Abstract
The plant growth-promoting rhizobacteria (PGPR) can improve the biotic or abiotic stress condition by exploiting the productivity and plant growth of the plants under stressful conditions. This study examines the role of a rhizospheric bacterial isolate Kosakonia pseudosacchari TCPS-4 isolated from cluster bean plant (Cyamopsis tetragonoloba) under dryland condition. The low-cost media engineering was evaluated, and the phosphate-solubilizing and IAA-producing abilities of Kosakonia pseudosacchari TCPS-4 were improved using a hybrid statistical tool viz. Multi-objective Genetic Algorithm (MOGA). Further, the effect of carbon and nitrogen media constituents and their interactions on IAA production and phosphate solubilization were also confirmed by a single-factor experiment assay. This revealed that MOGA-based model depicted 47.5 mg/L inorganic phosphate as the highest phosphate concentration in media containing 45 g/L carbon source, 12 g/L nitrogen source and 0.20 g/L MgSO4. The highest IAA production was 18.74 mg/L in media containing 45 g/L carbon source, 12 g/L nitrogen source and 0.2 g/L MgSO4. These values were also confirmed and measured by the experiments with phosphate solubilization of 45.71 mg/L and IAA production of 18.71 mg/L with 1012 cfu/mL. This concludes that effective media engineering using these statistical tools can enhance the phosphate and IAA production by each model. A good correlation between measured and predicted values of each model confirms the validity of both responses. The present study gives an insight on media engineering for phosphate and IAA production by Kosakonia pseudosacchari TCPS-4.
Keywords: IAA, Kosakonia pseudosacchari, Multi-objective Genetic Algorithm (MOGA), PGPR, Stress tolerance
Introduction
Plant growth-promoting rhizobacteria enhance plant growth, nutrient absorption, plant rooting and photosynthesis process (Singh et al. 2019). These bacteria form a well microbial diversity and help in maintaining soil texture, soil quality, ecosystem function and help in sustainable agriculture (Essel et al. 2019). It is well known that only 2–4% of bacteria help in plant growth promotion. The rhizospheric region is the hotspot region for microbial interactions (Pandey et al. 2019). In the field, plant roots release exudates that are used by microbes and in turn, these microbes aid in plant growth promotion (Wang et al. 2018; Xu et al. 2020). Consequently, the selection and screening of the beneficial rhizobacterial and their exploitation in agricultural practices help in the sustainability of agro-ecosystems. More than 154.2 million tonnes of chemical fertilizers are used for the increase in crop yield. Despite their effectiveness in plant growth promotion, these chemicals are proved as hazardous for human beings and the animal population. The soil fertility and biological disease control can be maintained by applying microbial inoculants or inorganic fertilizers in place of chemical fertilizers. Various indirect or direct mechanisms are employed in the rhizospheric region for the improvement in plant health and growth (Guo et al. 2020; Zheng et al. 2020). In the present study, Kosakonia pseudosacchari TCPS-4 was isolated from dry land and identified based on their biochemical and morphological properties, in addition to its phylogenetic analysis done by 16S rDNA (Watts et al. 2017; Panchami et al. 2020). The research team reported the IAA-producing and phosphate-solubilizing capability of Kosakonia pseudosacchari TCPS-4 and found that this isolate enhances the phosphate solubilization and IAA concentration.
Due to the high cost of yeast and beef extract, the optimization rate for the IAA and phosphate solubilization rate for Kosakonia pseudosacchari TCPS-4 becomes too expensive. Though, the industrial use of TCPS-4 strain can be made economically less by using low-cost media such as soybean meal and corn flour as by-products in agricultural practices. Various types of low-cost inorganic and organic nitrogen sources were used in a previous study by replacing the expensive sources (Ozdemir et al. 2009). It was reported that PGPR produced more mannitol by using corn flour and soybean meal as compared to yeast and beef extract (Chandra et al. 2018). A statistical design experiment was used to develop a low-cost medium by corn flour (Taiwo et al. 2018). This type of study gives valuable insights into the low-cost nutritionally effective media for the large-scale production of PGPR and its use for sustainable agriculture. In the present study, the culture medium was optimized by the response surface methodology (RSM) and Multi-objective Genetic Algorithm (MOGA) for the low-cost process. It obtained a high bacterial cell concentration, IAA content and phosphate-solubilizing capability by the low-cost medium. Nowadays, RSM is used for the optimization of various processes in the agricultural and fermentation process for the media optimization. RSM uses both statistical and mathematical techniques that are utilized for the analysis and modeling of problematic data (Saini et al. 2020; Qin et al. 2012). Various optimization techniques used in the previous study for media optimization are described in Table 1. First, the different concentrations of nitrogen, carbon and inorganic salts were screened and then BBD (Box Behnken Design) used to measure the IAA content and phosphate-solubilizing capability in a low-cost medium (Peng et al. 2019). The main motive of this study is to optimize the low-cost media components for IAA production and phosphate solubilization using Kosakonia pseudosacchari TCPS-4. This study gives a better foundation for agricultural practices on a large scale by high-density culture.
Table 1.
Some notable studies representing various media optimization techniques and the metabolites produced by different microorganisms
| Techniques | Design | Microorganisms used | Metabolite | Reference |
|---|---|---|---|---|
| RSM | CCD | Bacillus subtilis | Jiean-peptide | Zhong et al. 2014 |
| RSM | CCD | Streptomyces | Oxytetracycline | Singh and Rai 2012 |
| NMDS, ANN | CCD | Streptomyces | Actinomycin D | Tripathi et al. 2012 |
| RSM | CCD | Xenorhabdus | Antibiotic | Wang et al. 2011 |
| GA, ANN | BBD | Lactobacillus | Nisin | Guo et al. 2010 |
| RSM | BBD, PBD | Streptomyces | Antibiotic | Rajeswari et al. 2014 |
| RSM | CCD, PBD | Streptomyces olivaceus | Olivanic acid | Singh and Tripathi 2008 |
| RSM | BBD, PBD | Streptomyces | Milbemycin | Baoxin et al. 2011 |
Materials and methods
Sample collection and isolation of rhizobacteria
The rhizospheric soil sample of the cluster bean plant was collected from the field of CCS Haryana Agricultural University, Hisar and stored at 4 °C. The soil sample was used to isolate the most predominant rhizobacterial species using the serial dilution method on nutrient agar medium (beef extract 5 g, peptone 10 g, NaCl 5 g, agar 20 g, H2O 1000 mL at 7 pH) (Ahmad et al. 2019; Verma and Pal, 2020). The agar plates were incubated at 28 ± 2 °C for 2–4 days. The pure and selected isolate was identified by their morphological and biochemical properties and transferred on NA agar slants for further analysis.
Single-factor experimental design
Due to the high expenditure of yeast and beef extract, soybean meal and corn flour were used as Nitrogen and Carbon source for this study (Li et al. 2019). The primary 1-mL culture of Kosakonia pseudosacchari TCPS-4 inoculum was inoculated into 100 mL of media with 10, 15, 20 and 25 g/L soybean meal as the nitrogen content. In the same way, 1 mL of primary inoculum was inoculated into another media with 15, 30, 45 and 60 g/L of corn powder as carbon content in 250 mL flask at 28 °C and 160 rpm for 84 h. In both carbon and nitrogen source medium, MnSO4 (0.2 g/L), MgSO4 (0.2 g/L), CaCO3 (1 g/L), K2HPO4 (2 g/L) and FeCl3 (0.2 g/L) were used as inorganic salt content (Wu et al. 2008). The cell concentration at the initial level in the low-cost medium was measured as 7.2 × 1010 cfu/mL on the nutrient agar plate after whole night incubation at 28 °C. After 84 h of the incubation period, the IAA production, cell density and phosphate solubilization of K. pseudosacchari TCPS-4 were calculated. As we know, soybean meal has tryptophan, protein, lysine, cysteine and corn meal has riboflavin, carbohydrates, carotene and other content that are useful for bacterial growth.
Measurement of IAA production by Kosakonia pseudosacchari TCPS-4
The production of IAA (indole acetic acid) by the selected isolate was determined by the colorimetric assay described by Tang and Bonner method (Tang and Bonner 1947). After 72 h of cultivation, the bacterial cells were collected by centrifugation at 10,000 g for 12 min. The IAA concentration was determined using the Salkowski method. Two milliliters of culture supernatant were mixed with 2 mL of Salkowski reagent and left this mixture in dark for 25 min at room temperature and the optical density measured at 530 nm. The total amount of IAA was estimated using the standard curve of known IAA concentration (10–200 µg/mL). The negative control was taken by mixing 2 mL of uninoculated broth with 2 mL of Salkowski reagent (Liu et al. 2019).
Measuring the ability Kosakonia pseudosacchari TCPS-4 to solubilize phosphate
For the inorganic phosphate solubilization, a qualitative test was carried out on Pikovskaya agar medium plate containing Ca3 (PO4)2 (Pikovskaya 1948; Mehta and Nautiyal 2001). For this, the selected culture was spotted on the Pikovskaya agar plate and incubated at 30 °C for 5–6 days. The occurrence of a clear halo zone around the spotted culture indicates the ability of phosphate solubilization. Kosakonia pseudosacchari TCPS-4 seed liquid culture was inoculated in media with different concentrations of soybean meal, corn flour and inorganic salts for 72 h. After that, 1% of primary inocula was transferred into 40 mL of Pikovskaya medium in 150-mL flask and incubated at 28 °C at 160 rpm for 3 days. After the incubation period, the bacterial sample was collected and centrifuged at 10,000 g for 12 min. The soluble phosphate concentration in the supernatant was determined using the molybdate blue colorimetric method (Debruyne 1983).
Experimental design by RSM and its statistical analysis
A total of 20 experiments has been performed using RSM-based FCCD matrix as represented in Table 2. Similar experiment is used for the training and validation of the designed model. RSM strategy has been too much utilized for modeling and studying various factors based on the experimental plan. It depends on the standards of statics and arithmetic for improvement, investigation, approval and optimization of target value with a given design matrix of process parameters (Bezerra et al. 2008; Liu and Wang 2007). FCCD is utilized since it offers complex favorable circumstances other than RSM design counting its capacity to decrease the figure of keeps running when contrasted with full factorial RSM plans. Every one of the three input factors is changed at four levels as demonstrated in Table 3.
Table 2.
RSM-based FCCD experimental design matrix for three factors
| Sr. no | Carbon source (g/L) | Nitrogen source (gm/L) | MgSO4 (g/L) | Phosphate (mg/L) | IAA (mg/L) |
|---|---|---|---|---|---|
| 1 | 45 | 12 | 0.20 | 44.99 | 16.99 |
| 2 | 75 | 12 | 0.20 | 45.61 | 16.45 |
| 3 | 45 | 12 | 0.25 | 45.93 | 18.61 |
| 4 | 45 | 8 | 0.20 | 45.20 | 18.00 |
| 5 | 63 | 8 | 0.25 | 46.20 | 18.22 |
| 6 | 63 | 8 | 0.15 | 44.82 | 18.22 |
| 7 | 27 | 8 | 0.15 | 43.91 | 16.67 |
| 8 | 63 | 17 | 0.15 | 45.41 | 18.22 |
| 9 | 45 | 12 | 0.20 | 45.71 | 18.71 |
| 10 | 45 | 12 | 0.20 | 46.30 | 18.30 |
| 11 | 45 | 12 | 0.20 | 45.50 | 18.50 |
| 12 | 27 | 17 | 0.15 | 44.50 | 16.67 |
| 13 | 27 | 17 | 0.25 | 44.88 | 16.67 |
| 14 | 45 | 12 | 0.20 | 43.75 | 17.70 |
| 15 | 63 | 17 | 0.25 | 46.13 | 18.22 |
| 16 | 15 | 12 | 0.20 | 42.09 | 13.83 |
| 17 | 45 | 20 | 0.20 | 45.95 | 18.66 |
| 18 | 45 | 12 | 0.15 | 45.95 | 18.62 |
| 19 | 27 | 8 | 0.25 | 43.29 | 16.67 |
| 20 | 45 | 12 | 0.20 | 45.85 | 18.55 |
Table 3.
Input factors with their ranges and levels
| Input parameters | Minimum value (g/L) | Maximum value (g/L) | Level |
|---|---|---|---|
| Carbon | 15 | 75 | 4 |
| Nitrogen | 8 | 20 | 4 |
| MgSO4 | 0.15 | 0.25 | 3 |
MOGA optimization
A very few researchers applied MOGA as an optimization tool in microbiology. In the present study, we conclude that neural network-MOGA is more effective and efficient than RSM tool (Chau et al. 2019; Selvakumar and Ravikumar 2018). In the present study, MOGA was applied for the optimization of IAA and Phosphate production. For this purpose, three input parameters, i.e., Carbon source, Nitrogen source and MgSO4 salt are used within pre-defined limits. Similar FCCD design matrix is used for the training and validation of artificial neural network model. MOGA tool can give much sensitive analysis as compare to RSM model. The machine-learning model based on neural network is fed in to MOGA to find out optimal input factors with target to maximize the IAA and Phosphate production. The important parameters for MOGA have been set as follows:
Population Type = Double Vector
Creation Function = Custom
Crossover Function = Crossover two point
Mutation Function = Mutation uniform
Crossover Fraction = 0.8
Elite Count = 2
Population Size = 100
Generations = 10
Plot Function = gaplotpareto
Multi-objective Genetic Algorithm has been applied to find out the best combination of Carbon source, Nitrogen source and MgSO4 salt for maximum production of IAA and Phosphate. As there are two objectives functions, linear Pareto front plot has been considered for evaluations of results, which is easy to understand and also less time-consuming compared to the 3D surface plot. The Pareto front diagram indicates the optimal solution both the objective function simultaneously without affecting each other. In addition, the results are compared with the RSM results.
Results and discussion
Effect of different soybean constituent
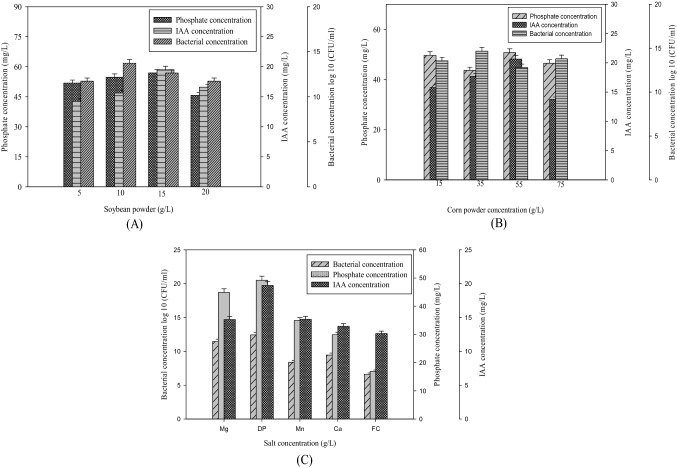
Different concentration of soybean meal as nitrogen source added to the medium to show the effect of strain on IAA production, inorganic phosphate solubilization and cell density in Kosakonia pseudosacchari TCPS-4. Figure 1a shows the highest production of IAA (21 mg/L) and phosphate solubilization (55 mg/L) at different soybean concentration by strain TCPS-4 at 20 g/L in the low-cost medium. Therefore, 20 g/L of soybean flour was used as the main nitrogen content for this medium. In the same way, some previous studies have also shown that 20 g/L concentration as a nitrogen source is suitable for IAA production and phosphate solubilization (Battini et al. 2016).
Fig. 1.
Variation in the IAA production, phosphate solubilization and cell concentration of Kosakonia pseudosacchari TCPS-4 in different media with different concentration. a Soybean powder concentration. b Corn powder concentration. c Inorganic salt concentrations (Mg; DP; Mn; Ca; FC). The experiment was carried by inoculating 1% of test culture into media at 30 °C for 72 h at 160 rpm
Effect of different corn flour constituents on K. pseudosacchari TCPS-4
The number of feasible bacterial cells in a medium with different corn flour constituent was almost similar. This result reveals that the different corn constituent has a minute or no effect on bacterial growth. The PGPR isolate, K. pseudosacchari TCPS-4 produced a 5.4 mm halo zone on the Pikovskaya agar plate after 5 days of incubation at 30 °C. This zone showed the ability of strain in solubilizing inorganic phosphate. The TCPS-4 strain had different phosphate solubilization capabilities under different corn flour concentration. Figure 1b shows maximum phosphate-dissolving capability under concentration 45 g/L of corn powder was 50 mg/L, which is more than the other corn powder concentration. In some previous studies, it is also reported that many plant growth-promoting isolates has been recognized for the cytokinin, IAA and other plant growth hormones in the rhizospheric soil (Yadav et al. 2017). These hormones help in the improvement of plant growth and lessen the effect of growth inhibitors. This study showed that the IAA concentration was 18 mg/L at the 50 g/L corn concentration, which is higher than the other concentration. This shows that this concentration is effective for better IAA production for the TCPS-4 strain. A related effect observed by Tang, who used corn and barley flour for the optimization of media by this design (Tang et al. 2004). Hence, 50 g/L concentration of corn flour was selected for the carbon source in low-cost medium for further test.
Effects of inorganic salts
To know the effect of various inorganic salts (FeCl3, MnSO4, MgSO4, CaCO3 and K2HPO4) on IAA production, cell density and phosphate solubilization of K. pseudosacchari TCPS-4, the various concentrations of inorganic salts were added to the low-cost medium (Peng et al. 2014). Figure 1c demonstrates that the IAA production and phosphate solubilization were 12 and 55 mg/L in the media contain K2HPO4. Meanwhile, the IAA production and phosphate solubilization of the medium containing MgSO4 was 12 and 52 mg/L, respectively. In addition, the other inorganic salts such as FeCl3, MnSO4 and CaCO3 have little or no effect on the cell density of culture medium. In addition, the IAA production and phosphate-dissolving capability of K. pseudosacchari TCPS-4 was observed in the medium containing these two salts. Therefore, MgSO4 and K2HPO4 were selected as the inorganic salts for further tests.
RSM model details
For the optimization of IAA and Phosphate production, the RSM second-order quadratic model has been derived and analyzed. All model details such as F-value, P-value, and Lack of fit for both response functions are shown in Tables 4 and 5. Experimental data have been modeled at best recommend lambda 1, which means no need for any power-law transformation. The regression equation for the Phosphate and IAA are derived using RSM to analyze the model is represented by the following equations, respectively. The significance of the model for the IAA and phosphate response is validated by the P value of < 0.0001 and the lack of fit tends to 1 as shown in Tables 4 and 5, respectively. The plot between actual vs. predicted values for both the response functions shows that there is a close relation between actual and predicted values.
Table 4.
ANOVA for Quadratic RSM model details for the IAA production by Kosakonia pseudosacchari TCPS-4
| Source | Sum of squares | Df | Mean square | F-value | p value | |
|---|---|---|---|---|---|---|
| Model | 25.79 | 9 | 2.87 | 11.96 | 0.0003 | Significant |
| P-Carbon | 8.24 | 1 | 8.24 | 34.40 | 0.0002 | |
| Q-Nitrogen | 0.0451 | 1 | 0.0451 | 0.1883 | 0.6735 | |
| R-MgSO4 | 0.0000 | 1 | 0.0000 | 0.0000 | 1.0000 | |
| PQ | 0.0000 | 1 | 0.0000 | 0.0000 | 1.0000 | |
| PR | 0.0000 | 1 | 0.0000 | 0.0000 | 1.0000 | |
| QR | 0.0000 | 1 | 0.0000 | 0.0000 | 1.0000 | |
| P2 | 17.12 | 1 | 17.12 | 71.52 | 0.0001 | |
| Q2 | 0.0562 | 1 | 0.0562 | 0.2345 | 0.6386 | |
| R2 | 0.5295 | 1 | 0.5295 | 2.21 | 0.1679 | |
| Residual | 2.39 | 10 | 0.2395 | |||
| Lack of fit | 0.2316 | 5 | 0.0463 | 0.1071 | 0.1071 | Not significant |
| Pure error | 2.16 | 5 | 0.4326 | |||
| Cor total | 28.18 | 19 |
Table 5.
ANOVA for Quadratic RSM model details for the Phosphate production by Kosakonia pseudosacchari TCPS-4
| Source | Sum of squares | Df | Mean square | F-value | p value | |
|---|---|---|---|---|---|---|
| Model | 18.28 | 9 | 2.03 | 4.00 | 0.0207 | Significant |
| P-Carbon | 10.35 | 1 | 10.35 | 20.40 | 0.0011 | |
| Q-Nitrogen | 1.24 | 1 | 1.24 | 2.45 | 0.1485 | |
| R-MgSO4 | 0.3394 | 1 | 0.3394 | 0.6685 | 0.4326 | |
| PQ | 0.3414 | 1 | 0.3414 | 0.6724 | 0.4313 | |
| PR | 0.6830 | 1 | 0.6830 | 1.35 | 0.2731 | |
| QR | 0.0144 | 1 | 0.0144 | 0.0285 | 0.8694 | |
| P2 | 5.16 | 1 | 5.16 | 10.16 | 0.0097 | |
| Q2 | 0.0460 | 1 | 0.0460 | 0.0906 | 0.7696 | |
| R2 | 0.1802 | 1 | 0.1802 | 0.3550 | 0.5645 | |
| Residual | 5.08 | 10 | 0.5077 | |||
| Lack of fit | 1.08 | 5 | 0.2165 | 0.2711 | 0.9109 | Not significant |
Analysis of IAA and Phosphate production for different input parameters
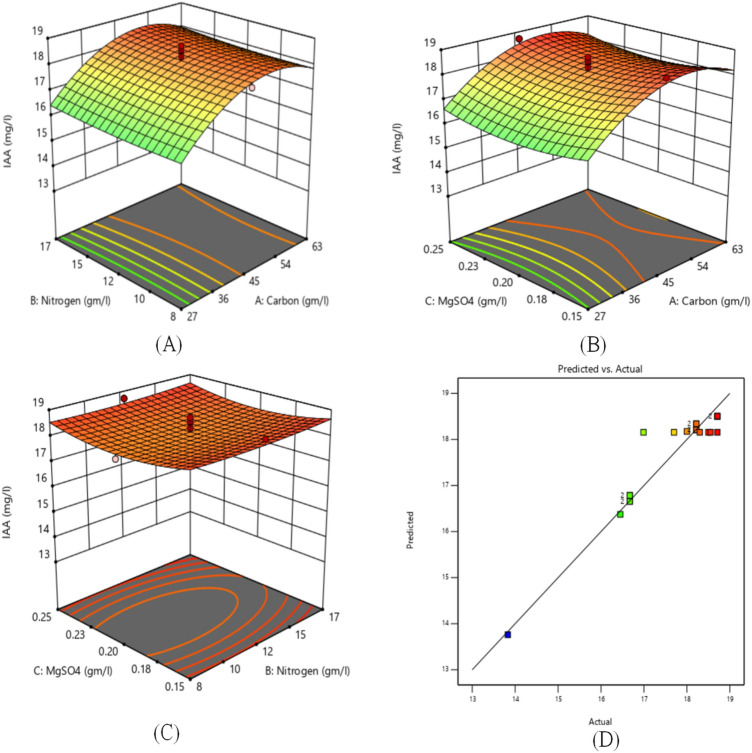
The 3-Dimensional surface plot is used to show the effect of different factors on IAA and Phosphate production. It is found that the carbon source has a significant effect on IAA and Phosphate production. The effect of carbon source, nitrogen source and inorganic salt on IAA and Phosphate production with other factors is represented by 3D surface plots as shown in Figs. 2 and 3, respectively. Figure 2a shows the 3D response surface of IAA production between the carbon source and nitrogen source. The plot represents that IAA production is increasing with increase in the value of carbon sources. The maximum IAA production is obtained at 45 g/L of carbon source and 20 g/L nitrogen source. The effect of inorganic salts very less as compared to carbon source and nitrogen source as no variation is seen in IAA production value with an increase in the value of inorganic salts from 0.15 to 0.2 g/L. The same can be observed in Table 2 also. Similarly, the combined effect of carbon source and nitrogen source with inorganic salt on the IAA production is analyzed using 3D plots as shown in Fig. 2b and c, respectively. Figure 2d indicates the plot between indicated and predicted values. The close adherence between these values along the line validates the designed RSM model.
Fig. 2.
3D surface plot of a Nitrogen source and Carbon source. b Inorganic salt and Carbon sources. c Inorganic salt and Nitrogen sources. d Actual vs. predicted values plot for the IAA production
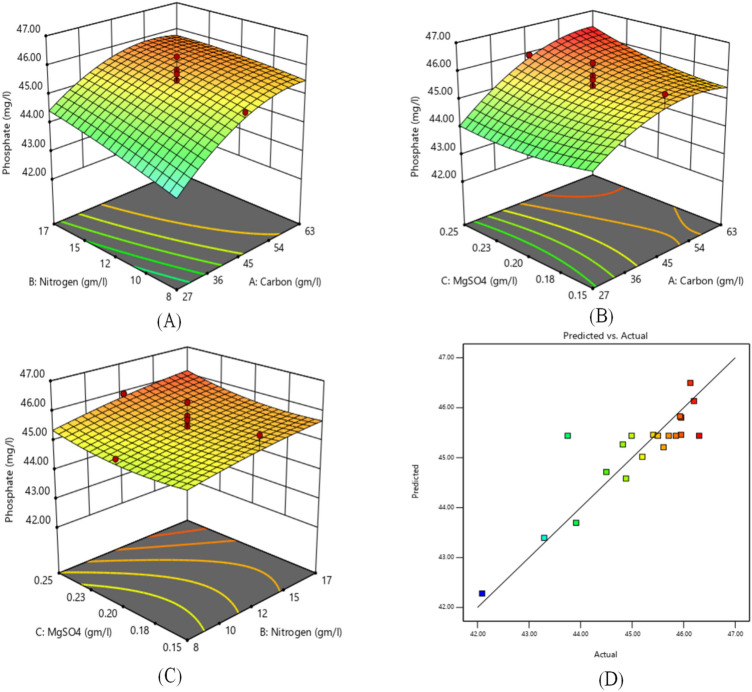
Fig. 3.
3D surface plot of a Nitrogen source and Carbon source. b Inorganic salt and Carbon sources. c Inorganic salt and Nitrogen sources. d Actual vs. predicted values plot for the Phosphate production
Similarly, the effect of carbon source, nitrogen source and inorganic salt on Phosphate production with other factors is represented by 3D surface plots as shown in Fig. 3. Figure 3a shows the 3D response surface of Phosphate production between the carbon source and nitrogen source. The plot represents that Phosphate production is increasing with the increase in the value of carbon source. The maximum Phosphate production was obtained at 45 g/L of carbon source and 12 g/L nitrogen source. The effect of inorganic salts is very less as compared to carbon source and nitrogen source as no variation is seen in Phosphate production value with an increase in the value of inorganic salts from 0.15 to 0.2 g/L. The same can be observed in Table 2 also. Similarly, the combined effect of carbon source and nitrogen source with inorganic salt on the Phosphate production is analyzed using 3D plots as shown in Fig. 3b and c, respectively. Figure 3d indicates the plot between indicated and predicted values. The close adherence between these values along the line validates the designed RSM model.
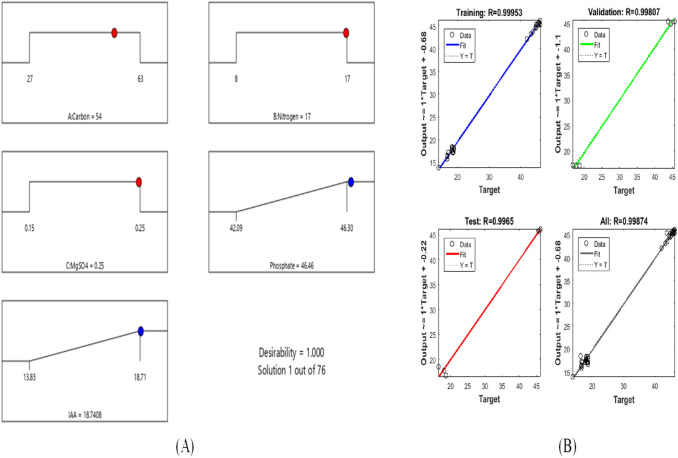
The RSM model calculated the 76 optimal solutions at desirability 1 based on Table 2. Out of which number 1 is the best optimal solution corresponding to which, the IAA and Phosphate production is highest. As shown in Fig. 4a, the maximum production of IAA and phosphate is 18.74 and 46.46 mg/L, respectively, at a carbon content of 54 g/L, nitrogen content 17 g/L and inorganic salt 0.25 g/L.
Fig. 4.
a Optimization result for Phosphate and IAA production using RSM. b Regression plot of MOGA-ANN
MOGA optimization
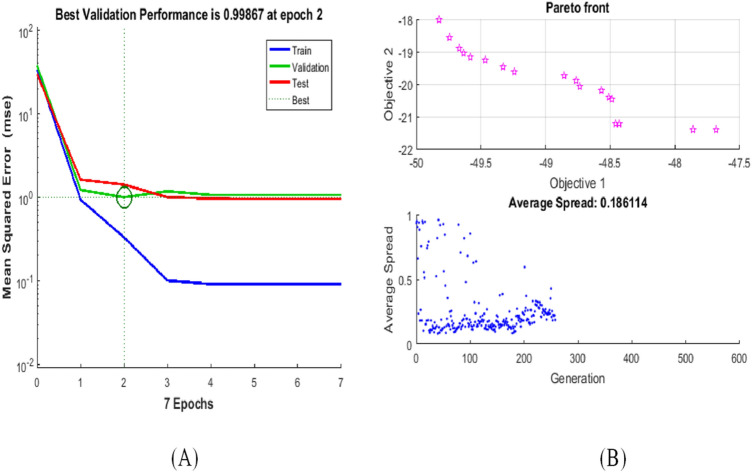
Central Composite Design, a Factorial Design tool is used to design an input matrix with three input parameters having 14 non-center and 6 center points, in which each numeric factor is set to four levels as well as plus alpha and minus alpha points are defined as per the range of input parameters. The artificial neural network has been trained with these 20 datasets of three input process parameters (carbon sources, nitrogen source and inorganic salt) and two output response (IAA and Phosphate production) using MOGA algorithms. The best value of overall Regression coefficient (R) (0.99874) has been obtained as shown in Fig. 4b. Best validation performance at epoch 2 for the designed model is 0.99867 out of total seven epoch as shown in Fig. 5a. Such a high value of best validation performance indicates that the designed model is trained and tested with a minimum mean square error that produced the best-optimized results.
Fig. 5.
a Mean square error plot for the designed model. b Optimization plot for Objective 1 (Phosphate Production) and Objective 2 (IAA production) using MOGA
The machine-learning model based on neural network is used as a data source for MOGA as shown in above (Table 2). MOGA calculated the 275 optimal generations with an average spread of 0.186114 for the objective functions, i.e., IAA and Phosphate production as shown in Fig. 5a.
MOGA calculated the 275 generations within the limits of input parameters as shown in (Table 3) and provide the best optimal solution for Objective 1, i.e., Phosphate Production and Objective 2, i.e., IAA production of 47.5 and 18 g/L as shown in Fig. 5a. The optimized results of MOGA are better than the RSM model but with a minor difference as the regression coefficients of MOGA are comparatively higher than the RSM model. As there are two objectives functions Objective 1 (Phosphate Production) and Objective 2 (IAA production), linear Pareto front plot has been considered for evaluations of results as shown in Fig. 5b. Pareto front diagram indicates the optimal solution both the objective function simultaneously without affecting each other. As per results, MOGA can optimize the IAA and Phosphate production better than RSM.
Conclusion
In this work, both conventional and advanced techniques of media optimization are discussed which revealed that all these statistical approaches can improve the phosphate and IAA production in Kosakonia pseudosacchari TCPS-4 and save experimental time. The quadratic model for both IAA and Phosphate production with different concentrations of diverse ingredients was investigated by RSM statistical analysis tool. A high correlation between actual and predicted values justifies the validity of both models. In addition to RSM, MOGA statistical analysis tool was also used for the optimization of carbon source, nitrogen source and inorganic salts to maximize the IAA and Phosphate production. This MOGA-based model depicted 47.5 mg/L inorganic phosphate as the highest phosphate concentration in media containing 45 gm/L carbon source, 12 g/L nitrogen source and 0.20 g/L MgSO4. The highest IAA production was 18.74 mg/L in media containing 45 g/L carbon source, 12 g/L nitrogen source and 0.2 g/L MgSO4. In addition, in recent years, these integrated approaches are being used for microbial cultivation during both aerobic and anaerobic condition. In a study, Peng et al. (2014) have also applied this approach for Pseudomonas putida Rs-198 for the optimization of low-cost medium, however, the MOGA tool as used in the present study has more advantages than RSM due to its high efficiency and the regression coefficient of the MOGA tool is higher than RSM. Therefore, from the present study, it can be concluded that the MOGA optimization tool is a more effective method than RSM to maximize the IAA and Phosphate production. This study definitely gives some future prospects of such media engineering tools for IAA and Phosphate production by Kosakonia pseudosacchari TCPS-4.
Acknowledgements
TC acknowledges Maharshi Dayanand University, Rohtak, India for University Research Scholarship (URS). PS acknowledges Department of Science and Technology, New Delhi, Govt. of India, FIST grant (Grant No. 1196 SR/FST/LS-I/2017/4) and Department of Biotechnology, Government of India (Grant No. BT/PR27437/BCE/8/1433/2018). PS also acknowledges, the Lab Infrastructure grant by BHU, Varanasi (F(C)/XVIII-Spl.Fund/Misc/Infrastructure/Instt.Sc/2019-2020/10290) and BTISNET- Sub-Distributed Information Centre, funded by DBT, Govt. of India at the School of Biotechnology, Banaras Hindu University, Varanasi, India
Authors' contributions
TC wrote the manuscript with contributions from DY. The manuscript was edited by PS, RG and DC.
Compliance with ethical standards
Ethical statement
This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors do not have any conflict of interest.
References
- Ahmad F, Ahmad I, Altaf M, Khan M, Shouche YS. Characterization of Paenibacillus durus (PNF16) a new isolate and its synergistic interaction with other isolated rhizobacteria in promoting growth and yield of chickpea. J Microbial Biotechnol Food Sci. 2019;2019:345–350. doi: 10.15414/jmbfs.2016.5.4.345-350. [DOI] [Google Scholar]
- Baoxin Z, Xiangjing W, Wensheng X. Optimization of fermentation medium for enhanced production of milbemycin by a mutant of Streptomyces bingchenggensis BC-X-1 using response surface methodology. Afr J Biotech. 2011;10:7225–7235. doi: 10.5897/AJB11.077. [DOI] [Google Scholar]
- Battini F, Cristani C, Giovannetti M, Agnolucci M. Multifunctionality and diversity of culturable bacterial communities strictly associated with spores of the plant beneficial symbiont Rhizophagus intraradices. Microbiol Res. 2016;183:68–79. doi: 10.1016/j.micres.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76(5):965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Chandra S, Askari K, Kumari M. Optimization of indole acetic acid production by isolated bacteria from Stevia rebaudiana rhizosphere and its effects on plant growth. J G E B. 2018;16(2):581–586. doi: 10.1016/j.jgeb.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne I. Inorganic phosphate determination: colorimetric assay based on the formation of a rhodamine B-phosphomolybdate complex. Anal Biochem. 1983;130(2):454–460. doi: 10.1016/0003-2697(83)90615-2. [DOI] [PubMed] [Google Scholar]
- Essel E, Xie J, Deng C, Peng Z, Wang J, Shen J, Xie J, Coulter JA, Li L. Bacterial and fungal diversity in rhizosphere and bulk soil under different long-term tillage and cereal/legume rotation. Soil Till Res. 2019;194:104302. doi: 10.1016/j.still.2019.104302. [DOI] [Google Scholar]
- Guo WI, Zhang YB, Lu J-h, Ly J, Teng LR, Wang Y. Optimization of fermentation medium for nisin production from Lactococcus lactis subsp. lactis using response surface methodology (RSM) combined with artificial neural network-genetic algorithm (ANN-GA) Afr J Biotechnol. 2010;9:6264–6272. [Google Scholar]
- Guo J, Muhammad H, Lv X, Wei T, Ren X, Jia H, Atif S, Hua L. Prospects and applications of plant growth promoting rhizobacteria to mitigate soil metal contamination: a review. Chemosphere. 2020;246:125823. doi: 10.1016/j.chemosphere.2020.125823. [DOI] [PubMed] [Google Scholar]
- Le Chau N, Le HG, Dao TP. Design and optimization for a new compliant planar spring of upper limb assistive device using hybrid approach of RSM–FEM and MOGA. Arab J Sci Eng. 2019;44(9):7441–7456. doi: 10.1007/s13369-019-03795. [DOI] [Google Scholar]
- Li Y, Xu Y, Li W, Yang Y, Wang L, Yu J, Wang C, Li X. Study on optimizing nutrition and fermentation conditions for compound Bacillus spp. Am J MolBiol. 2019;9(2):75–84. doi: 10.4236/ajmb.2019.92007. [DOI] [Google Scholar]
- Liu GQ, Wang XL. Optimization of critical medium components using response surface methodology for biomass and extracellular polysaccharide production by Agaricus blazei. Appl Microbiol Biot. 2007;74:78–83. doi: 10.1007/s00253-006-0661-6. [DOI] [PubMed] [Google Scholar]
- Liu X, Li Q, Li Y, Guan G, Chen S. Paenibacillus strains with nitrogen fixation and multiple beneficial properties for promoting plant growth. Peer J. 2019;7:7445. doi: 10.7717/peerj.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, Nautiyal CS. An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol. 2001;43(1):51–56. doi: 10.1007/s002840010259. [DOI] [PubMed] [Google Scholar]
- Ozdemir S, Giiven K, Baysal Z, Uyar F. Screening of various organic substrates and the development of a suitable low-cost fermentation medium for the production of amylase by Bacillus subtilis. Food Technol Biotech. 2009;47:264–369. [Google Scholar]
- Panchami PS, Thanuja KG, Karthikeyan S. Isolation and characterization of indigenous plant growth-promoting rhizobacteria (PGPR) from cardamom rhizosphere. Curr Microbiol. 2020 doi: 10.1007/s00284-020-02116-x. [DOI] [PubMed] [Google Scholar]
- Pandey A, Tripathi A, Srivastava P, Choudhary KK, Dikshit A. Plant growth-promoting microorganisms in sustainable agriculture. In: Kumar A, Singh AS, Choudhary KK, editors. Role of plant growth promoting microorganisms in sustainable agriculture and nanotechnology. Cambridge: Woodhead Publishing; 2019. pp. 1–19. [Google Scholar]
- Peng Y, He Y, Wu Z, Lu J, Li C. Screening and optimization of low-cost medium for Pseudomonas putida Rs-198 culture using RSM. Braz J Microbiol. 2014;45(4):1229–1237. doi: 10.1590/S1517-83822014000400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Sun Y, Sun R, Zhou Y, Tsang DC, Chen Q. Optimizing the synthesis of Fe/Al (Hydr) oxides-Biochars to maximize phosphate removal via response surface model. J Clean Prod. 2019;237:117770. doi: 10.1016/j.jclepro.2019.117770. [DOI] [Google Scholar]
- Pikovskaya RI. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiologiya. 1948;17:362–370. [Google Scholar]
- Qin SH, Wu ZS, Rasool A, Li C. Synthesis and characterization of slow-release nitrogen fertilizer with water absorbency: based on poly (acrylic acid-acrylic amide)/Na-bentonite. J Appl Polym Sci. 2012;126:1687–1697. doi: 10.1002/app.37007. [DOI] [Google Scholar]
- Rajeswari P, Arul Jose P, Amiya R, Jebakumar SRD. Characterization of saltern based Streptomyces sp. and statistical media optimization for its improved antibacterial activity. Front Microbiol. 2014;5:753. doi: 10.3389/fmicb.2014.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini DK, Yadav D, Pabbi S, Chhabra D, Shukla P. Phycobiliproteins from Anabaena variabilis CCC421 and its production enhancement strategies using combinatory evolutionary algorithm approach. Bioresour Technol. 2020;309:123347. doi: 10.1016/j.biortech.2020.123347. [DOI] [PubMed] [Google Scholar]
- Selvakumar S, Ravikumar R. A novel approach for optimization to verify RSM model by using multi-objective genetic algorithm (MOGA) Mater Today Proc. 2018;5(5):11386–11394. doi: 10.1016/j.matpr.2018.02.106. [DOI] [Google Scholar]
- Singh N, Rai V. Optimization of cultural parameters for antifungal and antibacterial metabolite from microbial isolate; Streptomyces rimosus MTCC 10792 from soil of Chhattisgarh. Int J Phar Pharm Sci. 2012;4:94–101. [Google Scholar]
- Singh V, Tripathi C. Production and statistical optimization of a novel olivanic acid by Streptomyces olivaceus MTCC 6820. Pro Biochem. 2008;43:1313–1317. doi: 10.1016/j.procbio.2008.07.015. [DOI] [Google Scholar]
- Singh D, Ghosh P, Kumar J, Kumar A. Plant Growth-Promoting Rhizobacteria (PGPRs): functions and benefits. In: Singh DP, Gupta VK, Prabha R, editors. Microbial interventions in agriculture and environment. Singapore: Springer; 2019. pp. 205–227. [Google Scholar]
- Taiwo AE, Ojumu TV, Madzimbamuto TN. Statistical optimization of acetoin production using corn steep liquor as a low-cost nitrogen source by Bacillus Subtilis CICC 10025. Renew Res Biorefineries. 2018 doi: 10.5772/intechopen.79353. [DOI] [Google Scholar]
- Tang XJ, He GQ, Chen QH, Zhang XY, Mokhtar AMA. Medium optimization for the production of thermal stable -glucanase by Bacillus subtilis ZJF-1A5 using response surface methodology. Bioresour Technol. 2004;93:175–181. doi: 10.1016/j.biortech.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Tang YW, Bonner J. The enzymatic inactivation of indoleacetic acid; some characteristics of the enzyme contained in pea seedlings. Arch Biochem. 1947;13:11–25. [PubMed] [Google Scholar]
- Tripathi CK, Khan M, Praveen V, Khan S, Srivastava A. Enhanced antibiotic production by Streptomyces sindenensis using artificial neural networks coupled with genetic algorithm and nelder-mead downhill simplex. J Microbiol Biotechnol. 2012;22:939–946. doi: 10.4014/jmb.1109.09018. [DOI] [PubMed] [Google Scholar]
- Verma T, Pal P. Isolation and Screening of Rhizobacteria for various plant growth promoting attributes. J Pharmacogn Phytochem. 2020;9(1):1514–1517. doi: 10.22271/phyto.2020.v9.i1z.10678. [DOI] [Google Scholar]
- Wang Y, Fang X, An F, Wang G, Zhang X. Improvement of antibiotic activity of Xenorhabdus bovienii by medium optimization using response surface methodology. Microb Cell Fact. 2011;10:1–15. doi: 10.1186/1475-2859-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao X, Guo Z, Jia Z, Wang S, Ding K. Response of soil microbes to a reduction in phosphorus fertilizer in rice-wheat rotation paddy soils with varying soil P levels. Soil till res. 2018;181:127–135. doi: 10.1016/j.still.2018.04.005. [DOI] [Google Scholar]
- Watts DB, Runion GB, Balkcom KS. Nitrogen fertilizer sources and tillage effects on cotton growth, yield, and fiber quality in a coastal plain soil. Field Crops Res. 2017;201:184–191. doi: 10.1016/j.fcr.2016.11.008. [DOI] [Google Scholar]
- Wu Y, Tao J, Zhao SF. Optimizing the fermentation conditions for Bacillus sp. combination CL-8 thalli with cooperative and synergistic action and its biocontrol efficacy. Trans CSAE. 2008;24:204–208. [Google Scholar]
- Xu P, Liu Y, Zhu J, Shi L, Fu Q, Chen J, Hu H, Huang Q. Influence mechanisms of long-term fertilizations on the mineralization of organic matter in Ultisol. Soil till res. 2020;201:104594. doi: 10.1016/j.still.2020.104594. [DOI] [Google Scholar]
- Yadav AN, Verma P, Kour D, Rana KL, Kumar V, Singh B, Chauahan VS, Sugitha T, Saxena AK, Dhaliwal HS. Plant microbiomes and its beneficial multifunctional plant growth promoting attributes. Int J Environ Sci Nat Resour. 2017;3(1):1–8. doi: 10.19080/IJESNR.2017.03.555601. [DOI] [Google Scholar]
- Zheng L, Chen H, Wang Y, Mao Q, Zheng M, Su Y, Xiao K, Wang K, Li D. Responses of soil microbial resource limitation to multiple fertilization strategies. Soil till res. 2020;196:104474. doi: 10.1016/j.still.2019.104474. [DOI] [Google Scholar]
- Zhong J, Zhang X, Ren Y, Yang J, Tan H, Zhou J. Optimization of Bacillus subtilis cell growth effecting jiean-peptide production in fed batch fermentation using central composite design. Electron J Biotechnol. 2014;17:132–136. doi: 10.1016/j.ejbt.2014.04.010. [DOI] [Google Scholar]