Abstract
Polycystic ovary syndrome (PCOS) women have a hypercoagulable state; however, whether this is intrinsically due to PCOS or, alternatively, a consequence of its metabolic complications is unclear. We determined plasma coagulation pathway protein levels in PCOS (n = 146) and control (n = 97) women recruited to a PCOS biobank. Circulating levels of a panel of 18 clotting pathway proteins were determined by Slow Off-rate Modified Aptamer-scan plasma protein measurement. Cohorts were age matched, though PCOS had elevated body mass index (p < 0.001), insulin (p < 0.001) and C-reactive protein (CRP) (p < 0.0001). Eight pro-coagulation proteins were elevated in PCOS: plasminogen activator inhibitor-1 (p < 0.0001), fibrinogen (p < 0.01), fibrinogen gamma chain (p < 0.0001), fibronectin (p < 0.01), von Willebrand factor (p < 0.05), D-dimer (p < 0.0001), P-selectin (p < 0.05), and plasma kallikrein (p < 0.001). However, two anticoagulant proteins, vitamin K-dependent protein-S (p < 0.0001) and heparin cofactor-II (p < 0.001) were elevated and prothrombin was decreased (p < 0.05). CRP, as a marker of inflammation, and insulin resistance (HOMA-IR) correlated with 11 and 6 of the clotting proteins, respectively (p < 0.05). When matched for BMI < 25 (16 PCOS, 53 controls) HOMA-IR remained elevated (p < 0.05) and heparin cofactor-II was increased (p < 0.05). In a multivariate analysis accounting for inflammation, insulin resistance and BMI, there was no correlation of PCOS with any of the coagulation proteins. The hypercoagulable state in PCOS is not intrinsic to the disease as it can be fully accounted for by BMI, inflammation and insulin resistance.
Subject terms: Endocrinology, Endocrine system and metabolic diseases, Obesity, Pre-diabetes
Introduction
PCOS has been recognized as a reproductive-metabolic disorder given the excess prevalence of type 2 diabetes, hypertension, and cardiovascular diseases in this population at a later stage in life1. Polycystic ovary syndrome (PCOS) patients have increased platelet aggregation and decreased plasma fibrinolytic activity, resulting in a prothrombotic propensity2,3. Elevated coagulation markers have been reported in PCOS in comparison to controls4 and the coagulation parameters including prothrombin time, thrombin time and fibrin degradation products may be predictive of PCOS5. It has been reported that coagulation proteins such as thrombin-activatable fibrinolysis inhibitor, PAI-1, D-dimer, Antithrombin III and thrombomodulin are significantly increased in women with PCOS compared with age- and BMI-matched controls4. This suggests that PCOS, independent of its metabolic features, may be a risk factor for a hypercoagulable state.
This study was undertaken to determine the parameters contributing to the hypercoagulable state reported for PCOS.
Materials and methods
We determined plasma coagulation pathway protein levels in PCOS (n = 146) and control (n = 97) women recruited to a PCOS biobank (ISRCTN70196169). The Newcastle & North Tyneside Ethics committee approved this study which was conducted according to the Declaration of Helsinki. All patients gave written informed consent. Clinical data and samples were accessed from the PCOS Genetic Biobank in the UK, therefore the Newcastle & North Tyneside Ethics committee serves as a national center to provide ethical approval for these Biobank samples.
All women were Caucasian. The diagnosis of PCOS was based on at least two out of three of the diagnostic criteria of the Rotterdam consensus as detailed previously6; namely clinical and biochemical evidence of hyperandrogenism (Ferriman-Gallwey score > 8; free androgen index > 4, total testosterone > 1.5 nmol/L), oligomenorrhea or amenorrhoea and polycystic ovaries on transvaginal ultrasound. Nonclassical 21-hydroxylase deficiency, hyperprolactinemia, Cushing’s disease and androgen secreting tumors were excluded by appropriate tests. The baseline study measurements have been described in detail previously7 and the demographic data for the PCOS and control women is shown in Table 1. All the control women had regular periods, no clinical or biochemical hyperandrogenism, no polycystic ovaries on ultrasound, no significant background medical history and none of them were on any medications including oral contraceptive pills or over the counter medications.
Table 1.
Demographics, baseline, hormonal and metabolic parameters of the PCOS subjects and controls.
| Baseline demographics | PCOS (n = 146) | Controls (n = 97) | p value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 29.1 (6.1) | 29.6 (6.5) | 0.09 |
| BMI (Kg/m2) | 34.1 (7.5) | 26.7 (6.6) | < 0.0001 |
| Weight (Kg) | 96.5 (23.7) | 74.4 (18.4) | < 0.0001 |
| Insulin (IU/ml) | 10.2 (6.1) | 6.2 (3.2) | 0.001 |
| HOMA-IR | 3.8 (0.6) | 1.6 (0.2) | < 0.005 |
| CRP (mg/L) | 4.4 (4.2) | 2.4 (3.9) | 0.0008 |
| SHBG (nmol/L) | 42.5 (39.6) | 77.5 (78.4) | 0.0003 |
| Testosterone (nmol/l) | 1.6 (1.0) | 1.05 (0.48) | < 0.0001 |
BMI Body mass index, HOMA-IR Homeostasis model of assessment-insulin resistance, CRP C reactive protein, SHBG Sex hormone binding globulin.
Circulating levels of clotting pathway proteins were determined by Slow Off-rate Modified Aptamer (SOMA)-scan plasma protein measurement, the details of which have been previously reported8. Normalization of raw intensities, hybridization, median signal and calibration signal were performed based on the standard samples included on each plate, as previously described9.
We used version 3.1 of the SOMAscan Assay, specifically targeting those proteins involved in the coagulation cascade and fibrinolysis pathway in the SOMAscan panel of 18 proteins, in line with a previous report for those involved in a hypercoagulable state and alteration in coagulation proteins10: antithrombin III, heparin cofactor 2, fibrinogen gamma chain, D-Dimer, P-selectin, fibronectin, fibronectin fragment 3, fibronectin fragment 4, vitamin K dependent protein S, alpha 2 antiplasmin, fibrinogen, von Willebrand factor, plasma kallikrein, prothrombin, coagulation factor Xa, tissue factor, coagulation factor XI and angiostatin. Those proteins in the panel that inform us of a hypercoagulable state include fibrinogen and fibronectin as well as inhibition of the fibrinolytic pathway with plasminogen activator inhibitor-1 and evidence of activated fibrinolysis with D-Dimer levels10–12 (Table 2).
Table 2.
Correlation of coagulation proteins with (A) Body mass index (BMI), (B) inflammation (C reactive protein; CRP) and (C) insulin resistance (HOMA-IR), (D) shows the results of the multivariate analysis taking into account BMI, CRP and HOMA-IR.
| A. BMI | B. CRP | C. HOMA-IR | D. CRP + BMI + HOMA-IR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| p value | r2 | p value | r2 | p value | r2 | p value | r2 | ||||
| Antithrombin.III | < 0.00001 | 0.154 | Antithrombin III | < 0.00001 | 0.202 | Antithrombin III | < 0.00001 | 0.140 | D.dimer | 0.06 | 0.151 |
| Fibrinogen gamma chain | 0.00004 | 0.101 | Heparin cofactor.2 | 0.00002 | 0.116 | Heparin cofactor 2 | 0.0007 | 0.070 | Fibrinogen gamma chain | 0.07 | 0.179 |
| Vitamin.K dependent protein S | 0.0007 | 0.070 | Fibrinogen gamma chain | 0.00003 | 0.111 | P.selectin | 0.001 | 0.066 | Fibrinogen | 0.07 | 0.089 |
| D.dimer | 0.0008 | 0.068 | D.dimer | 0.00012 | 0.094 | Fibronectin | 0.009 | 0.042 | Plasma kallikrein | 0.09 | 0.051 |
| Fibronectin | 0.0008 | 0.068 | P.selectin | 0.0012 | 0.067 | Vitamin.K dependent protein.S | 0.015 | 0.037 | Fibronectin Fragment.4 | 0.12 | 0.064 |
| Prothrombin | 0.007 | 0.044 | Fibronectin | 0.0014 | 0.066 | Alpha.2 antiplasmin | 0.02 | 0.032 | Vitamin.K dependent protein.S | 0.14 | 0.089 |
| Heparin cofactor.2 | 0.009 | 0.042 | Fibronectin Fragment.3 | 0.006 | 0.050 | Prothrombin | 0.05 | 0.024 | von Willebrand factor | 0.14 | 0.035 |
| Fibrinogen | 0.013 | 0.038 | Fibronectin Fragment.4 | 0.007 | 0.047 | Fibronectin Fragment.3 | 0.09 | 0.018 | Coagulation factor.Xa | 0.19 | 0.030 |
| Angiostatin | 0.014 | 0.037 | Vitamin K dependent protein.S | 0.02 | 0.037 | Plasma kallikrein | 0.18 | 0.011 | Antithrombin.III | 0.19 | 0.264 |
| Fibronectin Fragment.3 | 0.03 | 0.031 | Alpha.2 antiplasmin | 0.02 | 0.036 | Tissue Factor | 0.20 | 0.010 | Fibronectin | 0.24 | 0.094 |
| P.selectin | 0.04 | 0.026 | Fibrinogen | 0.02 | 0.035 | Fibrinogen gamma chain | 0.24 | 0.009 | Fibronectin Fragment.3 | 0.31 | 0.060 |
| Alpha.2 antiplasmin | 0.16 | 0.012 | von Willebrand factor | 0.12 | 0.016 | Fibronectin Fragment.4 | 0.32 | 0.006 | Tissue Factor | 0.40 | 0.030 |
| Fibronectin Fragment.4 | 0.18 | 0.011 | Plasma kallikrein | 0.18 | 0.012 | D.dimer | 0.43 | 0.004 | Angiostatin | 0.41 | 0.056 |
| Tissue.Factor | 0.19 | 0.011 | Prothrombin | 0.19 | 0.011 | Angiostatin | 0.50 | 0.003 | Prothrombin | 0.42 | 0.048 |
| von.Willebrand factor | 0.26 | 0.008 | Coagulation factor.Xa | 0.22 | 0.010 | Coagulation Factor.XI | 0.81 | 0.000 | Alpha.2 antiplasmin | 0.42 | 0.052 |
| Coagulation factor Xa | 0.49 | 0.003 | Tissue.Factor | 0.71 | 0.001 | Coagulation factor.Xa | 0.83 | 0.000 | Heparin cofactor 2 | 0.58 | 0.133 |
| Coagulation Factor.XI | 0.66 | 0.001 | Coagulation Factor.XI | 0.73 | 0.001 | von Willebrand factor | 0.87 | 0.000 | Coagulation Factor.XI | 0.70 | 0.005 |
| Plasma kallikrein | 0.97 | 0.000 | Angiostatin | 0.74 | 0.001 | Fibrinogen | 0.93 | 0.000 | P.selectin | 0.71 | 0.096 |
Bolded numbers indicate significant differences between PCOS and control groups, at the p < 0.05 level.
Statistics
Measured protein data were log transformed to ascertain normality. Proteins were regressed on the continuous variables CRP, HOMA-IR and BMI in separate models to assess the extent of association with each trait. A multivariate linear model incorporating all three traits and PCOS status was performed to evaluate the relationship between the measured proteins and PCOS whilst correcting for the traits. All analyses were performed using R version 4. P values were corrected for multiple testing using the false discovery rate (FDR).
Ethics approval
The Newcastle & North Tyneside Ethics committee approved this study which was conducted according to the Declaration of Helsinki. All patients gave written informed consent. Clinical data and samples were accessed from the PCOS Genetic Biobank in the UK, therefore the Newcastle & North Tyneside Ethics committee serves as a national center to provide ethical approval for these Biobank samples.
Consent for publication
All authors gave their consent for publication.
Results
Cohorts were age matched, though PCOS had elevated BMI (p < 0.001), fasting glucose (p < 0.05), insulin (p < 0.001), C-reactive protein (p < 0.0001) and platelet number (p < 0.01).
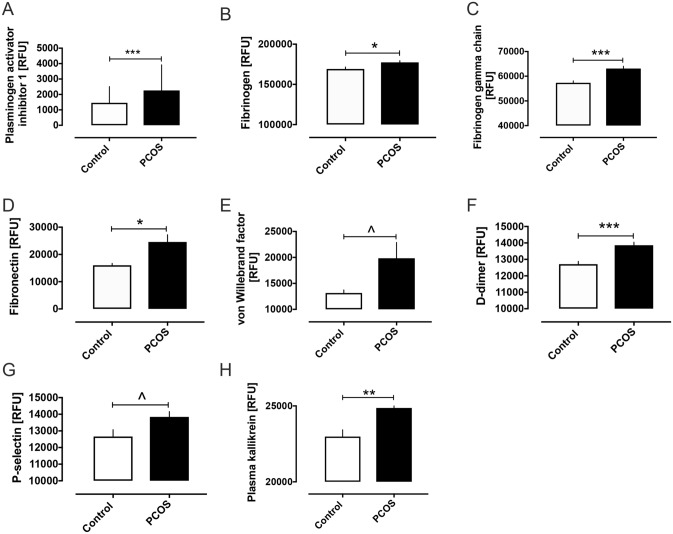
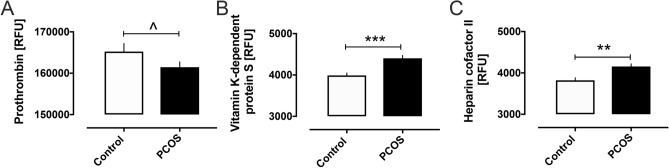
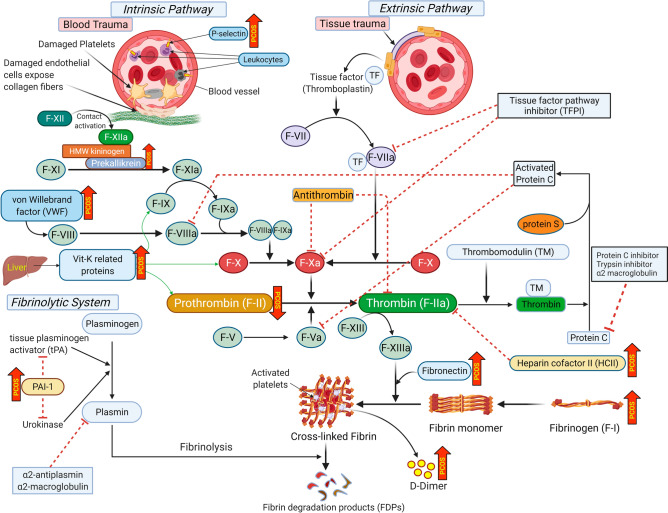
Pro-coagulation proteins elevated in PCOS are shown in Fig. 1 and include plasminogen activator inhibitor-1 (PAI-1) (2259 ± 137 vs 1457 ± 107 RFU, PCOS vs control, p < 0.0001), fibrinogen (177,423 ± 2108 vs 169,230 ± 2425 RFU, PCOS vs control, p < 0.01), fibrinogen gamma chain (63,118 ± 946 vs 57,328 ± 830 RFU, p < 0.0001), fibronectin (24,594 ± 2627 vs 16,041 ± 698 RFU, p < 0.01), von Willebrand factor (19,849 ± 3038 vs 13,159 ± 595 RFU, p < 0.05), D-dimer (13,860 ± 185 vs 12,708 ± 172 RFU, p < 0.0001), P-selectin (13,843 ± 317 vs 12,660 ± 412 RFU, p < 0.05), and plasma kallikrein (24,868 ± 376 vs 22,981 ± 447 RFU, p < 0.001). Prothrombin levels were decreased in PCOS (161,458 ± 1275 vs 165,233 ± 1958 RFU, p < 0.05) and the anticoagulant vitamin K-dependent protein S (4403 ± 69 vs 3989 ± 59 RFU, p < 0.0001) and heparin cofactor II (HCII) (4156 ± 64 vs 3821 ± 63 RFU, p < 0.001) were increased (Fig. 2). A schematic illustration of all the blood coagulation proteins altered in PCOS is shown in Fig. 3.
Figure 1.
Pro-coagulation clotting pathway proteins that were increased in women with polycystic ovary syndrome (PCOS). Levels of plasma plasminogen activator inhibitor 1 (A), fibrinogen (B), fibrinogen gamma chain (C), fibronectin (D), von Willebrand factor (E), D-dimer (F), P-selectin (G) and plasma kallikrein (H) in women with and without polycystic ovary syndrome (PCOS). RFU, relative fluorescent units. Graphs shown as mean ± SEM. ^p < 0.05, *p < 0.01, **p < 0.001, ***p < 0.0001.
Figure 2.
Anti-coagulation clotting pathway proteins that were altered in women with polycystic ovary syndrome (PCOS). Levels of plasma prothrombin (A), Vitamin K dependent Protein S (B) and heparin cofactor II in women with and without polycystic ovary syndrome (PCOS). RFU, relative fluorescent units. Graphs shown as mean ± SEM. ^p < 0.05, **p < 0.001, ***p < 0.0001.
Figure 3.
Schematic illustration showing altered coagulation pathway proteins in polycystic ovary syndrome (PCOS). Cellular stimulation factors and proteins involved in blood coagulation pathways [intrinsic (top left), extrinsic (top right) and fibrinolytic system (bottom left)] are illustrated. Proteins increased in women with PCOS are indicated by upward red arrows (labelled ‘PCOS’) with the protein decreased in PCOS (prothrombin) indicated by a downward red arrow. Red dotted lines indicate the inhibitory actions of the proteins in blood coagulation pathways.
Significant correlations of coagulation proteins with body mass index are shown in Table 2 and include antithrombin III (p < 0.0001), fibrinogen gamma chain (p < 0.0001), vitamin K dependent protein S (p < 0.001), D-dimer (p < 0.001), fibronectin (p < 0.001), prothrombin (p < 0.01), heparin cofactor 2 (p < 0.01), fibrinogen (p < 0.05), angiostatin (p < 0.05), fibrinogen fragment 3 (p < 0.05) and P-selectin (p < 0.05). Significant correlations of coagulation proteins with CRP, as a marker of inflammation, are shown in Table 2, that included antithrombin III (p < 0.0001), heparin cofactor 2 (p < 0.0001), fibrinogen gamma chain (p < 0.0001), D-dimer (p < 0.0001), P-selectin (p < 0.001), fibronectin (p < 0.001), and its fragments 3 and 4 (p < 0.01, respectively), vitamin K dependent protein S, alpha 2 antiplasmin and fibrinogen (p < 0.05, respectively). Significant correlations of coagulation proteins with insulin resistance, as determined by HOMA-IR, were also seen for antithrombin III (p < 0.0001), heparin cofactor 2 (p < 0.001), P-selectin (p < 0.0001), fibronectin (p < 0.01), vitamin K dependent protein S and alpha 2 antiplasmin (p < 0.05, respectively) (Table 2). However, in a multivariate analysis accounting for BMI, inflammation (CRP) and insulin resistance (HOMA-IR), there was no correlation with the coagulation proteins (Table 2).
To eliminate the confounding effect of obesity, a subset of women with BMI ≤ 25 kg/m2 (16 PCOS and 53 controls) were compared. Here, HOMA-IR remained elevated in PCOS (1.6 ± 1.2 vs 1.1 ± 0.5, p < 0.05), CRP did not differ, whilst heparin cofactor 2 (3979 ± 649 vs 3613 ± 585 RFU, p < 0.05) was elevated in the normal weight PCOS group (Table 3).
Table 3.
Demographics, baseline, hormonal and metabolic parameters of the normal weight (BMI ≤ 25 kg/m2) PCOS subjects and controls (16 PCOS and 53 control women).
| Baseline demographics | PCOS (n = 16) | Controls (n = 53) | p value |
|---|---|---|---|
| Mean (SEM) | Mean (SEM) | ||
| Age (years) | 27.8 (1.7) | 29.5 (0.9) | 0.37 |
| BMI (Kg/m2) | 22.7 (0.5) | 22.8 (0.3) | 0.87 |
| Weight (Kg) | 64.1 (1.9) | 63.0 (1.0) | 0.59 |
| Insulin (IU/ml) | 8.9 (2.4) | 5.0 (0.5) | 0.02 |
| HOMA-IR | 1.6 (0.3) | 1.1 (0.09) | 0.04 |
| CRP (mg/L) | 1.2 (0.2) | 0.9 (0.1) | 0.22 |
| SHBG (nmol/L) | 72.7 (15.6) | 95.9 (12.2) | 0.33 |
| Testosterone (nmol/l) | 1.2 (0.1) | 1.1 (0.1) | 0.29 |
BMI Body mass index, HOMA-IR Homeostasis model of assessment-insulin resistance, CRP C reactive protein, SHBG Sex hormone binding globulin.
Discussion
These data show that the hypercoagulable state in PCOS can be completely accounted for by BMI and its associated inflammation, and enhanced insulin resistance. In comparison to the normal controls, overall 10 pro-coagulation proteins were elevated in PCOS; plasminogen activator inhibitor-1 (PAI-1), fibrinogen, fibrinogen gamma chain, fibronectin, von Willebrand factor, D-dimer, P-selectin, plasma kallikrein. anticoagulant vitamin K-dependent protein S and heparin cofactor II, whilst prothrombin was decreased. These results are in accord with others who have reported changes in coagulation proteins in PCOS4,13, but underlying pathophysiology has not been previously described and shows that in PCOS alterations in the coagulation factors appears complex and multifactorial. When normal weight (BMI ≤ 25) PCOS patients were compared with normal weight control subjects, insulin resistance remained elevated and heparin cofactor 2, that is protective and inactivates thrombin in tissues, differed. These data are in accord with the association of heparin cofactor 2 with insulin resistance14, indicating that normal weight PCOS subjects likely have no additional risk associated with a hypercoagulable state; however, obesity with associated inflammation markedly exaggerates the hypercoagulable state with an increased number of clotting parameters altered. The multivariate analysis showed that all of the changes in the coagulation proteins could be accounted for by BMI, inflammation and insulin resistance.
It is well recognized that obesity causes inflammation and increased insulin resistance15,16 and is associated with changes in coagulation parameters. For example, fibronectin is correlated to BMI17 and obesity is associated with increased PAI-1 in PCOS18. Others have reported, using a repeated fibrin formation and degradation functional assay, that “overall hemostatic potential” was BMI-dependent and not associated with PCOS19. Central fat mass has been associated with fibrinogen, CRP, coagulation factor XIII, waist-to-hip ratio, plasminogen, PAI-1, plasmin inhibitor, and thrombin activatable fibrinolysis inhibitor20).
Conversely, thrombin-activatable fibrinolysis inhibitor, PAI-1, D-dimer, Antithrombin III and thrombomodulin were reported to be significantly increased in women with PCOS compared with age- and BMI-matched controls, suggesting that alterations in these proteins are BMI-independent and due to other factors such as inflammation and insulin resistance, as reported here4.
Inflammation (CRP) correlated significantly with antithrombin III, heparin cofactor 2, fibrinogen gamma chain, D-dimer, P-selectin, fibronectin, and its fragments 3 and 4, vitamin K dependent protein S, alpha 2 antiplasmin and fibrinogen. Inflammation crosstalk with coagulation leading to increased coagulopathy is well recognized; however, with the initiation of coagulation, the coagulation proteases may then modulate the inflammatory response21,22. In PCOS, both CRP and fibrinogen are predicted by BMI in accord with obesity initiating the increased inflammation23 and particularly CRP, PAI-1, D-dimer, Antithrombin III with central fat mass as noted above20.
In this study, insulin resistance (HOMA-IR) correlated with Antithrombin III, heparin cofactor 2, P-selectin, fibronectin, vitamin K dependent protein S and alpha 2 antiplasmin. It is recognized that insulin resistance is associated with enhanced thrombogenesis24; however, it is difficult to determine the contribution of insulin resistance alone to its association with obesity and inflammation metabolic syndrome lipid parameters25–27.
As noted above, there are reports of changes in coagulation proteins in PCOS4,13 and changes in functional assays2,3; however, conversely others have not found changes in the coagulation proteins between PCOS and controls28. It can be seen from the data presented here that the likely reason for these discrepancies are due to the patient population being studied with the results dependent on the degree of obesity, inflammation and insulin resistance present. In addition, the PCOS phenotype may have an important role, with those having all three of the diagnostic criteria exhibiting the metabolic phenotype with increased insulin resistance in comparison to those with only two of the three diagnostic criteria29.
The hypercoagulation state is in homeostasis with the pro-coagulation protein changes seen here in PCOS being balanced by the reduction in prothrombin and increased vitamin K-dependent protein S and heparin cofactor II that we also report.
Limitations of this study include that it was a cross sectional study, but this was mitigated by the large number of subjects. As all study subjects were Caucasian, these results may not be generalizable to other ethnic populations. Only BMI, rather than an assessment of visceral fat, was available for this study population. In addition, only the proteins involved in the coagulation cascade and fibrinolysis pathway were measured. It would have been optimal to have included prothrombin fragments 1 and 2 as markers of activated coagulation, but these were not available in the SOMAscan proteomics panel. Likewise, the thrombin generation assay would have been a good indicator of an increase in activated coagulation; however, no functional assays were undertaken in this study. Whilst there was no single good marker for activated coagulation it has well been recognised that indicators found here, such as high plasma fibrinogen, factor VII/VIIa, tissue-type plasminogen activator and plasminogen activator inhibitor levels, have been associated with at least as great a risk of developing myocardial (re)infarction or sudden death as high cholesterol levels, especially in the young11.
In conclusion, the hypercoagulable state in PCOS can be fully accounted for by BMI, inflammation and insulin resistance. This is an important finding because, whilst the prothrombotic propensity of women with PCOS has previously been reported, it was assumed to be intrinsic to the PCOS condition. Here, we show that this is, in fact, not the case, but is a consequence of the increased BMI, inflammation and insulin resistance that often accompany the PCOS condition; the impact of directed therapy needs to be determined in the future. Further studies in lean PCOS women would help to clarify our findings.
Author contributions
A.S.M.M. and A.E.B. analyzed the data and wrote the manuscript. T.S. supervised clinical studies and edited the manuscript. I.D. performed the statistical analysis. S.L.A. and M.A.E. contributed to study design, data interpretation and the writing of the manuscript. All authors reviewed and approved the final version of the manuscript. A.E.B. is the guarantor of this work.
Data availability
All the data for this study will be made available upon reasonable request to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Alexandra E. Butler and Stephen L. Atkin.
References
- 1.Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome—a position statement of the Androgen Excess Society. J. Clin. Endocrinol. Metab. 2007;92(12):4546–4556. doi: 10.1210/jc.2007-1549. [DOI] [PubMed] [Google Scholar]
- 2.Mak W, Dokras A. Polycystic ovarian syndrome and the risk of cardiovascular disease and thrombosis. Semin. Thromb. Hemost. 2009;35(7):613–620. doi: 10.1055/s-0029-1242715. [DOI] [PubMed] [Google Scholar]
- 3.Yildiz BO, Haznedaroğlu IC, Kirazli S, Bayraktar M. Global fibrinolytic capacity is decreased in polycystic ovary syndrome, suggesting a prothrombotic state. J. Clin. Endocrinol. Metab. 2002;87(8):3871–3875. doi: 10.1210/jcem.87.8.8716. [DOI] [PubMed] [Google Scholar]
- 4.Oral B, Mermi B, Dilek M, Alanoğlu G, Sütçü R. Thrombin activatable fibrinolysis inhibitor and other hemostatic parameters in patients with polycystic ovary syndrome. Gynecol. Endocrinol. 2009;25(2):110–116. doi: 10.1080/09513590802549874. [DOI] [PubMed] [Google Scholar]
- 5.Sun Q, Yang Y, Peng X, Zhang Y, Gao Y, Wang F, et al. Coagulation parameters predictive of polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;240:36–40. doi: 10.1016/j.ejogrb.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Sathyapalan, T., Al-Qaissi, A., Kilpatrick, E. S., Dargham, S. R. & Atkin, S. L. Anti-Mullerian hormone measurement for the diagnosis of polycystic ovary syndrome. Clin. Endocrinol. 88(2), 258–262. 10.1111/cen.13517 (2017). [DOI] [PubMed]
- 7.Sathyapalan T, Al-Qaissi A, Kilpatrick ES, Dargham SR, Adaway J, Keevil B, et al. Salivary testosterone measurement in women with and without polycystic ovary syndrome. Sci. Rep. 2017;7(1):3589. doi: 10.1038/s41598-017-03945-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahal H, Halama A, Aburima A, Bhagwat AM, Butler AE, Grauman J, et al. Effect of induced hypoglycemia on inflammation and oxidative stress in type 2 diabetes and control subjects. Sci. Rep. 2020;10(1):4750. doi: 10.1038/s41598-020-61531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraemer S, Vaught JD, Bock C, Gold L, Katilius E, Keeney TR, et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: a SOMAmer-based, streamlined multiplex proteomic assay. PLoS ONE. 2011;6(10):e26332. doi: 10.1371/journal.pone.0026332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller SK, Nocera AL, Dillon ST, Wu D, Libermann TA, Bleier BS. Highly multiplexed proteomic analysis reveals significant tissue and exosomal coagulation pathway derangement in chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2018;8(12):1438–1444. doi: 10.1002/alr.22189. [DOI] [PubMed] [Google Scholar]
- 11.Rossi ML, Merlini PA, Ardissino D. Laboratory markers of hypercoagulability. Ital. Heart J. 2001;2(7):490–494. [PubMed] [Google Scholar]
- 12.Nieuwdorp M, Stroes ES, Meijers JC, Büller H. Hypercoagulability in the metabolic syndrome. Curr. Opin. Pharmacol. 2005;5(2):155–159. doi: 10.1016/j.coph.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Targher G, Zoppini G, Bonora E, Moghetti P. Hemostatic and fibrinolytic abnormalities in polycystic ovary syndrome. Semin. Thromb. Hemost. 2014;40(5):600–618. doi: 10.1055/s-0034-1384512. [DOI] [PubMed] [Google Scholar]
- 14.Kurahashi K, Inoue S, Yoshida S, Ikeda Y, Morimoto K, Uemoto R, et al. The role of heparin cofactor II in the regulation of insulin sensitivity and maintenance of glucose homeostasis in humans and mice. J. Atheroscler. Thromb. 2017;24(12):1215–1230. doi: 10.5551/jat.37739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017;127(1):1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 17.Nácul AP, Andrade CD, Schwarz P, de Bittencourt PI, Jr., Spritzer PM. Nitric oxide and fibrinogen in polycystic ovary syndrome: associations with insulin resistance and obesity. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007;133(2):191–196. doi: 10.1016/j.ejogrb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Atiomo WU, Fox R, Condon JE, Shaw S, Friend J, Prentice AG, et al. Raised plasminogen activator inhibitor-1 (PAI-1) is not an independent risk factor in the polycystic ovary syndrome (PCOS) Clin. Endocrinol. 2000;52(4):487–492. doi: 10.1046/j.1365-2265.2000.00946.x. [DOI] [PubMed] [Google Scholar]
- 19.Rakusa M, Jensterle M, Božič-Mijovski M, Janez A. Increased coagulation and decreased fibrinolysis as measured with overall hemostatic potential are dependent on bmi and not associated with PCOS. Metab. Syndr. Relat. Disord. 2017;15(4):194–198. doi: 10.1089/met.2016.0148. [DOI] [PubMed] [Google Scholar]
- 20.Godtfredsen ACM, Sidelmann JJ, Gram JB, Andersen M, Glintborg D. Fibrin lysability is associated with central obesity and inflammation in women with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2020;99(8):1078–1084. doi: 10.1111/aogs.13825. [DOI] [PubMed] [Google Scholar]
- 21.Levi M, van der Poll T. Inflammation and coagulation. Crit. Care Med. 2010;38(2 Suppl):S26–S34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 22.Esmon CT. The interactions between inflammation and coagulation. Br. J. Haematol. 2005;131(4):417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 23.Mažibrada I, Djukić T, Perović S, Plješa-Ercegovac M, Plavšić L, Bojanin D, et al. The association of hs-CRP and fibrinogen with anthropometric and lipid parameters in non-obese adolescent girls with polycystic ovary syndrome. J. Pediatr. Endocrinol. Metab. 2018;31(11):1213–1220. doi: 10.1515/jpem-2017-0511. [DOI] [PubMed] [Google Scholar]
- 24.Baalbaki HA, Bell DS. Insulin resistance and thrombogenesis: recent insights and therapeutic implications. Endocr. Pract. 2007;13(6):679–686. doi: 10.4158/EP.13.6.679. [DOI] [PubMed] [Google Scholar]
- 25.Lallukka S, Luukkonen PK, Zhou Y, Isokuortti E, Leivonen M, Juuti A, et al. Obesity/insulin resistance rather than liver fat increases coagulation factor activities and expression in humans. Thromb. Haemost. 2017;117(2):286–294. doi: 10.1160/TH16-09-0716. [DOI] [PubMed] [Google Scholar]
- 26.Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122(20):3415–3422. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ragab A, Abousamra NK, Higazy A, Saleh O. Relationship between insulin resistance and some coagulation and fibrinolytic parameters in patients with metabolic syndrome. Lab. Hematol. 2008;14(1):1–6. doi: 10.1532/LH96.07017. [DOI] [PubMed] [Google Scholar]
- 28.Kebapcilar L, Taner CE, Kebapcilar AG, Sari I. High mean platelet volume, low-grade systemic coagulation and fibrinolytic activation are associated with androgen and insulin levels in polycystic ovary syndrome. Arch. Gynecol. Obstet. 2009;280(2):187–193. doi: 10.1007/s00404-008-0884-0. [DOI] [PubMed] [Google Scholar]
- 29.Neven ACH, Laven J, Teede HJ, Boyle JA. A summary on polycystic ovary syndrome: diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin. Reprod. Med. 2018;36(1):5–12. doi: 10.1055/s-0038-1668085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data for this study will be made available upon reasonable request to the corresponding author.