Abstract
Background/Objectives
Sarcopenia is defined as the gradual age-associated loss of both muscle quantity and strength in older adults, and is associated with increased mortality, falls, fractures and hospitalisations. Current sarcopenia criteria use dual-energy X-ray absorptiometry (DXA) measures of muscle mass, a test that cannot be performed at the bedside, unlike point-of-care ultrasound (PoCUS). We examined the association between ultrasonic measures of muscle thickness (MT, vastus medialis muscle thickness) and measures of muscle quantity and strength in older adults.
Methods
A total of 150 older adults (age ≥ 65; mean age 80.0 ± 0.5 years, 66 women, 84 men) were recruited sequentially from geriatric medicine clinics. Each subject had lean body mass (LBM, by bioimpedance assay), grip strength, mid-arm biceps circumference (MABC), gait speed and MT measured. All initial models were adjusted for biological sex.
Results
In our final parsimonious models, MT showed a strong significant correlation with all measures of muscle mass, including LBM (Standardised β = 0.204 ± 0.058, R2 = 0.577, P < 0.001) and MABC (Standardised β = 0.141 ± 0.067, R2 = 0.417, P = 0.038). With respect to measures of muscle quality, there was a strong significant correlation with grip strength (Standardised β = 0.118 ± 0.115, R2 = 0.511, P < 0.001) but not with subject performance (gait speed).
Conclusions
MT showed strong correlations with both measures of muscle mass (LBM and MABC) and with muscle strength (grip strength). Although more work needs to be done, PoCUS shows potential as a screening tool for sarcopenia in older adults.
Keywords: geriatric medicine, older people, point-of-care ultrasound, sarcopenia
Key points
Sarcopenia is common in older adults.
There is a need for a quick and accurate bedside screening test for this condition.
Ultrasonic measures of muscle thickness showed strong associations with measures of both muscle mass and muscle strength.
This suggests that that point-of-care ultrasound could be a potential bedside marker for sarcopenia in older adults.
Introduction
The ageing process results in a gradual decline in both muscle quantity and muscle quality. Sarcopenic patients are three times more likely to fall over 2 years and sarcopenia is strongly established as a risk factor for both fractures and hospitalisation [2]. Each older adult diagnosed with sarcopenia costs the healthcare system an additional $3,000 (USD) per year, which would work out to approximately 26 billion dollars per year overall [3]. The most commonly (and most recently updated) definition of sarcopenia was developed by the European Working Group on Sarcopenia in Older People (EWGSOP) [4]. This defines sarcopenia as having both low muscle mass on dual-energy X-ray absorptiometry (DXA) measures of lean body mass (LBM) and low muscle strength (grip strength).
The combination of acute illness and sarcopenia can be quite a devastating combination resulting in increased mortality and increased intensive care days during acute illness [5]. Ideally, we should be broadly screening for sarcopenia so that each patient’s status is known at baseline. This poses difficulties in an outpatient setting, since DXA scans are not portable and are only approved in most countries for the diagnosis of osteoporosis [6]. Other methods of measuring muscle mass, such as bioelectrical impedance analysis (BIA) have shown large inaccuracies with altered food intake, hydration, fluid distribution (such as with diuretic use) and vascular perfusion [7], all of which can be highly deranged in older adult patients. Grip strength also has a large number of potential confounders [8] including sedating medications, altered mental status/confusion and the constitutional weakening effects of acute illness.
One potential solution [9, 10] is the use of point-of-care ultrasound (PoCUS), which is quickly becoming a standard part of the physical examination as a quick marker for both muscle mass and quality. The current study examined the relationship between ultrasound measures of muscle mass (vastus medialis thickness, MT) and other measures of muscle quantity (LBM and mid-arm biceps circumference, MABC). We also examined the association between MT and measures of muscle strength (grip strength) and muscle performance (gait speed) in an older adult population.
Methods
Subjects
Our design was a cross-sectional observational study. All subjects had to be 65 years of age or over, and were referred to one of five outpatient geriatric medicine clinics by a primary care physician (Vancouver General Hospital, Vancouver, Canada). Haemodialysis patients were excluded, due to potential for excessive fluid shifts with dialysis. Patients using chronic oral corticosteroids were also excluded, due to potential medication-induced muscle atrophy. Any patient with hemiparesis due to a stroke or paresis of the lower limbs was excluded, as well as any subject with pitting edema on physical exam (due to liver, renal or heart failure). Any patient with severe dehydration, myositis, systemic connective tissue disorders, systemic atrophies affecting the central nervous system (CNS) and CNS demyelinating diseases were also excluded. All subjects gave written consent and our study protocol received approval by the Human Subjects Committee of the University of British Columbia.
Grip strength
Handgrip strength was measured using a digital grip strength dynamometer (Sammons Preston, Nottinghamshire, UK). This measurement was carried out three times using the subject’s dominant hand, and then averaged.
Muscle mass measurement (LBM and MABC)
LBM was measured by BIA using an HBF-510 W Full Body Composition Monitor (Omron, Seoul, Korea). MABC was measured to the nearest millimetre using a tape measure.
Gait speed
Gait speed was measured in metres per second. Each subject was given instructions to walk from a standing start, at their usual pace for 6 m.
Point-of-care ultrasound (MT)
Vastus medialis tissue thickness was assessed in the supine position, with knees resting comfortably in extension using a portable ultrasound device (Vscan with Dual Probe, GE Healthcare, IL). Measures were taken in B-mode, which allowed us to obtain cross-sectional images of the femoral quadriceps, as per current standards [11]. MT was measured using the on-screen callipers. All ultrasonic measures were obtained by a single operator (BF).
Statistical methods
Our response variables consisted of two measures of muscle mass (LBM and MABC), one measure of muscle strength (grip strength) and one performance measure (gait speed). Our predictor variables were age, biological sex, body mass index (BMI) and MT. Any predictors that demonstrated skewing were logarithmically transformed (base 10) prior to both univariate and multivariate analyses. After our initial model, model simplification for model parsimony was accomplished through a stepwise method, by removing the least significant predictor with a P value of greater than 0.10. After each predictor was removed, Akaike’s Information Criterion (AIC) was calculated [12]. Tolerance values and variance inflation factors (VIFs) were examined for multicollinearity. The R core software package version 3.6.0 was used for statistical analysis with a significance level of P < 0.05 [13]. All data analysis was done in a blinded fashion and the format mean ± standard error was used to express results.
Results
Subject characteristics (Table 1)
Table 1.
Subject characteristics
| Measure | Subjects (n = 149) |
|---|---|
| Age (years) | 80.0 ± 0.5 |
| Biological sex | 66 women, 84 men |
| BMI (kg/m2) | 26.5 ± 0.4 |
| Waist circumference (cm) | 92 ± 1 |
| MT (mm) | 19 ± 0 |
| Mean grip strength (kg) | 24.7 ± 0.7 |
| Gait speed (m/s) | 0.89 ± 0.2 |
| MABC (cm) | 25.6 ± 0.3 |
| Rockwood frailty index | 3.1 ± 0.1 |
| LBM (kg) | 21.3 ± 0.4 |
A total of 150 subjects were recruited sequentially from geriatric medicine clinics (66 women and 84 men). One subject dropped out of the study prior to completing all measures (one woman). Subject characteristics are shown in Table 1. Men had higher grip strength (P < 0.001), higher LBM (P < 0.001) and higher waist circumferences (P = 0.004) than women (see Appendix 1).
Univariate analysis
No skewing was detected on inspection of the predictor variable density plots and no transformation was required prior to the analyses. In our initial univariate analysis, ultrasound measures of muscle thickness (MT) showed a significant correlation with LBM (P < 0.001), MABC (P < 0.001) and grip strength (P = 0.002). However, MT did not show any significant correlation with gait speed (P = 0.561).
Multivariate analysis—LBM (Table 2)
Table 2.
Multivariate regression analysis (n = 149)
| R2 | Unstandardised β (SE) | Standardised β | P value | |
|---|---|---|---|---|
| LBM, MEM F(3,145) = 65.83 | 0.577 | <0.001* | ||
| Biological sex | 5.746 (0.509) | 1.231 (0.109) | <0.001* | |
| BMI | 0.263 (0.055) | 0.277 (0.058) | <0.001* | |
| MT | 1.847 (0.522) | 0.204 (0.058) | <0.001* | |
| Grip Strength, MEM F(3,145) = 50.18 | 0.511 | <0.001* | ||
| Age | −0.296 (0.080) | 0.019 (0.118) | <0.001* | |
| Biological sex | 10.867 (0.964) | −0.535 (0.222) | <0.001* | |
| MT | 2.487 (0.930) | 0.118 (0.115) | <0.001* | |
| MABC, MEM F(2,146) = 52.18 | 0.417 | <0.001* | ||
| BMI | 0.437 (0.050) | 0.583 (0.067) | <0.001* | |
| MT | 1.008 (0.480) | 0.141 (0.067) | 0.038* | |
| Gait speed, MEM F(1,147) = 25.26 | 0.520 | <0.001* | ||
| Age | −0.014 (0.003) | −0.394 (0.079) | <0.001* |
F, F Statistic; MEM. Minimal Effective Model; R2, coefficient of determination; SE, standard error; β, beta-coefficient; *, P < 0.05
The continuous predictors (age, BMI and MT) and the logistic predictor (biological sex) were entered into a multivariate regression model that initially explained 58% of the variance in LBM. VIFs were checked in the initial model; the highest VIF was 1.16 (MT) indicating that there were no issues with multicollinearity. As shown in Table 2, our most parsimonious model demonstrated a significant association between LBM with male biological sex (P < 0.001), BMI (P < 0.001) and MT (P < 0.001).
Multivariate analysis—grip strength (Table 2, Figure 1)
Figure 1.
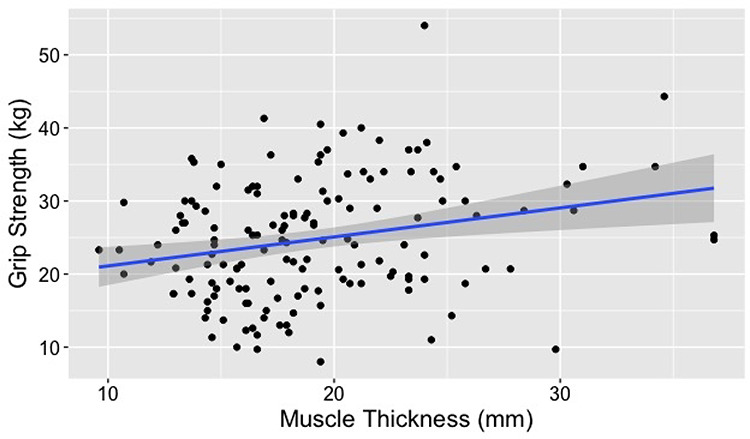
Association between vastus medialis thickness (MT) and grip strength: We were able to demonstrate a significant association between MT and grip strength (P < 0.001 in adjusted model) in our older adult subjects (n = 150).
Our initial model (containing age, BMI, MT and biological sex) initially explained 50% of the variation in grip strength. VIFs were checked in the initial model; the highest VIF was 1.03 (age) indicating that there were no issues with multicollinearity. As shown in Table 2, our minimum effective model demonstrated a significant association between grip strength and age (P < 0.001), male biological sex (P < 0.001) and MT (P < 0.001).
Multivariate analysis—MABC (Table 2, Appendix 1, Figure 2)
Figure 2.
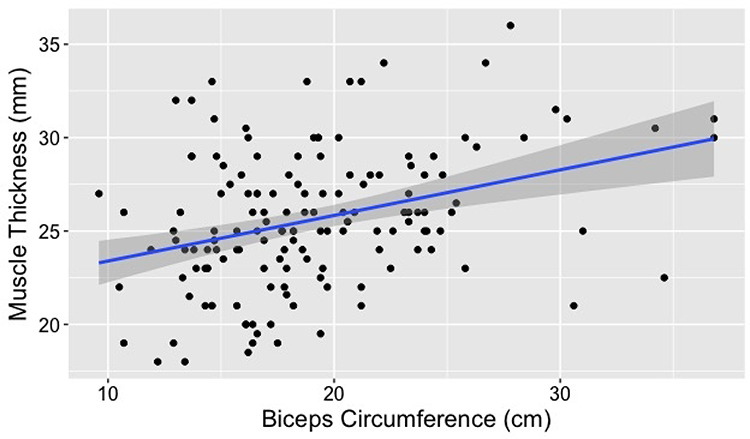
Association between vastus medialis thickness (MT) and MABC: There was a significant association between MT and MABC (P = 0.038 in adjusted model) in our older adult subjects (n = 150).
Our initial model initially explained 42% of the variation in MABC. VIFs were checked in the initial model; the highest VIF was 1.13 (BMI) indicating that there were no issues with multicollinearity. As shown in Table 2, our minimum effect model demonstrated a significant association between MABC and BMI (P < 0.001) and MT (P = 0.038).
Multivariate analysis—gait speed (Table 2)
Our initial model initially explained 15% of the variation in gait speed. As shown in Table 2, our most parsimonious model demonstrated a significant association between gait speed and age (P < 0.001).
Discussion
Principal findings
Sarcopenia is characterised as a loss of both muscle mass and muscle performance. Bedside ultrasound measures of vastus medialis MT showed strong correlations with overall LBM, as well as muscle circumference in the upper limb (MABC). MT also showed a strong association with one of our performance measures (grip strength), but no correlation with subject gait speed.
Clinical uses for PoCUS
PoCUS is emerging as a paradigm in which clinicians use portable ultrasonic devices to answer specific point-of-care questions that are incorporated into their bedside history and physical exam, similar to the current use of a stethoscope. What started out as a new bedside device used by emergency medicine physicians has been gradually expanded to examine patients to ask immediate clinical questions, including volume status [14], spleen size [15] and the presence of synovitis [16].
Previous work
Although there is not a single universally accepted definition for sarcopenia, all definitions involve an objective measure of muscle mass, as measured by DXA and an objective measure of muscle quality/function (usually by a measure of muscle strength or a measure of physical performance) [18]. The most commonly accepted definition is the recently revised EWGSOP2, which defines sarcopenia by biological sex-specific cutoffs for low muscle mass [18]. Unfortunately, it is difficult to use the current diagnostic criteria to screen for this condition in acutely ill patients, given that DXA scans are not portable, are expensive and involve the use of radiation. They are also not commonly used to screen for low muscle mass, due to the cost issues of screening such large numbers of patients. The current proposal suggests that we could reduce the number of DXA scans by using bedside ultrasound as a tool to screen for decreased muscle mass in older adults. To date the most established bedside methods of measuring muscle mass have been based on a screening grid based on age and BMI [19], and a prediction equation using anthropometric measures [20].
Similar to measurements of muscle mass, there is not a single universally accepted definition for reduced muscle performance [18]. The most commonly accepted definition again is found in the recently revised (EWGSOP2) criteria, which defines reduced muscle performance by biological sex-specific cutoffs for grip strength [18], a measure that is not commonly performed at the bedside. Grip strength has significant limitations in the setting of acute and chronic illness, is difficult to assess in a patient with delirium or dementia, and can be greatly affected by sedative medications [21].
The multifactorial nature of the pathophysiology of sarcopenia makes it extremely unlikely that a single blood biomarker can be used for diagnosis [22]. A previous attempt to develop a multifactorial tool to predict sarcopenia examined a panel of potential inflammatory, vascular adhesion and growth factors [23], and was only able to show an association with slower gait speeds [22, 23]; additionally, the mechanisms underlying sarcopenia are poorly characterised, posing a barrier to finding a panel of potential biomarkers for this condition [22].
Given the limited options for bedside tests for muscle mass and performance, as well as the limitations on blood-test based biomarkers, some recent work has examined the possibility of using PoCUS as a measure of both muscle quantity and muscle quantity. Congruent with our findings, work done by Strasser et al. [24] has shown that ultrasonic measures of MT in the quadriceps muscle have a highly significant correlation with maximal voluntary contraction. Other work done by Miron-Mombiela et al. [25] has shown that a measure of muscle echointensity (a measure of muscle quality which requires additional image processing) also shows a high correlation with grip strength. To our knowledge, the current study is the first to demonstrate that a simple bedside test of MT shows strong correlations with both overall muscle mass (LBM), upper limb muscle mass (MABC) and muscle strength (grip strength). This suggests that bedside ultrasound, which is increasingly becoming a mainstream component of the standard physical exam, could be used as a single marker muscle mass and muscle quality. We were unable to show any correlations between MT and our performance measure (gait speed); this is not surprising given that gait speed is affected by many other factors than physical strength, including depressed mood, aerobic capacity and physical health status [26].
Clinical implications
Sarcopenia is a source of increased morbidity, increased mortality and increased health care costs in the older adult population. There are currently several non-pharmacological treatments recommended for this condition, but more significantly there are several potential therapies under development [27] that have yet to be trialled in humans. This condition remains clinically neglected, mainly due to the fact that the standard components of sarcopenia criteria (grip strength, DXA scanning) are either almost never performed clinically to assess muscle health or impossible to perform at the bedside, especially in the setting of acute illness. Although much more work needs to be done, the establishment of an accurate bedside screening test for low muscle mass will greatly increase the awareness of this long neglected condition, and will help facilitate future randomised controlled trials of potential treatments for sarcopenia. There are challenges to incorporating PoCUS measures in geriatric medicine assessments; a previous survey of geriatrician interest in incorporating PoCUS into their daily clinical practice revealed understandable concerns about the cost of the device, uncertainty of how to undergo training, worries about liability with respect to incorrect radiological diagnoses and a lack of reimbursement [30].
Limitations and future research
The technique used in our study to measure LBM was BIA, which is not the gold standard measure of muscle mass (DXA scans). Future work on establishing PoCUS-based markers of muscle mass will need to be validated against more gold standard muscle measures, such as DXA. Such measures will be necessary to determine the sensitivity and specificity of these measures compared to current gold standard EWGSOP criteria.
Main conclusions
PoCUS measures of MT showed strong correlations with overall muscle mass (LBM), upper limb muscle mass (MABC) and muscle performance (grip strength).
Supplementary Material
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
The Allan M. McGavin Foundation.
References
- 1. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 2000; 88: 1321–6. [DOI] [PubMed] [Google Scholar]
- 2. Cederholm T, Cruz-Jentoft AJ, Maggi S. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med 2013; 49: 111–7. [PubMed] [Google Scholar]
- 3. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 2004; 52: 80–5. [DOI] [PubMed] [Google Scholar]
- 4. Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moisey LL, Mourtzakis M, Cotton BA et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care 2013; 17: R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res 2016; 28: 1047–60. [DOI] [PubMed] [Google Scholar]
- 7. Kushner RF, Gudivaka R, Schoeller DA. Clinical characteristics influencing bioelectrical impedance analysis measurements. Am J Clin Nutr 1996; 64: 423S–7. [DOI] [PubMed] [Google Scholar]
- 8. Maurissen JPJ, Marable BR, Andrus AK, Stebbins KE. Factors affecting grip strength testing. Neurotoxicol Teratol 2003; 25: 543–53. [DOI] [PubMed] [Google Scholar]
- 9. Bartley JM, Studenski SA. Muscle ultrasound as a link to muscle quality and frailty in the clinic. J Am Geriatr Soc 2017; 65: 2562–3. [DOI] [PubMed] [Google Scholar]
- 10. Watanabe Y, Yamada Y, Fukumoto Y et al. Echo intensity obtained from ultrasonography images reflecting muscle strength in elderly men. Clin Interv Aging 2013; 8: 993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perkisas S, Baudry S, Bauer J et al. Application of ultrasound for muscle assessment in sarcopenia: towards standardized measurements. Eur Geriatr Med 2018; 9: 739–57. [DOI] [PubMed] [Google Scholar]
- 12. Crawley MJ. Statistics: An Introduction using R. West Sussex, England: Wiley, 2011. [Google Scholar]
- 13.R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (3 January 2020, date last accessed).
- 14. Stawicki SP, Braslow BM, Panebianco NL et al. Intensivist use of hand-carried ultrasonography to measure IVC collapsibility in estimating intravascular volume status: correlations with CVP. J Am Coll Surg 2009; 209: 55–61. [DOI] [PubMed] [Google Scholar]
- 15. Lee M, Roberts JM, Chen L et al. Estimation of spleen size with hand-carried ultrasound. J Ultrasound Med 2014; 33: 1225–30. [DOI] [PubMed] [Google Scholar]
- 16. van den Berg PJ, Daoudi K, Bernelot Moens HJ, Steenbergen W. Feasibility of photoacoustic/ultrasound imaging of synovitis in finger joints using a point-of-care system. Photoacoustics 2017; 8: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alpert JS, Mladenovic J, Hellmann DB. Should a hand-carried ultrasound machine become standard equipment for every internist? Am J Med 2009; 122: 1–3. [DOI] [PubMed] [Google Scholar]
- 18. Cruz-Jentoft AJ, Bahat G, Bauer J et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goodman MJ, Ghate SR, Mavros P et al. Development of a practical screening tool to predict low muscle mass using NHANES 1999--2004. J Cachexia Sarcopenia Muscle 2013; 4: 187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu S, Appleton S, Chapman I et al. An anthropometric prediction equation for appendicular skeletal muscle mass in combination with a measure of muscle function to screen for sarcopenia in primary and aged care. J Am Med Dir Assoc 2015; 16: 25–30. [DOI] [PubMed] [Google Scholar]
- 21. Martin S, Neale G, Elia M. Factors affecting maximal momentary grip strength. Hum Nutr Clin Nutr 1985; 39: 137–47. [PubMed] [Google Scholar]
- 22. Calvani R, Marini F, Cesari M et al. Biomarkers for physical frailty and sarcopenia: state of the science and future developments. J Cachexia Sarcopenia Muscle 2015; 6: 278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marzetti E, Landi F, Marini F et al. Patterns of circulating inflammatory biomarkers in older persons with varying levels of physical performance: a partial least squares-discriminant analysis approach. Front Med 2014; 1: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age 2013; 35: 2377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mirón Mombiela R, Facal de Castro F, Moreno P, Borras C. Ultrasonic echo intensity as a new noninvasive in vivo biomarker of frailty. J Am Geriatr Soc 2017; 65: 2685–90. [DOI] [PubMed] [Google Scholar]
- 26. Buchner DM, Cress ME, Esselman PC et al. Factors associated with changes in gait speed in older adults. J Gerontol A Biol Sci Med Sci 1996; 51: M297–302. [DOI] [PubMed] [Google Scholar]
- 27. J-PG C, Petersen MC, Abudukadier A et al. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc Natl Acad Sci U S A 2016; 113: 2212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hardee JP, Lynch GS. Current pharmacotherapies for sarcopenia. Expert Opin Pharmacother 2019; 1–13. [DOI] [PubMed] [Google Scholar]
- 29. Gillman LM, Kirkpatrick AW. Portable bedside ultrasound: the visual stethoscope of the 21st century. Scand J Trauma Resusc Emerg Med 2012; 20: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leone AF, Schumacher SM, Krotish DE, Eleazer GP. Geriatricians’ interest to learn bedside portable ultrasound (GEBUS) for application in the clinical practice and in education. J Am Med Dir Assoc 2012; 13: e7–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


