine, 3-methyl-1-phenyl-1H-pyrazole-5-amine) derivatives. As shown in Table 2, the obtained results indicated that Fe3O4@Co(BDC)-NH2 is appropriate for the preparation of target molecules in high to excellent yields (65–90%) with in relatively short reaction times (20–40 min). Furthermore, the model reaction is tested by the reaction of 3-methyl-1H-pyrazole-5-amine, and 3-methyl-1H-pyrazole-5-amine to give a mixture of the corresponding pyridine and 1,4-dihydropyridine respectively.
Table 2.
Synthesis of (a) 3-methyl-1,4-diphenyl-1,8-dihydro-5H-pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-5,7(6H)-dione derivatives, (b) 3-Methyl-4-phenyl-1,4,8,9-tetrahydro-5H-pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-5,7(6H)-dione derivatives, (c) Bis and tris 3-methyl-1,4-diphenyl-1,8-dihydro-5H-pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-5,7(6H)-dione derivatives using Fe3O4@Co(BDC)-NH2 under ultrasonic irradiation.
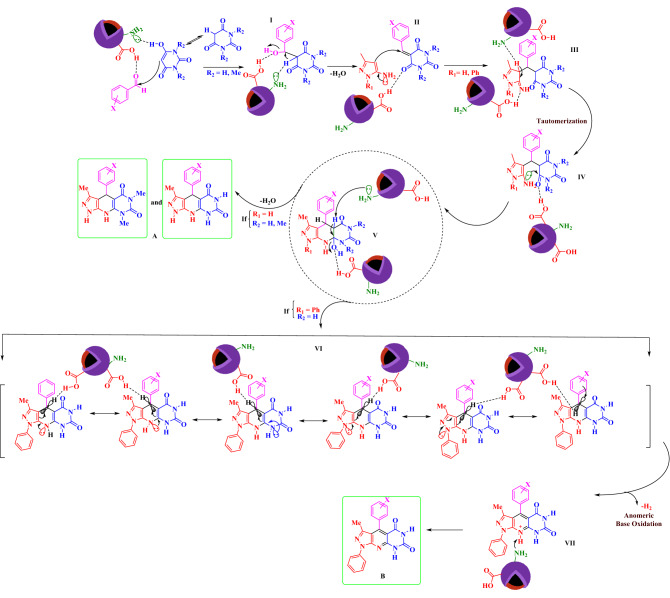
In the proposed mechanism, the aldehyde is activated by Fe3O4@Co(BDC)-NH2. In the initial step, intermediate (I) is produced by the reaction of pyrimidine (R2 = H, Me) and activated aldehyde. In the next step, intermediate (II) is prepared with losing one molecule of H2O. In the third step, pyrazole-5-amine (R1 = H, Ph) derivatives react with intermediate (II) to gives intermediate (IV) after tautomerization. Then, intermediate (IV) gives intermediate (V) after intramolecular cyclization and losing another molecule of H2O. In the last step, the lone pair electrons of N atoms of 1,4-dihydropyridine (VI) interacts through C–C double bonds with a vacant anti-bonding orbital of C–H bond (nN→ σC–H* and πC=C→ σC–H*) and weaken it, so that is favoring for hydride transfer and H2 releasing from intermediate VI to generate its corresponding pyridinium salt. The achieved data from the optimization of described reaction under argon and nitrogen atmospheres verified our suggestion for oxidation and aromatization of intermediate VI. On the other hand, 1,4-dihydropyridine (VI) is converted to its corresponding pyridinium intermediate (VII), via a cooperative vinylogous anomeric based oxidation and releasing a hydrogen molecule (–H2)47–55. Finally, the desired pyridine fused with pyrazole and pyrimidine moiety (B) is obtained via removing a proton from the pyridinium intermediate (VII). When, 3-methyl-1H-pyrazole-5-amine was used instead of 3-methyl-1-phenyl-1H-pyrazole-5-amine after intramolecular cyclization and losing a molecule of water, intermediate (VI) is converted to product (A) (Scheme 3). Interestingly, the 1,4-dihydropyridines (A) did not convert to their corresponding pyridines.
Scheme 3.
Proposed mechanism for the preparation of novel fused pyridines and 1,4-dihydropyridines with pyrazole and pyrimidine moieties via a cooperative vinylogous anomeric based oxidation.
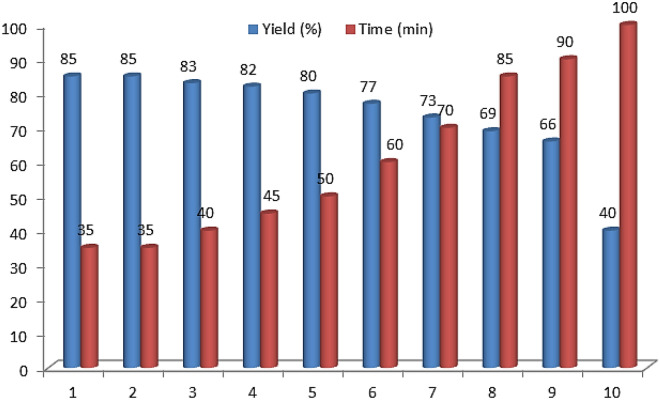
The reusability of Fe3O4@Co(BDC)-NH2 was also investigated. The reaction of 4-nitro benzaldehyde (1.0 mmol, 0.151 g), 3-methyl-1-phenyl-1H-pyrazole-5-amine (1.0 mmol, 0.174 g) and pyrimidine-2,4,6(1H,3H,5H)-trione (1.0 mmol, 0.128 g) was selected as a model reaction under ultrasonic irradiation. The nanomagnetic metal–organic frameworks (NMMOFs) catalyst was separated by an external magnet, washed with DMF and dried. The results indicated that the catalyst could be utilized for nine runs without any significant loss of its initial catalytic activity, which can be ascribed to the high stability of the synthesized catalyst (Fig. 8). Then, the reused catalyst was also characterized by FT-IR spectrum (Figure S6 see supporting information), N2 adsorption–desorption isotherm (BET) and scanning electron microscope (SEM) images. The obtained spectra are as same as the corresponding spectra of fresh catalyst (Figures S7, S8 see supporting information), Furthermore, to compare the performance of nanomagnetic metal–organic frameworks (NMMOFs) catalyst for the synthesis of desired fused pyridines and 1,4-dihydropyridines with pyrazole and pyrimidine moieties via a cooperative vinylogous anomeric based oxidation, we have used various organic and inorganic acid catalysts for condensation reaction between 4-nitro benzaldehyde (1.0 mmol, 0.151 g), 3-methyl-1-phenyl-1H-pyrazole-5-amine (1.0 mmol, 0.174 g) and pyrimidine-2,4,6(1H,3H,5H)-trione (1.0 mmol, 0.128 g). As Table 3 indicates, Fe3O4@Co(BDC)-NH2 is the best of choice for the synthesis of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-5,7(6H)-dione (Table 3).
Figure 8.
Recyclability of Fe3O4@Co(BDC)-NH2 as a catalyst in the synthesis pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-5,7(6H)-dione.
Table 3.
Synthesis of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-5,7(6H)-dionein the presence of various catalysts under ultrasonic irradiation.
| Entry | Catalyst | (mol%) | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | FeCl3 | 10 | 120 | 25 |
| 2 | [PVI-SO3H]FeCl56 | 10 | 120 | 48 |
| 3 | Fe3O4 | 10 mg | 120 | Trace |
| 4 | NH4NO3 | 10 | 120 | Trace |
| 5 | SSA57,58 | 10 mg | 120 | 35 |
| 6 | Nano-SB-[PSIM]Cl59 | 10 mg | 120 | Trace |
| 7 | NaHSO4 | 10 | 120 | – |
| 8 | GTBSA60 | 10 | 120 | 55 |
| 9 | SBA-15/(CH2)3 N(CH2PO3H2)261 | 10 mg | 120 | 35 |
| 10 | [Py-SO3H]Cl62 | 10 | 120 | 35 |
| 11 | p-TSA | 10 | 120 | 25 |
| 12 | Et3N | 10 | 120 | – |
| 13 | MIL-100(Cr)/NHEtN(CH2PO3H2)263 | 10 mg | 120 | 63 |
| 14 | APVPB64 | 10 mg | 120 | 40 |
| 15 | MHMHPA65 | 10 | 120 | 55 |
| 16 | GTMPA66 | 10 | 120 | 40 |
| 17 | Co(NO3)3·6H2O | 10 | 120 | 36 |
| 18 | H2BDC-NH2 | 10 | 120 | 42 |
| 19 | Fe3O4@Co(BDC)-NH2 | 10 mg | 50 | 72a |
aThis work.
Conclusion
In summary, a novel core–shell nanomagnetic metal–organic frameworks Fe3O4@Co(BDC)-NH2 as a new catalyst was prepared and fully characterized. This catalyst was applied for the synthesis of a range of novel fused pyridines and 1,4-dihydropyridines with pyrazole and pyrimidine moieties with good yields via a cooperative vinylogous anomeric based oxidation mechanism under ultrasonic irradiation. The obtained biological-based compounds are suitable candidates for biological studies. The described catalyst is reusable and easily separated by an external magnet.
Supplementary Information
Acknowledgements
We thank the Bu-Ali Sina University and Iran National Science Foundation (INSF) (Grant Number: 98001912) for financial support.
Author contributions
H.S., M.Z. and S.B.: methodology, validation, investigation, writing the original draft. M.A.Z.: supervision, resources, project administration, funding acquisition, conceptualization, writing—review and editing. S.R.: advisor of project and editing of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mahmoud Zarei, Email: mahmoud8103@yahoo.com.
Mohammad Ali Zolfigol, Email: zolfi@basu.ac.ir.
Sadegh Rostamnia, Email: rostamnia@iust.ac.ir.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-84005-2.
References
- 1.Amaniampong PN, Jerome F. Catalysis under ultrasonic irradiation: A sound synergy. Curr. Opin. Chem. 2020;22:7–12. [Google Scholar]
- 2.Amaro-Gahete J, Klee R, Esquivel D, Ruiz JR, Jiménez-Sanchidrián C, Romero-Salguero FJ. Fast ultrasound-assisted synthesis of highly crystalline MIL-88A particles and their application as ethylene adsorbents. Ultrason. Sonochem. 2019;50:59–66. doi: 10.1016/j.ultsonch.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Bigdeli F, Rouhani F, Morsali A, Ramazani A. Ultrasonic-assisted synthesis of the nanostructures of a Co(II) metal organic framework as a highly sensitive fluorescence probe of phenol derivatives. Ultrason. Sonochem. 2020;62:104862. doi: 10.1016/j.ultsonch.2019.104862. [DOI] [PubMed] [Google Scholar]
- 4.Ke F, Yuan YP, Qiu LG, Shen YH, Xie AJ, Zhu JF, Tianc XY, Zhang LD. Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J. Mater. Chem. 2011;21:3843–3848. doi: 10.1039/c0jm01770a. [DOI] [Google Scholar]
- 5.Li JR, Kuppler RJ, Zhou HC. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009;38:1477–1504. doi: 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]
- 6.Ricco R, Malfatti L, Takahashi M, Hill AJ, Falcaro P. Applications of magnetic metal–organic framework composites. J. Mater. Chem. A. 2013;1:13033–13045. doi: 10.1039/c3ta13140h. [DOI] [Google Scholar]
- 7.Hu X, Liu Q, Wu Y, Deng Z, Long J, Deng C. Magnetic metal–organic frameworks containing abundant carboxylic groups for highly effective enrichment of glycopeptides in breast cancer serum. Talanta. 2019;204:446–454. doi: 10.1016/j.talanta.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Rostamnia S, Alamgholiloo H, Jafari M. Ethylene diamine post-synthesis modification on open metal site Cr-MOF to access efficient bifunctional catalyst for the Hantzsch condensation reaction. Appl. Organomet. Chem. 2018;32:e4370. doi: 10.1002/aoc.4370. [DOI] [Google Scholar]
- 9.Alamgholiloo H, Rostamnia S, Hassankhani A, Banaei R. Synthesis of a zeoliticimidazolate-zinc metal–organic framework and the combination of its catalytic properties with 2, 2, 2-trifluoroethanol for N-formylation. Synlett. 2018;29:1593–1596. doi: 10.1055/s-0037-1610159. [DOI] [Google Scholar]
- 10.Alamgholiloo H, Rostamnia S, Hassankhani A, Khalafy J, Baradarani MM, Mahmoudi G, Liu X. Stepwise post-modification immobilization of palladium schiff-base complex on to the OMS-Cu (BDC) metal–organic framework for Mizoroki–Heck cross-coupling reaction. Appl. Organomet. Chem. 2018;32:e4539. doi: 10.1002/aoc.4539. [DOI] [Google Scholar]
- 11.Rostamnia S, Jafari M. Metal–organic framework of amine-MIL-53(Al) as active and reusable liquid-phase reaction inductor for multicomponent condensation of Ugi-type reactions. Appl. Organomet. Chem. 2016;3:1–6. [Google Scholar]
- 12.Rostamnia S, Mohsenzad F. Nanoarchitecturing of open metal site Cr-MOFs for oxodiperoxo molybdenum complexes [MoO(O2)2@En/MIL-100(Cr)] as promising and bifunctional catalyst for selective thioether oxidation. Mol. Catal. 2018;445:12–20. doi: 10.1016/j.mcat.2017.11.003. [DOI] [Google Scholar]
- 13.Hu ML, Safarifard V, Doustkhah E, Rostamnia S, Morsali A, Nouruzi N, Beheshti S, Akhbari K. Taking organic reactions over metal–organic frameworks as heterogeneous catalysis. Microporous Mesoporous Mater. 2018;256:111–127. doi: 10.1016/j.micromeso.2017.07.057. [DOI] [Google Scholar]
- 14.Chen MN, Mo LP, Cui ZS, Zhang ZH. Magnetic nanocatalysts: Synthesis and application in multicomponent reactions. Curr. Opin. Chem. 2019;15:27–37. [Google Scholar]
- 15.Bai S, Li R, Su G, Duan X, Wang S, Ren NQ, Ho SH. Magnetic biochar catalysts from anaerobic digested sludge: Production, application and environment impact. Environ. Int. 2019;126:302–308. doi: 10.1016/j.envint.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Maleki B, Reiser O, Esmaeilnezhad E, Choi HJ. SO3H-dendrimer functionalized magnetic nanoparticles (Fe3O4@DNH(CH2)4SO3H): Synthesis, characterization and its application as a novel and heterogeneous catalyst for the one-pot synthesis of polyfunctionalizedpyrans and polyhydroquinolines. Polyhedron. 2019;162:129–141. doi: 10.1016/j.poly.2019.01.055. [DOI] [Google Scholar]
- 17.Koukabi N, Kolvari E, Zolfigol MA, Khazaei A, Shaghasemi BS, Fasahati B. A magnetic particle-supported sulfonic acid catalyst: Tuning catalytic activity between homogeneous and heterogeneous catalysis. Adv. Synth. Catal. 2012;354:2001–2008. doi: 10.1002/adsc.201100352. [DOI] [Google Scholar]
- 18.Abu-Dief AM, Abdel-Fatah SM. Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Suef Univ. J. Basic Appl. Sci. 2018;7:55–67. doi: 10.1016/j.bjbas.2017.05.008. [DOI] [Google Scholar]
- 19.Yao Y, Cai Y, Lu F, Wei F, Wang X, Wang S. Magnetic recoverable MnFe2O4 and MnFe2O4-graphene hybrid as heterogeneous catalysts of peroxymonosulfate activation for efficient degradation of aqueous organic pollutants. J. Hazard. Mater. 2014;270:61–70. doi: 10.1016/j.jhazmat.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Lu N, He X, Wang T, Liu S, Hou X. Magnetic solid-phase extraction using MIL-101(Cr)-based composite combined with dispersive liquid-liquid microextraction based on solidification of a floating organic droplet for the determination of pyrethroids in environmental water and tea samples. Microchem. J. 2018;137:449–455. doi: 10.1016/j.microc.2017.12.009. [DOI] [Google Scholar]
- 21.Gao G, Di JQ, Zhang HY, Mo LP, Zhang ZH. A magnetic metal organic framework material as a highly efficient and recyclable catalyst for synthesis of cyclohexenone derivatives. J. Catal. 2020 doi: 10.1016/j.jcat.2020.04.013. [DOI] [Google Scholar]
- 22.Safari M, Yamini Y, Masoomi MY, Morsali A. Magnetic metal–organic frameworks for the extraction of trace amounts of heavy metal ions prior to their determination by ICP-AES. Microchim. Acta. 2017;184:1555–1564. doi: 10.1007/s00604-017-2133-3. [DOI] [Google Scholar]
- 23.Yamini Y, Safari M, Morsali A, Safarifard V. Magnetic frame work composite as an efficient sorbent for magnetic solid-phase extraction of plasticizer compounds. J. Chromatogr. A. 2018;1570:38–46. doi: 10.1016/j.chroma.2018.07.069. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Ji G, Liu W, Quan B, Liang X, Shang C, Du Y. Thermal conversion of an Fe3O4@metal–organic framework: A new method for an efficient Fe-Co/nanoporous carbon microwave absorbing material. Nanoscale. 2015;7:12932–12942. doi: 10.1039/C5NR03176A. [DOI] [PubMed] [Google Scholar]
- 25.Huo JB, Xu L, Chen X, Zhang Y, Yang JCE, Yuan B, Fu ML. Direct epitaxial synthesis of magnetic Fe3O4@UiO-66 composite for efficient removal of arsenate from water. Microporous Mesoporous Mater. 2019;276:68–75. doi: 10.1016/j.micromeso.2018.09.017. [DOI] [Google Scholar]
- 26.Fan L, Wu H, Wu X, Wang M, Cheng J, Zhang N, Sun K. Fe-MOF derived jujube pit like Fe3O4/C composite as sulfur host for lithium-sulfur battery. Electrochim. Acta. 2019;295:444–451. doi: 10.1016/j.electacta.2018.08.107. [DOI] [Google Scholar]
- 27.Gao Y, Liu G, Gao M, Huang X, Xu D. Recent advances and applications of magnetic metal–organic frameworks in adsorption and enrichment removal of food and environmental pollutants. Crit. Rev. Anal. Chem. 2019 doi: 10.1080/10408347.2019.1653166. [DOI] [PubMed] [Google Scholar]
- 28.Nadar SS, Rathod VK. Magnetic-metal organic framework (magnetic-MOF): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 2018;120:2293–2302. doi: 10.1016/j.ijbiomac.2018.08.126. [DOI] [PubMed] [Google Scholar]
- 29.Wang R, Zhang C, Wang S, Zhou Y. Synthesis and application of magnetic metal–organic frameworks. Prog. Chem. 2015;27:945–952. [Google Scholar]
- 30.Grivsky EM, Lee S, Sigel CW, Duch DS, Nichol CA. Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido [2,3-d] pyrimidine. J. Med. Chem. 1980;23:327–329. doi: 10.1021/jm00177a025. [DOI] [PubMed] [Google Scholar]
- 31.Deb ML, Bhuyan PJ. Synthesis of novel classes of pyrido[2,3-d]-pyrimidines, Pyrano[2,3-d]pyrimidines, and pteridines. Synth. Commun. 2006;36:3085–3090. doi: 10.1080/00397910600775622. [DOI] [Google Scholar]
- 32.Devi I, Borah HN, Bhuyan PJ. Studies on uracils: A facile one-pot synthesis of oxazino[4,5-d]−, pyrano[2,3-d]−, pyrido[2,3-d]− and pyrimido[4,5-d] pyrimidines using microwave irradiation in the solid state. Tetrahedron Lett. 2004;45:2405–2408. doi: 10.1016/j.tetlet.2004.01.094. [DOI] [Google Scholar]
- 33.Lee HM, Chiu PL, Hu CH, Lai CL, Chou YC. Synthesis and structural characterization of metal complexes based on pyrazole/imidazolium chlorides. J. Organomet. Chem. 2005;690:403–414. doi: 10.1016/j.jorganchem.2004.09.053. [DOI] [Google Scholar]
- 34.Allais C, Grassot JM, Rodriguez J, Constantieux T. Metal-free multicomponent synthesis of pyridines. Chem. Rev. 2014;114:10829–10868. doi: 10.1021/cr500099b. [DOI] [PubMed] [Google Scholar]
- 35.Tabacchi G, Vanoni MA, Gamba A, Fois E. Does negative hyperconjugation assist enzymatic dehydrogenations. Chem. Phys. Chem. 2007;8:1283–1288. doi: 10.1002/cphc.200700085. [DOI] [PubMed] [Google Scholar]
- 36.Hamasaka G, Tsuji H, Uozumi Y. Organoborane-catalyzed hydrogenation of unactivated aldehydes with a Hantzsch ester as a synthetic NAD(P)H analogue. Synlett. 2015;26:2037–2041. doi: 10.1055/s-0034-1378846. [DOI] [Google Scholar]
- 37.He T, Shi R, Gong Y, Jiang G, Liu M, Qian S, Wang Z. Base-promoted cascade approach for the preparation of reduced knoevenagel adducts using hantzsch esters as reducing agent in water. Synlett. 2016;27:1864–1869. doi: 10.1055/s-0035-1562099. [DOI] [Google Scholar]
- 38.Bai CB, Wang NX, Xing Y, Lan XW. Progress on chiral NAD(P)H model compounds. Synlett. 2017;13:402–414. [Google Scholar]
- 39.Zhao X, Xiao J, Tang W. Enantioselective reduction of 3-substituted quinolines with a cyclopentadiene-based chiral Brønsted acid. Synthesis. 2017;49:3157–3164. doi: 10.1055/s-0036-1589012. [DOI] [Google Scholar]
- 40.Moradi S, Zolfigol MA, Zarei M, Alonso DA, Khoshnood A, Tajally A. An efficient catalytic method for the synthesis of pyrido[2,3-d] pyrimidines as biologically drug candidates by using novel magnetic nanoparticles as a reusable catalyst. Appl. Organomet. Chem. 2017;32:e4043. [Google Scholar]
- 41.Rajabi-Salek M, Zolfigol MA, Zarei M. Synthesis of a novel DABCO-based nanomagnetic catalyst with sulfonic acid tags: Application to the synthesis of diverse spiropyrans. Res. Chem. Intermed. 2018;44:5255–5269. doi: 10.1007/s11164-018-3421-1. [DOI] [Google Scholar]
- 42.Zhang Y, Guo J, Shi L, Zhu Y, Hou K, Zheng Y, Tang Z. Tunable chiral metal organic frameworks toward visible light-driven asymmetric catalysis. Sci. Adv. 2017;3(8):e1701162. doi: 10.1126/sciadv.1701162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Lin R, Ge L, Hou L, Bernhardt P, Rufford TE, Zhu Z. Synthesis and characterization of three amino-functionalized metal–organic frameworks based on the 2-aminoterephthalic ligand. Dalton Trans. 2015;44:8190–8197. doi: 10.1039/C4DT03927K. [DOI] [PubMed] [Google Scholar]
- 44.Li C, Chen T, Xu W, Lou X, Pan L, Chen Q, Hu B. Mesoporous nanostructured Co3O4 derived from MOF template: A high-performance anode material for lithium-ion batteries. J. Mater. Chem. 2015;3:5585–5591. doi: 10.1039/C4TA06914E. [DOI] [Google Scholar]
- 45.Bhamini B, Shanmugam SA, Tan M. Study of aluminium doped transition metal ferrite nanomaterials as supercapacitor electrodes. J. Eng. Res. 2014;2:1–10. doi: 10.7603/s40632-014-0001-4. [DOI] [Google Scholar]
- 46.Shi F, Zhou D, Tu S, Li C, Cao L, Shao Q. Pot atom and step economic synthesis of fused three heterocyclic ring compounds under microwave irradiation in water. J. Heterocycl. Chem. 2008;45:1305–1310. doi: 10.1002/jhet.5570450508. [DOI] [Google Scholar]
- 47.Yarie M. Catalytic anomeric based oxidation. Iran. J. Catal. 2017;7:85–88. [Google Scholar]
- 48.Yarie M. Catalytic vinylogous anomeric based oxidation. Iran. J. Catal. 2020;10:79–83. doi: 10.1039/d0ra04461j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babaee S, Zolfigol MA, Zarei M, Zamanian J. 1,10-Phenanthroline-based molten salt as a bifunctionalsulfonic acid catalyst: Application to the synthesis of N-heterocyclecompounds via anomericbased oxidation. ChemistrySelect. 2018;3:8947–8954. doi: 10.1002/slct.201801476. [DOI] [Google Scholar]
- 50.Babaee S, Zarei M, Sepehrmansourie H, Zolfigol MA, Rostamnia S. Synthesis of metal–organic frameworks MIL-101(Cr)-NH2 containing phosphorous acid functional groups: Application for the synthesis of N-amino-2-pyridone and pyrano [2,3-c]pyrazole derivatives via a cooperative vinylogous anomeric based oxidation. ACS Omega. 2020;5:6240–6249. doi: 10.1021/acsomega.9b02133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghasemi P, Yarie M, Zolfigol MA, Taherpour AA, Torabi M. Ionically tagged magnetic nanoparticles with urea linkers: Application for preparation of 2-aryl-quinoline-4-carboxylic acids via an anomeric-based oxidation mechanism. ACS Omega. 2020;5:3207–3217. doi: 10.1021/acsomega.9b03277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karimi F, Yarie M, Zolfigol MA. A convenient method for synthesis of terpyridines via a cooperative vinylogous anomeric based oxidation. RSC Adv. 2020;10:25828–25835. doi: 10.1039/D0RA04461J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karimi F, Yarie M, Zolfigol MA. Synthesis and characterization of Fe3O4@SiO2@(CH2)3NH(CH2)2O2P(OH)2 and its catalytic application in the synthesis of benzo-[h]quinoline-4-carboxylic acids via a cooperative anomeric based oxidation mechanism. Mol. Catal. 2020;489:110924. doi: 10.1016/j.mcat.2020.110924. [DOI] [Google Scholar]
- 54.Karimi F, Yarie M, Zolfigol MA. Fe3O4@SiO2@(CH2)3-urea-thiourea: A novel hydrogen-bonding and reusable catalyst for the construction of bipyridine-5-carbonitriles via a cooperative vinylogous anomeric based oxidation. Mol. Catal. 2020;489:111201. doi: 10.1016/j.mcat.2020.111201. [DOI] [Google Scholar]
- 55.Dashteh M, Zolfigol MA, Khazaei A, Baghery S, Yarie M, Makhdoomi S, Safaiee M. Synthesis of cobalt tetra-2,3-pyridiniumporphyrazinato with sulfonic acid tags as an efficient catalyst and its application for the synthesis of bicyclic ortho-aminocarbonitriles, cyclohexa-1,3-dienamines and 2-amino-3-cyanopyridines. RSC Adv. 2020;10:27824–27834. doi: 10.1039/D0RA02172E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sepehrmansourie H, Zarei M, Taghavi R, Zolfigol MA. Mesoporous ionically tagged cross-linked poly (vinyl imidazole) s as novel and reusable catalysts for the preparation of N-heterocycle spiropyran. ACS Omega. 2019;4:17379–17392. doi: 10.1021/acsomega.9b02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zolfigol MA. Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions. Tetrahedron. 2001;57:9509–9511. doi: 10.1016/S0040-4020(01)00960-7. [DOI] [Google Scholar]
- 58.Sepehrmansourie H. Silica sulfuric acid (SSA): As a multipurpose catalyst. Iran. J. Catal. 2020;10:175–179. [Google Scholar]
- 59.Zare A, Merajoddin M, Moosavi-Zare AR, Zarei M, Beyzavi MH, Zolfigol MA. Design and characterization of nano-silica-bonded 3-n-propyl-1-sulfonic acid imidazolium chloride nano-SB-[PSIM]Cl as a novel, heterogeneous and reusable catalyst for the condensation of arylaldehydes with β-naphthol and alkyl carbamates. Res. Chem. Intermed. 2016;42:2365–2378. doi: 10.1007/s11164-015-2154-7. [DOI] [Google Scholar]
- 60.Zarei M, Sepehrmansourie H, Zolfigol MA, Karamian R, Moazzami Farida SH. Novel nano-size and crab-like biological-based glycoluril with sulfonic acid tags as a reusable catalyst: Its application to the synthesis of new mono- and bis-spiropyrans and their in vitro biological studies. New. J. Chem. 2018;42:14308–14317. doi: 10.1039/C8NJ02470G. [DOI] [Google Scholar]
- 61.Jalili F, Zarei M, Zolfigol MA, Rostamnia S, Moosavi-Zare AR. SBA-15/PrN(CH2PO3H2)2 as a novel and efficient mesoporous solid acid catalyst with phosphorous acid tags and its application on the synthesis of new pyrimido[4,5-b]quinolones and pyrido[2,3-d]pyrimidines via anomeric based oxidation. Microporous Mesoporous Mater. 2020;294:109865. doi: 10.1016/j.micromeso.2019.109865. [DOI] [Google Scholar]
- 62.Moosavi-Zare AR, Zolfigol MA, Zarei M, Khakyzadeh V, Hasaninejad A. Design, characterization and application of new ionic liquid 1-sulfopyridinium chloride as an efficient catalyst for tandem Knoevenagel-Michael reaction of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one with aldehydes. Appl. Catal. A Gen. 2013;467:61–68. doi: 10.1016/j.apcata.2013.07.004. [DOI] [Google Scholar]
- 63.Sepehrmansourie H, Zarei M, Zolfigol MA, Moosavi-Zare AR, Rostamnia S, Moradi S. Multilinker phosphorous acid anchored En/MIL-100 (Cr) as a novel nanoporous catalyst for the synthesis of new N-heterocyclic pyrimido [4, 5-b] quinolones. Mol. Catal. 2020;481:110303. doi: 10.1016/j.mcat.2019.01.023. [DOI] [Google Scholar]
- 64.Noroozizadeh E, Moosavi-Zare AR, Zolfigol MA, Zarei M, Karamian R, Asadbegy M, Yari S, Moazzami Farida SH. Synthesis of bis-coumarins over acetic acid functionalized poly(4-vinylpyridinum) bromide (APVPB) as a green efficient catalyst under solvent-free conditions their biological activity. J. Iran. Chem. Soc. 2018;15:471–481. doi: 10.1007/s13738-017-1247-1. [DOI] [Google Scholar]
- 65.Afsar J, Zolfigol MA, Khazaei A, Zarei M, Gu Y, Alonso DA, Khoshnood A. Synthesis and application of melamine-based nano catalyst with phosphonic acid tags in the synthesis of (3′-indolyl)pyrazolo[3,4-b]pyridines via vinylogous anomeric based oxidation. Mol. Catal. 2020;482:110666. doi: 10.1016/j.mcat.2019.110666. [DOI] [Google Scholar]
- 66.Moradi S, Zolfigol MA, Zarei M, Alonso DA, Khoshnood A. Synthesis of a biological-based glycoluril with phosphorous acid tags as a new nanostructured catalyst: Application for the synthesis of novel natural henna-based compounds. ChemistrySelect. 2018;3:3042–3047. doi: 10.1002/slct.201702544. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.