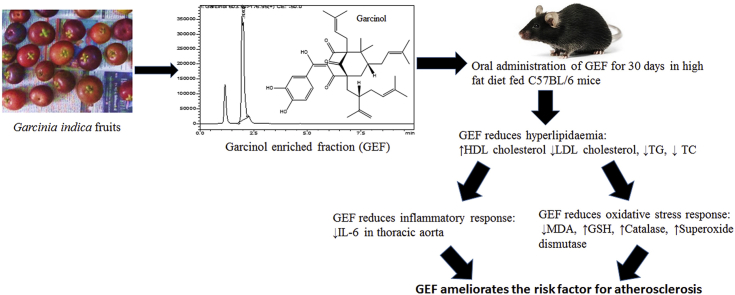
Graphical abstract
Keywords: Anti-hyperlipidemic, Anti-inflammatory, Atherosclerosis, Camboginol, Kokum, Oxidative-stress
Highlights
-
•
Garcinol enriched fraction (GEF) was prepared from the fruit rind of Garcinia indica.
-
•
GEF corrects dyslipidemia, inflammation and oxidative stress in experimental mice.
-
•
GEF ameliorates a major risk factor involved in progression of atherosclerosis.
1. Introduction
Atherosclerosis is a major cause of many cardiovascular diseases (CVD’s) causing considerable mortality and morbidity globally. It is responsible for 50% of the deaths in westernized society.1,2 Formation of atherosclerotic plaque begins with abnormalities in lipid metabolism, advent of inflammatory reactions and endothelial cell dysfunction. As opposed to the earlier belief of association of high lipid levels with the risk of CVD’s, now it is well accepted that atherosclerosis is also a chronic inflammatory disease.3,4 From a pathological view point, all stages of the atherosclerotic plaque (i.e., initiation, growth, and complication) can be considered as an inflammatory response to injury.4Current strategies to prevent atherosclerosis are to block the lipid accumulation in arterial smooth muscle cells and to inhibit inflammation.
Ayurveda, a traditional system of medicine in India has listed Garcinia indica in the class of drugs used for the treatment of CVD’s (Hridyvarga).5 Fruit rinds of Garcinia indica contain a phytochemical named Garcinol, which exerts anti-inflammatory effect by modulating arachidonic acid metabolism, suppressing activation of nuclear factor kappa B (NF-κB) and expression of cyclooxygenase-2 (COX-2) through interruption of lipopolysachharide (LPS) binding to toll-like receptors,6,7 acts as an anti-oxidant8 and is a P300/CBP-associated factor (PCAF) inhibitor reducing local inflammation, vascular smooth muscle cell proliferation and intimal hyperplasia after arterial injury.9
In addition to the above mentioned activities if Garcinol shows anti-hyperlipidemic effects, it could be a potential molecule in not only ameliorating the risk factor but also correcting the underlying pathological mechanism of atherosclerosis.
Thus, this study was undertaken to investigate the effects of GEF (Garcinol enriched fraction) against hyperlipidemia in diet induced hyperlipidemic C57BL/6 mice.
2. Materials and methods
2.1. Chemicals and plant materials
Standard Garcinol was purchased from Cayman Chemical Company-USA. Methanol (HPLC grade) was purchased from SD Fine chemical Limited (Mumbai, India). N-hexane, toluene, ethyl acetate, formic acid, and other chemical were purchased from Loba Chemie (Mumbai, India). Thiobarbituric acid, trichloro acetic acid, 5,5′-Dithiobis (2-nitrobenzoic acid), Hydrogen peroxide, hydroxylamine hydrochloride, Triton-X-100 and Nitrobluetetrazolium (NBT) were purchased from Sigma Aldrich, MUMBAI. Simvastatin was received as a gift sample from Micro Labs Ltd., MUMBAI. High density cholesterol (HDL-C), Low density cholesterol (LDL-C), Triglyceride (TG) and Total Cholesterol (TC) estimation kits were purchased from ERBA Diagnostics Mannheim, MUMBAI. IL6 kit was purchased from Krishgen biosystems, MUMBAI. The fresh fruits of Garcinia indica were procured from Dr. Badhe Waadi, Phoolpada Road, Virar (East) located at 19.45 north latitude and 72.81 east longitude and were authenticated from Agharkar Research Institute, Pune, Maharashtra, India. A voucher specimen with identification number-15-147, has been deposited at the same institute. The plant name has been checked with http://www. theplantlist.org on 2nd November 2018.
2.2. Preparation of Garcinol enriched fraction (GEF)
The hexane extract of the fruit rinds was prepared in the laboratory.10 The extract (1g) was loaded on a pre-packed silica column and eluted with hexane and ethyl acetate in increasing order of polarity to get various fractions using flash chromatography(Telydyne Isco, USAModel: COMBIFLASH RF) with flow rate of 15 ml/min 12 ml fractions were collected and identified for the presence of Garcinol by thin layer chromatography using silica gel G f254 as stationary phase, Toluene: Ethyl acetate: Formic acid (4 : 1: 0.5) as the mobile phase, vanillin sulphuric acid as spraying reagent and standard Garcinol as reference standard. Fractions showing the presence of Garcinol were pooled and taken up for efficacy studies. This process of flash chromatography was repeated thrice to obtain sufficient amount of GEF.
2.3. LC-MS/MS analysis for identification and quantification of Garcinol
GEF was subjected to LC-MS/MS (Shimadzu LC-MS 8040) analysis for identification and quantification of Garcinol using a reported method.10
2.3.1. In-vivo studies
The study was approved by the Institutional Animal Ethics committee (Protocol no. CPCSEA/IAEC/SPTM/P-55/2016) and were done in accordance with guidelines of Committee for the Purpose and Supervision of Experiments on Animals (CPCSEA).
2.4. Animals and treatment
C57BL/6 male mice of 6–8 weeks of age and body weight range 15–22 g were procured from Bharat Serum, Pune, Maharashtra, India and used in the study. All mice were kept in standard environmental conditions [23 °C ± 5, 60% ± 5 RH, and12:12hdark and light cycle], allowed free access to drinking water and standard diet during the period of acclimatization (one week). The animals were randomly divided into 6 groups of 6 animals each. The animals in Group 1 were given standard feed and vehicle for 16 weeks and served as normal control. Animals in all other groups were given modified western diet comprising of standard feed added with 21% milk powder, 34% Sucrose, 0.2% Cholesterol, 20%Vanaspati ghee and 10% Pork lard for 16 weeks.11 Animals in Group 2 served as disease control. For last 4 weeks animals in group 3, 4, 5 and 6 were administered with Simvastatin (8 mg/kg), GEF (25 mg/kg), GEF (50 mg/kg) and GEF (100 mg/kg) respectively by oral gavage along with Western diet. The body weights were measured on a weekly basis.
Blood was withdrawn from animals of all the groups through retro-orbital puncture, initially at zero week i.e. just before the start of diet, 12th week and 16th week after start of modified western diet. The plasma was separated and analyzed for HDL-C (High density lipoprotein cholesterol), LDL-C (Low density lipoprotein cholesterol), TG (Triglyceride) and TC (Total cholesterol). After the treatment schedule, i.e. at the end of 16th week, mice were euthanized. The thoracic aorta was isolated for histopathological examination, measurement of inflammatory markers and oxido-redox state parameters.
2.5. Measurement of lipid levels
Estimation of HDL-C, LDL-C, TG and TC was done in the plasma using commercial kits as per the manufacturer’s instructions in the respective kits and employing a blood analyzer for measurements.
2.6. Measurement of IL 6 in aorta homogenates and plasma
IL-6 (Mouse IL-6 KRISHGEN Biosystems) one of the important inflammatory marker was evaluated in the plasma and tissue homogenate using ELISA method. The thoracic aorta of animals of each group were removed by dissection and rinsed with ice cold saline. The tissues were weighed and homogenized (10%w/v) in ice cold phosphate buffer (pH 7.4) using probe homogenizer (Polytron®, France), centrifuged in high speed cooling centrifuge. IL-6 was measured in the supernatant as per the manufacturer’s instructions.
2.7. Measurement of endogenous anti-oxidants in aorta homogenates
The protein content of the homogenate as prepared above was measured for determination of activity per mg protein.12 Lipid peroxides and reduced Glutathione (GSH) were measured in homogenate as prepared above. Activity of Catalase and superoxide dismutase (SOD) were measured in post nuclear supernatant (obtained on centrifugation of homogenate at 2500g for 20 min at 4 °C) and post mitochondrial supernatant (obtained on centrifugation of homogenate at 10,000g for 20 min at 4 °C) respectively.
2.7.1. Lipid peroxide assay
100 μl tissue homogenate was mixed with 100 μl of sodium dodecyle sulphate solution (8.1%), 750 μl of glacial acetic acid (20%), 750 μl of thiobarbituric acid solution (0.8%), and 300 μl of distilled water. The reaction mixture was thoroughly mixed and incubated at 100 °C for 1 h. The solution was then centrifuged for 10 min at 10,000 rpm, absorbance of the supernatant was recorded at 532 nm. Concentration of lipid peroxides in samples was calculated using a calibration curve of standard.13,14
2.7.2. GSH assay
Reaction mixture containing 300 μl of tissue homogenate and 300 μl trichloro acetic acid solution (10%) was allowed to stand for 1 h at 4 °C, followed by centrifugation at 8000 rpm for 20 min. 2700 μl of phosphate buffer (7.4 pH) and 200 μl of 100 mM DTNB (5, 5′-Dithiobis (2-nitrobenzoic acid)) solution was added to 100 μl of supernatant. The solution was mixed and the absorbance of the solution was measured at 412 nm. GSH concentration in samples was calculated using a calibration curve of standard.15
2.7.3. Catalase assay
25 μl of sample and 3 ml of phosphate buffer with hydrogen peroxide was kept for 30 s. Decrease in optical density was measured at 240 nm for 2 min at 1 min interval. Catalase activity was calculated in terms of micromole of H2O2 decomposed/min/mg protein using molar extinction coefficient of 71 M−1 cm−116
2.7.4. SOD assay
Mixture containing 1.3 ml of 50 mM sodium carbonate solution (containing 0.1 mM EDTA, pH 10.0), 0.5 ml of 96 96 μM NBT solution and 0.1 ml of 0.6% Triton-X-100 solution was incubated at 37 °C for 10 min. The reaction was initiated by addition of 0.1 ml of 20 mM of hydroxylamine hydrochloride (pH 6.0). NBT reduction was measured at 560 nm. Following this, 0.05 ml supernatant was added to the mixture and NBT reduction in the presence of enzyme was measured again at 560 nm for 2 min at 30 s interval. Percentage inhibition in the rate of NBT reduction was calculated.17,18
2.8. Measurement of atherosclerosis index19
Various indices predicting the risk of atherosclerosis and subsequent cardiovascular disease were calculated using lipid values as follows:
Atherogenic index of plasma (AIP) = (log (TG/HDL-C))
Cardiac risk ratio (CRR) = (TC/HDL-C).
Atherogenic coefficient (AC) = (TC–HDL-C/HDL-C).
2.9. Histopathological studies
After the mice were euthanized, tissue sections of thoracic aorta were washed with physiological saline and fixed in a 10% buffered formalin solution. Tissues were embedded in paraffin, sectioned into 5 μM thick slices and stained with Haematoxylin and Eosin (H & E) to observe the smooth muscle cells. The slides were blindly examined under a light microscope by an experienced pathologist. Congestion, hemorrhage with loss of histological architecture of aorta, degenerative changes in the layers of aorta were observed and the lesions were scored for overall pathological changes (Lesion score) as follows: Score 0 = no change, Score1 = minimal changes, Score 2 = mild changes, Score 3 = moderate changes and Score 4 = severe changes.
2.10. Statistical analysis
Statistical analysis was performed using two-way ANOVA and one way ANOVA as suitable followed by Bonferroni’s multiple comparison, using Graphpad Prism Version 6.01 software for windows (Graph Pad Software Inc.,). P value of less than 0.05 was considered statistically significant.
3. Results
3.1. LC-MS/MS analysis for identification and quantification of Garcinol
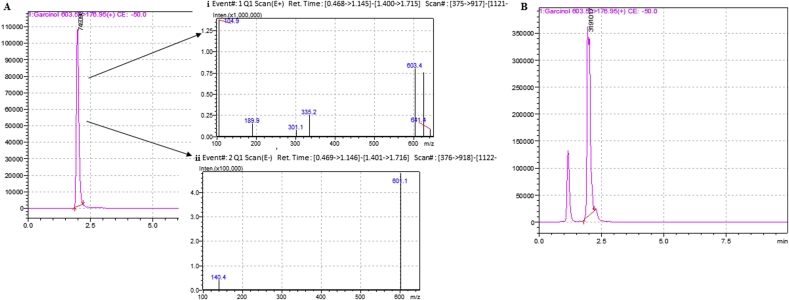
GEF contained 85.98% w/w of Garcinol (Fig. 1).The remaining 14.02% w/w of the fraction comprised of phenols and flavonoids as evident by qualitative phytochemical tests performed on the fraction.
Fig. 1.
(A)LC-MS chromatogram of standard Garcinol. (i) Mass spectra at Rt = 1.987 showing a peak at m/z 603 in positive ionisation mode (ii) Mass spectra at Rt = 1.987 showing a peak at m/z 601 in negative ionisation mode (B) LC-MS chromatogram of GEF (Rt = 1.957).
3.2. Effect of GEF treatment on body weight
Table 1 shows the effect of the various treatments on the body weight of experimental animals. It was observed that there was significant increase (p < 0.05&p < 0.01) in body weights of animals of all groups at 12th week when compared to normal control. The body weight of diseased animals kept on increasing significantly (p < 0.05) till 16th week as compared to normal animals. However, the body weight of animals receiving Simvastatin and GEF significantly reduced (p < 0.001 and p < 0.0001 respectively) from 12th week to 16th week. This effect might be related to the lipid lowering effect as discussed below and certainly is a desired one for treating hyperlipidemia.
Table 1.
Effect on body weight of animals.
| Animal groups | Body weight (g) |
||
|---|---|---|---|
| Week 0 | Week 12 | Week 16 | |
| Normal control | 18.11 ± 1.57 | 21.39 ± 0.83 | 24.81 ± 0.61 |
| Disease control | 19.68 ± 1.09 | 27.21 ± 1.4## | 30.17 ± 0.15# |
| Disease + Simvastatin | 21.95 ± 1.18 | 26.98 ± 2.17# | 23.18 ± .024*** |
| Disease + GEF 25 mg/kg | 21.10 ± 0.73 | 26.41 ± 0.49# | 21.80 ± 0.2**** |
| Disease + GEF 50 mg/kg | 22.14 ± 0.8 | 27.03 ± 1.80## | 21.91 ± 0.13**** |
| Disease + GEF 100 mg/kg | 21.26 ± 1.04 | 26.78 ± 2.04# | 22.09 ± 0.35**** |
Each value represent Mean ± S.E.M. #p < 0.05, ##p < 0.01 when compared with normal control, ***p < 0.001, ****p < 0.0001 when compare with disease control using two way ANOVA followed by Bonferroni’s multiple comparison.
3.3. Effect of GEF treatment on lipid levels
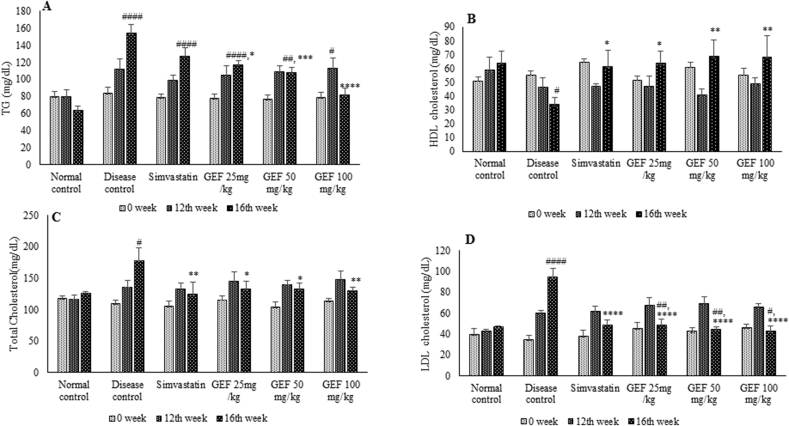
Animals fed for 16 weeks with modified western diet showed significant negative changes in the lipid profile indicating induction of the disease (Fig. 2). Administration of GEF and Simvastatin positively modified the lipid profile in a dose dependent manner, thus attenuating one of the risk factors of atherosclerosis. GEF not only increased the HDL-C (p < 0.05 and p < 0.01) but also simultaneously reduced TG concentration (p < 0.05, p < 0.0001 and p < 0.0001) (Fig. 2A and 2B). GEF treatment also lowered LDL-C (p < 0.0001) and TC (p < 0.05 and p < 0.01) (Fig. 2C and 2D).
Fig. 2.
Lipid profile of experimental animals, A-Triglycerides, B-HDL cholesterol, C-total cholesterol and D-LDL cholesterol. Each value represent Mean ± S.E.M. #p < 0.05, ##p < 0.01, ####p < 0.0001 when compared to normal control,*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 when compared to disease control, using two way ANOVA followed by Bonferroni’s multiple comparison.
3.4. Effect of GEF treatment atherosclerosis markers
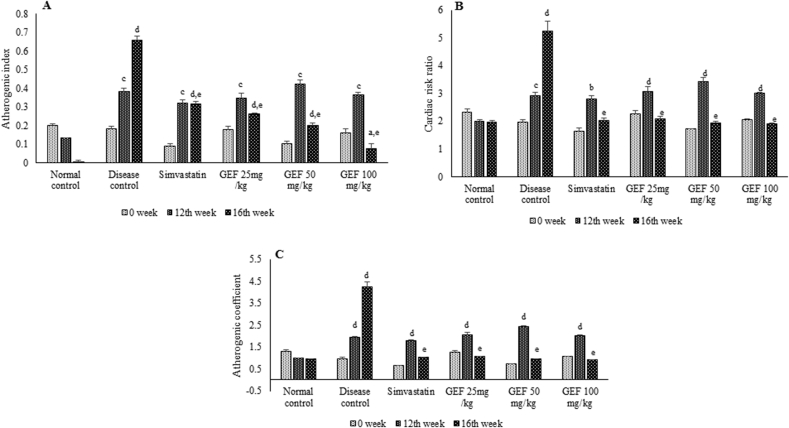
All the animals fed on modified western diet had significantly higher values (p < 0.01, p < 0.001 and p < 0.0001) of AIP, CRR and AC at the end of 12th week (Fig. 3). Treatment with Simvastatin and GEF at all doses significantly reduced (p < 0.0001) these scores, almost normalizing the values, thus reducing the risk of atherosclerosis.
Fig. 3.
Markers predicting the risk of atherosclerosis and cardiovascular disease in experimental animals, A-Atherogenic index, B-Cardiac risk ratio and C- Atherogenic coefficient. Each value represent Mean ± S.E.M. ap<0.05, bp < 0.01, cp < 0.001, dp < 0.0001 when compared to normal control, ep < 0.0001 when compared to disease control, using two way ANOVA followed by Bonferroni’s multiple comparison.
3.5. Effect of GEF treatment on IL-6 in aorta homogenates and plasma
At the end of 16th week, IL-6 levels in the plasma and thoracic aorta, were significantly increased (p < 0.0001 and p < 0.001 respectively) in disease control animals, indicating the presence of inflammation. IL-6 levels in the tissue were significantly lowered(p < 0.01) in animals treated with Simvastatin and GEF at the dose of 50 and 100 mg/kg and the effect was found to be dose dependent, whereas IL-6 levels in the plasma were significantly reduced (p < 0.0001) by all three doses of GEF and Simvastatin (Table 2).
Table 2.
IL-6 levels in experimental animals.
| Animal groups | IL6 in plasma (pg/ml) | IL6 in aorta (pg/mg tissue protein) |
|---|---|---|
| Normal control | 15.56 ± 1.600 | 40.29 ± 3.930 |
| Disease control | 38.01 ± 7.585#### | 494.55 ± 25.660### |
| Disease + Simvastatin | 13.7 ± 0.845**** | 68.14 ± 5.400** |
| Disease + GEF 25 mg/kg | 33.195 ± 0.845**** | 304.93 ± 13.100 |
| Disease + GEF 50 mg/kg | 18.35 ± 0.345**** | 37.67 ± 0.250** |
| Disease + GEF 100 mg/kg | 11.645 ± 0.155**** | 34.28 ± 2.530** |
Each value represent Mean ± S.E.M. ###p < 0.001, ####p < 0.0001 when compared to normal control, *p < 0.05,**p < 0.01, ***p < 0.001, ****p < 0.0001 when compared to disease control, using one way ANOVA followed by Bonferroni’s multiple comparison.
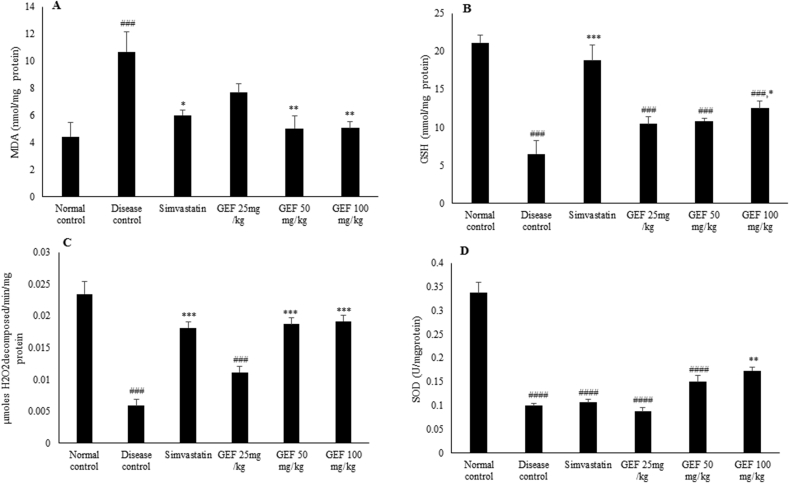
3.6. Effect of GEF treatment on endogenous anti-oxidant
Changes in lipid peroxidation and ox-redox status were measured by both enzymatic and non-enzymatic markers. Lipid peroxide, a marker of oxidative stress was significantly higher (p < 0.001) in disease control animals. Simvastatin and GEF (50 and 100 mg/kg) significantly lowered (p < 0.05 and p < 0.01 respectively) this increase in lipid peroxidation, though it was on higher side as compared to normal animals (Fig. 4A). GSH was significantly depleted (p < 0.001), in disease control animals. Simvastatin and GEF (100 mg/kg) treatment significantly increased (p < 0.001 and p < 0.05 respectively) GSH concentration, though it did not reach the concentration as that of normal control animals (Fig. 4B).
Fig. 4.
Oxido-redox state in the tissues of experimental animals, A-Malondialdehyde concentrations, B-GSH (Reduced Glutathione) concentrations, C-Activity of Catalase and D-Activity of Superoxide dismutase. Each value represent Mean ± S.E.M. ###p < 0.001, ####p < 0.0001 when compared to normal control,*p < 0.05, **p < 0.01, ***p < 0.001 when compared to disease control, using one way ANOVA followed by Bonferroni’s multiple comparison.
Activity of catalase and SOD were significantly lowered (p < 0.001 and p < 0.0001 respectively) in disease control animals. Treatment with Simvastatin and GEF (50 and 100 mg/kg) was able to significantly (p < 0.001) restore the reduction in Catalase and the activity was comparable to that of normal control animals (Fig. 4C). Only 100 mg/kg dose of GEF could significantly increase (p < 0.01) the activity of SOD (Fig. 4D). GEF thus reduced the oxidative stress response.
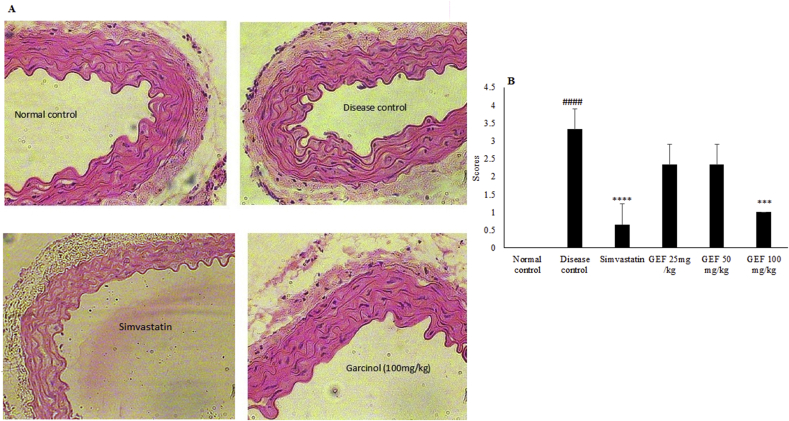
3.7. Effect of GEF treatment on histology of aorta
The histological architecture and thickness of all layers of the aorta was intact in normal control animals. Also there was no fatty deposit or cellular debris on the inner lining of tunica interna in this group. The histopathology of aorta for disease control animals showed thickening of vessel wall. The thickness of the tunica media was much more than that of tunica interna due to proliferation of smooth muscle cells (Fig. 5A). Treatment with Simvastatin and GEF (100 mg/kg) reduced these pathological changes and the histological architecture of thoracic aorta was found to be normal (Fig. 5A). The damage to aorta was based on a scoring system (Fig. 5B). The disease control animals had a score of 3.33. Treatment with GEF (25 and 50 mg/kg) improved the score to 2.33 whereas Simvastatin and GEF (100 mg/kg) treatment showed completely normal vascular structure with a score of 0.66 and 1 respectively, indicating that the treatment improves the vascular architecture and thus halts the process of atherosclerosis.
Fig. 5.
Effect of various treatments on (A) histopathological changes and (B) scores in thoracic aorta of mice using H&E stain (magnification x 400). Each value represent Mean ± S.E.M. ####p < 0.0001 when compared to normal control, ***p < 0.001, ****p < 0.0001 when compared to disease control, using one way ANOVA followed by Bonferroni’s multiple comparison.
4. Discussion
The key finding of the study is that GEF had a positive effect in correcting dyslipidemia caused due to administration of modified western diet in experimental animals. It also improved the lipid ratios indicating its usefulness in reducing the risk of atherosclerosis. Secondly, GEF showed anti-inflammatory effect as it significantly lowered the tissue and plasma levels of IL-6, which has direct involvement in exacerbating early atherosclerosis in mice.20 Lastly, GEF displayed anti-oxidant activity as it increased the levels of GSH, enhanced the activity of Catalase and SOD and lowered MDA levels in the tissue. Bearing in mind the fact that GEF also has anti-inflammatory and antioxidant, it can be useful to halt the progression and possibly reverse the symptoms of atherosclerosis.
Lee et al.21 have reported the weight lowering effect of Garcinol in high fat diet fed mice. On similar lines GEF also reduces the body weight of animals in this study which points to its anti-obesity effect and is not suggestive of toxicity of GEF.
GEF was able to reverse the hyperlipidemic changes in mice. It is a well-known fact that HDL has an inverse correlation with atherosclerosis. Low HDL-C reflects an increase in triglyceride rich lipoprotein (TRL), the main carriers of TG. Elevated levels of both TG and TRL are risk factors for CVD’s and vascular inflammation.22 Therapy with statins may not always lower the TG levels,23 which was also observed during this study (Fig. 2A). Any novel therapy therefore, should act dually to decrease the TRL/TG and improve HDL cholesterol.24This study has shown that GEF acts in this dual manner (Fig. 2A and 2B). GEF treatment also lowered LDL-C, an effect known to decrease the risk of atherosclerosis and subsequent cardiovascular events.25
Inflammation is one of the mechanism involved in progression of atherosclerosis. After the induction of dyslipidemia in experimental animals, inflammatory pathways get activated,26 which in turn can initiate formation of atheroma and associated complications.3 Several inflammatory markers are reported to be involved in progression of atherosclerosis, however none is specific. Though, they just represent a non-specific inflammatory state, targeting inflammation has been reported to be beneficial in reducing atherosclerosis progression.27,28 In this study, the effect of GEF on a major inflammatory marker IL-6 involved in atherosclerosis progression was investigated. IL-6 is an upstream cytokine responsible for the formation of many downstream inflammatory mediators responsible for atherosclerosis. Higher levels of IL-6 found in this condition causes the release of acute phase reactants, endothelial cell injury, pro-thrombotic effect on platelets, and promotion of lymphocyte & smooth muscle proliferation and differentiation and macrophage lipid accumulation.29,30 Thus, targeting IL-6 would be favorable in treatment of atherosclerosis. GEF treatment significantly lowered the plasma as well as tissue levels of IL-6, thus reducing the inflammatory response and exerting beneficial effect against atherosclerosis progression.
Modified western diet triggered oxidative stress response in experimental animals and consequently increased the lipid peroxidation and reduced antioxidant enzymes- Catalase and SOD. Administration of antioxidants can attenuate this oxidative stress and reduce atherosclerotic lesions.31We observed that GEF treatment showed a significant decrease in lipid peroxidation and a marked increase in GSH, Catalase and SOD. Due to this antioxidant capacity of GEF, it effectively reduced the oxidative stress response and possibly the atherosclerotic lesions.
Lipid deposition in the vessel wall and smooth muscle cell proliferation contributed to increased thickness of the aorta, which is regarded as another risk factor for atherosclerosis.32 GEF mediated regression of dyslipidemia reduced the deposition of lipids in the vessel wall and consequently reduced the thickness of the intimal and medial layer of the aorta (Fig. 5A). Thus, GEF attenuated one more risk factor for atherosclerosis.
5. Conclusions
GEF showed a dose dependent amelioration of hyperlipidemia, a risk factor for atherosclerosis and also modified the underlying pathological mechanisms namely oxidative stress and inflammation, in modified high fat diet fed experimental animals. 100 mg/kg dose was the most effective dose to control all risk factors. Thus, GEF could be a promising candidate in the treatment of atherosclerosis due to its lipid lowering, antioxidant and anti-inflammatory effect.
Natural Products
Taxonomy (classification by EVISE): Traditional herbal medicine, Ayurveda, Cardiovascular disease, Hyperlipidemia, Atherosclerosis.
Declaration of competing interest
The author declares no conflict of interest.
Acknowledgements
The author would like to acknowledge Shobhaben Pratapbhai Patel School of Pharmacy and Technology management SVKM’s NMIMS, Mumbai , India for funding the project, Dr. Lokesh Bhatt, Associate Professor, SVKM’s Dr. Bhanuben Nanvati College of Pharmacy, Mumbai University and Mr. Sachin Suryavanshi, Shobhaben Pratapbhai Patel School of Pharmacy and Technology management, SVKM’s NMIMS, for the timely support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.11.001.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Weber C., Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 2.Pahwa R., Jialal I. StatPearls [Internet], Treasure Island (FL): StatPearls Publishing. 2019. Atherosclerosis.https://www.ncbi.nlm.nih.gov/books/NBK507799/P Available from: [Google Scholar]
- 3.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. 115–126. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous . Charak Samhita. 2019. Sutra sthana.http://www.carakasamhitaonline.com/mediawiki-1.32.1/index.php?title=Shadvirechanashatashritiya_Adhyaya#Group_VII:_Five_subgroups [Google Scholar]
- 6.Hong J., Sang S., Park H.J. Modulation of arachidonic acid metabolism and nitric oxide synthesis by garcinol and its derivatives. Carcinogenesis. 2006;27:278–286. doi: 10.1093/carcin/bgi208. [DOI] [PubMed] [Google Scholar]
- 7.Koeberle A., Northoff H., Werz O. Identification of 5-lipoxygenase and microsomal prostaglandin E 2 synthase-1 as functional targets of the anti-inflammatory and anti- carcinogenic garcinol. Biochem Pharmacol. 2009;77:1513–1521. doi: 10.1016/j.bcp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Padhye S., Ahmad A., Oswal N., Sarkar F.H. Emerging role of Garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs. J Hematol Oncol. 2009;1:2. doi: 10.1186/1756-8722-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong R.C.M., Ewing M.M., de Vries M.R. The epigenetic factor PCAF regulates vascular inflammation and is essential for intimal hyperplasia development. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0185820. 0185820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warriar P., Barve K., Prabhakar B. Anti-arthritic effect of garcinol enriched fraction against adjuvant induced arthritis. Recent Pat Inflamm Allergy Drug Discov. 2019;13:49–56. doi: 10.2174/1872213X12666181120091528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao L., Heuser-Baker J., Herlea-Pana O. Bone marrow endothelial progenitors augment atherosclerotic plaque regression in a mouse model of plasma lipid lowering. Stem Cells. 2012;30:2720–2731. doi: 10.1002/stem.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin. J Biol Chem. 1951;193:265–275. doi: 10.1016/0304-3894(92)87011-4. [DOI] [PubMed] [Google Scholar]
- 13.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Shaikh S., Kumar L., Barve K. Attenuation of isoproterenol-induced cardiotoxicity in rats by Narirutin rich fraction from grape fruit. Phytomedicine. 2019;55:222–228. doi: 10.1016/j.phymed.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 15.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 16.Catalase Luck H. In: Methods of Enzymatic Analysis. Bergmeyer H.S., editor. Academic Press; New York and London: 1965. pp. 885–894. [Google Scholar]
- 17.Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 18.Paoletti F., Mocali A., Aldinucci D. Superoxide-driven NAD(P)H oxidation induced by EDTA-manganese complex and mercaptoethanol. Chem Biol Interact. 1990;76:3–18. doi: 10.1016/0009-2797.(90)90030-Q. [DOI] [PubMed] [Google Scholar]
- 19.Sozański T., Kucharska A.Z., Szumny A. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine. 2014;21:1774–1784. doi: 10.1016/j.phymed.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Huber S.A., Sakkinen P., Conze D., Hardin N., Tracy R. Interleukin-6 exacerbates early arteriosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. https://www.ahajournals.org/doi/10.1161/01.ATV.19.10.2364 [DOI] [PubMed] [Google Scholar]
- 21.Lee P.S., Teng C.Y., Kalyanam N., Ho C.T., Pan M.H. Garcinol reduces obesity in high-fat-diet-fed mice by modulating gut Microbiota composition. Mol Nutr Food Res. 2019;63 doi: 10.1002/mnfr.201800390. e1800390. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz E.A., Reaven P.D. Lipolysis of triglyceride-rich lipoproteins, vascular inflammation, and atherosclerosis. Biochim Biophys Acta Mol Cell Biol Lipids. 2012;1821:858–866. doi: 10.1016/j.bbalip.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118:138–145. doi: 10.1016/j.amjcard.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Parhofer K.G. Increasing HDL-cholesterol and prevention of atherosclerosis: a critical perspective. Atheroscler. 2015;18:109–111. doi: 10.1016/j.atherosclerosissup.2015.02.020. Suppl. [DOI] [PubMed] [Google Scholar]
- 25.Baigent C., Blackwell L., Emberson J. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trial. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736.(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lira F.S., Rosa Neto J.C., Antunes B.M., Fernandes R.A. The relationship between inflammation, dyslipidemia and physical exercise: from the epidemiological to molecular approach. Curr Diabetes Rev. 2014;10:391–396. doi: 10.2174/1573399810666141122210135. [DOI] [PubMed] [Google Scholar]
- 27.Takeshi S., Masataka S. Inflamm. Biomark. Atheroscler. 2016;57(2):134–139. doi: 10.1536/ihj.15-346. [DOI] [Google Scholar]
- 28.Ruparelia N., Chai J.T., Fisher E.A., Choudhury R.P. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017;14(3):133–144. doi: 10.1038/nrcardio.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartman J., Frishman W.H. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22:147–151. doi: 10.1097/CRD.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 30.Reiss A.B., Siegart N.M., De Leon J. Interleukin-6 in atherosclerosis: atherogenic or atheroprotective? Clin Lipidol. 2017;12:14–23. doi: 10.1080/17584299.2017.1319787. [DOI] [Google Scholar]
- 31.Kaliora A.C., Dedoussis G.V.Z., Schmidt H. Dietary antioxidants in preventing atherogenesis. Atherosclerosis. 2006;187:1–17. doi: 10.1016/j.atherosclerosis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Sakakura K., Nakano M., Otsuka F., Ladich E., Kolodgie F.D., Virmani R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013;22:399–411. doi: 10.1016/j.hlc.2013.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.