Abstract
Objective
Assimilate evidence for interventions to ameliorate negative changes in physical performance, muscle strength and muscle quantity in hospitalised older adults.
Methods
We searched for articles using MEDLINE, Embase, CINAHL and Cochrane library using terms for randomised controlled trials, older adults, hospitalisation and change in muscle quantity, strength or physical performance. Two independent reviewers extracted data and assessed risk of bias. We calculated standardised mean differences for changes in muscle function/quantity pre- and post-intervention.
Results
We identified 9,805 articles; 9,614 were excluded on title/abstract; 147 full texts were excluded. We included 44 studies including 4,522 participants; mean age 79.1. Twenty-seven studies (n = 3,417) involved physical activity interventions; a variety were trialled. Eleven studies involved nutritional interventions (n = 676). One trial involved testosterone (n = 39), two involved Growth Hormone (n = 53), one involved nandrolone (n = 29), and another involved erythropoietin (n = 141). Three studies (n = 206) tested Neuromuscular Electrical Stimulation. Evidence for effectiveness/efficacy was limited. Strongest evidence was for multi-component physical activity interventions. However, all studies exhibited at least some concerns for overall risk of bias, and considering inconsistencies of effect sizes across studies, certainty around true effect sizes is limited.
Conclusion
There is currently insufficient evidence for effective interventions to ameliorate changes in muscle function/quantity in hospitalised older adults. Multiple interventions have been safely trialled in heterogeneous populations across different settings. Treatment may need to be stratified to individual need. Larger scale studies testing combinations of interventions are warranted. Research aimed at understanding pathophysiology of acute sarcopenia will enable careful risk stratification and targeted interventions.
Keywords: acute sarcopenia, systematic review, older people, interventions
Key points
A variety of interventions have been trialled for outcome measures relevant to acute sarcopenia.
There is currently insufficient evidence for effective interventions to treat acute sarcopenia.
Trials involving a combination of interventions in stratified individually are warranted.
Introduction
Sarcopenia is defined by low muscle strength with low muscle quantity/quality; additionally demonstrated low physical performance defines severe sarcopenia. Cut-offs are two standard deviations (SDs) below means of young healthy reference populations [1]. Acute sarcopenia (acute muscle insufficiency) particularly affects hospitalised older adults [2,3]. Normally proceeded by stressor events, it is defined by acute declines in muscle quantity/quality and/or function (strength or physical performance) producing incident sarcopenia [1,3]. Previous reviews considered chronic sarcopenia treatment/prevention [4–6]; strongest evidence exists for physical activity. Resistance training improves muscle quantity, strength and physical performance in community-dwelling populations [7]. Some trials demonstrated enhanced benefit of nutritional supplementation alongside [8]. Large studies are underway evaluating combined nutritional and exercise interventions for chronic sarcopenia [9].
It is unknown whether chronic sarcopenia interventions can treat acute sarcopenia. Mechanisms differ, which may affect treatment efficacy. Acute sarcopenia is associated with greater systemic inflammation and immune-endocrine dysregulation. Inflammation (acute or chronic) may blunt response to exercise or protein challenges (anabolic resistance), but this may be acutely/severely upregulated in acute sarcopenia [10]. Acute sarcopenia follows an accelerated course [3]; traditional treatments may not work fast enough. Additionally, community interventions may be unfeasible in hospital. This review aimed to identify trialled interventions for ameliorating negative changes in muscle quantity, strength or physical performance in hospitalised older adults, and to summarise/synthesise findings.
Methods
Protocol and registration
Protocol was agreed by all researchers and registered with Prospective Register of Systematic Reviews—CRD42018112021. Reporting is consistent with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidance.
Eligibility criteria
We included randomised controlled trials (RCTs) and quasi-RCTs involving hospitalised patients ≥65 years-old, where pre- and post-intervention measurements of muscle quantity, strength or physical performance were available. Post-intervention measures until 28 days post-intervention were included. We included physical activity, nutritional, pharmaceutical or Neuromuscular Electrical Stimulation (NMES) trials. Exclusion criteria were: degenerative neuromuscular disorders; acute stroke; trials of parenteral nutrition, surgical technique/invasive procedure, chemotherapy/radiotherapy, or anaesthetic agents/techniques; no control group; lengths of stay less than 2 days. We included studies that measured muscle quantity using computed tomography (CT), magnetic resonance imaging (MRI), dual energy X-ray absorptiometry (DXA), bioelectrical impedance analysis (BIA), or ultrasound, muscle strength using handgrip strength, knee flexion, or knee extension, or physical performance using short physical performance battery (SPPB), gait speed, timed up and go (TUG), or 6-Minute Walking Test (6MWT). There were no date or language restrictions.
Information sources
We searched electronic databases (MEDLINE, Embase, CINAHL, CENTRAL) on 16 January 2019; search repeated on 3 April 2020. Grey literature was identified through Web of Science, Google Scholar, Clinicaltrials.gov, article references and protocol citations. We contacted authors for information where necessary, including requesting age breakdown of data. If no response was obtained, a decision was made to include studies where mean age was one SD > 65.
Search strategy
We used published and unpublished terms for study design (RCTs), population (older adults AND hospitalised) and outcome measures (muscle mass OR muscle strength OR physical performance) in our search. Full search strategy is available in the online supplement; this was reviewed and agreed with an information specialist.
Study selection
Citations were imported into Microsoft Excel 2016. Duplicates were removed automatically/manually. Two reviewers independently screened titles and abstracts for inclusion (CW, ZM). Disagreements were resolved through discussion. Full texts were reviewed independently by the same reviewers; disagreements were resolved through discussion or third review (TAJ).
Data extraction
Data were extracted independently by two reviewers (CW, ZM) using a template (Microsoft Excel 2016). Extracted data were country, study design, sample size and dropouts, sample characteristics (age, ethnicity, body mass index—BMI, sex), speciality, intervention description (type of intervention, how delivered), intervention characteristics (timing of intervention, dosage), control group, outcome data, length of stay and adverse events. Outcome data at baseline and follow-up to include muscle quantity, muscle strength and physical performance were extracted.
Risk of bias
Two reviewers (CW, ZM) independently assessed risk of bias using Cochrane risk of bias tool. Conflicts were resolved by discussion. Risk of bias was collated using RevMan version 5.3 [11].
Synthesis of results
We summarised study and participant characteristics, and outcome data at baseline and follow-up using means/SDs in text and tables. Interventions were grouped by subtype and outcomes. All studies were included in narrative synthesis. If sufficient information was available to estimate standardised mean differences (SMDs) of change scores, effect sizes were evaluated as described in statistical analysis section. Certainty of interventions with large effect sizes was evaluated using Grading of Recommendations, Assessment, Development and Evaluations [12].
Statistical analysis
Correlations for outcome measures were calculated from studies reporting SDs of change scores and baseline/follow-up measures [13]. Mean correlation for each outcome was used to estimate SD of change in outcomes in studies where this was not available. We calculated SMDs of change scores by dividing difference in change score between comparison and intervention groups by SD of change score in comparison group [14]. Effect sizes were calculated to one decimal place and classified as no effect (≤0.1), small (0.2–0.4), medium (0.5–0.7) or large (0.8 or greater) [15]. If more than one effect size was available for a single trialled intervention and outcome type, the larger was included. Meta-analysis was not performed due to high heterogeneity of interventions and outcomes.
Results
Study selection
We identified 9,805 articles after duplicates removal. We excluded 9,613 following title/abstract screening; 192 full texts assessed for eligibility. We excluded 148 full text articles due to mean age not more than one SD above 65 (n = 56), no control group (n = 10), follow-up over 28 days (n = 12), no baseline measures (n = 6), no measures meeting inclusion criteria (n = 20), duplicate data (n = 11), unable to obtain necessary data from authors (n = 24), other intervention type (n = 2), and non-hospitalised population (n = 6) (Figure 1). We included 44 studies in narrative synthesis and 32 studies in effect size evaluation.
Figure 1.
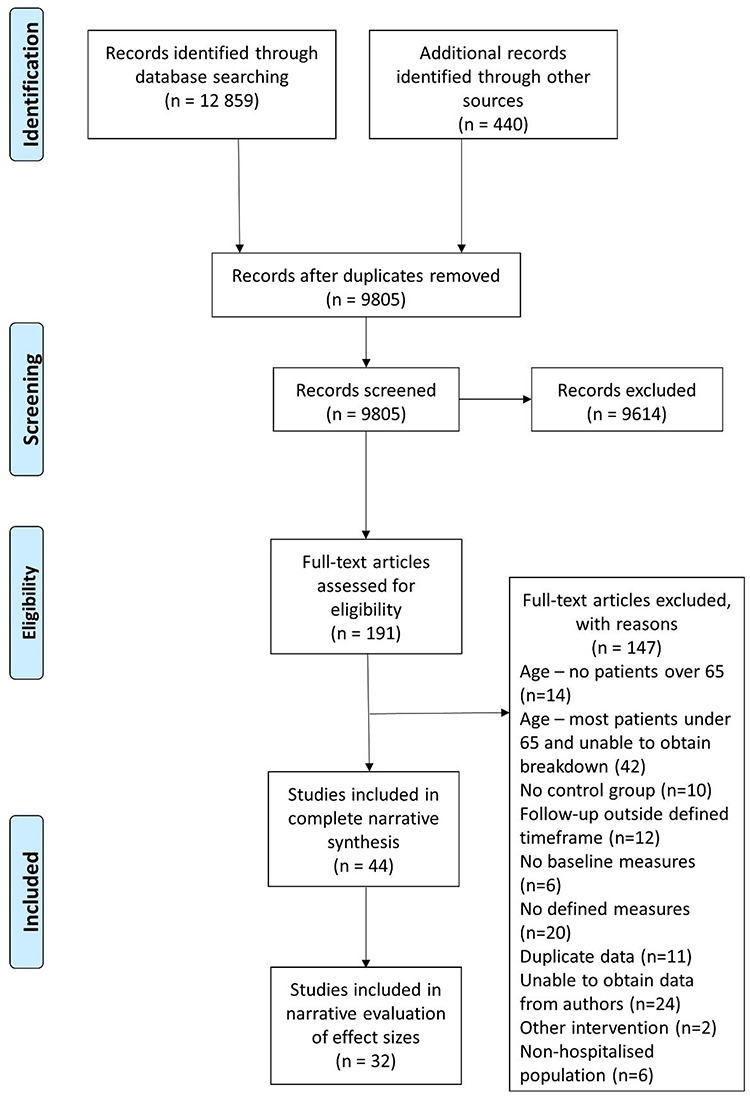
Flowchart demonstrating identification of included studies. All stages of screening and inclusion/exclusion were performed in duplicate. Reasons for exclusion of articles reviewed as full texts are specified.
Study characteristics
This review included 4,522 participants (2,160 control, 2,362 intervention). Sample size per arm ranged from 7 to 232. Most studies were small; 52% (23/44) [17–40] included 30 or fewer participants per arm; only 9% (4/44) [41–43] included over 100 participants in both arms. Mean age across all studies was 79.1 years; 59% female. Of studies reporting BMI, 74% (20/27) [17, 18, 21, 23, 26–28, 31, 34, 35, 38, 43–51] reported mean overweight (≥25) BMI; three studies reported mean obese (≥30) BMI in at least one arm [23, 39, 52]. One study reported data on ethnicity [38]. Two studies [45, 50] reported frailty prevalence in control and intervention arms by recognised definitions; a third reported mean frailty indices [37]. Table 1 shows included studies’ details; full study characteristics and results are available online. Table 2 shows effect sizes separated by interventions and outcomes.
Table 1.
Characteristics of all studies included in narrative synthesis
| Author, date | Setting | N (control/intervention) | Intervention | Outcomes |
|---|---|---|---|---|
| Physical activity | ||||
| Busch [44], 2012 | Cardiac surgery | 64/57 | Resistance and balance training | TUG 6MWT Knee extension |
| Blanc-Bissonb [24], 2008 | Geriatric medicine | 24/22 | Early physiotherapy | Handgrip |
| Braun [37], 2019 | Geriatric medicine | 18/17 | Augmented Prescribed Exercise Programme | Gait speed TUG 6MWT |
| de Morton [41], 2007 | General medicine | 126/110 | Physiotherapy-designed exercises | TUG |
| Deer [38], 2019 | General medicine | 20/21 | Chair-based and resistance exercise | SPPB DXA FFM |
| Fioreb [20], 2017 | Elective colorectal surgery | 22/25 | Early mobilisation | 6MWT |
| Giangregorio [19], 2009 | Orthopaedic rehabilitation | 7/14 | Body weight supported treadmill training | TUG |
| Henriksenb [25)], 2002 | Elective colorectal surgery | 12/13 | Enhanced recovery | Handgrip Knee extension |
| Houborg [46], 2006 | Elective colorectal surgery | 59/60 | Strength training programme | Gait speed Handgrip strength Knee extension |
| Jones [53], 2006 | General medicine | 80/80 | Individualised progressive exercise | TUG |
| Martinez-Velilla [43], 2019 | Geriatric medicine | 185/185 | Multi-component physical exercise | Gait speed SPPB Handgrip |
| McCullagh [45], 2017 | General medicine | 95/95 | Augmented prescribed exercise programme | Gait speed SPPB Handgrip |
| McGowanb [36], 2018 | Acute medicine for older people | 25/25 | Pedal exerciser | Knee extension Knee flexion |
| Moseley [54], 2009 | Orthopaedic rehabilitation | 80/80 | Weight-bearing exercise | Gait speed Knee extension |
| Ortiz-Alonso [50], 2019 | Geriatric medicine | 131/150 | Chair-based exercise and walking | SPPB |
| Opasich [55], 2010 | Cardiac surgery | 80/160 | Individualised physical training programme | TUG 6MWT |
| Prasciene [40], 2019 | Cardiac surgery | 15/14 | Balance and resistance training | SPPB 6MWT |
| Rahmann [18], 2009 | Elective orthopaedic | 20/24 | Aquatic physiotherapy | TUG Knee extension |
| 24/21 | Water exercise | TUG Knee extension |
||
| Raymond [42], 2017 | Geriatric medicine | 232/236 | High-intensity group exercises | TUG |
| Saidb [22], 2012 | Geriatric rehabilitation | 24/22 | Enhanced physical activity | TUG |
| Saidb [56], 2018 | Geriatric rehabilitation | 93/98 | Multimodal exercise programme | Gait speed TUG |
| Sano [47], 2018 | Elective orthopaedic | 41/40 | Seated side-tapping training | Gait speed TUG Knee extension Knee flexion |
| Schwenk [57], 2014 | Geriatric rehabilitation | 74/74 | Individualised physical training programme | Gait speed Handgrip |
| Sherrington [58], 2003 | Orthopaedic rehabilitation | 39/41 | Weight-bearing exercise | Gait speed Knee extension |
| Tal-Akabib [21], 2007 | Orthopaedic rehabilitation | 29/33 | High-intensity exercise | TUG |
| Torres-Sánchez [23], 2017 | Respiratory | 29/29 | Pedal exerciser | Knee extension |
| Wnuk [17], 2016 | Vascular | 16/15 | Backward walking | 6MWT |
| 16/16 | Forward walking | 6MWT | ||
| Nutrition | ||||
| Beelen [48], 2017 | General medicine | 39/36 | Protein-enriched familiar foods | SPPB Handgrip Knee extension |
| Bouillanne [30], 2018 | Geriatric rehabilitation | 14/13 | Citrulline amino acid | DXA ASMM |
| Deer [38], 2019 | General medicine | 20/20 | Whey protein | SPPB DXA FFM |
| 20/20 | Whey protein and exercise | SPPB DXA FFM |
||
| Ekinci [59], 2016 | Orthopaedic surgery | 37/38 | Beta-hydroxy-beta-methylbutyrate | Handgrip |
| Files [39], 2020 | Critical care | 11/11 | Nitrate-rich beetroot juice | SPPB |
| Gade [49], 2019 | General medicine | 82/83 | Protein-enriched milk supplement | Gait speed Handgrip BIA FFM |
| Hermanky [27], 2017 | Orthopaedic surgery | 20/20 | Nutritional consultation and exercise | Handgrip BIA FFM |
| Niccoli [26], 2017 | Geriatric medicine | 26/26 | Whey protein | Gait speed TUG Handgrip Knee extension |
| Ogasawara [29], 2018 | Respiratory medicine | 21/21 | EPA-enriched oral nutritional supplements | BIA SMI |
| Pedersen [51], 2019 | General medicine | 42/43 | Protein and exercise | Gait speed Handgrip |
| Saudny-Unterberger [28], 1997 | Respiratory medicine | 16/17 | Oral nutritional supplements | Handgrip |
| Pharmaceutical | ||||
| Deer [38], 2019 | General medicine | 20/19 | Testosterone | SPPB DXA FFM |
| Hedström [32], 2004 | Orthopaedic surgery | 9/11 | Growth hormone | Knee extension DXA LBM |
| Sloan [33], 1992 | Orthopaedic surgery | 14/15 | Nandrolone | BIA FFM |
| Weissberger [31], 2003 | Orthopaedic surgery | 16/17 | Growth hormone | Knee flexion CT thigh CSA |
| Zhang [60], 2019 | Orthopaedic surgery | 33/44 | EPO injections (females) | DXA ASM |
| 25/39 | EPO injection (males) | DXA ASM | ||
| Neuromuscular electrical stimulation | ||||
| Lopez-Lopez [52], 2019 | General medicine | 47/48 | NMES and exercise combined | SPPB |
| Martin-Salvador [35], 2016 | Respiratory medicine | 20/24 | Exercise and NMES combined | Knee extension |
| Zinglersen [34], 2018 | Geriatric medicine | 48/20 | Chair-based functional exercise | Gait speed |
| 8/12 | NMES and functional training | Gait speed | ||
N, participant numbers; ASMM, appendicular skeletal muscle mass; FFM, fat-free mass; SMI, skeletal muscle index; LBM, lean body mass; CSA, cross-sectional area; LoS, length of hospital stay; COPD, chronic obstructive pulmonary disease.
bUnpublished data.
Table 2.
Summary of intervention effect by intervention type, outcome type and effect size
| Physical performance | Muscle strength | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect sizea | N (con/exp) | Risk of Biasb | Study | Effect sizea | N (con/exp) | Risk of Biasb | Study | ||
| Physical activity | Strength and balance training | + ++ ++ +++ |
93/98 64/57 20/21 15/14 |
+ +/−—- |
[56] [44] [38] [40] |
− | 64/57 | +/− | [44] |
| Early/increased mobilisation, or additional physiotherapy | -—++ ++ - |
24/22 22/25 16/16 126/110 131/150 |
- + +/−—- |
[22] [20] [17] [41] [50] |
- +++ |
24/22 12/13 |
- - |
[24] [25] |
|
| Water exercise and physiotherapy | + | 20/24 | +/− | [18] | + | 20/24 | +/− | [18] | |
| Seated side-tapping | +++ | 41/40 | − | [47] | − | 41/40 | − | [47] | |
| Seated pedal exercises | No data | + ++ |
25/25 29/29 |
- +/− |
[36] [23] |
||||
| Progressive weight-bearing exercise | + + +++ |
39/41 80/80 7/14 |
+/− +/− - |
[58] [54] [19] |
- - |
39/41 80/80 |
+/− +/− |
[58] [54] |
|
| Individualised physical training programme | - + + +++ +++ |
95/95 74/74 80/160 185/185 18/17 |
+/− +/−—+/− +/− |
[45] [57] [55] [43] [37] |
-—+++ | 95/95 74/74 185/185 |
+/− +/− +/− |
[45] [57] [43] |
|
| Nutrition | Protein-enriched foods | +++ ++ |
26/26 20/20 |
+/− - |
[26] [38] |
+ +++ |
39/36 26/26 |
+/− +/− |
[48] [26] |
| Protein and exercise | +++ | 20/20 | − | [38] | + | 42/43 | − | [51] | |
| β-Hydroxy-β-MethylButyrate | No data | − | 37/38 | +/− | [59] | ||||
| Oral nutritional supplementation and snacks | +++ | 16/17 | +/− | [28] | |||||
| Nutrition consultation combined with exercise | + | 20/20 | +/− | [27] | |||||
| Drugs | Testosterone | +++ | 20/19 | − | [36] | [38] No data |
|||
| Growth hormone | No data | − | 9/11 | − | [32] | ||||
| NMES | NMES in combination with exercise | +++ | 47/48 | +/− | [52] | + | 20/24 | +/− | [35] |
N, participant numbers; con, comparison group; exp, intervention group.
aEffect sizes categorised as: no effect [−] (≤0.1), small [+] (0.2–0.4), medium [++] (0.5–0.7), or large [+++] (0.8 or greater).
bRisk of Bias categorised according to overall risk as: low [+], some concerns [+/−], or high [−].
Physical activity interventions
Most studies (61%, 27/44) reported physical activity interventions. Eighty-nine percent (24/27) included physical performance [17–22, 34, 37, 38, 40–47, 50, 53–58] and 44% (12/27) included muscle strength [18, 23–25, 36, 43, 44, 46, 47, 54, 57, 58]. One study reported muscle quantity change [38], a multi-arm trial including nutritional and pharmaceutical interventions. Trials were conducted in various settings including elective orthopaedic [18, 47], colorectal [20, 25, 46], orthopaedic rehabilitation [19, 21, 54, 58], vascular [17], and cardiac surgery [40, 44, 55], and geriatric [22, 24, 34, 36, 37, 42, 43, 56, 57], respiratory [23] and general medicine [38, 41, 45, 53].
A range of physical activity interventions were trialled; evidence for effect was limited. Interventions included strength and balance training [21, 38, 40, 44, 46, 56], early and/or increased mobilisation [17, 20, 22, 24, 25, 41], group exercise [42], water exercise/physiotherapy [18], chair-based exercise [34, 38], seated side-tapping [47], pedal exercisers [23, 36] and progressive weight-bearing exercise in orthopaedic rehabilitation [19, 54, 58], using specialised harnesses where appropriate. An individualised multimodal physical training programme involving resistance exercise using machines and/or weights and gait/balance training substantially improved physical performance (gait speed and SPPB) and muscle strength in one of the largest studies [43]. Other trials of individualised physical training programmes (strength with or without aerobic exercise stratified by frailty/functional status) showed small effects on physical performance [37, 45, 53, 55, 57]. Differences may relate to how interventions were delivered or adherence. The trial with the largest effect size reported adherence rates of 83.4–95.8% (≥90% exercises successfully performed each session) [43] compared to 59.7% (>3 sessions attended per week; offered daily) in another [57].
Interventions that ameliorated reductions in physical performance in trial populations included backward walking [17], progressive exercises stratified by frailty [55], resistance and balance training [44], chair-based resistance exercise [38], individually progressed lower limb and core strengthening exercise [45], individualised progressive resistance, balance, and walking exercises [43] and seated side-tapping [47]. Interventions that ameliorated reductions in muscle strength included pedal exercise [23], individualised progressive resistance, balance and walking exercises [43] and early mobilisation with enhanced recovery after surgery [25]. A high-intensity physiotherapy-led group exercise programme was as efficacious as individual sessions; group exercise resulted in improved therapist efficiency [42]. Group exercise was embedded into a multimodal physical training trial [43].
Nutritional interventions
Eleven nutrition trials were identified. Populations included orthopaedic surgery [27, 59], geriatric [26, 30], general [38, 48, 49, 51], and respiratory medicine [28, 29] and critical care [39]. Six studies reported physical performance change [26, 38, 39, 48, 49, 51], seven muscle strength change [26–28, 48, 49, 51, 59] and four muscle quantity change [27, 29, 30, 38]. Most studies were small; only one included more than 45 patients per arm. Interventions included protein-enriched foods [26, 48] or supplements [38, 49, 51], β-Hydroxy-β-MethylButyrate [59], oral nutritional supplementation [28], eicosapentaenoic acid [29], citrulline [30], nitrate-rich beetroot juice [49] and nutritional consultation [27]. Three trials combined nutritional consultation to reach specified caloric/protein intake) with strength/resistance training [27, 38, 51]. One study of progressive strength training followed by immediate protein supplementation showed statistically significant improved handgrip strength [51]. Statistically significant improvements in physical performance were demonstrated comparing all interventions in a multi-arm study to placebo, including whey protein with/without exercise [38].
Pharmaceutical interventions
Five trials involved pharmaceuticals; four in orthopaedic surgery populations. Pharmaceuticals included growth hormone (GH) [31, 32], steroid (nandrolone) [33], testosterone [38] and erythropoietin injections [60]. All studies measured muscle quantity (DXA, CT or BIA) and both GH trials measured muscle strength. The only study that measured physical performance was the multi-arm study including physical activity and nutritional interventions [38]. One GH trial showed statistically significant amelioration in muscle quantity loss by DXA [32] and the other showed statistically significant amelioration in knee flexion strength loss [31]. Adverse events were similar between control and intervention arms [31]; one study showed slightly higher peripheral oedema rates amongst GH recipients [32]. The nandrolone trial did not report statistically significant results [33]. Erythropoietin induced a small statistically significant amelioration in muscle quantity loss after orthopaedic surgery, not related to haemoglobin changes. Testosterone was safe in the multi-arm study, with statistically significant amelioration in physical performance demonstrated comparing all intervention groups to placebo [38].
Neuromuscular electrical stimulation
Three trials involved NMES [34, 35, 52]; all combined NMES with exercise. One trial (geriatric medicine population) tested functional training alone against functional training with NMES [34]. No statistically significant different change in gait speed between groups was demonstrated. Another trial (respiratory medicine population) showed significant lesser decline in knee extension strength with NMES [35]. The third trial (general medicine population) resulted in significant improvements in physical performance with NMES [52].
Risk of bias and certainty across studies
Figure 2 shows overall risk of bias across studies. Full risk of bias details is shown in the Supplementary Appendix. There were at least some concerns for overall risk of bias across most studies. Adherence to trial intervention was associated with lowest risk and selection of reported outcome with highest risk. Most common reason for high risk of bias related to randomisation processes. Over half of studies exhibited at least some concerns for selection of reported result. Table 3 shows assessment of certainty for two interventions (individualised physical training programmes and protein supplementation) across studies.
Figure 2.
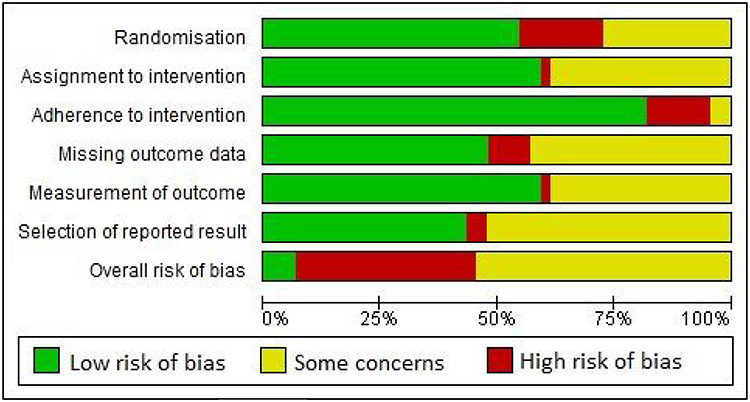
Risk of bias results across all included studies.
Table 3.
GRADE domain certainty for individual physical training programmes and protein supplementation
| GRADE Domain | Certainty | Comments |
|---|---|---|
| Individual physical training programme | ||
| Risk of bias | Moderate | RCTs assessed were mainly considered to have some concerns for overall risk of bias; no studies with high risk of bias. |
| Imprecision | Moderate | Meta-analysis of effect sizes across RCTs was not performed, although larger sample sizes in included studies. |
| Inconsistency | Low | Inconsistency of effect sizes across studies. |
| Indirectness | High | All but one study in geriatric medicine setting; all in older adults. All patients able to ambulate pre-admission and at risk of functional decline. |
| Publication bias | High | Publication bias of RCTs unlikely, particularly as mixed results presented. Inclusion of thesis and conference abstract for another physical activity intervention included. |
| Protein supplementation (with or without exercise) | ||
| Risk of bias | Moderate | RCTs assessed were considered to have either low risk or some concerns for overall risk of bias. |
| Imprecision | Low | Overall small number of studies with low sample sizes. |
| Inconsistency | Moderate | Similar effect sizes demonstrated in small numbers of studies. |
| Indirectness | High | All studies performed in general/geriatric medicine setting in older adults. |
| Publication bias | High | Publication bias of RCTs unlikely, particularly considering identification of studies with low sample size. |
Discussion
Interpretation of findings
Physical activity interventions were investigated more commonly than others. However, this mostly relates to studies with physical performance outcomes; only four trials not involving physical activity interventions measured physical performance [26, 39, 48, 49]. Conversely, many physical activity trials reported muscle strength change but only one measured muscle quantity change, a multi-arm study also involving nutritional/pharmaceutical interventions. Nutritional and pharmaceutical trials focused on muscle strength and quantity changes rather than physical performance. This suggests disconnect in how physical activity interventions are trialled compared to other interventions; physical performance declines may not be prioritised as organ insufficiency markers in need of urgent treatment.
Only nine trials reported muscle quantity change. This relates to historical reduced availability of feasible serial assessment tools; DXA, CT and MRI remain gold-standard, but ultrasound is increasingly utilised [1,16]. As sarcopenia definition has developed, measures of muscle function are considered more important than muscle quantity [1]. However, in acute sarcopenia, early muscle quantity declines may not be associated with muscle strength declines [3]; preventing this may be important to prevent longer term deteriorations. Additionally, muscle strength may be affected by fatigue/effort during acute illness making testing of efficacy/effectiveness challenging [17]. Muscle quantity may be an appropriate treatment target in hospitalised patients; future trials of interventions for acute sarcopenia should consider incorporating in outcomes. Measurement of muscle quantity is also important to show biological effectiveness/mechanistic action.
We identified several physical activity interventions that stratified treatment protocols individually (e.g. by frailty) [37, 43, 45, 53, 55]. Most substantial and significant effects on muscle strength and physical performance were demonstrated in the highest reported adherence trial [43]. Although this demonstrates high adherence of hospitalised older adults to complex trial designs is possible, effectiveness is expected to be reduced in clinical environments with limited compliance. Increasing mobilisation alone may be insufficient to prevent/treat acute sarcopenia [17, 20, 22, 24, 41], although this is safe to do when possible and should be commended [17, 20, 24, 25, 41]. Physical activity interventions can be multidimensional and include resistance exercise [43, 44]; it is safe and feasible to use machines/weights during acute phase of illness in hospitalised older patients [43, 44, 57]. Pedal exercises [23, 36] and seated side-tapping [47] are simple, cheap, feasible and potentially effective; these may be implemented as part of multidimensional stratified interventions. Group exercise may be as effective as individual exercise but more cost-effective [42]. Group exercise has additional benefits of improving social interaction, and potentially improving motivation [18] and adherence [43].
Several nutritional interventions were trialled. Although few trials showed statistically significant results, all trials were small and may have been under-powered for efficacy. Three trials combined nutritional intervention with physical activity [27, 38, 51]. Research in chronic sarcopenia suggested additional protein supplementation may be most effective when combined with targeted physical activity i.e. resistance exercise [19]. As inflammation and anabolic resistance are heightened with acute illness [3], greater doses (i.e. greater protein/amino acid intake) may be warranted in hospitalised older adults.
Few studies tested pharmaceuticals. There is suggestion from GH trials that this may be effective in ameliorating reductions in muscle quantity and strength [31, 32]. Further research is needed, including longer term outcomes. Benefits of GH supplementation need to be balanced against adverse effects, although supplementation was safe in dosages used in these small studies. Research is ongoing into novel pharmaceutical agents for use in acute and chronic sarcopenia [20]. Studies assessing correlations between immune-endocrine biomarkers and phenotypic changes in muscle quantity, quality or function will enable stratified treatments and direct potential drug pathways.
Trials of NMES showed conflicting results. NMES involves delivery of controlled electrical stimuli to superficial muscles via self-adhesive skin electrodes. These stimuli evoke muscle contractions, recruiting motor units and activating muscle fibres [21]. NMES has been shown to ameliorate reductions in muscle quantity and function in healthy young volunteers during bed rest [22]. It is plausible that NMES may treat acute sarcopenia in hospitalised older adults. However, in establishing effectiveness in clinical practice, adherence, physical activity impact, and which muscle groups to stimulate should be considered.
What are the limitations of this review?
This review included hospitalised adults over 65 years-old. We excluded younger adults to focus towards most vulnerable patients, who are most likely to benefit from targeted interventions. More studies were excluded for participant age than were included (56 versus 44). This suggests persistent bias against involvement of older people in clinical trials, particularly those with frailty. Considering we included search terms for older people in our search, it is likely more trials involving younger adults were not identified, as well as trials excluded through abstract screening. Trials conducted in younger adults may be useful when developing interventions for acute sarcopenia in older adults, but caution should be taken extrapolating results from younger less heterogeneous populations.
It is important to consider only three studies reported frailty status in both control and intervention arms [37, 45, 50]. Frailty was measured in intervention arms but rates were not reported in studies that stratified by frailty [55, 57]. Although important measures, handgrip strength and gait speed alone may be insufficient to diagnose pre-morbid frailty during acute illness [42]. Recording levels of frailty prior to hospitalisation can ensure control and intervention arms are matched and enable sub-group analysis assessing treatment effect in individuals with and without frailty [45]. Only one study reported ethnicity amongst participants [38]. Normative values of muscle quantity may vary according to ethnicity [23], and muscle echotexture may differ [24]. Further research is needed to assess effects of genetics and environment on ethnic differences, and how these relate to differences in muscle function and responsiveness to interventions. Without information on ethnicity within published trials, it is not possible to assess for between group differences.
As described, majority of trials were small; many may have been underpowered to detect changes. Due to high heterogeneity in populations, interventions, and outcome measures, it was not possible to conduct meta-analyses. Some interventions that were not shown to be effective in small individual trials may be effective in larger powered studies. Additionally, most studies exhibited some concerns for risk of bias overall, and due to inconsistencies in effect sizes across different studies, there is limited certainty around true effect sizes. Many different outcome measures were also assessed across different RCTs. We consider that standardisation of assessment and outcome measures within geriatric medicine research will enable greater ease of knowledge transfer, sharing of datasets and future meta-analyses of RCTs in ageing.
It is important to consider that none of the included trials specifically included the presence of (acute or chronic) sarcopenia as inclusion criteria, or stratified treatment by sarcopenia. However, we consider that results of identified RCTs identified will be pivotal towards designing trials for prevention and/or treatment of acute sarcopenia. Acute sarcopenia is a rapidly progressing research area and therapeutic target. Twenty-two percent of studies (10/44) included in this review were published in the last 18 months. This demonstrates how rapidly progressive this area is, with increasing numbers of studies measuring muscle quantity and function as outcome measures.
Conclusion
Deteriorations in muscle quantity, strength and physical performance are problematic in older adults following hospitalisation. However, insufficient evidence exists to enable targeted prevention/treatment strategies. A number of interventions have been trialled and shown to be safe for heterogeneous populations across various settings. Multidimensional physical activity interventions which are individually tailored (e.g. for frailty) have been trialled [43, 45, 55]; the trial with most substantial effect size reported excellent adherence [43]. Large scale multi-arm studies assessing effectiveness of combined interventions including physical activity [23, 43, 45, 47, 55], NMES [34], nutrition [59] and pharmaceuticals [31, 32] are warranted. Treatment may be most effective when stratified according to individual need. Treatment is likely to be guided by a combination of clinical and biological factors (e.g. immune-endocrine markers). Further research aimed at understanding pathophysiology of acute sarcopenia will enable risk stratification and targeted interventions.
Supplementary Material
Acknowledgements
The authors acknowledge Mrs Susan Bayliss, information specialist in the Institute of Applied Health Research at the University of Birmingham for kindly reviewing and advising on our search strategy.
Contributor Information
Carly Welch, Institute of Inflammation and Ageing, University of Birmingham, Birmingham B15 2TT, UK; Medical Research Council and Versus Arthritis Centre for Musculoskeletal Ageing Research, University of Birmingham and University of Nottingham, Nottingham, UK; University Hospitals Birmingham NHS Trust, Birmingham, UK.
Zeinab Majid, Institute of Inflammation and Ageing, University of Birmingham, Birmingham B15 2TT, UK; University Hospitals Birmingham NHS Trust, Birmingham, UK.
Carolyn Greig, Medical Research Council and Versus Arthritis Centre for Musculoskeletal Ageing Research, University of Birmingham and University of Nottingham, Nottingham, UK; University Hospitals Birmingham NHS Trust, Birmingham, UK; School of Sport, Exercise, and Rehabilitation Sciences, University of Birmingham, Birmingham B15 2TT, UK.
John Gladman, Medical Research Council and Versus Arthritis Centre for Musculoskeletal Ageing Research, University of Birmingham and University of Nottingham, Nottingham, UK; National Institute for Health Research Birmingham Biomedical Research Centre, University Hospitals Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK.
Tahir Masud, Medical Research Council and Versus Arthritis Centre for Musculoskeletal Ageing Research, University of Birmingham and University of Nottingham, Nottingham, UK; National Institute for Health Research Nottingham Biomedical Research Centre: Musculoskeletal Disease theme, Nottingham, UK; Healthcare of Older People, Queens Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK.
Thomas Jackson, Institute of Inflammation and Ageing, University of Birmingham, Birmingham B15 2TT, UK; Medical Research Council and Versus Arthritis Centre for Musculoskeletal Ageing Research, University of Birmingham and University of Nottingham, Nottingham, UK; University Hospitals Birmingham NHS Trust, Birmingham, UK.
Declaration of Sources of Funding
This has been supported by the Medical Research Council and Versus Arthritis Centre for Musculoskeletal Ageing Research in the form of a PhD studentship awarded to Dr Carly Welch. Dr Zeinab Majid is funded by a National Institute for Health Research (NIHR) Academic Clinical Fellowship and Dr Thomas Jackson is funded by the West Midlands NIHR Clinical Research Network. The sponsors did not contribute to the design, execution, analysis, and interpretation of data or writing of the study. The views are those of the authors and not necessarily those of the NIHR, National Health Service, or Department of Health.
Declaration of Conflicts of Interest
None declared.
References
- 1. Cruz-Jentoft AJ, Bahat G, Bauer J et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. The Lancet 2019;393:2636–46. [DOI] [PubMed] [Google Scholar]
- 3. Welch C, Hassan-Smith Z, Greig C, Lord J, Jackson T. Acute sarcopenia secondary to hospitalisation - an emerging condition affecting older adults. Aging Dis 2018;9:151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for treating sarcopenia: a systematic review and meta-analysis of randomized controlled studies. J Am Med Dir Assoc. 2017;18:553.e1-.e16. [DOI] [PubMed] [Google Scholar]
- 5. Beaudart C, Dawson A, Shaw SC, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int 2017;28:1817–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beckwee D, Delaere A, Aelbrecht S, et al. Exercise interventions for the prevention and treatment of sarcopenia. A systematic umbrella review. J Nutr Health Aging 2019;23:494–502. [DOI] [PubMed] [Google Scholar]
- 8. Denison HJ, Cooper C, Avan AS, Robinson SM. Prevention and optimal management of sarcopenia: a review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin Interv Aging 2015; 10: 859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marzetti E. The SPRINTT Project: Tackling Physical Frailty and Sarcopenia to Prevent Disability in the Elderly. Free Radical Biology and Medicine Conference: 19th Biennial Meeting for the Society for Free Radical Research International, SFRRI 2018 Portugal; 2018;120:S16. [Google Scholar]
- 10. Morton RW, Traylor DA, Weijs PJM, Phillips SM. Defining anabolic resistance: implications for delivery of clinical care nutrition. Curr Opin Crit Care 2018;24:124–30. [DOI] [PubMed] [Google Scholar]
- 11. The Cochrane Collaboration . Review Manager (RevMan). 5.3 edition. Copenhagen: The Nordic Cochrane Centre The Cochrane Collaboration, 2014. [Google Scholar]
- 12. Siemieniuk RG, Gordon. What is GRADE? : BMJ Best Practice. Available at: https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/. [Google Scholar]
- 13. The Cochrane Collaboration . Imputing standard deviations for changes from baseline. In: Sg JPTH, ed. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0; ed2011.
- 14. The Cochrane Collaboration . The standardized mean difference. In: Sg JPTH, ed. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0; ed2011.
- 15. Cohen J. A power primer. Psychol Bull 1992;112:155–9. [DOI] [PubMed] [Google Scholar]
- 16. Wilson DV, Moorey H, Stringer H et al. Bilateral anterior thigh thickness: a new diagnostic tool for the identification of low muscle mass? J Am Med Dir Assoc 2019. [DOI] [PubMed] [Google Scholar]
- 17. Van Ancum JM, Scheerman K, Jonkman NH et al. Change in muscle strength and muscle mass in older hospitalized patients: a systematic review and meta-analysis. Exp Gerontol 2017; 92: 34–41. [DOI] [PubMed] [Google Scholar]
- 18. Fuller LM, Button B, Tarrant B, et al. Patients' expectations and experiences of rehabilitation following lung transplantation. Clin Transplant 2014;28:252–8. [DOI] [PubMed] [Google Scholar]
- 19. Martone AM, Marzetti E, Calvani R et al. Exercise and protein intake: a synergistic approach against sarcopenia. Biomed Res Int 2017; 2017: 2672435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hardee JP, Lynch GS. Current pharmacotherapies for sarcopenia. Expert Opin Pharmacother 2019;20:1645–57. [DOI] [PubMed] [Google Scholar]
- 21. Maffiuletti NA, Green DA, Vaz MA, Dirks ML. Neuromuscular electrical stimulation as a potential countermeasure for skeletal muscle atrophy and weakness during human spaceflight. Front Physiol 2019; 10: 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dirks ML, Wall BT, Snijders T, Ottenbros CLP, Verdijk LB, van Loon LJC. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiologica 2014;210:628–41. [DOI] [PubMed] [Google Scholar]
- 23. Silva AM, Shen W, Heo M, et al. Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol 2010;22:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melvin MN, Smith-Ryan AE, Wingfield HL, Fultz SN, Roelofs EJ. Evaluation of muscle quality reliability and racial differences in body composition of overweight individuals. Ultrasound Med Biol 2014;40:1973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


