Abstract
Background
Parity is associated with decreased risk of invasive ovarian cancer; however, the relationship between incomplete pregnancies and invasive ovarian cancer risk is unclear. This relationship was examined using 15 case-control studies from the Ovarian Cancer Association Consortium (OCAC). Histotype-specific associations, which have not been examined previously with large sample sizes, were also evaluated.
Methods
A pooled analysis of 10 470 invasive epithelial ovarian cancer cases and 16 942 controls was conducted. Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between incomplete pregnancies and invasive epithelial ovarian cancer were estimated using logistic regression. All models were conditioned on OCAC study, race and ethnicity, age, and education level and adjusted for number of complete pregnancies, oral contraceptive use, and history of breastfeeding. The same approach was used for histotype-specific analyses.
Results
Ever having an incomplete pregnancy was associated with a 16% reduction in ovarian cancer risk (OR = 0.84, 95% CI = 0.79 to 0.89). There was a trend of decreasing risk with increasing number of incomplete pregnancies (2-sided Ptrend < .001). An inverse association was observed for all major histotypes; it was strongest for clear cell ovarian cancer.
Conclusions
Incomplete pregnancies are associated with a reduced risk of invasive epithelial ovarian cancer. Pregnancy, including incomplete pregnancy, was associated with a greater reduction in risk of clear cell ovarian cancer, but the result was broadly consistent across histotypes. Future work should focus on understanding the mechanisms underlying this reduced risk.
Parity is associated with a decreased risk of ovarian carcinoma (cancer) in a dose-dependent manner (1-3). Compared to nulliparous women, women with 1 birth have an approximate 24% (95% confidence interval [CI] = 12% to 34%) decrease in risk, and women with 2 or more births have an approximate 42% (95% CI = 35% to 49%) risk reduction (1).
However, the association between incomplete pregnancies (induced and spontaneous abortions) and ovarian cancer risk is unclear. Some studies (4-7) and 1 pooled analysis of 6 population-based case-control studies (8) have reported a decreased risk. However, a number of studies have reported a null association (9-15), and 1 reported an increased risk (16), but there was no adjustment for any potential confounders in this study. Whether the association might differ by histotype has not been adequately studied because of limited numbers. Because different histotypes likely represent distinct diseases with different risk factors (17), understanding the association by histotype may provide insight into their etiologies.
Given the equivocal literature, we evaluated the relationship between incomplete pregnancies and ovarian cancer risk using data from 15 case-control studies with data on incomplete pregnancies from the Ovarian Cancer Association Consortium (OCAC); some data from 2 of these studies have been published previously (10,12). We have included 10 470 women with ovarian cancer and 16 942 controls. This is the largest analysis of this relationship, allowing us to consider histotype-specific associations with sufficient sample sizes.
Methods
Study Populations
We included data from 15 case-control studies (14 population based and 1 clinic based) in the OCAC. These studies were conducted in the United States (n = 10) (3,18-26), the United Kingdom (n = 1) (27), Europe (n = 3) (28-30), and Australia (n = 1) (31). Each study received institutional review board approval, and written informed consent was provided by all women included in this analysis. Eligible cases had a pathologically confirmed invasive epithelial ovarian cancer.
Statistical Analysis
A complete pregnancy was defined as any pregnancy that lasted 6 months or longer. This variable was created by summing the number of live births lasting 6 or more months and the number of still births (defined as pregnancies lasting 6 or more months, including late-term pregnancy terminations). An incomplete pregnancy was defined as the number of reported pregnancies minus the number of complete pregnancies. It is possible that a small number of pregnancies lasted less than 6 months, but resulted in live births; these pregnancies were included in the incomplete pregnancy category based on their duration.
We categorized women as ever or never having an incomplete pregnancy as well as according to the number of incomplete pregnancies (0, 1, ≥2). The number of complete pregnancies (0, 1, 2, and ≥3), duration of oral contraceptive use (never, <1, 1 to <5, 5 to <10, ≥10 years), duration of breastfeeding (never, <12 months, 12 to <24 months, ≥24 months), race and ethnicity (non-Hispanic White, Hispanic White, Black, Asian, and other), age (in 5-year categories from <30 to ≥80 years), and education level (less than high school, high school, some college, college graduate) was considered important a priori confounders and were included in every model.
Additional potential confounders were added one at a time to the model previously described, and the impact on the incomplete pregnancy–ovarian cancer association was evaluated. These variables included a personal history of endometriosis (yes or no), body mass index (<18.5, 18.5 to <25, 25 to <30, ≥30 kg/m2), age at menarche (continuous), a first-degree family history of ovarian cancer (yes or no), tubal ligation (yes or no), and a previous diagnosis of a cancer other than nonmelanoma skin cancer (yes or no). None of these variables materially affected the incomplete pregnancy–ovarian cancer relationship and were not included in the final model.
To evaluate the association between incomplete pregnancies and risk of ovarian cancer, we first conducted logistic regression and calculated odds ratios (ORs) and 95% confidence intervals (CIs) for each OCAC study site; all models were conditioned on race and ethnicity, age, and education level and adjusted for number of complete pregnancies, oral contraceptive use, and breastfeeding (all fitted as previously described). Because we did not observe heterogeneity in the study-specific effect estimates, we pooled the individual-level data across the 15 OCAC studies and used logistic regression as previously described with the addition of conditioning on OCAC study site. This pooled approach was also used for histotype-specific analyses. Tests for trend were carried out using a grouped ordinal variable both with and without the reference group included. Multinomial logistic regression was used to evaluate whether the results across histotypes were different from each other.
Individuals with missing data (8% of controls and 6% of cases) for any of the variables included in the model were dropped from the analysis. All P values quoted are 2-sided and considered statistically significant if P is less than .05.
In addition to the usual standard joint analysis of complete (0, 1, 2, ≥3) and incomplete (0, 1, ≥2) pregnancies assuming a multiplicative relationship of their odds ratios, we also evaluated whether there was a statistical or qualitative departure from multiplicativity. The statistical assessment was carried out by fitting an interaction term between complete and incomplete pregnancies. The qualitative assessment was carried out by modeling complete and incomplete pregnancies as a single variable having 12 levels with nulligravid women as the reference group. To qualitatively assess whether there was evidence of a departure from multiplicativity, we calculated the difference between what was observed from the standard joint analysis to the model with a single variable.
Meta-Analysis
We identified 13 published reports encompassing 18 independent datasets (4-16). Of the reports, 3 excluded nulligravid women (13-15), therefore, their results for incomplete pregnancies are in part a comparison of the effect of incomplete pregnancies to ever having a complete pregnancy and cannot be compared to ours or to those of the other published papers. One study did not adjust for any confounders (16). Two of the studies from the published literature (10,12) are subsets of data included in the present OCAC analysis (AUS and NEC); the remaining 7 reports (4-9,11) were included in a meta-analysis with the individual OCAC study results. Meta-analysis was carried out following the methods described by Higgins and Thompson (32). Fixed effects results are presented because they were very close to the random effects results.
Results
The 15 studies included 10 470 ovarian cancer cases and 16 942 controls. Among the cases, 32.3% reported ever having had an incomplete pregnancy compared with 38.0% of the controls. Table 1 shows the number of ovarian cancer cases and controls by OCAC study site and the percentages of participants with an incomplete pregnancy. The number of control women and those with ovarian cancer by number of incomplete pregnancies (overall and by histotype) are shown in Supplementary Table 1 (available online).
Table 1.
Number of ovarian cancer cases and controls with the percent of women ever having an incomplete pregnancy by OCAC study site
| OCAC site | Geographic location | Diagnosis years | Controls (n = 16 942)a | Cases (n = 10 470)a,b | High-grade serous (n = 5029)a | Low-grade serous (n = 438)a | Mucinous (n = 714)a | Endometrioid (n = 1630)a | Clear cell (n = 810)a |
|---|---|---|---|---|---|---|---|---|---|
| AUS | Australia | 2001-2006 | 1445 (35.4) | 1104 (30.0) | 596 (32.9) | 46 (39.1) | 44 (28.3) | 131 (27.5) | 86 (17.4) |
| CON | Connecticut, USA | 1999-2003 | 421 (38.2) | 300 (29.0) | 150 (31.3) | 6 (16.7) | 17 (23.5) | 58 (32.8) | 26 (26.9) |
| DOV | Washington, USA | 2002-2009 | 1845 (41.5) | 1140 (35.9) | 559 (40.8) | 17 (70.6) | 33 (48.5) | 184 (25.0) | 87 (29.9) |
| GER | Germany | 1993-1998 | 527 (23.3) | 225 (20.9) | 83 (25.3) | 15 (13.3) | 25 (28.0) | 26 (15.4) | 6 (16.7) |
| HAW | Hawaii, USA | 1993-2008 | 1103 (39.9) | 709 (30.9) | 279 (35.1) | 11 (54.6) | 71 (39.4) | 117 (21.4) | 82 (20.7) |
| HOP | Western Pennsylvania, Northeast Ohio, Western New York, USA | 2003-2009 | 1802 (33.3) | 622 (31.0) | 331 (31.1) | 22 (31.8) | 37 (24.3) | 94 (30.9) | 45 (22.2) |
| MAL | Denmark | 1994-1999 | 1552 (41.8) | 543 (32.8) | 225 (35.1) | 90 (32.2) | 50 (34.0) | 75 (32.0) | 42 (11.9) |
| NCO | North Carolina, USA | 1999-2008 | 1050 (38.0) | 840 (32.6) | 399 (31.3) | 47 (31.9) | 45 (40.0) | 135 (34.8) | 87 (21.8) |
| NEC | New Hampshire, Eastern Massachusetts, USA | 1992-2008 | 2079 (37.6) | 1419 (30.8) | 783 (35.9) | 45 (33.3) | 91 (35.2) | 315 (31.1) | 68 (20.6) |
| NJO | New Jersey, USA | 2002-2009 | 442 (36.0) | 224 (33.5) | 103 (36.9) | 9 (33.3) | 12 (41.7) | 31 (38.7) | 30 (20.0) |
| POL | Poland | 2000-2004 | 516 (46.1) | 209 (42.8) | 59 (37.3) | 2 (100.0) | 15 (40.0) | 29 (41.4) | 7 (42.9) |
| STA | Northern California, USA | 1997-2002 | 567 (43.4) | 495 (35.4) | 224 (40.6) | 25 (36.0) | 42 (38.1) | 61 (26.2) | 49 (24.5) |
| UCI | California, USA | 1995-2005 | 298 (36.2) | 384 (34.6) | 177 (39.0) | 16 (31.3) | 28 (32.1) | 70 (40.0) | 36 (27.8) |
| UKO | United Kingdom | 2006-2009 | 786 (21.8) | 439 (16.2) | 191 (18.3) | 13 (0.0) | 43 (18.6) | 74 (13.5) | 43 (18.6) |
| USC | Los Angeles, California, USA | 1993-2008 | 2509 (43.2) | 1817 (35.3) | 870 (37.4) | 74 (27.0) | 161 (39.8) | 230 (30.9) | 116 (26.7) |
OCAC = Ovarian Cancer Association Consortium. Site abbreviations. AUS = Australian Ovarian Cancer Study; CON = Connecticut Ovary Study; DOV = Diseases of the Ovary and their Evaluation Study; GER = German Ovarian Cancer Study; HAW = Hawaii Ovarian Cancer Study; HOP = Hormones and Ovarian Cancer Prediction; MAL = Malignant Ovarian Cancer Study; NCO = North Carolina Ovarian Cancer Study; NEC = New England Case-Control Study; NJO = New Jersey Ovarian Cancer Study; POL = Polish Ovarian Cancer Case-Control Study; STA = Genetic Epidemiology of Ovarian Cancer Study; UCI = University of California, Irvine Ovarian Cancer Study; UKO = United Kingdom Ovarian Cancer Population Study; USC = University of Southern California, Study of Lifestyle and Women's Health.
Total number (percentage reporting an incomplete pregnancy).
The sum of high-grade serous, low-grade serous, mucinous, endometrioid, and clear cell is lower than the total number of cases because some cases were not classified as 1 of those 5 histotypes.
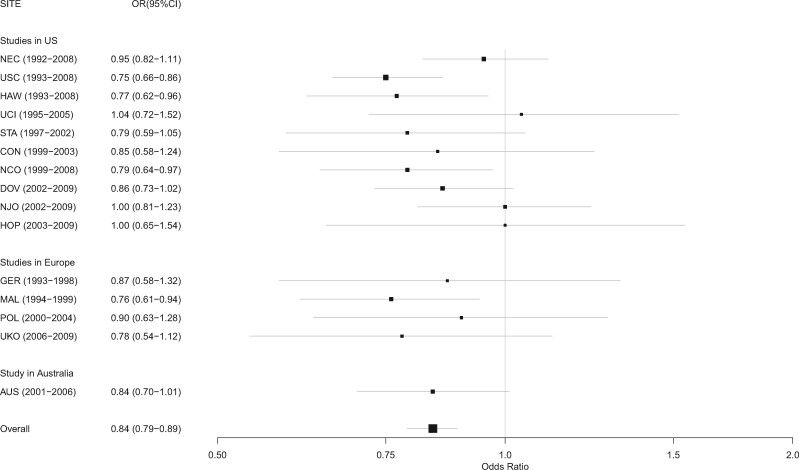
There was a statistically significant inverse association between ever having had an incomplete pregnancy and ovarian cancer overall (OR = 0.84, 95% CI = 0.79 to 0.89; P < .001; Pheterogeneity across studies = .59; Figure 1). This inverse association was observed in 11 of the 15 studies with results from 4 of the 11 studies reaching statistical significance; the results for the remaining 4 of the 15 studies were null (with ORs ranging from 0.95 to 1.04). The results from all studies were compatible with the overall odds ratio of 0.84 (Figure 1).
Figure 1.
Association between incomplete pregnancy and ovarian cancer by study site. CI = confidence interval; OR = odds ratio. Site abbreviations are as follows: AUS = Australian Ovarian Cancer Study; CON = Connecticut Ovary Study; DOV = Diseases of the Ovary and their Evaluation Study; GER = German Ovarian Cancer Study; HAW = Hawaii Ovarian Cancer Study; HOP = Hormones and Ovarian Cancer Prediction; MAL = Malignant Ovarian Cancer Study; NCO = North Carolina Ovarian Cancer Study; NEC = New England Case-Control Study; NJO = New Jersey Ovarian Cancer Study; POL = Polish Ovarian Cancer Case-Control Study; STA = Genetic Epidemiology of Ovarian Cancer Study; UCI = University of California, Irvine Ovarian Cancer Study; UKO = United Kingdom Ovarian Cancer Population Study; USC = University of Southern California, Study of Lifestyle and Women's Health.
Women who reported 1 incomplete pregnancy had a 14% decreased risk (OR = 0.86, 95% CI = 0.81 to 0.92), and women who reported 2 or more incomplete pregnancies had a 20% decreased risk (OR = 0.80, 95% CI = 0.74 to 0.87; Table 2). Having an incomplete pregnancy was also associated with decreased risk of ovarian cancer among women who had never had a complete pregnancy (Table 3, Observed Model column). Among women who had no complete pregnancies, having 1 incomplete pregnancy was associated with a 16% decreased risk of ovarian cancer (OR = 0.84, 95% CI = 0.72 to 0.99; Table 3, Observed Model column). Similarly, for women who had no complete pregnancies and 2 incomplete pregnancies, a 31% decreased risk was observed (OR = 0.69, 95% CI = 0.57 to 0.83).
Table 2.
Association between ovarian cancer risk and incomplete and complete pregnancies by histotype
| No. of incomplete and complete pregnancies | All cases |
High-grade serous |
Low-grade serous |
Mucinous |
Endometrioid |
Clear cell |
|---|---|---|---|---|---|---|
| ORa (95% CI) | ORa (95% CI) | ORa (95% CI) | ORa (95% CI) | ORa (95% CI) | ORa (95% CI) | |
| Incomplete pregnancies | ||||||
| 0 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 1 | 0.86 (0.81 to 0.92) | 0.94 (0.87 to 1.03) | 0.90 (0.70 to 1.15) | 0.94 (0.77 to 1.15) | 0.77 (0.66 to 0.89) | 0.68 (0.55 to 0.84) |
| ≥2 | 0.80 (0.74 to 0.87) | 0.93 (0.84 to 1.03) | 0.68 (0.49 to 0.95) | 0.77 (0.59 to 1.00) | 0.71 (0.59 to 0.84) | 0.39 (0.28 to 0.53) |
| P trend with ref.b | <.001 | .09 | .02 | .06 | <.001 | <.001 |
| P trend without ref.b | .15 | .55 | .32 | .27 | .91 | .012 |
| Complete pregnancies | ||||||
| 0 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 1 | 0.75 (0.68 to 0.83) | 0.93 (0.81 to 1.06) | 0.69 (0.48 to 1.00) | 0.68 (0.50 to 0.92) | 0.59 (0.49 to 0.71) | 0.49 (0.38 to 0.64) |
| 2 | 0.59 (0.54 to 0.65) | 0.81 (0.72 to 0.91) | 0.49 (0.35 to 0.70) | 0.54 (0.41 to 0.71) | 0.38 (0.32 to 0.46) | 0.27 (0.21 to 0.35) |
| ≥3 | 0.51 (0.47 to 0.57) | 0.70 (0.62 to 0.79) | 0.45 (0.31 to 0.66) | 0.50 (0.37 to 0.67) | 0.28 (0.23 to 0.34) | 0.19 (0.15 to 0.26) |
| P trend with ref.b | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| P trend without ref.b | <.001 | <.001 | .015 | .040 | <.001 | <.001 |
Model included both incomplete and complete pregnancies, conditioned on study site, age, race and ethnicity, and education level and adjusted for oral contraceptive use and breastfeeding. CI = confidence interval; OR=odds ratio.
P trend with ref. includes the referent group; Ptrend without ref. excludes the referent group and represents the trend among exposed women.
Table 3.
Evaluation of the multiplicative relationship between ovarian cancer and incomplete and complete pregnancies
| No. of complete pregnancies | No. of incomplete pregnancies | Expected modela | Observed modelb | Difference |
|---|---|---|---|---|
| ORjoint(expected) | ORjoint(observed) (95% CI) | ORjoint(expected) - ORjoint(observed) | ||
| 0 | 0 | 1.00 | 1.0 | |
| 1 | 0.86 | 0.84 (0.72 to 0.99) | 0.02 | |
| ≥2 | 0.80 | 0.69 (0.57 to 0.83) | 0.11 | |
| 1 | 0 | 0.75 | 0.76 (0.67 to 0.85) | −0.01 |
| 1 | 0.65 | 0.63 (0.54 to 0.75) | 0.02 | |
| ≥2 | 0.60 | 0.55 (0.46 to 0.67) | 0.05 | |
| 2 | 0 | 0.59 | 0.59 (0.53 to 0.65) | 0.00 |
| 1 | 0.51 | 0.47 (0.41 to 0.53) | 0.04 | |
| ≥2 | 0.47 | 0.51 (0.44 to 0.60) | −0.04 | |
| ≥3 | 0 | 0.51 | 0.49 (0.44 to 0.54) | 0.02 |
| 1 | 0.44 | 0.47 (0.41 to 0.57) | −0.03 | |
| ≥2 | 0.41 | 0.42 (0.36 to 0.49) | −0.01 |
Expected model calculated as ORcomplete* ORincomplete, using OR estimates from Table 2 and assuming multiplicativity, eg, expected OR for 2 complete and 1 incomplete = 0.59 * 0.86 = 0.51. CI = confidence interval; OR = odds ratio.
Observed model included a single variable with all combinations of incomplete and complete pregnancy categories (total 12 categories).
When we more finely categorized incomplete pregnancies (0, 1, 2, 3, ≥4), there was a 34% decreased risk in the 4 or more group (OR = 0.66, 95% CI = 0.54 to 0.80; Ptrend = .049 among those with at least 1 incomplete pregnancy). The inverse association with incomplete pregnancy was seen for each histotype; the 2 or more incomplete pregnancies odds ratios show that the magnitude of the association was weakest for high-grade serous (OR = 0.93, 95% CI = 0.84 to 1.03) and strongest for clear cell (OR = 0.39, 95% CI = 0.28 to 0.53; Table 2). The association between having 2 or more incomplete pregnancies and clear cell ovarian cancer was statistically significantly different from that observed with high-grade serous, low-grade serous, mucinous, and endometrioid cancers (P < .05 for all comparisons).
The magnitude of the decreased risk for an incomplete pregnancy was weaker than that for a complete pregnancy. The reduction in risk for a single incomplete pregnancy compared with a single complete pregnancy was 14% compared with 25%. We modeled all joint categories of having 0, 1, 2, or 3 or more complete pregnancies and 0, 1, or 2 or more incomplete pregnancies, taking nulligravid women as the reference group. This analysis showed that the assumption of a multiplicative relationship provided a close estimate of the joint estimate of complete and incomplete pregnancies (Table 3). Similarly, no departure from multiplicativity was observed in a model with an interaction term between complete and incomplete pregnancies (P > .05)
Of the 10 published reports that are comparable to our OCAC analysis, 5 reported a decreased risk (4-8) including the pooled analysis (8), 4 reported no association (9-12), and 1 reported an increased risk (16), however there was no adjustment for potential confounders in this study. Of the null studies, 2 are subsets of data included in the present OCAC analysis (10,12). One of these studies (AUS) shows an inverse association in the present analysis, whereas the other (NEC) continues to be null (Figure 1). Meta-analysis of the existing comparable published studies (excluding the AUS and NEC published studies as well as the study that did not adjust for any confounders) with the results from the individual OCAC studies yielded a pooled odds ratio of 0.87 for ever having an incomplete pregnancy (95% CI = 0.81 to 0.92; P < .001; Pheterogeneity = .13).
Discussion
We have carried out a comprehensive analysis of the relationship between incomplete pregnancies and risk of invasive ovarian cancer in a large number of women with ovarian cancer and controls from 15 OCAC studies. We found a statistically significant reduced risk of ovarian cancer among women who have had an incomplete pregnancy. Of the 15 studies, 11 showed an inverse association, and the results from all studies were compatible with each other. The published literature is also consistent with our findings. This inverse association was present for each histotype but most apparent for clear cell cancer.
The inverse association for an incomplete pregnancy was weaker than that of a complete pregnancy. The biologic mechanism(s) underlying the association between complete and incomplete pregnancies and ovarian cancer is not clear. The original mechanism proposed for the association with parity was thought to be through the cessation of recurrent breakdown and repair of the ovarian surface epithelium as a result of ovulation suppression during pregnancy (33). However, given that the ovarian surface epithelium is no longer believed to be the site of origin for most high-grade serous ovarian cancers (the most common histotype), this can only provide a small part of the explanation. It has also been suggested that stopping ovulation reduces the exposure of fallopian tube fimbria, endosalpingiosis, and endometriosis—the presumed cells of origin of most ovarian cancers—to inflammatory follicular fluid from within the ovary (34). In our analysis, the odds ratio for an incomplete pregnancy (OR = 0.86) is approximately what would be expected based on an odds ratio for a complete pregnancy (OR = 0.75) given the difference in duration. Thus, our results suggest that the effects of an incomplete pregnancy are no less than would be expected based on the duration of the pregnancy. More research is needed to elucidate the mechanism through which pregnancy is protective for ovarian cancer.
Reporting bias is a potential concern in case-control studies. However, one might expect controls to be less likely to report incomplete pregnancies than cases, which has been observed in previous breast cancer case-control studies (35), and such a scenario would produce a positive association between incomplete pregnancy and ovarian cancer risk rather than the inverse association we observed. In addition, there is the possibility that women may be more likely to report induced abortions as spontaneous because stigma, but because our data focused on any type of incomplete pregnancy, the effect of this type of misreporting is likely to be mitigated. There are 2 cohort studies that have examined the incomplete pregnancy–ovarian cancer relationship; these studies would be free of differential reporting bias. One did observe a positive association with 4 or more incomplete pregnancies (9), but this was not observed in the other cohort study that found a protective effect overall and particularly for 3 or more incomplete pregnancies (5), which is in line with our results. Also, 2 previously published studies carried out in China where there may be less stigma surrounding the reporting of incomplete pregnancies given its past one-child policy found an inverse association (4,7). Lastly, because the incomplete pregnancy variable is calculated from the total number of pregnancies and the number of pregnancies lasting 6 months or longer, it is possible that a few pregnancies lasting less than 6 months resulted in a live birth. In our analysis, such births were included in the incomplete pregnancy group; this is unlikely to affect our results because such births are very uncommon and given their duration, they may be more likely to mirror the association with incomplete pregnancies.
Considering the evidence from all of the studies on incomplete pregnancies, having an incomplete pregnancy appears to be associated with decreased risk of ovarian cancer. Interestingly, this inverse association with incomplete pregnancies has also been observed in endometrial cancer (36), which shares similar risk factors with ovarian cancer. Future research should focus on understanding the mechanisms underlying the reduced risk associated with complete and incomplete pregnancies to shed light on ovarian cancer etiology.
Funding
OCAC funding: This work was supported by a grant from the Ovarian Cancer Research Fund thanks to donations by the family and friends of Kathryn Sladek Smith (PPD/RPCI.07). The scientific development and funding for this project were in part supported by the US National Cancer Institute at the National Institutes of Health GAME-ON Post-GWAS Initiative (grant number U19-CA148112). AUS: This work was supported by the US Army Medical Research and Materiel Command (grant number DAMD17-01–1-0729), Cancer Councils of New South Wales, Victoria, Queensland, South Australia, and Tasmania, Cancer Foundation of Western Australia (Multi-State Applications 191, 211 and 182), and the National Health and Medical Research Council of Australia (grant numbers ID199600, ID400413, ID400281). CON: This work was supported by the National Institutes of Health (grant numbers R01-CA063678, R01-CA074850; R01-CA080742). DOV: This work was supported by National Institutes of Health (grant numbers R01-CA112523 and R01-CA87538). HRH is supported by the National Institutes of Health (grant number K22 CA193860). GER: This work was supported by the German Federal Ministry of Education and Research, Programme of Clinical Biomedical Research (grant number 01 GB 9401) and the German Cancer Research Center (DKFZ). HAW: This work was supported by the US National Institutes of Health (grant numbers R01-CA58598, N01-CN-55424, N01-PC-67001). HOP: This work was supported by the Department of Defense (grant number DAMD17-02–1-0669) and the National Cancer Institute at the National Institutes of Health (grant numbers K07-CA080668, R01-CA95023, P50-CA159981). MAL: This work was supported by the National Cancer Institute at the National Institutes for Health (grant number R01- CA61107), the Danish Cancer Society (grant number 94 222 52), and the Mermaid I and III projects. NCO: This work was supported by the National Institutes of Health (grant number R01-CA76016) and the Department of Defense (grant number DAMD17-02–1-0666). NEC: This work was supported by the National Institutes of Health (grant numbers R01-CA54419, P50-CA105009) and the Department of Defense (grant number W81XWH-10–1-02802). NJO: This work was supported by the National Cancer Institute at the National Institutes for Health (grant number NIH-K07 CA095666, R01-CA83918, NIH-K22-CA138563, P30-CA072720) and the Cancer Institute of New Jersey. POL: This work was supported by the Intramural Research Program of the National Cancer Institute. STA: This work was supported by the National Institutes of Health (grant numbers U01 CA71966, U01 CA69417). UCI: This work was supported by the National Institutes of Health (grant number R01-CA058860) and the Lon V Smith Foundation (grant number LVS-39420). UKO: This work was supported by The Eve Appeal (The Oak Foundation) and the National Institute for Health Research University College London Hospitals Biomedical Research Centre. USC: This work was supported by the National Institutes of Health (grant numbers P01CA17054, P30CA14089, R01CA61132, N01PC67010, R03CA113148, R03CA115195, N01CN025403) and California Cancer Research Program (grant numbers 00–01389 V-20170, 2II0200). MCP was supported in part through the NIH/NCI Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center. AWL was supported in part through a Scientific Scholar Award from the Rivkin Center for Ovarian Cancer.
Notes
Role of the funders: The study sponsors had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: The authors declare no conflicts of interest regarding the publication of this article.
Role of the authors: MCP, PMW, and CLP conceived and designed the study. UM, HA, ASW, WS, NW, EVB, KLT, DWC, JMS, AB, SKK, RBN, FM, KM, MTG, JC, MR, HRH, JAD, HAR, AHW, MCP, PMW, and CLP acquired the data. AWL, SR, AW, and CLP analyzed the data. All authors contributed to data interpretation as well as critical revision and final approval of the manuscript.
OCAC acknowledgments: We thank study participants, doctors, nurses, clinical and scientific collaborators, health-care providers, and health information sources who have contributed to the participating studies. The Australian Ovarian Cancer Study Group (www.aocstudy.org) acknowledges the cooperation of the participating institutions in Australia and the contributions of the study nurses, research assistants, and all clinical and scientific collaborators of the study. The Connecticut Ovary Study gratefully acknowledges the cooperation of the 32 Connecticut hospitals, including Stamford Hospital, in allowing patient access; this study was approved by the Connecticut State Department of Public Health Human Investigation Committee and certain data used in this study were obtained from the Connecticut Tumor Registry in the Connecticut Department of Public Health; the authors assume full responsibility for analyses and interpretation of these data.
Data Availability
The data underlying this article will be shared upon approval of a data request form by the Ovarian Cancer Association Consortium Data Access Coordinating Committee and with appropriate human subjects approval and data transfer agreements.
Supplementary Material
References
- 1. Pearce CL, Rossing MA, Lee AW, et al. ; for Australian Cancer Study (Ovarian Cancer). Combined and interactive effects of environmental and GWAS-identified risk factors in ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(5):880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsilidis KK, Allen NE, Key TJ, et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2011;105(9):1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu AH, Pearce CL, Lee AW, et al. Timing of births and oral contraceptive use influences ovarian cancer risk. Int J Cancer. 2017;141(12):2392–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, Wu PC, Lang JH, et al. Risk factors for epithelial ovarian cancer in Beijing, China. Int J Epidemiol. 1992;21(1):23–29. [DOI] [PubMed] [Google Scholar]
- 5. Kvale G, Heuch I, Nilssen S, et al. Reproductive factors and risk of ovarian cancer: a prospective study. Int J Cancer. 1988;42(2):246–251. [DOI] [PubMed] [Google Scholar]
- 6. Riman T, Dickman PW, Nilsson S, et al. Risk factors for invasive epithelial ovarian cancer: results from a Swedish case-control study. Am J Epidemiol. 2002;156(4):363–373. [DOI] [PubMed] [Google Scholar]
- 7. Shu XO, Brinton LA, Gao YT, et al. Population-based case-control study of ovarian cancer in Shanghai. Cancer Res. 1989;49(13):3670–3674. [PubMed] [Google Scholar]
- 8. Whittemore AS, Harris R, Itnyre J.. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136(10):1184–1203. [DOI] [PubMed] [Google Scholar]
- 9. Braem MG, Onland-Moret NC, Schouten LJ, et al. Multiple miscarriages are associated with the risk of ovarian cancer: results from the European Prospective Investigation into Cancer and Nutrition. PLoS One. 2012;7(5):e37141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dick ML, Siskind V, Purdie DM, et al. Incomplete pregnancy and risk of ovarian cancer: results from two Australian case-control studies and systematic review. Cancer Causes Control. 2009;20(9):1571–1585. [DOI] [PubMed] [Google Scholar]
- 11. Risch HA, Marrett LD, Howe GR.. Parity, contraception, infertility, and the risk of epithelial ovarian cancer. Am J Epidemiol. 1994;140(7):585–597. [DOI] [PubMed] [Google Scholar]
- 12. Titus-Ernstoff L, Perez K, Cramer DW, et al. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer. 2001;84(5):714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen MT, Cook LS, Daling JR, et al. Incomplete pregnancies and risk of ovarian cancer (Washington, United States). Cancer Causes Control. 1996;7(4):415–420. [DOI] [PubMed] [Google Scholar]
- 14. Gierach GL, Modugno F, Ness RB.. Relations of gestational length and timing and type of incomplete pregnancy to ovarian cancer risk. Am J Epidemiol. 2005;161(5):452–461. [DOI] [PubMed] [Google Scholar]
- 15. Rosenblatt KA, Gao DL, Ray RM, et al. Induced abortions and the risk of all cancers combined and site-specific cancers in Shanghai. Cancer Causes Control. 2006;17(10):1275–1280. [DOI] [PubMed] [Google Scholar]
- 16. Newhouse ML, Pearson RM, Fullerton JM, et al. A case control study of carcinoma of the ovary. Br J Prev Soc Med. 1977;31(3):148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karnezis AN, Cho KR, Gilks CB, et al. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer. 2017;17(1):65–74. [DOI] [PubMed] [Google Scholar]
- 18. Bandera EV, King M, Chandran U, et al. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health. 2011;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lurie G, Wilkens LR, Thompson PJ, et al. Combined oral contraceptive use and epithelial ovarian cancer risk: time-related effects. Epidemiology. 2008;19(2):237–243. [DOI] [PubMed] [Google Scholar]
- 20. McGuire V, Felberg A, Mills M, et al. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. Am J Epidemiol. 2004;160(7):613–618. [DOI] [PubMed] [Google Scholar]
- 21. Moorman PG, Calingaert B, Palmieri RT, et al. Hormonal risk factors for ovarian cancer in premenopausal and postmenopausal women. Am J Epidemiol. 2008;167(9):1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ness RB, Dodge RC, Edwards RP, et al. Contraception methods, beyond oral contraceptives and tubal ligation, and risk of ovarian cancer. Ann Epidemiol. 2011;21(3):188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Risch HA, Bale AE, Beck PA, et al. PGR +331 A/G and increased risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1738–1741. [DOI] [PubMed] [Google Scholar]
- 24. Rossing MA, Cushing-Haugen KL, Wicklund KG, et al. Risk of epithelial ovarian cancer in relation to benign ovarian conditions and ovarian surgery. Cancer Causes Control. 2008;19(10):1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terry KL, De Vivo I, Titus-Ernstoff L, et al. Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res. 2005;65(13):5974–5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ziogas A, Gildea M, Cohen P, et al. Cancer risk estimates for family members of a population-based family registry for breast and ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(1):103–111. [PubMed] [Google Scholar]
- 27. Balogun N, Gentry-Maharaj A, Wozniak EL, et al. Recruitment of newly diagnosed ovarian cancer patients proved challenging in a multicentre biobanking study. J Clin Epidemiol. 2011;64(5):525–530. [DOI] [PubMed] [Google Scholar]
- 28. Garcia-Closas M, Brinton LA, Lissowska J, et al. Ovarian cancer risk and common variation in the sex hormone-binding globulin gene: a population-based case-control study. BMC Cancer. 2007;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glud E, Kjaer SK, Thomsen BL, et al. Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med. 2004;164(20):2253–2259. [DOI] [PubMed] [Google Scholar]
- 30. Royar J, Becher H, Chang-Claude J.. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001;95(6):370–374. [DOI] [PubMed] [Google Scholar]
- 31. Merritt MA, Green AC, Nagle CM, et al. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122(1):170–176. [DOI] [PubMed] [Google Scholar]
- 32. Higgins JPT, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 33. Fathalla MF. Incessant ovulation—a factor in ovarian neoplasia? Lancet. 1971;2(7716):163. [DOI] [PubMed] [Google Scholar]
- 34. Emori MM, Drapkin R.. The hormonal composition of follicular fluid and its implications for ovarian cancer pathogenesis. Reprod Biol Endocrinol. 2014;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rookus MA, van Leeuwen FE.. Induced abortion and risk for breast cancer: reporting (recall) bias in a Dutch case-control study. J Natl Cancer Inst. 1996;88(23):1759–1764. [DOI] [PubMed] [Google Scholar]
- 36. Husby A, Wohlfahrt J, Melbye M.. Pregnancy duration and endometrial cancer risk: nationwide cohort study. BMJ. 2019;366:l4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon approval of a data request form by the Ovarian Cancer Association Consortium Data Access Coordinating Committee and with appropriate human subjects approval and data transfer agreements.