Abstract
Circadian misalignment remains a distinct challenge for night shift workers. Variability in individual sleep-wake/light-dark patterns might contribute to individual differences in circadian alignment in night shift workers. In this simulation study, we compared the predicted phase shift from a mathematical model of the effect of light on the human circadian pacemaker to the observed melatonin phase shift among individuals who completed one of four interventions during simulated night shift work. Two inputs to the model were used to simulate circadian phase: sleep-wake/light-dark patterns measured from a wrist monitor (Simulation 1) and sleep-wake/light-dark patterns measured from a wrist monitor enhanced by known light levels measured at the level of the eye during simulated night shifts (Simulation 2). The estimated phase shift from the model was within 2 hours of the observed phase shift in ~80% of night shift workers for both simulations; none of the model-predicted phase shifts was more than ~3 hours from the observed phase shift. Overall, the root mean square error between observed and predicted phase shifts was better for Simulation 1. The light input from the wrist monitor informed by actual light level measured at the eye performed better in the sub-group exposed to bright light during their night shifts. The findings from this simulation study suggest that using a mathematical model combined with sleep-wake and light exposure data from a wrist monitor can facilitate the design of shift work schedules to enhance circadian alignment, which is expected to improve sleep, alertness and performance.
Keywords: shiftwork, mathematical model, circadian phase, phase shift, light exposure, DLMO, melatonin phase, actigraphy
Introduction
Shift work has become more and more common as our 24/7 global society has required an increasing number of workers to do their jobs at night and/or at irregular hours. According to the National Health Interview Survey (NHIS), in 2010 approximately 28.7% of the American workforce was engaged in work outside a regular day shift (outside 09:00-to17:00h; Alterman et al. 2013). Shift workers are exposed to atypical or irregular sleep-wake schedules which can lead to misalignment between the endogenous circadian timing system and the sleep–wake cycle. This misalignment leads to disruption of physiological rhythms and may contribute to the development of the adverse health effects associated with night shift work, such as increased risk for cardiovascular disease, metabolic syndrome, depression, and other health problems (Boivin and Boudreau 2014; Kecklund and Axelsson 2016; Matheson et al. 2014).
Circadian misalignment, when an individual’s circadian rhythm timing is out of sync to his/her sleep-wake times and/or with the natural light-dark cycle, remains a distinct challenge for night shift workers, who do not show complete circadian adaptation, even when working permanent night shifts (Folkard 2008; Mitchell et al. 1997). The primary factors that contribute to this lack of adaptation are thought to be exposure to outdoor light during the morning commute home and adopting a night-sleep, and day-active schedule on days off (Bjorvatn et al. 2002; Dumont et al. 2001; Mitchell et al. 1997). Melatonin is a robust marker of the endogenous circadian clock that is heavily influenced by light exposure patterns, and is widely used to determine the circadian phase/alignment in shift workers (Benloucif et al. 2005).
There are individual differences in the amount/rate of circadian adaptation/alignment as reported in both laboratory- and field-based studies (Chinoy et al. 2016; Dijk et al. 2012; Folkard 2008; Rahman et al. 2017). As light is the strongest zeitgeber for the synchronization of the circadian timing system, variability in individual light exposure patterns/light exposure history (outside work hours) might be an important factor for the individual differences found in circadian alignment in night shift workers. Night shift workers are not only exposed to different environmental light levels compared to day workers, there are also inter-individual differences within shift workers, influenced by chronotype, age, and other non-physiological factors, such as the presence of children at home (Rabstein et al. 2019).
Previous studies have demonstrated that controlling the pattern of light and dark exposure throughout the day constitutes an important factor affecting the adjustment of circadian phase (Czeisler et al., 1990; Buxton et al. 2000; Crowley et al. 2003; Duffy et al. 1996; Dumont et al. 2001; Eastman et al. 1994; Santhi et al. 2005). In our recent simulated shift work study, we found large inter-individual differences in circadian adaptation, i.e., in the dim-light melatonin onset (DLMO) phase shifts between baseline (Day shift) and after 3 night shifts [see Figure 4A in (Chinoy et al. 2016) and Figure 4 in (Isherwood et al. 2020)]. Because the participants left the laboratory after their work shifts, each participant probably experienced different patterns of light exposure in their real-life setting. Thus, we were interested in whether the patterns of light exposure could explain the individual differences in phase shift between the participants.
Therefore, our research question is: Can we explain the individual differences in phase shift in response to a night shift schedule (with various interventions) based on individual light exposure patterns by applying a mathematical model of the effects of light on the circadian system? To investigate this, we used light exposure and rest-activity data collected from a wrist-worn device used by participants in our previous study (Chinoy et al. 2016; Isherwood et al. 2020) as input to a mathematical model of photic and non-photic effects on the circadian system (St. Hilaire et al. 2007). The model estimates circadian phase based on sleep-wake timing and the timing and intensity of light exposure, and has been validated with data from experimental and field-based settings (Flynn-Evans et al. 2016; Jewett 1997; Klerman et al. 2016). Here, we tested the ability of the model to estimate melatonin phase resetting within an individual among a group of healthy older participants randomized to different sleep timing and light exposure conditions during their transition from day to night shift.
Materials and Methods
Participants and Study Schedule
Data used in the present analyses were collected in a series of 10 d simulated shift work studies (Chinoy et al. 2016; Isherwood et al. 2020). The study was reviewed and approved by the Partners Health Care Human Research Committee, and participants provided written informed consent prior to their participation. The protocol conformed to the international ethical standards for biological rhythm research studies as outlined in Portaluppi et al. (2010).
Participants were 36 healthy non-shift working adults (mean age 58, range 50–66 y; 12 women, 24 men) living in greater Boston (USA). They each wore a wrist activity-light monitor on their non-dominant arm for ~one week immediately before their study began and throughout the 10 d laboratory-field study.
Each study began with four simulated day shifts (07:00–15:00h), followed by an off day, and then four simulated night shifts (23:00–07:00h). Participants were in controlled lighting conditions in the laboratory throughout each simulated shift as well as for ~6 h immediately before the first night shift and 24 h after the final night shift so that their circadian timing could be assessed (see below). At all other times, participants were outside the laboratory. When working day shifts, participants worked in ordinary indoor room light (see below) and instructed to spend 8 h in bed attempting to sleep before each shift.
After the fourth day shift, each participant was randomized into one of four intervention groups for the following 4 night shifts: control (C); 8 h afternoon-evening sleep timing and night shift bright light (ST+L); 8 h afternoon-evening sleep timing (ST); ad libitum afternoon-evening sleep timing (SA). The Control group was given no instructions about their sleep between night shifts; the ST+L and ST groups were instructed to go to bed between 13:00 and 14:00h and spend 8 h in bed attempting to sleep between their night shifts; while the SA group was instructed to not go to bed before 13:00h, with no further instructions about how long they should sleep.
In-Laboratory Lighting
All lighting was administered from ceiling-mounted fluorescent lamps (4100 K; Philips Lighting, Eindhoven, the Netherlands) and measured from 137 cm above the floor in the direction the participant was facing while sitting at the desk where he/she most of the shift. Participants in all four groups were exposed to typical indoor room lighting (0.23 W/m2 (~89 lux) at 137 cm from the floor in the horizontal angle with a maximum of 0.48 W/m2 (~150 lux) at 187 cm from the floor in the horizontal angle anywhere in the room.) throughout all day shifts. Participants in the C, ST, and SA groups had the same lighting throughout their first three Night shifts. Participants in the ST+L group had the same lighting for the first half (23:00-03:00h) of their first three Night shifts, and then lighting was increased to ~2209 ± 342 lux (~4.87 W/m2) for the latter half (03:00–07:00h) of those Night shifts. During the circadian phase assessments, lighting for all four groups was reduced (~3.3 lx, ~0.0087 W/m2) to allow for dim-light melatonin onset (DLMO) assessment.
Circadian Phase Assessment
Circadian phase of the salivary DLMO was assessed before the first Night shift and during/after last (fourth) Night shift. During these phase estimation procedures, hourly saliva samples were collected while the participant remained in dim lighting. Assessment 1 was on study day 5 from 17:00 to 23:00h (just before the first Night shift) and Re-assessment was from 23:00 on study d 8 (start of the fourth Night shift) through 24:00h on study d 9. Saliva samples were frozen and shipped to Solidphase (Portland, Maine, USA), where melatonin levels were assayed via direct saliva melatonin radioimmunoassay (BÜHLMANN Laboratories AG, Schönenbuch, Switzerland). DLMO was calculated as the clock time at which melatonin levels reached a 3.0 pg/mL threshold, using linear interpolation between adjacent samples. In cases where melatonin levels did not reach 3.0 pg/mL [2 control group, 1 ST+L group, 2 ST group participants], a 1.0 pg/mL DLMO threshold was used. Phase shifts were calculated as the difference in clock time of DLMO from assessment to re-assessment.
Actigraphy
All participants wore an activity-light monitor on their non-dominant wrist for approximately one week before the study began and throughout the study. Twenty-nine participants wore a MotionWatch8 (CamNTech, Cambridge, UK), five wore the Actiwatch-L (Mini Mitter Respironics, Bend, Oregon, USA), and two wore the Actiwatch Spectrum (Philips Respironics, Eindhoven, the Netherlands). Actigraphy data were collected in 1 min epochs.
Model Simulations
For simulations, we used a mathematical model of photic and non-photic effects on the circadian system (St. Hilaire et al. 2007), which was coded and run in MATLAB version 2015b (The MathWorks; Natick, MA USA). No changes to the model equations or parameters were made. All activity data (regardless of device used for collection) were scored for sleep or wakefulness per 1 min epoch using Motionware (V.1.1.20, CAmNTech, Cambridge, UK) using the high-sensitivity setting. Bed and wake times determined from sleep diaries and voicemail call-ins were used as input for sleep-wakefulness analysis. All light data from the wrist monitor were read into the model in 1 min epochs. The scored sleep was used in the model to determine whether each epoch corresponded to sleep or wake. Two simulations were run for each participant using the following assumptions: Simulation 1: The raw light level for each 1 min epoch recorded by the wrist monitor was read directly into the model as light input. Simulation 2: The raw light level for each 1 min epoch recorded by the wrist monitor was read directly in the model as light input, except during the 8 h simulated night shifts in the lab; during these times, the actual light level at the desk where the participant was working (measured in a horizontal direction at 137 cm from the floor) was used as the light input to the model.
For missing epochs, the wrist monitor-recorded light level from the last non-missing epoch was used. The simulation of each schedule was preceded by 180 d of the participant’s habitual pre-inpatient sleep-wake schedule to adjust the starting phase of the model. The primary model output was core body temperature minimum (CBTmin), which was rescaled to DLMO based on a scaling factor of −6.67 h (St. Hilaire et al. 2007). Phase shifts were calculated from the model output as final DLMO phase subtracted from initial DLMO phase on the nights corresponding to the times when DLMO was measured in the laboratory.
Statistical Analysis
We constructed Bland-Altman plots, including the mean difference (i.e., fixed bias) and 95% limits of agreement (Bland and Altman 1999), to show the difference between the observed phase shift and predicted phase shift for Simulation 1 and Simulation 2 in OriginPro 8.5 (OriginLab, Northampton, MA, USA). We computed the root mean square error (RMSE) between observed and predicted phase shifts for each simulation. We also computed the proportion of phase shifts predicted by each simulation that fell within 30, 60, or 120 min of the observed phase shift.
Results
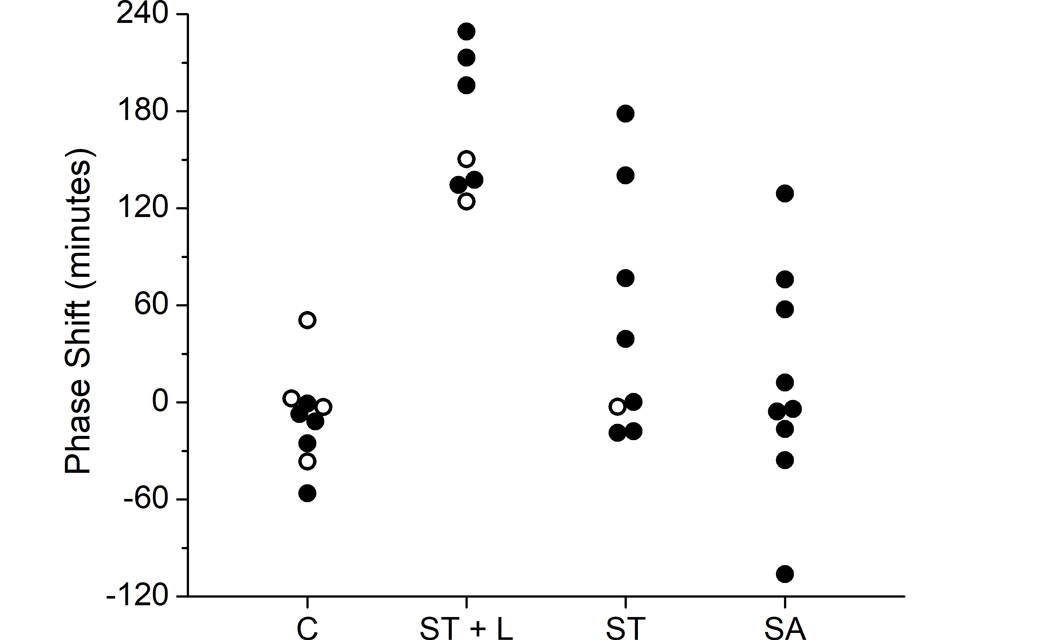
Of the 36 participants who took part in the studies, three had insufficient melatonin data to calculate DLMO phase shifts, six had missing activity and/or light data for conducting model simulations, and one had a suspected failure of their Actiwatch-L, leaving 26 for the modelling analysis (three who wore an Actiwatch-L and 23 who wore a MotionWatch8). This included five participants in the control group (1 Actiwatch-L), five in the ST + L group (1 Actiwatch-L), seven in the ST group (1 Actiwatch-L), and nine in the SA group. Twelve of 26 participants had missing epochs of data, ranging from 4–17 consecutive min in 10 of the 12 participants (average 6.5 min). The duration of missing epochs was more notable in two participants, one of whom was missing 83 consecutive min of data and one who was missing more than a day of data (1866 min). Overall, the number of missing epochs represented less than ~0.65% of all epochs. Figure 1 shows the observed DLMO phase shifts by group for all included participants.
Figure 1. Observed phase shifts by group.
Phase shifts (in min) were calculated as initial phase minus final phase based on salivary dim light melatonin onset (DLMO) for each participant within each intervention group. Participants excluded from the present analysis due to missing data are indicated by open symbols. C: control; ST + L: 8 h afternoon-evening sleep timing and night shift bright light; ST: 8 h afternoon-evening sleep timing; and SA: ad lib afternoon-evening sleep timing. These data were originally reported in (Chinoy et al. 2016 and Isherwood et al. 2020).
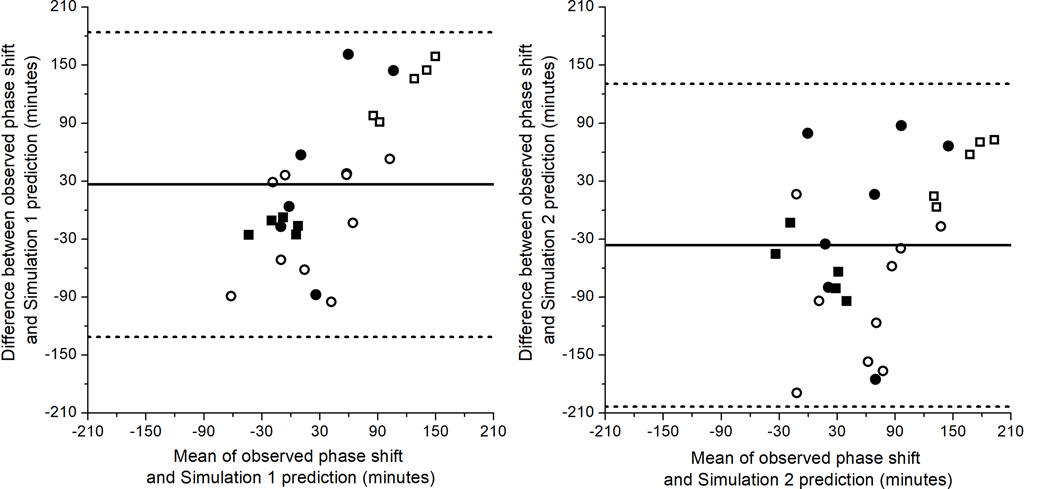
Bland-Altman plots of the differences between observed phase shifts and predicted phase shifts for Simulations 1 and 2 are shown in Figure 2. Figure 2 indicates that Simulation 1 tends to underpredict phase shifts overall and Simulation 2 tends to overpredict phase shifts overall, with fixed biases of +26.44 min and –36.24 min, respectively. The RMSEs were 81.73 and 89.53 for Simulation 1 and Simulation 2, respectively, indicating that Simulation 1 generated better fits to the data overall. This finding is supported by the mean absolute prediction error and standard deviation, which was 64.92 ± 50.63 min for Simulation 1 and 73.43 ± 52.22 min for Simulation 2. Notably, however, Simulation 2 performs better than Simulation 1 on the ST + L group, who were exposed to bright light during the latter half of their night shifts. The RMSEs and mean absolute prediction errors and standard deviation overall and by group are reported in Table 1.
Figure 2. Bland-Altman plots of differences between observed and predicted phase shifts.
Bland-Altman plots show the differences between observed and predicted phase shifts for Simulation 1 (left) and Simulation 2 (right). The mean (i.e., fixed bias; solid line) was +26.44 min for Simulation 1 and –36.24 min for Simulation 2. The confidence intervals for 95% limits of agreement (dashed lines) are −131.28 to 184.17 for Simulation 1 and −203.21 to 130.74 for Simulation 2. Control = filled squares (■); ST+L = open squares (□); ST = filled circles (●); SA = open circles (❍).
Table 1.
Comparison of performance of Simulation 1 and Simulation 2 overall and by group. RMSE: root mean square error; LOA: limits of agreement; MAE (SD): mean absolute error and standard deviation; C: control; ST + L: 8 h afternoon-evening sleep timing and night shift bright light; ST: 8 h afternoon-evening sleep timing; and SA: ad lib afternoon-evening sleep timing.
| Simulation 1 | Simulation 2 | ||
|---|---|---|---|
| Overall | RMSE | 81.73 | 89.53 |
| Fixed Bias (LOA) | 26.44 (−128.13, 181.01) | −36.24 (−199.87, 127.40) | |
| MAE (SD) | 64.92 (50.63) | 73.43 (52.22) | |
| Control | RMSE | 18.64 | 65.90 |
| Fixed Bias (LOA) | −17.13 (−33.20, −1.06) | −59.49 (−121.61, 2.62) | |
| MAE (SD) | 17.13 (8.20) | 59.49 (31.69) | |
| ST + L | RMSE | 128.58 | 52.43 |
| Fixed Bias (LOA) | 125.80 (67.50, 184.09) | 43.60 (−20.21, 107.41) | |
| MAE (SD) | 125.80 (29.74) | 43.60 (32.56) | |
| ST | RMSE | 92.17 | 90.15 |
| Fixed Bias (LOA) | 42.82 (−129.98, 215.61) | −5.87 (−196.32, 184.58) | |
| MAE (SD) | 72.68 (61.22) | 77.09 (50.48) | |
| SA | RMSE | 57.54 | 113.55 |
| Fixed Bias (LOA) | −17.28 (−131.37, 96.81) | −91.28 (−231.68, 49.11) | |
| MAE (SD) | 51.61 (26.98) | 94.91 (66.11) | |
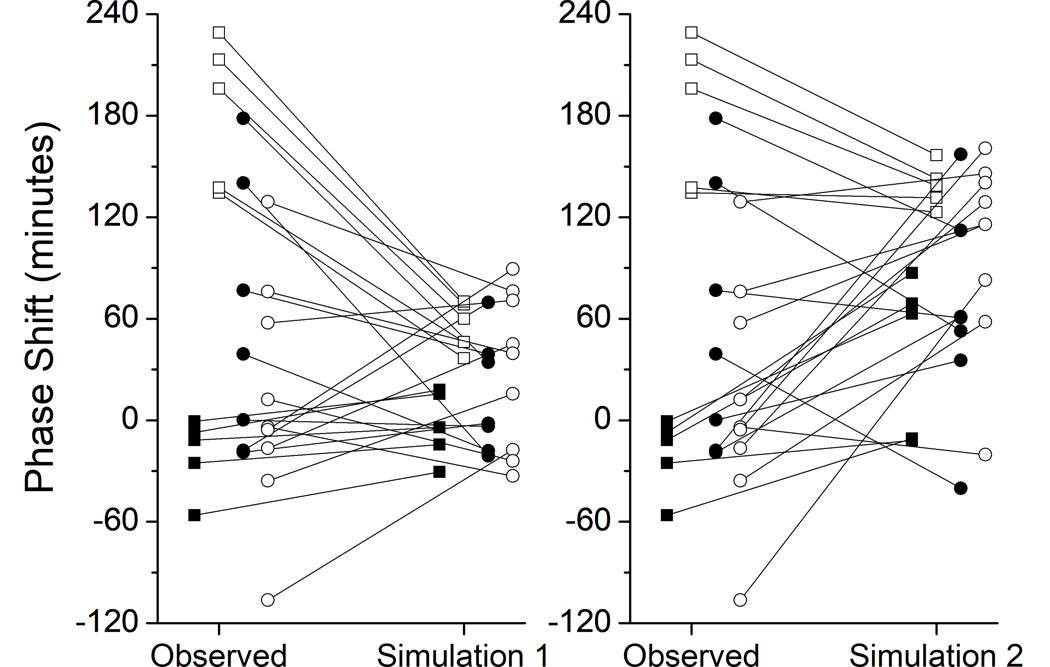
The observed and predicted phase shifts for each participant for each simulation are shown in Figure 3. Simulation 2, but not Simulation 1, underpredicts the magnitude of phase delays observed in the Control group, and in three participants predicts advances instead of delays. In the ST + L group, both simulations, particularly Simulation 1, underpredict the magnitude of phase advances. In the ST group, Simulation 1 and Simulation 2 predict the opposite direction of phase shift in 4 participants and 3 participants, respectively. Similarly, in the SA group, Simulation 1 and Simulation 2 predict the opposite direction of phase shift in 4 participants each.
Figure 3. Observed versus predicted phase shifts by group.
The observed and predicted phase shift (in min) for each participant is shown. Left panel: Observed phase shift vs. Simulation 1; Right panel: Simulation 2. Control = filled squares (■); ST+L = open squares (□); ST = filled circles (●); SA = open circles (❍).
For Simulation 1, 35% of the predicted phase shifts were within 30 min of the actual phase shift, compared with 23% for Model 2; 58% and 42% were within 60 min, and 81% and 85% were within 120 min for Simulation 1 and Simulation 2, respectively. No prediction for either model was more than 189 min from the observed phase shift.
Discussion
We used raw light levels (in lux) from a wrist monitor and actigraphy-scored sleep-wake information as input to a mathematical model of the effects of light exposure on the human circadian pacemaker to predict DLMO phase shifts in healthy older participants. Simulations that used the raw light level measured at the wrist (Simulation 1) yielded similar results to simulations that used the light levels measured at the eye during the night shift (Simulation 2), except for the group that received bright light exposure, in which Simulation 2 yielded significantly better predictions. The findings from this simulation study, therefore, suggest that this mathematical model combined with light exposure and activity data from a wrist monitor can facilitate the design of shift work schedules to enhance circadian alignment, and thereby optimize sleep, alertness and performance.
The original mathematical model was developed based on group-averaged data and was not intended to simulate individual-level data. Nevertheless, the findings from this study suggest that the model can yield reasonable estimates of circadian phase shifts when individual light level data from a wrist monitor are used as input to the model. Approximately 80% of the simulations yielded phase shift estimates within 2 h of the observed phase shift, and none of the model-predicted phase shifts differed by more than ~3 h from the observed phase shift. Our current findings are consistent with a recent study (Stone et al. 2019) that used the same model (St. Hilaire et al. 2007) to estimate circadian phase from wrist monitor-derived sleep-wake and light-dark patterns among 25 nursing and medical staff during their transition from day/evening shifts to 3–5 consecutive night shifts. In that study, investigators observed a mean absolute prediction error and standard deviation of 66.6 ± 52.8 min, and 80% of predictions within 120 min of the observed phase shifts. Further development of the model will be needed to yield more accurate phase shift estimates using individual-level data.
The major strength of the approach we used is that the mathematical model incorporates multiple experimental findings on the effects of the intensity, duration, and timing of light on the circadian system as well as non-photic effects into its prediction (St. Hilaire et al. 2007). The results suggest that the model can provide an efficient way to estimate the effect of a night shift work schedule on predicted circadian phase. Because the model is linked to a mathematical model of neurobehavioral performance and alertness (Jewett and Kronauer 1999), this approach also can be used to predict times of anticipated performance impairments.
There are also some limitations to our approach. The model does not take into account all factors that may be encountered in an operational setting, such as use of caffeine, which may have independent effects on phase shifts (Burke et al. 2015). Participants in this study were instructed to not use caffeine, and, therefore, we do not believe that the prediction error was due to a phase-shifting effect of caffeine. In addition, the model was not accurate for all individuals; for approximately 20% of the individuals in our study, predicted phase shifts differed by more than 2 h different from the observed phase shifts, which is consistent with the findings from other studies that have used this model to simulate data from actual shift workers (Stone et al. 2019). The model currently assumes an intrinsic circadian period of 24.2 h for all participants; however, there is significant inter-individual variability in intrinsic period (Czeisler et al. 1999; Duffy et al. 2011) which likely introduces some error. Although we did not know the intrinsic period of our participants, varying intrinsic period input into the model would be expected to have improved its predictions of phase shifts. The model also does not account for individual differences in physiological light sensitivity.
In conclusion, the findings from this study suggest that mathematical models combined with light data from wrist monitors can be useful for developing best practices in work scheduling, as part of an integrated approach where light and sleep interventions are used to improve adaptation to night shifts.
Acknowledgments
The authors wish to thank Drs. Min Ju Kim and Jee Hyun Kim for serving as project leaders for some of the studies; Joyce Hong, Michael P. Harris, Audra S. Murphy, and John C. Wise for assistance with participant recruitment and data processing; and Joseph M. Ronda, M.S. for technical assistance.
The studies were supported by NIH grant R01 AG044416 and the laboratory segments were carried out in the Brigham and Women’s Hospital Center for Clinical Investigation, part of Harvard Catalyst (Harvard Clinical and Translational Science Center) and supported by NIH Award UL1 TR001102 and financial contributions from Brigham and Women’s Hospital and from Harvard University and its affiliated academic healthcare centers. EDC was supported by NIH fellowship T32 HL007901 during the time of data collection. MSH is supported by NIH grant R21 NR018974 and NASA grant 80NSSC20K0576.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- Alterman T, Luckhaupt SE, Dahlhamer JM, Ward BW, Calvert GM. 2013. Prevalence rates of work organization characteristics among workers in the U.S.: Data from the 2010 National Health Interview Survey. Am J Ind Med. 56:647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’Hermite-Balériaux M, Zee PC. 2005. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 20:178–188. [DOI] [PubMed] [Google Scholar]
- Bjorvatn B, Gronli J, Hamre F, Sorensen E, Fiske E, Bjorkum AA, Portas CM, Ursin R. 2002. Effects of sleep deprivation on extracellular serotonin in hippocampus and frontal cortex of the rat. Neuroscience. 113:323–330. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. 1999. Measuring agreement in method comparison studies. Stat Methods Med Res. 8:135–60. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Boudreau P. 2014. Impacts of shift work on sleep and circadian rhythms. Pathol Biol (Paris). 62:292–301. [DOI] [PubMed] [Google Scholar]
- Burke TM, Markwald RR, McHill AW, Chinoy ED, Snider JA, Bessman SC, Jung CM, O’Neill JS, Wright KP, Jr. 2015. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci Transl Med. 7:305ra146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton OM, L’Hermite-Balériaux M, Turek FW, Van Cauter E. 2000. Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol Regul Integr Comp Physiol. 278:R373–R382. [DOI] [PubMed] [Google Scholar]
- Chinoy ED, Harris MP, Kim MJ, Wang W, Duffy JF. 2016. Scheduled evening sleep and enhanced lighting improve adaptation to night shift work in older adults. Occup Environ Med. 73:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. 2003. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 18:513–523. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. 1990. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med. 322:1253–1259. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. 1999. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 284:2177–2181. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Silva EJ, Shanahan TL, Boivin DB, Czeisler CA. 2012. Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans. PLoS ONE. 7:e30037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang AM, Phillips AJK, Münch MY, Gronfier C, Wyatt JK, Dijk D, Wright KP, Czeisler CA. 2011. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA. 108:15602–15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Kronauer RE, Czeisler CA. 1996. Phase-shifting human circadian rhythms: Influence of sleep timing, social contact and light exposure. J Physiol (Lond). 495:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M, Benhaberou-Brun D, Paquet J. 2001. Profile of 24-h light exposure and circadian phase of melatonin secretion in night workers. J Biol Rhythms. 16:502–511. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Stewart KT, Mahoney MP, Liu L, Fogg LF. 1994. Shiftwork: Dark goggles and bright light improve circadian rhythm adaptation to night-shift work. Sleep. 17:535–543. [DOI] [PubMed] [Google Scholar]
- Flynn-Evans E, Barger LK, Kubey A, Sullivan JP, Czeisler CA. 2016. Circadian misalignment affects sleep and medication use before and during spaceflight. NPJ Microgravity. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkard S 2008. Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int. 25:215–224. [DOI] [PubMed] [Google Scholar]
- Isherwood CM, Chinoy ED, Murphy AS, Kim JH, Wang W, Duffy JF. 2020. Scheduled afternoon-evening sleep leads to better night shift performance in older adults. Occup Environ Med. 77:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett ME. (1997). Models of circadian and homeostatic regulation of human performance and alertness. [PhD Dissertation] Cambridge, MA: Harvard University. [Google Scholar]
- Jewett ME, Kronauer RE. 1999. Interactive mathematical models of subjective alertness and cognitive throughput in humans. J Biol Rhythms. 14:588–597. [DOI] [PubMed] [Google Scholar]
- Kecklund G, Axelsson J. 2016. Health consequences of shift work and insufficient sleep. BMJ. 355:i5210. [DOI] [PubMed] [Google Scholar]
- Kervezee L, Kosmadopoulos A, Boivin DB. 2020. Metabolic and cardiovascular consequences of shift work: The role of circadian disruption and sleep disturbances. Eur J Neurosci. 51:396–412. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Beckett SA, Landrigan CP. 2016. Applying mathematical models to predict resident physician performance and alertness on traditional and novel work schedules. BMC Med Educ. 16:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson A, O’Brien L, Reid JA. 2014. The impact of shiftwork on health: a literature review. J Clin Nurs. 23:3309–3320. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Hoese EK, Liu L, Fogg LF, Eastman CI. 1997. Conflicting bright light exposure during night shifts impedes circadian adaptation. J Biol Rhythms. 12:5–15. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. 2010. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 27:1911–1929. [DOI] [PubMed] [Google Scholar]
- Rabstein S, Burek K, Lehnert M, Beine A, Vetter C, Harth V, Putzke S, Kantermann T, Walther J, Wang-Sattler R, Pallapies D, Bruning T, Behrens T. 2019. Differences in twenty-four-hour profiles of blue-light exposure between day and night shifts in female medical staff. Sci Total Environ. 653:1025–1033. [DOI] [PubMed] [Google Scholar]
- Rahman SA, St Hilaire MA, Chang AM, Santhi N, Duffy JF, Kronauer RE, Czeisler CA, Lockley SW, Klerman EB. 2017. Circadian phase resetting by a single short-duration light exposure. JCI Insight. 2:e89494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhi N, Duffy JF, Horowitz TS, Czeisler CA. 2005. Scheduling of sleep/darkness affects the circadian phase of night shift workers. Neurosci Lett. 384:316–320. [DOI] [PubMed] [Google Scholar]
- St Hilaire MA, Gronfier C, Zeitzer JM, Klerman EB. 2007. A physiologically-based mathematical model of melatonin including ocular light suppression and interactions with the circadian pacemaker. J Pineal Res. 43:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Hilaire MA, Klerman EB, Khalsa SBS, Wright Jr. KP, Czeisler CA, Kronauer RE. 2007. Addition of a non-photic component to a light-based mathematical model of the human circadian pacemaker. J Theoretical Biol. 247:583–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JE, Aubert XL, Maass H, Phillips AJK, Magee M, Howard ME, Lockley SW, Rajaratnam SMW, Sletten TL. 2019. Application of a limit-cycle oscillator model for prediction of circadian phase in rotating night shift workers. Sci Rep. 9:11032. [DOI] [PMC free article] [PubMed] [Google Scholar]