Abstract
Background
Over the past decades, many regions have experienced a steady increase in the incidence of cutaneous melanoma. Here, we report on incidence trends for subsequent primary melanoma.
Methods
In this nationwide population-based study, patients diagnosed with a first primary cutaneous melanoma reported to the Swedish Cancer Registry were followed for up to 10 years for a diagnosis of subsequent primary melanoma. Patients were grouped with patients diagnosed with first melanoma in the same decade (1960s, 1970s, 1980s, 1990s, and 2000s, respectively). Frequencies, incidence rates (IRs), standardized incidence ratios (SIRs), and 95% confidence intervals (CIs) for second melanomas were calculated. All tests of statistical significance were 2-sided.
Results
Of patients with melanoma, 54 884 were included and 2469 were diagnosed, within 10 years, with subsequent melanomas. Over the 5 decades, there was a statistically significant steady increase in the frequency, IR, and SIR for second primary melanoma. For example, in the 1960s cohort, less than 1% (IR = 1.0, 95% CI = 0.5 to 1.7, and IR = 1.1, 95% CI = 0.5 to 1.9 per 1000 person-years in women and men, respectively) had second primary melanoma, and this rose to 6.4% (IR = 7.5, 95% CI = 6.8 to 8.3, per 1000 person-years) in the women and 7.9% (IR = 10.3, 95% CI = 9.3 to 11.2, per 1000 person-years) in the men in the 2000s cohort. This rise was seen independent of age, sex, invasiveness, or site of the melanoma. Further, in patients diagnosed with a second melanoma, the frequency of those having more than 2 melanomas increased statistically significantly and was 0.0% in the 1960s and rose to 18.0% in the 2000s (P < .001).
Conclusions
This is the first study to evaluate and report on a rising trend for subsequent primary melanoma. Additional primary melanomas worsen the patients’ survival, and precautions are needed to turn this steep upgoing trend.
Over the past decades, there has been a well-documented steady increase in the incidence of cutaneous melanoma in many countries in Europe, Oceania, and North America (1–4). In recent years, an indication of an incidence stabilization, particularly in younger age groups, has been observed in some countries, including Australia, the United States, and Norway, although besides Norway, such a stabilization has not yet been seen in most European countries (1–3,5). Patients with melanoma are at increased risk of additional primaries; however, there have been large variations in the reported incidence with frequencies ranging from 1% to 13% (6–17). This variation likely stems from different study designs concerning included patient cohorts and lengths of follow-up. It is also possible that some of the variation stems from the patients having been diagnosed in different time epochs; however, there is limited knowledge on how the incidence of second primary melanomas has changed over time. In this study, we use the comprehensive Swedish Cancer Register to address the question of how the incidence of second primaries has evolved in the past decades. In Sweden, there is still a stable 5% annual increase in the melanoma incidence (18). Sweden currently has an annual age standardized (world) incidence of 24.7 per 100 000 inhabitants and has the sixth highest melanoma incidence of the world’s countries, preceded by Australia, New Zealand, Norway, the Netherlands, and Denmark (19).
Materials and Methods
Data
The Swedish Cancer Register was used to obtain information on registered cases of cutaneous melanoma. The register, maintained by the National Board of Health and Welfare, was founded in 1958 and covers the whole population of Sweden. The Swedish Cancer Register is considered to be highly accurate and complete. It is estimated to cover more than 96% of diagnosed cancers in the country, and 99% of tumors are morphologically verified (20). The database records cancers according to the International Classification of Diseases 7th revision (ICD-7) and later revisions and by WHO histological classification of neoplasms (WHO/HS/CANC/24.1) codes and later revisions. The register derives information on age, sex, census, and causes of death from the Swedish national personal identification number, the Swedish Population Register, and the Swedish Cause of Death Register. The current population size of the country is 10.2 million inhabitants, and the majority is Caucasian of Scandinavian descent. There has been a growing immigrant population with less than 1% of inhabitants in the year 1960 and 8% in the year 2010 born outside northern Europe; however, of the melanomas diagnosed from 1990-2007, only 1% occurred in individuals with origin outside northern Europe (21, 22).
Patient Selection
Patients with a diagnosis of cutaneous melanoma were identified through the ICD-7 codes (190 for melanoma of the skin) and WHO/HS/CANC/24.1 codes (176 and 173 or 174) for invasive and in situ melanoma, respectively) that are available for all patients in the registry. All data were anonymized, and the study was approved by the Swedish ethical review authority. The body site (head and neck area, trunk, upper extremity, or lower extremity) of the melanoma was deducted from the ICD-7 code. We followed newly diagnosed melanoma patients from January 1, 1960, until December 31, 2014, for diagnosis of second primary melanoma. Because the objective of the study was to investigate time trends in the diagnoses of multiple primary melanoma, the data were analyzed by decades, with each melanoma patient accounted for once and grouped with patients diagnosed with first melanoma in the same decade (1960s, 1970s, 1980s, 1990s, and 2000s, respectively). Each patient was followed for a maximum of 10 years or shorter if the patient died or emigrated within 10 years from the first primary melanoma diagnosis. To warrant up to 10 years of follow-up for all patients, the 2000s decade included patients diagnosed in 2000-2004. Because the 1950s decade encompassed only the years 1958-1959, these 2 years were not included for further analyses, and melanoma patients diagnosed in 1960 or later that were found to have their first melanoma in 1958-1959 were not included in the study (n = 6).
Statistical Analysis
Person-years at risk were calculated as the time from first primary melanoma until the diagnosis of a second primary melanoma, the date of death or emigration, and up to a maximum of 10 years of follow-up for each patient. Incidence rates (IRs) and 95% confidence intervals (CI) for second primaries were calculated per 1000 person-years among melanoma patients. The rate ratio (RR) and 95% confidence interval were calculated as the ratio between 2 incidence rates. To calculate P values, χ2 test (categorical variables) and Student t test (continuous variables) were used, and P values less than .05 were deemed statistically significant. When comparing incidence rates and rate ratios, nonoverlapping confidence intervals were also deemed as statistically significant. All statistical tests were 2-sided. Incidence rates for second primaries were adjusted for age using direct standardization by 5‐year age groups with the Swedish census population in the year 2000 as standard. Likewise, incidence rates for melanoma per 100 000 person-years in the Swedish population were adjusted for age using direct standardization by 5-year age groups with the Swedish census population in the year 2000 as standard. The standardized incidence ratio (SIR) was calculated as the ratio between the observed number of cases and expected number of cases in the Swedish population at each decade. A 95% confidence interval was calculated by assuming a Poisson distribution of the observed number of cases. Statistical tests were performed with StatSoft Statistica software, version 10.
Results
Patient Characteristics
From January 1, 1960, until December 31, 2004, 54 884 patients with cutaneous melanoma were identified from the Swedish Cancer Register; 44 729 had invasive melanoma, and 10 155 had in situ melanoma. Each person was followed up for a maximum of 10 years, adding up to a total of 428 793 person-years. Characteristics of the melanoma patients diagnosed with the first primary melanoma in the 1960s, 1970s, 1980s, 1990s, and 2000s decades, respectively, are outlined in Table 1. Over time, there has been a noticeable increase in the numbers of melanoma cases and also a statistically significant increase in the median age at diagnosis and the numbers and proportion of in situ melanomas (P < .001). In women, the lower extremities were the most common tumor localization, although there was, over time, a shift with a statistically significant decrease in the proportion of melanomas in this localization and an increase in the proportion of truncal and upper-extremity melanomas. In men, truncal melanomas were most frequent, and as for the women, the proportion of melanomas in the lower extremities receded and increased statistically significantly in the upper extremities.
Table 1.
Characteristics of melanoma patients diagnosed in Sweden over 5 decades, from the 1960s to 2000sa
| Decade |
P, 2000s decade vs |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | 1960s | 1970s | 1980s | 1990s | 2000sb | 1960s | 1970s | 1980s | 1990s |
| Characteristics, women | 1985 | 3887 | 6722 | 10 114 | 6236 | ||||
| Invasiveness of melanoma, No. (%) | |||||||||
| In situ | 64 (3.2) | 232 (6.0) | 1151 (17.1) | 2622 (25.9) | 1785 (28.6) | <.001 | <.001 | <.001 | <.001 |
| Invasive | 1921 (96.8) | 3655 (94.0) | 5571 (82.9) | 7492 (74.1) | 4451 (71.4) | ||||
| Age at diagnosis, median (range), y | |||||||||
| Invasive or in situ | 53 (1-97) | 56 (5-98) | 58 (9-100) | 59 (11-107) | 60 (2-104) | <.001 | <.001 | <.001 | <.001 |
| In situ | 50 (17-80) | 54 (14-91) | 57 (12-95) | 61 (12-101) | 63 (2-100) | <.001 | <.001 | <.001 | <.001 |
| Invasive | 53 (1-97) | 56 (5-98) | 58 (9-100) | 58 (11-107) | 59 (10-104) | <.001 | <.001 | <.001 | <.001 |
| Site of melanoma, No. (%) | |||||||||
| Head and neck | 357 (19.9) | 683 (19.4) | 1168 (19.3) | 1868 (19.4) | 1129 (18.8) | .32 | .50 | .52 | .38 |
| Trunk | 431 (24.0) | 829 (23.5) | 1505 (24.8) | 2611 (27.1) | 1681 (28.0) | .001 | <.001 | <.001 | .21 |
| Upper extremities | 269 (15.0) | 619 (17.6) | 1181 (19.5) | 1864 (19.3) | 1224 (20.4) | <.001 | .001 | .21 | .11 |
| Lower extremities | 740 (41.2) | 1393 (39.5) | 2206 (36.4) | 3294 (34.2) | 1967 (32.8) | <.001 | <.001 | <.001 | .07 |
| Unknown | 188 | 363 | 662 | 477 | 235 | ||||
| Characteristics, men | 1670 | 3218 | 6168 | 9210 | 5674 | ||||
| Invasiveness of melanoma, No. (%) | |||||||||
| In situ | 30 (1.8) | 158 (4.9) | 809 (13.1) | 1970 (21.4) | 1334 (23.5) | <.001 | <.001 | <.001 | .002 |
| Invasive | 1640 (98.2) | 3060 (95.1) | 5359 (86.9) | 7240 (78.6) | 4340 (76.5) | ||||
| Age at diagnosis, median (range), y | |||||||||
| Invasive or in situ | 54 (6-104) | 57 (1-97) | 60 (7-100) | 62 (10-100) | 64 (1-97) | <.001 | <.001 | <.001 | <.001 |
| In situ | 51 (16-81) | 53 (15-97) | 62 (14-94) | 65 (19-94) | 66 (6-95) | <.001 | <.001 | <.001 | <.001 |
| Invasive | 54 (6-104) | 57 (1-96) | 61 (7-100) | 63 (10-100) | 64 (1-97) | <.001 | <.001 | <.001 | <.001 |
| Site of melanoma, No. (%) | |||||||||
| Head and neck | 350 (23.2) | 552 (19.4) | 1026 (18.7) | 1705 (19.7) | 1106 (20.3) | .01 | .38 | .04 | .39 |
| Trunk | 747 (49.5) | 1511 (53.2) | 2958 (54.0) | 4541 (52.4) | 2813 (51.7) | .17 | .14 | .01 | .32 |
| Upper extremities | 143 (9.5) | 335 (11.8) | 788 (14.4) | 1397 (16.1) | 936 (17.2) | <.001 | <.001 | <.001 | .11 |
| Lower extremities | 270 (17.9) | 441 (15.5) | 710 (13.0) | 1028 (11.9) | 606 (11.1) | <.001 | <.001 | .003 | .17 |
| Unknown | 160 | 379 | 686 | 539 | 213 | ||||
Multiple primary melanoma patients are accounted for once, at their first melanoma diagnosis.
Includes the years 2000-2004.
Subsequent Primary Melanomas
Of the patients, 2469 (4.5%) were diagnosed, within 10 years, with a second primary invasive or in situ melanoma. Table 2 shows the time trends in frequency and incidence (per 1000 person-years) of second primaries stratified by sex, age, and invasiveness of tumors. For each of the strata, an increased incidence over time was deemed as statistically significant if there was a statistically significant difference between at least any 2 different decades. In the 1960s, second primaries (invasive or in situ) were diagnosed in less than 1% of melanoma patients, the frequency and incidence then increased for each decade and, in the 2000s, were diagnosed in 6.4% (IR = 7.5, 95% CI = 6.8 to 8.3) of the women and 7.9% (IR = 10.3, 95% CI = 9.3 to 11.2) of the men. There was a statistically significant incidence increase in both sexes and in both younger (55 years and younger) and older melanoma patients (invasive or in situ); however, a steeper increase was observed in male patients and in older patients. In patients with invasive melanomas, less than 1% were diagnosed with a second invasive melanoma in the 1960s, and the frequency has increased to 3.5% (IR = 4.2, 95% CI = 3.5 to 4.9) in the women and 5.0% (IR = 6.7, 95% CI = 5.8 to 7.6) in the men, and the increase was statistically significant in both sexes and in both younger and older patients. Among patients with invasive melanoma, there was also a statistically significant increase over time in the diagnosis of second in situ melanomas (Supplementary Table 1, available online). In patients with in situ melanoma, an increase over time was observed in the frequency and incidence of second in situ melanomas; however, the increase was only statistically significant in the women. In patients with in situ melanoma, there was not a statistically significant increase over time in second invasive tumors (Supplementary Table 1, available online).
Table 2.
Patients with second primary melanoma diagnosed within 10 years from first melanoma, diagnosed in the 1960s to the 2000s
| Decades |
P, 2000s decade vsc |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Second primary melanomas | 1960s | 1970s | 1980s | 1990s | 2000sa | 1960s | 1970s | 1980s | 1990s |
| Women with ≥2 primary melanomas (invasive or in situ) | |||||||||
| All ages | |||||||||
| Second primary melanoma, No. (%) | 14 (0.7) | 63 (1.6) | 226 (3.4) | 487 (4.8) | 401 (6.4) | ||||
| Incidence (95% CI)b | 1.0 (0.5 to 1.7) | 2.1 (1.6 to 2.7)d | 4.1 (3.6 to 4.7)d | 5.6 (5.1 to 6.2)d | 7.5 (6.8 to 8.3)d | <.001 | <.001 | <.001 | <.001 |
| Younger women with melanoma (≤55 years) | |||||||||
| Second primary melanoma, No. (%) | 10 (0.9) | 37 (2.0) | 93 (3.0) | 163 (3.6) | 130 (5.3) | ||||
| Incidence (95% CI) | 1.1 (0.5 to 2.0) | 2.2 (1.6 to 3.0)d | 3.2 (2.6 to 4.0)d | 3.8 (3.2 to 4.4) | 5.6 (4.6 to 6.6)d | <.001 | <.001 | <.001 | <.001 |
| Older women with melanoma (>55 years) | |||||||||
| Second primary melanoma, No. (%) | 4 (0.5) | 26 (1.3) | 133 (3.7) | 324 (5.8) | 271 (7.2) | ||||
| Incidence (95% CI) | 0.8 (0.2 to 2.0) | 1.9 (1.3 to 2.9) | 5.0 (4.2 to 6.0)d | 7.5 (6.7 to 8.4)d | 9.1 (8.0 to 10.2)d | <.001 | <.001 | <.001 | .02 |
| Women with ≥2 primary melanomas (invasive only) | |||||||||
| All ages | |||||||||
| Second primary invasive melanoma, No. (%) | 13 (0.7) | 46 (1.3) | 106 (1.9) | 202 (2.7) | 155 (3.5) | ||||
| Incidence (95% CI) | 1.0 (0.5 to 1.6) | 1.6 (1.2 to 2.2) | 2.4 (1.9 to 2.9)d | 3.2 (2.8 to 3.7)d | 4.2 (3.5 to 4.9)d | <.001 | <.001 | <.001 | .02 |
| Younger women with invasive melanoma (≤55 years) | |||||||||
| Second primary invasive melanoma, No. (%) | 10 (0.9) | 28 (1.6) | 42 (1.6) | 75 (2.2) | 52 (2.8) | ||||
| Incidence (95% CI) | 1.2 (0.6 to 2.1) | 1.8 (1.2 to 2.6) | 1.8 (1.3 to 2.4) | 2.3 (1.8 to 2.9) | 3.0 (2.2 to 3.9) | .006 | .03 | .01 | .15 |
| Older women with invasive melanoma (>55 years) | |||||||||
| Second primary invasive melanoma, No. (%) | 3 (0.4) | 18 (1.0) | 64 (2.1) | 127 (3.1) | 103 (4.0) | ||||
| Incidence (95% CI) | 0.6 (0.1 to 1.8) | 1.5 (0.9 to 2.3) | 3.0 (2.3 to 3.8)d | 4.2 (3.5 to 5.0)d | 5.2 (4.3 to 6.3) | <.001 | <.001 | <.001 | .12 |
| Women with ≥2 primary melanomas (in situ only) | |||||||||
| All ages | |||||||||
| Second primary in situ melanoma, No. (%) | 0 (0.0) | 2 (0.9) | 23 (2.0) | 94 (3.6) | 76 (4.3) | ||||
| Incidence (95% CI) | 0.0 (0.0 to 5.9) | 0.9 (0.1 to 3.3) | 2.2 (1.4 to 3.3) | 3.9 (3.2 to 4.5)d | 4.7 (3.9 to 5.9) | .21 | .02 | .001 | .23 |
| Younger women with in situ melanoma (≤55 years) | |||||||||
| Second primary in situ melanoma, No. (%) | 0 (0.0) | 1 (0.8) | 6 (1.1) | 16 (1.4) | 14 (2.3) | ||||
| Incidence (95% CI) | 0.0 (0.0 to 5.9) | 0.8 (0.0 to 4.6) | 1.1 (0.4 to 2.4) | 1.6 (0.9 to 2.4) | 2.3 (1.3 to 3.9) | .60 | .32 | .13 | .23 |
| Older women with in situ melanoma (>55 years) | |||||||||
| Second primary in situ melanoma, No. (%) | 0 (0.0) | 1 (0.9) | 17 (2.8) | 78 (5.0) | 62 (5.3) | ||||
| Incidence (95% CI) | 0.0 (0.0 to 20.3) | 1.0 (0.0 to 5.8) | 3.3 (1.9 to 5.3) | 5.9 (4.6 to 7.3)d | 6.2 (4.7 to 7.9) | .56 | .08 | .03 | .78 |
| Men with ≥2 primary melanomas (invasive or in situ) | |||||||||
| All ages | |||||||||
| Second primary melanoma, No. (%) | 10 (0.6) | 55 (1.7) | 242 (3.9) | 523 (5.7) | 448 (7.9) | ||||
| Incidence (95% CI) | 1.1 (0.5 to 1.9) | 2.7 (2.0 to 3.4)d | 5.5 (4.8 to 6.2)d | 7.7 (7.0 to 8.4)d | 10.3 (9.3 to 11.2)d | <.001 | <.001 | <.001 | <.001 |
| Younger men with melanoma (≤55 years) | |||||||||
| Second primary melanoma, No. (%) | 6 (0.7) | 24 (1.6) | 99 (4.1) | 148 (4.7) | 97 (5.7) | ||||
| Incidence (95% CI) | 1.0 (0.4 to 2.2) | 2.1 (1.3 to 3.1) | 4.9 (4.0 to 6.0)d | 5.1 (4.3 to 6.0) | 6.3 (5.1 to 7.6) | <.001 | <.001 | .09 | .13 |
| Older men with melanoma (>55 years) | |||||||||
| Second primary melanoma, No. (%) | 4 (0.5) | 31 (1.8) | 143 (3.8) | 375 (6.2) | 351 (8.8) | ||||
| Incidence (95% CI) | 1.1 (0.3 to 2.8) | 3.3 (2.2 to 4.6)d | 6.0 (5.0 to 7.0)d | 8.9 (8.0 to 9.8)d | 12.3 (11.0 to 13.7)d | <.001 | <.001 | <.001 | <.001 |
| Men with ≥2 primary melanomas (invasive only) | |||||||||
| All ages | |||||||||
| Second primary invasive melanoma, No. (%) | 8 (0.5) | 43 (1.4) | 144 (2.7) | 276 (3.8) | 218 (5.0) | ||||
| Incidence (95% CI) | 0.9 (0.4 to 1.7) | 2.2 (1.6 to 3.0)d | 3.9 (3.3 to 4.6)d | 5.1 (4.5 to 5.7)d | 6.7 (5.8 to 7.6)d | <.001 | <.001 | <.001 | <.001 |
| Younger men with invasive melanoma (≤55 years) | |||||||||
| Second primary invasive melanoma, No. (%) | 4 (0.5) | 20 (1.4) | 60 (2.9) | 76 (3.0) | 46 (3.5) | ||||
| Incidence (95% CI) | 0.7 (0.2 to 1.8) | 1.9 (1.2 to 2.9) | 3.5 (2.7 to 4.5)d | 3.3 (2.6 to 4.2) | 3.8 (2.8 to 5.1) | .001 | .008 | .61 | .43 |
| Older men with invasive melanoma (>55 years) | |||||||||
| Second primary invasive melanoma, No. (%) | 4 (0.5) | 23 (1.4) | 84 (2.6) | 200 (4.3) | 172 (5.7) | ||||
| Incidence (95% CI) | 1.1 (0.3 to 2.9) | 2.6 (1.6 to 3.9) | 4.2 (3.4 to 5.2)d | 6.4 (5.5 to 7.3)d | 8.4 (7.2 to 9.7)d | <.001 | <.001 | <.001 | .010 |
| Men with ≥2 primary melanomas (in situ only) | |||||||||
| All ages | |||||||||
| Second primary in situ melanoma, No. (%) | 0 (0.0) | 2 (1.3) | 22 (2.7) | 61 (3.1) | 55 (4.0) | ||||
| Incidence (95% CI) | 0.0 (0.0 to 14.7) | 1.5 (0.2 to 5.3) | 3.2 (2.0 to 4.8) | 3.6 (2.8 to 4.6) | 4.9 (3.7 to 6.4) | .55 | .11 | .13 | .13 |
| Younger men with in situ melanoma (≤55 years) | |||||||||
| Second primary in situ melanoma, No. (%) | 0 (0.0) | 0 (0.0) | 6 (2.0) | 10 (1.7) | 9 (2.5) | ||||
| Incidence (95% CI) | 0.0 (0.0 to 22.1) | 0.0 (0.0 to 4.5) | 2.1 (0.8 to 4.5) | 1.7 (0.8 to 3.1) | 2.6 (1.2 to 4.9) | .95 | .30 | .69 | .36 |
| Older men with in situ melanoma (>55 years) | |||||||||
| Second primary in situ melanoma, No. (%) | 0 (0.0) | 2 (2.7) | 16 (3.1) | 51 (3.7) | 46 (4.7) | ||||
| Incidence (95% CI) | 0.0 (0.0 to 43.9) | 3.7 (0.4 to 13.3) | 4.0 (2.3 to 6.4) | 4.7 (3.5 to 6.1) | 5.5 (4.0 to 7.4) | .97 | .56 | .24 | .29 |
First primary melanomas diagnosed in 2000-2004.
Incidence (95% confidence interval [CI]) of a second primary melanoma per 1000 person-years after the diagnosis of a first melanoma.
P value for the rate ratio of the incidence rate in the 2000s decade compared with the incidence in the 1990s, 1980s, 1970s, and 1960s.
Incidence rate was statistically significantly higher (P < .05) in this decade than in the closest preceding decade in the same strata (eg, in the 1990s vs the 1980s or in the 1970s vs the 1960s).
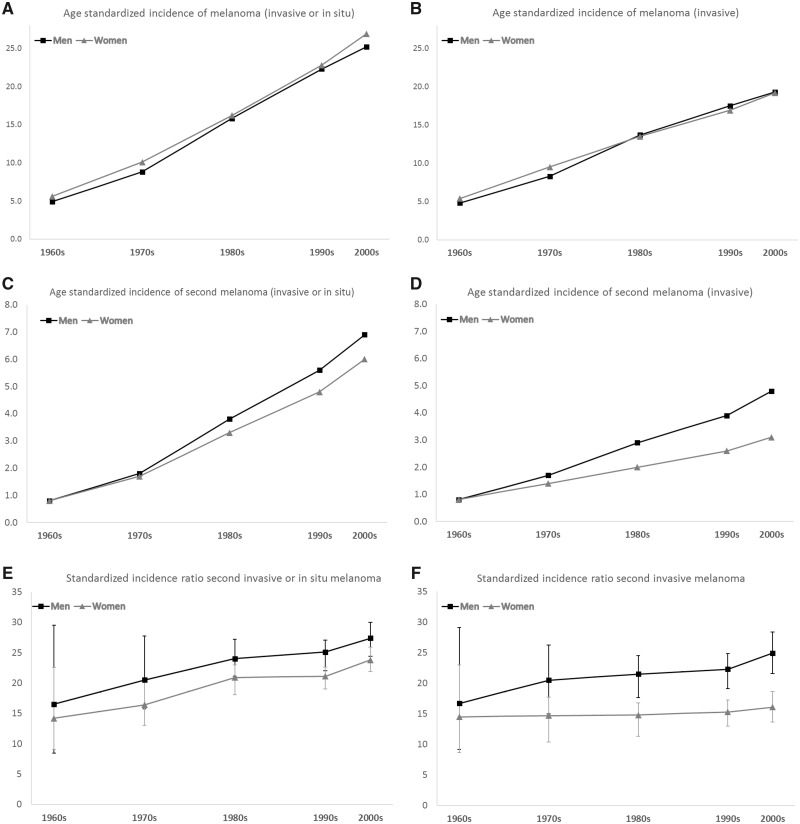
In Figure 1, the age standardized incidence rates of melanoma (per 100 000 person-years) in the Swedish population (Figure 1A for invasive or in situ tumors and Figure 1B for invasive tumors) and the age standardized incidence rates of second melanoma (per 1000 person-years) among melanoma patients (Figure 1C for invasive or in situ tumors and Figure 1D for invasive tumors) is shown alongside the standardized incidence ratio of the observed numbers of second melanoma to the expected number based on the incidence in the Swedish population (Figure 1E for invasive or in situ tumors and Figure 1F for invasive tumors) for each decade. Figure 1 shows that the incidence increase for second primary melanomas has occurred in parallel to the incidence increase for melanoma in the overall population. The standardized incidence ratio for invasive or in situ melanomas was 14.2 (95% CI = 9.1 to 22.6) in women and 16.5 (95% CI = 8.4 to 29.5) in men in the 1960s and 23.8 (95% CI = 21.8 to 25.9) in women and 27.4 (95% CI = 24.4 to 30.0) in men in the 2000s. For invasive melanomas, the standardized incidence ratio was 14.5 (95% CI = 8.7 to 22.9) in women and 16.7 (95% CI = 9.2 to 29.1) in men in the 1960s, and 16.1 (95% CI = 13.7 to 18.6.) in women and 24.9 (95% CI = 21.6 to 28.4) in men in the 2000s. In the whole cohort (women and men combined), there was a statistically significant increase in the standardized incidence ratio for invasive or in situ melanoma between the 1990s and the 2000s (SIR = 22.6, 95% CI = 21.3 to 24.0, vs SIR = 25.8, 95% CI = 24.1 to 27.6).
Figure 1.
Age standardized incidence rate of melanoma (per 100 000 person-years) in the Swedish population in the 1960s to the 2000s A) for invasive or in situ tumors and B) for invasive tumors. Age standardized incidence rate of second melanoma (per 1000 person-years) among melanoma patients in the 1960s to the 2000s C) for invasive or in situ tumors and D) for invasive tumors. Standardized incidence ratio of the observed numbers of second melanoma to the expected number based on the incidence in the Swedish population in the 1960s to the 2000s E) for invasive or in situ tumors and F) for invasive tumors.
Table 3 graphs the numbers and fraction of multiple primary melanoma patients diagnosed within 10 years with 2, 3, 4, or 5 or more primaries. In patients with multiple primary melanoma, the frequency of those with more than 2 primary tumors has increased statistically significantly over time (P < .001). In the 1960s, no patients were diagnosed with more than 2 melanomas compared with 18% (invasive or in situ melanoma) and 11% (invasive melanoma) in the 2000s.
Table 3.
Numbers of primary tumors diagnosed among patients with multiple primary melanoma in the 1960s to the 2000s
| Decade |
P, 2000s decade vsb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Multiple primary melanoma | No. of primaries | 1960s | 1970s | 1980s | 1990s | 2000sc | 1960s | 1970s | 1980s | 1990s |
| Women with multiple primaries, No. (%)a | ||||||||||
| Invasive or in situ | 2 | 14 (100.0) | 61 (96.8) | 214 (97.7) | 434 (89.7)d | 330 (82.3)d | .23 | .01 | <.001 | <.001 |
| 3 | 0 (0.0) | 2 (3.2) | 12 (5.3) | 40 (8.3) | 54 (13.5) | |||||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (1.0) | 12 (3.0) | |||||
| ≥5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (1.0) | 5 (1.2) | |||||
| Invasive | 2 | 13 (100.0) | 46 (100.0) | 105 (99.1) | 189 (93.6) | 139 (89.7) | .44 | .12 | .02 | .19 |
| 3 | 0 (0.0) | 0 (0.0) | 1 (0.9) | 12 (5.9) | 13 (8.4) | |||||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 2 (1.3) | |||||
| ≥5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | |||||
| Men with multiple primaries, No. (%) | ||||||||||
| Invasive or in situ | 2 | 10 (100.0) | 51 (92.7) | 231 (95.5) | 444 (84.9)d | 367 (81.9)d | .32 | .06 | <.001 | <.001 |
| 3 | 0 (0.0) | 3 (5.5) | 6 (2.5) | 59 (11.3) | 59 (13.5) | |||||
| 4 | 0 (0.0) | 1 (1.8) | 3 (1.2) | 13 (2.5) | 15 (3.3) | |||||
| ≥5 | 0 (0.0) | 0 (0.0) | 2 (0.8) | 7 (1.3) | 7 (1.6) | |||||
| Invasive | 2 | 8 (100.0) | 42 (97.7) | 141 (97.9) | 250 (90.9)d | 192 (88.1) | .56 | .10 | .004 | .31 |
| 3 | 0 (0.0) | 1 (2.3) | 2 (1.4) | 20 (7.3) | 22 (10.1) | |||||
| 4 | 0 (0.0) | 0 (0.0) | 1 (0.7) | 5 (1.8) | 3 (1.2) | |||||
| ≥5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | |||||
Fraction of multiple primary melanoma patients diagnosed within 10 years with 2, 3, 4, or 5 or more primaries.
P value for the frequency of multiple primary melanoma patients with more than 2 melanomas in the 2000s decade compared with the incidence in the 1990s, 1980s, 1970s, and 1960s.
First primary melanomas diagnosed in 2000-2004.
Frequency of multiple primary melanoma patients with more than 2 melanomas was statistically significantly higher (P < .05) in this decade than in the closest preceding decade.
Table 4 shows the occurrence of second melanomas diagnosed in the same or different body sites (head and neck, trunk, upper or lower extremities) as the first melanoma. For these analyses, only tumors with known first and second location were included. Because of a relatively low number of events for each of the strata in the first decades, patients diagnosed with first melanoma in the years 1960-1989 were grouped together. There was a statistically significant increase over time, both in patients diagnosed with a second melanoma in the same body site as the first melanom and in patients diagnosed with a second melanoma in a different body site as the first melanoma. However, the overall ratio of the incidence of concordant vs nonconcordant tumors did not change statistically significantly, and throughout the different time periods, around half of the melanomas were diagnosed in the same body site. In women, the head and neck area was the only site with a statistically significantly higher rate of second melanomas occurring in the same site. In the men, the trunk was the only site that consistently had a statistically significantly higher rate of second melanomas occurring in the same site. The head and neck area was the only site where a decline in the rate ratio for concordant vs nonconcordant tumors was noted; in the other sites, this ratio remined stable.
Table 4.
Occurrence of second primary melanoma in the same or different body site as the first melanoma (site concordance)
| Site | 1960s-1980s | 1990s | 2000sc | ||
|---|---|---|---|---|---|
| Women | |||||
| All sites | |||||
| Concordant site first and second melanoma, No. (%) | 114 (0.9) | 226 (2.2) | 183 (2.9) | ||
| Incidence concordant site (95% CI)a | 1.3 (1.0 to 1.5) | 2.8 (2.4 to 3.2)d | 3.6 (3.1 to 4.1)d | ||
| Incidence nonconcordant site (95% CI) | 1.3 (1.1 to 1.6) | 2.3 (2.0 to 2.6)d | 3.7 (3.1 to 4.2)d | ||
| RR concordant vs nonconcordant melanoma (95% CI)b | 1.0 (0.7 to 1.2) | 1.2 (1.0 to 1.4) | 1.0 (0.8 to 1.2) | ||
| Head and neck | |||||
| Concordant site first and second melanoma, No. (%) | 52 (2.4) | 107 (5.7) | 65 (5.8) | ||
| Incidence concordant site (95% CI) | 3.3 (2.4 to 4.3) | 7.4 (6.1 to 8.9)d | 7.5 (5.8 to 9.6) | ||
| Incidence nonconcordant site (95% CI) | 1.0 (0.6 to 1.6) | 2.3 (1.6 to 3.2)d | 3.8 (2.6 to 5.4)d | ||
| RR concordant vs nonconcordant melanoma (95% CI) | 3.2 (1.8 to 5.7) | 3.2 (2.2 to 4.8) | 2.0 (1.3 to 3.0) | ||
| Trunk | |||||
| Concordant site first and second melanoma, No. (%) | 15 (0.5) | 35 (1.3) | 47 (2.8) | ||
| Incidence concordant site (95% CI) | 0.7 (0.4 to 1.1) | 1.5 (1.1 to 2.1)d | 3.1 (2.3 to 4.2)d | ||
| Incidence nonconcordant site (95% CI) | 1.6 (1.1 to 2.2) | 2.3 (1.8 to 3.0) | 3.8 (2.9 to 4.9)d | ||
| RR concordant vs nonconcordant melanoma (95% CI) | 0.4 (0.2 to 0.8) | 0.6 (0.4 to 1.0) | 0.8 (0.6 to 1.2) | ||
| Upper extremities | |||||
| Concordant site first and second melanoma, No. (%) | 13 (0.6) | 27 (1.4) | 22 (1.8) | ||
| Incidence concordant site (95% CI) | 0.8 (0.4 to 1.3) | 1.7 (1.1 to 2.5)d | 2.1 (1.3 to 3.2) | ||
| Incidence nonconcordant site (95% CI) | 1.4 (0.9 to 2.1) | 2.8 (2.1 to 3.8)d | 3.7 (2.6 to 5.1) | ||
| RR concordant vs nonconcordant melanoma (95% CI) | 0.6 (0.3 to 1.1) | 0.6 (0.4 to 1.0) | 0.6 (0.5 to 1.4) | ||
| Lower extremities | |||||
| Concordant site first and second melanoma, No. (%) | 34 (0.1) | 57 (1.7) | 49 (2.5) | ||
| Incidence concordant site (95% CI) | 1.0 (0.7 to 1.3) | 2.0 (1.5 to 2.6)d | 2.9 (2.1 to 3.8) | ||
| Incidence nonconcordant site (95% CI) | 1.3 (0.9 to 1.7) | 1.9 (1.4 to 2.5)d | 3.4 (2.6 to 4.4)d | ||
| RR concordant vs nonconcordant melanoma (95% CI) | 0.8 (0.5 to 1.2) | 1.1 (0.7 to 1.5) | 0.8 (0.6 to 1.2) | ||
| Men | |||||
| All sites | |||||
| Concordant site first and second melanoma, No. (%) | 138 (1.2) | 233 (2.5) | 211 (3.7) | ||
| Incidence concordant site (95% CI) | 2.1 (1.7 to 2.4) | 3.5 (3.0 to 3.9)d | 5.0 (4.3 to 5.7)d | ||
| Incidence nonconcordant site (95% CI) | 1.7 (1.4 to 2.0) | 3.2 (2.8 to 3.7) | 5.0 (4.3 to 5.7) | ||
| RR concordant vs nonconcordant melanoma (95% CI) | 1.2 (1.0 to 1.6) | 1.1 (0.9 to 1.4) | 1.0 (0.8 to 1.2) | ||
| Head and neck | |||||
| Concordant site first and second melanoma, No. (%) | 38 (2.0) | 55 (3.2) | 34 (3.1) | ||
| Incidence concordant site (95% CI) | 3.2 (2.3 to 4.4) | 4.6 (3.5 to 6.0) | 4.3 (3.0 to 6.1) | ||
| Incidence nonconcordant site (95% CI) | 1.5 (0.9 to 2.4) | 4.3 (3.2 to 5.6)d | 5.6 (4.1 to 7.6)d | ||
| RR concordant vs nonconcordant melanoma (95% CI) | 2.1 (1.2 to 3.7) | 1.1 (0.7 to 4.6) | 0.8 (0.5 to 1.2) | ||
| Trunk | |||||
| Concordant site first and second melanoma, No. (%) | 84 (1.6) | 152 (3.3) | 147 (5.2) | ||
| Incidence concordant site (95% CI) | 2.3 (1.9 to 2.9) | 4.2 (3.6 to 4.9)d | 6.6 (5.6 to 7.7)d | ||
| Incidence nonconcordant site (95% CI) | 1.4 (1.0 to 1.8) | 2.3 (1.9 to 2.9)d | 4.2 (3.4 to 5.1)d | ||
| RR concordant vs nonconcordant melanoma (95% CI) | 1.7 (1.2 to 2.4) | 1.8 (1.4 to 2.4) | 1.6 (1.2 to 2.0) | ||
| Upper extremities | |||||
| Concordant site first and second melanoma, No. (%) | 7 (0.6) | 15 (1.1) | 21 (2.2) | ||
| Incidence concordant site (95% CI) | 0.8 (0.3 to 1.6) | 1.3 (0.7 to 2.2) | 2.8 (1.7 to 4.3)d | ||
| Incidence nonconcordant site (95% CI) | 2.7 (1.8 to 4.0) | 4.3 (3.1 to 5.7) | 7.0 (5.2 to 9.1)d | ||
| RR concordant vs nonconcordant melanoma (95% CI) | 0.3 (0.1 to 0.6) | 0.3 (0.2 to 0.6) | 0.4 (0.2 to 0.7) | ||
| Lower Extremities | |||||
| Concordant site first and second melanoma, No. (%) | 9 (0.6) | 11 (1.1) | 9 (1.5) | ||
| Incidence concordant site (95% CI) | 0.9 (0.4 to 1.8) | 1.4 (0.7 to 2.4) | 1.9 (0.9 to 3.5) | ||
| Incidence nonconcordant site (95% CI) | 2.0 (1.2 to 3.1) | 4.2 (2.9 to 5.9)d | 4.4 (2.7 to 6.7) | ||
| RR concordant vs nonconcordant melanoma (95% CI) | 0.5 (0.2 to 1.0) | 0.3 (0.2 to 0.6) | 0.4 (0.2 to 0.9) |
Incidence (95% confidence interval [CI]) of a second primary melanoma per 1000 person-years after the diagnosis of a first melanoma.
Rate ratio (RR) for the incidence rate of concordant (first and second melanoma in the same site) vs nonconcordant (first and second melanoma in different sites) primary tumors.
First primary melanomas diagnosed in 2000-2004.
Incidence rate was statistically significantly higher (P < .05) than in the preceding time period.
In the patients with second melanomas within 10 years, the fraction diagnosed each year after the initial diagnosis was calculated together with the incidence rate in individuals at risk at every time point (Table 5). In the 1960-1980s, 1990s, and 2000s cohorts, 20%-23% of second melanomas were diagnosed in the first year after the initial melanoma, 13%-14% in the second year, and 6%-10% in each year thereafter. The incidence rate for second primary melanoma was, in all cohorts, statistically significantly higher in the first year after the initial diagnosis compared with the following years, while no statistically significant changes were seen after the first year. The survival of the patients increased over time, where 47%, 42%, 37%, 37%, and 31% of the patients in the 1960s, 1970s, 1980s, 1990s, and 2000s cohort, respectively, died within the 10-year follow-up period. Table 5 shows that for each of the years after the initial diagnosis (involving only individuals alive and not emigrated at every time point), the incidence has increased statistically significantly over the decades.
Table 5.
Time (1-10 years) from the diagnosis of the first and the second primary melanoma
| Years from diagnosis of first melanoma | 1960s-1980s |
1990s |
2000sc |
|||
|---|---|---|---|---|---|---|
| %a | IR (95% CI)b | % | IR (95% CI) | % | IR (95% CI) | |
| 1 | 21.5 | 5.5 (4.6 to 6.6) | 23.1 | 11.9 (10.5 to 13.6)d | 19.6 | 14.0 (12.1 to 16.2) |
| 2 | 13.1 | 3.6 (2.8 to 4.4) | 12.7 | 6.8 (5.7 to 8.1)d | 14.0 | 10.3 (8.6 to 12.4)d |
| 3 | 8.9 | 2.6 (2.0 to 3.4) | 9.1 | 7.2 (6.0 to 8.5)d | 10.3 | 8.0 (6.4 to 9.9) |
| 4 | 9.8 | 3.2 (2.4 to 4.1) | 6.9 | 5.4 (4.3 to 6.6)d | 8.4 | 6.9 (5.4 to 8.7) |
| 5 | 8.0 | 2.8 (2.1 to 3.7) | 8.6 | 4.3 (3.4 to 5.4)d | 8.9 | 7.8 (6.2 to 9.8)d |
| 6 | 5.6 | 2.0 (1.4 to 2.8) | 8.0 | 5.6 (4.5 to 6.9)d | 8.8 | 8.1 (6.4 to 10.5)d |
| 7 | 8.9 | 3.4 (2.5 to 4.4) | 8.3 | 5.4 (4.3 to 6.7)d | 7.4 | 7.1 (5.5 to 9.1) |
| 8 | 7.2 | 2.9 (2.1 to 3.9) | 7.9 | 5.8 (4.6 to 7.1)d | 6.0 | 6.3 (4.7 to 8.3) |
| 9 | 8.9 | 3.6 (2.7 to 4.7) | 7.3 | 5.8 (4.6 to 7.2)d | 9.0 | 9.3 (7.3 to 11.7)d |
| 10 | 8.2 | 3.5 (2.6 to 4.7) | 8.1 | 5.5 (4.4 to 7.0)d | 7.5 | 8.3 (6.4 to 10.6) |
Fraction of multiple primary melanoma patients diagnosed with second primary melanoma in the first year, in the second year, and so on, up to 10 years after initial diagnosis.
Incidence (95% confidence interval [CI]) of a second primary melanoma per 1000 person-years in individuals at risk in each year after the first melanoma.
First primary melanomas diagnosed in 2000-2004.
Incidence rate (IR) in the first year, second year, and so on was statistically significantly higher (P < .05) than in the preceding time period (2000s vs 1990s or 1990s vs 1960s-1980s).
Discussion
The data from this study show that the incidence of subsequent primaries among melanoma patients has increased statistically significantly and mirrors the reported increase of new cases of melanoma in several populations. The increase was seen, independent of age and sex, although the steepest increase was observed in older patients and in males. Moreover, it has become increasingly common to be diagnosed with more than 2 melanomas. The increase has occurred for both invasive and in situ melanoma. It is possible that some of the increase of in situ melanoma may be attributed to a shift in the reporting of these premalignant melanocytic lesions, but the in situ melanoma patients nevertheless follow the same trend as the invasive melanomas. As the standardized incidence ratio increased over time, the incidence of second primaries appears to have had an even steeper increase than the melanoma incidence in the population. Hence, alongside the well-documented rise in the melanoma incidence, there has been a steeper increase in patients developing subsequent primaries. Further, the frequency, incidence, and standardized incidence ratio values for the second primary melanoma in the 2000s cohort is higher than what has been reported in previous studies involving patients diagnosed at earlier periods in, for example, Sweden, the Netherlands, Australia, or the United States (6,7,16,17).
Increased incidence of melanoma is, to a large extent, believed to be caused by lifestyle changes in recent decades with increased intermittent ultraviolet (UV) radiation exposure, with more tanning, travel to sunnier locations, and sunbed use (23). Increased awareness, surveillance, and early detection is also believed to have contributed to the incidence rise. Early detection, with thinner primary tumors, has also led to increased melanoma-specific survival, and in recent years, the emergence of novel oncologic treatments, including immunotherapy and targeted therapy, has further improved melanoma survival (3,4,24,25). It has been postulated that the observed surge of multiple primary melanomas is in part because of a general increase in the survival of melanoma patients, with more time at risk, but this study demonstrates that there has also been an actual increase in the incidence rate. Increased incidence of skin cancers has primarily affected individuals of European descent with fair pigmentation traits. Skin phenotypes are determined by several different genes, including MC1R, ASIP, TYR, and TYRP, and different variants in these genes are also associated with increased risks of skin cancers (26,27). Although such low-penetrance susceptibility gene variants are relatively common in the normal population, germline mutations in high-penetrance melanoma susceptibility gene CDKN2A are rare in the normal population but are found in 5%-20% of familial and multiple primary melanoma patients (28–32). Carriers of mutations in CDKN2A have much elevated risk of melanoma and 70%-80% develop, often multiple primary, melanoma (33,34). Other high-penetrance melanoma susceptibility genes are known, including CDK4, BAP1, POT1, ACD, TERF2IP, and TERT promoter, but such gene mutations are extremely rare (35–39). In the majority of high-risk individuals, such as those with multiple primaries and those belonging to melanoma-prone families, no high-penetrance susceptibility mutation is identified (40). Increased melanoma susceptibility often has a more complex etiology, attributed to a combination of low-penetrance susceptibility gene variants, pigmentation traits, nevus phenotype, and UV-exposure patterns (32,40–43). Such traits can probably act as effect modifiers, magnifying the effect of UV exposure, and when sun exposure habits have changed for individuals who are sensitive to melanoma, they have become increasingly prone to develop multiple primary tumors. It is also likely that increased skin cancer awareness and more dermatologic surveillance of melanoma patients have, at least in part, led to an increase in the diagnosis of additional melanomas.
In certain sites of the body, it was more common than in others to develop a second melanoma in the same site; for example, patients with melanoma in the usually more chronically UV-exposed head and neck area were more prone to develop second melanomas in the same site as compared with those with first melanoma in the usually more intermittently exposed extremities. In the women, those with first melanoma in the head and neck area were most likely to have second melanoma in the same site, whereas in the men, those with first melanoma on the usually more intermittently UV-exposed trunk were most likely to have second melanoma in the same site. Over time, the patients with head and neck melanoma became increasingly prone to develop melanoma also in other body sites. Melanoma in the head and neck area is more common in older patients and has been associated with chronic sun exposure seen among, for example, outside workers. The shift seen, with more second melanomas occurring in sites other than the head and neck area, is probably the effect of increased intermittent UV exposure to other parts of the body.
Melanoma survival has increased over the years, and this is mainly due to increased awareness, earlier detection, and to some extent, better treatments (3,44). Throughout the study period, the incidence of second melanoma was statistically significantly highest in the first year after initial diagnosis, which is probably related to many patients newly diagnosed with melanoma undergoing full dermatologic examination. A consequence of the higher mortality in the earlier cohorts is that in the pooled person-years from the earlier decades, there is a proportionally higher contribution of the first year after diagnosis (ie, the year with the highest incidence). Hence, a potential effect of the improving survival is that the incidence increase of second primaries within a 10-year follow-up period, is even somewhat underestimated.
The study design, with a registry-based approach, has both strengths and limits, with the former being the comprehensive and population-based nature of the study. The limitations to this design are that only register variables are available, and other relevant aspects, such as skin and nevus phenotype, UV-exposure patterns, family history, genetic information, melanoma pathology subtypes, or more precise information on the body site, cannot be addressed. The study includes all melanoma patients reported to the Swedish Cancer Register and is, hence, limited to Sweden, but we believe that the results, with increased incidence of second primary melanoma, are likely generalizable to other regions that have experienced similar rise in the melanoma incidence.
In summary, this population-based study views the incidence of multiple primary melanoma going back to the 1960s. To our knowledge, this is the first study to methodically investigate time trends in the occurrence of multiple primary melanoma. The steep increase in subsequent primaries is notable because additional invasive melanomas are associated with worse survival in melanoma patients (45–47). Precautions are needed to turn the upgoing trend for additional primary melanomas in melanoma patients. Currently, there is no evidence that supports population screening for skin cancer; however, surveillance should focus on high-risk populations, such as those with familial melanoma predisposition or multiple primary melanoma (48–51). In our opinion, dermatologic surveillance with a yearly full-body skin examination should be recommended to patients for at least 10 years after the initial melanoma diagnosis. Melanoma patients need to be informed about their risk to develop additional melanomas and thoroughly advised to avoid sunburns and tanning and, moreover, to seek medical help for suspicious lesions. Further, as the incidence increase of second primaries has been in parallel with the melanoma incidence in the whole population, it is likely that there would also be a similar trend if a stabilization or decrease in the incidence would be reached. It is therefore of great importance that authorities prioritize campaigns and other efforts aimed at melanoma prevention.
Funding
This work was supported by grants from the Swedish Cancer society (grant number CAN 2017/503) and the Cancer Research Funds of Radiumhemmet (grant number 194092).
Notes
Role of the funder: The funders were not involved in the design, conduct, or reporting of the study; the writing of the manuscript; or the decision to publish the manuscript.
Disclosures: The authors declare that they have no financial disclosures or conflicts of interest.
Author contributions: HH: Conceptualization; Supervision; Methodology; Data curation; Validation; Visualization; Writingã review & editing. KI: Formal analysis; Writing—review & editing. IF: Data curation; Methodology; Writing—review & editing. CI: Conceptualization; Writing—review & editing. JL: Conceptualization; Writing—review & editing. VH: Conceptualization; Writing—review & editing. JN-B: Validation; Visualization; Writing—review & editing. HO: Conceptualization; Data curation; Supervision; Writing—review & editing.
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Supplementary Material
References
- 1. Erdmann F, Lortet-Tieulent J, Schuz J, et al. International trends in the incidence of malignant melanoma 1953-2008–are recent generations at higher or lower risk? Int J Cancer. 2013;132(2):385–400. [DOI] [PubMed] [Google Scholar]
- 2. Sacchetto L, Zanetti R, Comber H, et al. Trends in incidence of thick, thin and in situ melanoma in Europe. Eur J Cancer. 2018;92:108–118. [DOI] [PubMed] [Google Scholar]
- 3. Tryggvadottir L, Gislum M, Hakulinen T, et al. Trends in the survival of patients diagnosed with malignant melanoma of the skin in the Nordic countries 1964-2003 followed up to the end of 2006. Acta Oncol. 2010;49(5):665–672. [DOI] [PubMed] [Google Scholar]
- 4. Nikolaou V, Stratigos AJ.. Emerging trends in the epidemiology of melanoma. Br J Dermatol. 2014;170(1):11–19. [DOI] [PubMed] [Google Scholar]
- 5. Aitken JF, Youlden DR, Baade PD, et al. Generational shift in melanoma incidence and mortality in Queensland, Australia, 1995-2014. Int J Cancer. 2018;142(8):1528–1535. [DOI] [PubMed] [Google Scholar]
- 6. van der Leest RJ, Liu L, Coebergh JW, et al. Risk of second primary in situ and invasive melanoma in a Dutch population-based cohort: 1989-2008. Br J Dermatol. 2012;167(6):1321–1330. [DOI] [PubMed] [Google Scholar]
- 7. Youlden DR, Youl PH, Soyer HP, et al. Distribution of subsequent primary invasive melanomas following a first primary invasive or in situ melanoma Queensland, Australia, 1982-2010. JAMA Dermatol. 2014;150(5):526–534. [DOI] [PubMed] [Google Scholar]
- 8. McCaul KA, Fritschi L, Baade P, et al. The incidence of second primary invasive melanoma in Queensland, 1982-2003. Cancer Causes Control. 2008;19(5):451–458. [DOI] [PubMed] [Google Scholar]
- 9. Titus-Ernstoff L, Perry AE, Spencer SK, et al. Multiple primary melanoma: two-year results from a population-based study. Arch Dermatol. 2006;142(4):433–438. [DOI] [PubMed] [Google Scholar]
- 10. Ferrone CR, Ben Porat L,, Panageas KS, et al. Clinicopathological features of and risk factors for multiple primary melanomas. JAMA. 2005;294(13):1647–1654. [DOI] [PubMed] [Google Scholar]
- 11. Levi F, Randimbison L, Te VC, et al. High constant incidence rates of second cutaneous melanomas. Int J Cancer. 2005;117(5):877–879. [DOI] [PubMed] [Google Scholar]
- 12. Bower MR, Scoggins CR, Martin RC 2nd, et al. Second primary melanomas: incidence and outcome. Am Surg. 2010;76(7):675–681. [PubMed] [Google Scholar]
- 13. Moore MM, Geller AC, Warton EM, et al. Multiple primary melanomas among 16,570 patients with melanoma diagnosed at Kaiser Permanente Northern California, 1996 to 2011. J Am Acad Dermatol. 2015;73(4):630–636. [DOI] [PubMed] [Google Scholar]
- 14. Uliasz A, Lebwohl M.. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46(6):575–577. [DOI] [PubMed] [Google Scholar]
- 15. Burden AD, Vestey JP, Sirel JM, et al. Multiple primary melanoma: risk factors and prognostic implications. BMJ. 1994;309(6951):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5)(suppl 1):S69–S77. [DOI] [PubMed] [Google Scholar]
- 17. Dong C, Hemminki K.. Multiple primary cancers of the colon, breast and skin (melanoma) as models for polygenic cancers. Int J Cancer. 2001;92(6):883–887. [DOI] [PubMed] [Google Scholar]
- 18. Ingvar C, Eriksson H.. Melanoma incidence in Sweden. Lakartidningen. 2017;114. [PubMed] [Google Scholar]
- 19.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2018. https://gco.iarc.fr/today. Accessed March 15, 2020. [Google Scholar]
- 20. Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33. [DOI] [PubMed] [Google Scholar]
- 21.Statistics Sweden (SCB). Summary of Population Statistics 1960-2018. https://www.scb.se/en/finding-statistics/statistics-by-subject-area/population/population-composition/population-statistics/pong/tables-and-graphs/yearly-statistics--the-whole-country/summary-of-population-statistics/. Accessed January 25, 2020.
- 22. Simberg-Danell C, Lyth J, Mansson-Brahme E, et al. Prognostic factors and disease-specific survival among immigrants diagnosed with cutaneous malignant melanoma in Sweden. Int J Cancer. 2016;139(3):543–553. [DOI] [PubMed] [Google Scholar]
- 23. D’Orazio J, Jarrett S, Amaro-Ortiz A, et al. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. [DOI] [PubMed] [Google Scholar]
- 25. Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381(7):626–636. [DOI] [PubMed] [Google Scholar]
- 26. Nan H, Kraft P, Hunter DJ, et al. Genetic variants in pigmentation genes, pigmentary phenotypes, and risk of skin cancer in Caucasians. Int J Cancer. 2009;125(4):909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoiom V, Tuominen R, Kaller M, et al. MC1R variation and melanoma risk in the Swedish population in relation to clinical and pathological parameters. Pigment Cell Melanoma Res. 2009;22(2):196–204. [DOI] [PubMed] [Google Scholar]
- 28. Hashemi J, Platz A, Ueno T, et al. CDKN2A germ-line mutations in individuals with multiple cutaneous melanomas. Cancer Res. 2000;60(24):6864–6867. [PubMed] [Google Scholar]
- 29. Monzon J, Liu L, Brill H, et al. CDKN2A mutations in multiple primary melanomas. N Engl J Med. 1998;338(13):879–887. [DOI] [PubMed] [Google Scholar]
- 30. Berwick M, Orlow I, Hummer AJ, et al. The prevalence of CDKN2A germ-line mutations and relative risk for cutaneous malignant melanoma: an international population-based study. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1520–1525. [DOI] [PubMed] [Google Scholar]
- 31. Puig S, Malvehy J, Badenas C, et al. Role of the CDKN2A locus in patients with multiple primary melanomas. J Clin Oncol. 2005;23(13):3043–3051. [DOI] [PubMed] [Google Scholar]
- 32. Helgadottir H, Tuominen R, Olsson H, et al. Cancer risks and survival in patients with multiple primary melanomas: association with family history of melanoma and germline CDKN2A mutation status. J Am Acad Dermatol. 2017;77(5):893–901. [DOI] [PubMed] [Google Scholar]
- 33. Bishop DT, Demenais F, Goldstein AM, et al. Geographical variation in the penetrance of CDKN2A mutations for melanoma. J Natl Cancer Inst. 2002;94(12):894–903. [DOI] [PubMed] [Google Scholar]
- 34. Helgadottir H, Hoiom V, Jonsson G, et al. High risk of tobacco-related cancers in CDKN2A mutation-positive melanoma families. J Med Genet. 2014;51(8):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aoude LG, Pritchard AL, Robles-Espinoza CD, et al. Nonsense mutations in the shelterin complex genes ACD and TERF2IP in familial melanoma. J Natl Cancer Inst. 2015;107(2):dju408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–961. [DOI] [PubMed] [Google Scholar]
- 37. Robles-Espinoza CD, Harland M, Ramsay AJ, et al. POT1 loss-of-function variants predispose to familial melanoma. Nat Genet. 2014;46(5):478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi J, Yang XR, Ballew B, et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat Genet. 2014;46(5):482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zuo L, Weger J, Yang Q, et al. Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nat Genet. 1996;12(1):97–99. [DOI] [PubMed] [Google Scholar]
- 40. Helgadottir H, Hoiom V, Tuominen R, et al. CDKN2a mutation-negative melanoma families have increased risk exclusively for skin cancers but not for other malignancies. Int J Cancer. 2015;137(9):2220–2226. [DOI] [PubMed] [Google Scholar]
- 41. Potjer TP, Bollen S, Grimbergen A, et al. ;on behalf of the Dutch Working Group for Clinical Oncogenetics. Multigene panel sequencing of established and candidate melanoma susceptibility genes in a large cohort of Dutch non-CDKN2A/CDK4 melanoma families. Int J Cancer. 2019;144(10):2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li C, Liu T, Tavtigian SV, et al. Targeted germline sequencing of patients with three or more primary melanomas reveals high rate of pathogenic variants. Melanoma Res. 2019;30(3):247–251. [DOI] [PubMed] [Google Scholar]
- 43. McMeniman EK, Duffy DL, Jagirdar K, et al. The interplay of sun damage and genetic risk in Australian multiple and single primary melanoma cases and controls. Br J Dermatol. 2020;183(2):357–366. [DOI] [PubMed] [Google Scholar]
- 44. Balch CM, Soong SJ, Milton GW, et al. Changing trends in cutaneous melanoma over a quarter century in Alabama, USA, and New South Wales, Australia. Cancer. 1983;52(9):1748–1753. [DOI] [PubMed] [Google Scholar]
- 45. Youlden DR, Baade PD, Soyer, HP, et al. Ten-year survival after multiple invasive melanomas is worse than after a single melanoma: a population-based study. J Invest Dermatol. 2016;136(11):2270–2276. [DOI] [PubMed] [Google Scholar]
- 46. Rowe CJ, Law MH, Palmer JM, et al. Survival outcomes in patients with multiple primary melanomas. J Eur Acad Dermatol Venereol. 2015;29(11):2120–2127. [DOI] [PubMed] [Google Scholar]
- 47. Utjes D, Lyth J, Lapins J, et al. Reduced disease-specific survival following a diagnosis of multiple primary cutaneous malignant melanomas–a nationwide, population-based study. Int J Cancer. 2017;141(11):2243–2252. [DOI] [PubMed] [Google Scholar]
- 48. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for skin cancer in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316(4):436–447. [DOI] [PubMed] [Google Scholar]
- 49. Haenssle HA, Vente C, Bertsch HP, et al. Results of a surveillance programme for patients at high risk of malignant melanoma using digital and conventional dermoscopy. Eur J Cancer Prev. 2004;13(2):133–138. [DOI] [PubMed] [Google Scholar]
- 50. Moloney FJ, Guitera P, Coates E, et al. Detection of primary melanoma in individuals at extreme high risk: a prospective 5-year follow-up study. JAMA Dermatol. 2014;150(8):819–827. [DOI] [PubMed] [Google Scholar]
- 51. Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67(1):e17–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.