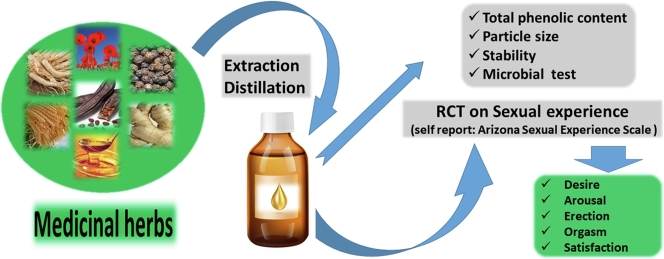
Abstract
Background and aim
An Aphrodisiac includes any drug and food that arouses sexual instinct, induces venereal desire, and increases pleasure and performance. The present study was designed to clinically evaluate efficacy and safety of Herbal Based Syrup (HBS) composed of Tribulus terrestris L., Panax ginseng C.A. Meyer., Zingiber officinale Rosc, Ceratonia siliqua L., Papaver rhoeas L., and Palm tree pollen on sexual experience of men.
Experimental procedure
The study was designed as a double-blind randomized clinical trial. The main outcome measures were the responses obtained from using the Arizona Sexual Experience Scale (ASEX). The ASEX was completed by 100 married and seemingly healthy men before and after taking one dose of HBS or placebo and at least one sexual intercourse. In addition, possible side effects were observed. A split-plot ANOVA (SPANOVA) design was used for statistical analysis.
Results
Results of analysis of data for each variable of the ASEX showed significantly lower scores in HBS-treated group compared to the placebo (control) group in items of desire, arousal, erection, orgasm and satisfaction (p < 0.05). No drug-related serious adverse events were observed.
Conclusion
Results of this study indicated a significant improvement in sexual experience of men following consumption of HBS. Due to various complications reported about the use of chemical sexual drive enhancers, HBS can be introduced as an alternative with fewer side effects.
Keywords: Natural product, Sexual desire, Libido, Traditional Persian medicine, Aphrodisiac
Graphical abstract
Highlights
-
•
The syrup enhances sexual desire, arousal, erection, orgasm and satisfaction.
-
•
The syrup may be a useful supplement to improve sexual experience of healthy men.
-
•
The syrup was stable more than 1 year in room temperature.
List of abbreviations
- ASEX
Arizona sexual experience scale
- Co. No
Company number
- TPC
Total phenolic content
- Min
Minutes
- RH
Relative humidity
- h
hours
- IRCT
Iranian Registry of Clinical Trials
- CTCAE
Common terminology criteria for adverse event
- GAE
Gallic acid equivalent
- TIM
Traditional Iranian medicine
1. Introduction
The human beings have looked for the compounds to increase their sexual desire, potency,and pleasure since ancient time.1 These compounds are commonly known as aphrodisiac agents and they are aften recommended as foods, supplements, or medicines.2 A Herbal Based Syrup (HBS) as a natural product comprising of sevaral medicinal plants including Tribulus terrestris L., Panax ginseng C.A. Meyer, Zingiber officinale Rosc, Ceratonia siliqua L., Papaver rhoeas L., and Palm tree pollen traditionally has been used to boost sexual drive or improve sexual experiences in some parts of Iran. HBS has been recommended to be used by men to improve quality of sexual relationship. Numerous studies have shown the effect of HBS components on sexual disorders.3,4 In Traditional Iranian Medicine (TIM), T. terrestris has been found to cause enhanced sexual potency, arousing sexual desire, and sperm production.3,5 Numerous studies have reported potential and positive effects of P. ginseng containing ginsenosides as its main active ingredient in improvement of various disorders including Erectile Dysfunction(ED).6 Results of a study have shown the effect of Z. officinale on sperm count and average sperm velocity.7 Also, in TIM, ginger is used to strengthen the stomach, liver, brain, and libido.8,9 The fruit of C. siliqua is known as a medication for treatment of infertility in traditional Turkish medicine, and recent evidence shows its positive effects on health factors and male fertilization.10,11 In TIM, fruit derivatives of carob alone or in combination with other herbs have been used to treat bleeding, gastrointestinal,and kidney problems.9,12 Extract of aerial parts of P. rhoeas contains alkaloids that have antioxidant properties, in this regard, previous studies demonstrated ovarian stimulation and sedative effects as a result of applying this plant on human and animal models.13,14 TIM references, have mentioned a number of digestive, diuretic, sedative,and topical anti-inflammatory properties for this plant.9,12 In TIM, Palm tree pollen (Phoenix dactylifera L.) has been expressed as an effective sexual arousal provoker.9,12 Recent investigations considerably support its effect on increasing fertility in men and even infertility treatment.15,16
Non-documented reports and pilot tests revealed that, HBS has a significantly positive effect on libido and sexual satisfaction of men. Noteworthily, no significant or serious complications have been reported after consumption of HBS. Therefore, the present study was conducted to evaluate the efficacy and safety of HBS on sexual experience of adult men.
2. Materials and methods
2.1. Preparation and physicochemical characterization of HBS
2.1.1. Preparation of materials
Herbal ingredients and Honey were purchased from traditional herbal shop as mentioned in Table 1. To assess quality of herbs, total ash and humidity content were determined. For syrup preparation, total water extracts of plants were prepared by maceration assay and required amounts of each component were mixed and packed in 60 mL bottles.
Table 1.
Characteristics of ingredients of HBS.
| Scientific name | Common name | Persian name | Family name | Used part | Type of extraction |
|---|---|---|---|---|---|
| Ceratonia siliqua | Carob | Kharnoob | Fabaceae | Seed | Water maceration |
| Honey | Honey | Asal | – | – | – |
| Panax ginseng | Ginseng | Ginseng | Araliaceae | Rhizome | Water maceration |
| Papaver rhoeas | Common poppy | Shaghayegh | Papaveraceae | Flower | Water maceration |
| Phoenix dactylifera | Date palm | Tamr | Arecaceae | Flower | Water maceration |
| Tribulus terrestris | Caltrop | Hasak | Zygophyllaceae | Aerial part | Distillate |
| Zingiber officinale | Ginger | Zanjafer | Zingiberaceae | Rhizome | Water maceration |
2.1.2. Determination of total phenol content
Total Phenolic Content (TPC) of HBS was determined spectrophotometrically using the Folin-Ciocalteu reagent (FCR) assay with gallic acid (150–500 μg/mL) as standard according to the method reported in the literature.17 In brief, 100 μL of sample was mixed with 500 μL of FCR. Afterwards, 400 μL of Na2CO3 (7.5% w/v) was added. After 30 min of incubation, absorbance was measured at 765 nm. To determine chemical stability of formulation, TPC was measured during 3 months after preparation.
2.1.3. Measurement of particle size
Syrup size was measured by laser light scattering method in fresh preparation and during 3 months after preparation for stability measurements. Fundamental particle size distribution derived from this technique was volume-based.18
2.1.4. Studying stability
Stability of selected formulations was assessed in terms of size, constituent separation, as well as TPC. As far as accelerated and intermediate stability testing condition is concerned, formulations were stored under two conditions including a temperature of 25 °C with Relative Humidity (RH) of 30%, and a temperature of 40 °C with RH of 70%. Then, suspensions were evaluated during 24 h, 1, and 3 months after preparation.
2.1.5. Preparation of placebo
The placebo was designed to be similar to HBS in terms of consistency, opacity, color, and packaging without any special odor coming from packed bottles. Placebo solution was prepared using water, mint distillate (1mL/60 mL), carboxymethyl cellulose powder (0.04 g/60 mL), and sugar (10 g/60 mL), and was packed in the bottles similar to those of HBS.
3. Experimental design
3.1. Participants
The current study was a double-blind randomized placebo-controlled clinical trial. This study was approved by the Medical Ethics Committee of Kerman University of Medical Sciences (IR.KMU.REC.1397.398) and was registered in the Iranian Registry of Clinical Trials (IRCT 20181025041462N1). The study was conducted in February 2019 at Nuriye Psychiatric Hospital (Kerman, Iran). Participants were included in the study through an announcement published on information boards of the hospital. An informed written consent was obtained from all the subjects before entering the study, and all the procedures were performed in accordance with the Declaration of Helsinki. Inclusion criteria were being male and married, having the age between 21 and 60 years old with a regular sexual activity. Subjects were excluded if they had severe or uncontrolled diseases, mental disorders, known case of sexual dysfunction disorder history of allergy to syrup components, or they used alcohol or narcotics in the last week before the study, and had any prohibition of sexual activity.
3.2. Randomization, hidden distribution, and blindness
For randomization, permuted block randomization was applied using quadruple blocks. The pharmacist gave the HBS and placebo to the interviewer along with specific codes and matched packages with a ratio of 1:1. The interviewer and participants were blinded to the allocation. Participants were not aware of group assignment. The questionnaires were decoded by the pharmacist at the end of the study.
3.3. Outcomes
The main outcome was sexual experience measured using the ASEX, which is a self-reporting questionnaire consisting of 5 items with a 6-point Likert scale ranging from 1(hyperfunction) to 6 (hypofunction).19 This questionnaire has been adopted in Persian context.20 The subjects completed the questionnaire twice, once at the beginning of the study, and once after consumption of one dose of HBS or placebo with having at least one sexual intercourse. To record Adverse events, a self-reported data collection form was attached to the second ASEX questionnaire. Accordingly, side effects were reported using the Common Terminology Criteria for Adverse Event (CTCAE) Version 5. There was no alteration or adjustment in the methods after trial beginning.
3.4. Intervention
After face-to-face interview and evaluating eligibility of the volunteers for participation in the study and presenting complementary explanations by the interviewer, the participants were asked to sign the consent form and complete the Arizona Sexual Experience Scale (ASEX). Afterwards, one bottle of HBS or placebo was given to the individuals. They were advised to consume HBS/placebo 7–12 h before sexual intercourse. Every 3 days, the subjects were contacted by phone by a researcher and they were asked whether or not they used HBS/placebo. In this regard, participants who did not receive HBS/placebo for up to 3 weeks were excluded from the study. After consuming HBS/placebo, each participant was asked to complete the ASEX questionnaire for second time.
3.4.1. Sample size
Considering Type I and Type II errors as 5% and 20%, and effect size as 30% on reduction of an average score of ASEX, sample size in each group was considered as 50 using G Power software (V 3.1.3 Franz Faul, Universität Kiel, Germany).
3.5. Statistical analysis
Subjects were randomly assigned to HBS or placebo groups. Before entering the study, the two groups were compared via Chi-Square test in terms of available demographic information including education level and age. A split-plot ANOVA (SPANOVA) design was used with respect to the research objective, nature of variables under study, and the need for combining a between-subject independent variable and a within-subject dependent variable. Basic assumptions of the analysis included homogeneity of variances and normal distribution of dependent variable. The sphericity assumption was tested using Mauchly’s test of sphericity. The compound symmetry assumption was examined and alternative approaches were ignored considering adoption of this assumption. The inter-group, intra-group, and their interaction were reported as the main results. All statistical tests were done with SPSS version 22.00.
4. Safety assessment
All subjects were informed about the side effects and benefits of the medication during the study. A printed form was given to all participants to record possible adverse events after consuming of the HBS or placebo. Patients brought this form with them on follow up visits. They were also given the telephone number of the first author and the interviewer to report adverse effects in emergency states.
5. Results
5.1. Physicochemical properties of HBS
Table 2 presents physical properties of HBS ingredients. TPC of HBS was equal to 133.29 ± 2.02 mg GAE/1.0 mL. In addition, mean volume diameter of HBS particles was equal to 67.05 ± 2.23. A little degradation was observed in the size attributing to dissolution of large or aggregated particles (Table 3).
Table 2.
Physical characteristics of ingredients in HBS.
| Plants name | The amount used (%) | Total ash (%) | Humidity content (%) |
|---|---|---|---|
| Caltrop | 20 | 5.8 ± 1.3 | 3.5 ± 0.8 |
| Ginseng | 20 | 2.7 ± 0.4 | 7.14 ± 1.1 |
| Ginger | 20 | 4.9 ± 0.6 | 5.41 ± 0.6 |
| Carob | 10 | 4.0 ± 0.4 | 5.2 ± 0.7 |
| Common poppy | 10 | 4.6 ± 0.5 | 5.6 ± 0.6 |
| Date palm | 5 | 6.4 ± 1.0 | 3.2 ± 0.5 |
| Honey | 15 | – | – |
Table 3.
Effect of storage on changes in size and total phenolic content of HBS at different time intervals after preparation.
| Storage condition | Total phenolic content (mg GAE±SD) |
Particle size (μm±SD) |
||||
|---|---|---|---|---|---|---|
| 24 h | 1month | 3months | 24 h | 1month | 3months | |
| 25 °C, 30%RH | 133.29 ± 2.02 | 131.40 ± 2.91 | 123.54 ± 3.10 | 67.05 ± 2.23 | 62.51 ± 5.32 | 59.70 ± 6.63 |
| 40 °C, 70%RH | – | 130.12 ± 1.67 | 119.32 ± 2.05 | – | 58.12 ± 3.44 | 41.66 ± 7.32 |
μm: micrometer, GAE: gallic acid equivalent, SD: standard deviation, RH: relative humidity.
5.2. Experimental results
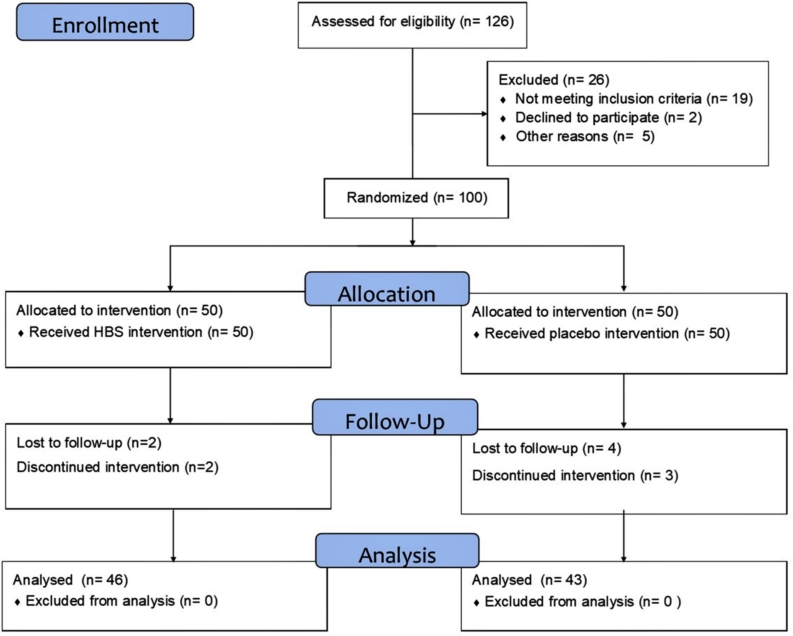
Among 126 volunteers, 100 individuals were qualified to participate in the study. Finally, 7 people in the placebo group and 4 people in HBS-treated group were excluded. 5 participants (2 people in HBS-treated group and 3 people in the placebo group) refused to answer the phone call or return to the center after receiving HBS/placebo. 4 participants (2 people in the HBS-treated group and 2 people in the placebo group) did not consume HBS/placebo. 2 people in the placebo group did not have sexual intercourse for 3 weeks after starting the study (Fig. 1).
Fig. 1.
The flow diagram of progress in parallel-randomized trial according to the CONSORT Guidelines.
Table 4 presents results of Chi-Square test regarding no statistical differences between the two study groups in terms of their education level and age.
Table 4.
Socio-demographic characteristics of the participants.
| Characteristic | Placebo(%)(N = 43) | HBS(%)(N = 46) | P value | ||
|---|---|---|---|---|---|
| Age | 20–29 | 5(11.6) | 3(6.5) | 0.293 | |
| 30–39 | 18(41.9) | 15(32.6) | |||
| 40–49 | 9(20.9) | 18(39.1) | |||
| 50–60 | 11(25.6) | 10(21.7) | |||
| Education | Under diploma | 8(18.6) | 6(13) | 0.703 | |
| Diploma | 20(46.5) | 19(41.3) | |||
| Post-diploma | 9(20.9) | 11(23.9) | |||
| License & up | 6(14) | 10(21.7) | |||
As shown in Table 5, there was a statistically significant difference between HBS-treated and placebo groups in items of sexual desire, arousal, erection, orgasm, and satisfaction (P < 0.05).
Table 5.
Comparison of scores obtained for each variable in the ASEX in two study groups before and after consumption of HBS with at least one sexual intercourse.
| Index | Pre-test (Mean ± SD) |
Post-test (Mean ± SD) |
Mean Square | df | F | P value | ||
|---|---|---|---|---|---|---|---|---|
| Placebo | HBS | Placebo | HBS | |||||
| Desire | 3.46 ± 1.27 | 3.25 ± 1.25 | 3.39 ± 1.21 | 1.56 ± 0.58 | 29.731 | 1.00 | 21.234 | 0.000 |
| Arousal | 3.32 ± 1.32 | 3.13 ± 1.30 | 3.36 ± 1.23 | 1.60 ± 0.74 | 17.085 | 1.00 | 9.644 | 0.003 |
| Erection | 3.02 ± 1.07 | 2.90 ± 0.94 | 3.30 ± 1.20 | 1.73 ± 0.80 | 12.513 | 1.00 | 12.035 | 0.001 |
| Orgasm | 3.30 ± 0.98 | 3.11 ± 1.09 | 3.15 ± 1.03 | 1.84 ± 0.86 | 23.946 | 1.00 | 18.143 | 0.000 |
| Satisfaction | 3.41 ± 1.11 | 3.20 ± 1.03 | 3.15 ± 1.03 | 1.80 ± 0.95 | 8.874 | 1.00 | 5.854 | 0.018 |
Pre-test: Results obtained before consuming of HBS and placebo, Post-test: Results obtained after consuming HBS and placebo, ASEX: Arizona Sexual Experience Scale Questionnaire.
Table 6 presents the results of split-plot ANOVA (SPANOVA) analysis of variance to investigate of within-group effects (before and after intervention), inter-group effects (HBS and placebo), and interactive effects aimed to investigate the effect of using HBS on various sexual experience items in men of different ages. There was a statistically significant difference between the two groups in the effect of HBS and placebo on items of sexual desire, arousal, satisfaction, erection and orgasm (p < 0.05). No statistical difference was observed in the obtained results regarding the effect on age between HBS-treated and placebo groups. There was a statistically significant difference between mean scores of ASEX items in all age groups after consuming HBS (p < 0.05). A statistically significant difference was also found in all studied items between HBS-treated and placebo groups (p < 0.05). (Table 6).
Table 6.
Comparison of scores obtained for each variable in the ASEX in two study groups based on age before and after consumption of HBS with at least one sexual intercourse.
| Index | Age | Pre-test (Mean ± SD) |
Post-test (Mean ± SD) |
|||||
|---|---|---|---|---|---|---|---|---|
| Placebo | HBS | Placebo | HBS | |||||
| Desire | 20–30 | 3.40 ± 1.14 | 2.67 ± 1.15 | 3.00 ± 0.71 | 1.33 ± 0.58 | <0.001 | <0.001 | <0.001 |
| 30–40 | 2.83 ± 0.71 | 2.73 ± 0.80 | 2.56 ± 0.62 | 1.33 ± 0.62 | ||||
| 40–50 | 3.22 ± 1.72 | 3.17 ± 0.92 | 3.22 ± 1.56 | 1.61 ± 0.50 | ||||
| 50–60 | 4.73 ± 0.79 | 5.00 ± 0.82 | 4.55 ± 1.04 | 1.90 ± 0.57 | ||||
| Arousal | 20–30 | 3.20 ± 1.78 | 1.33 ± 0.57 | 2.40 ± 1.51 | 2.00 + 1.00 | <0.001 | 0.001 | <0.001 |
| 30–40 | 3.22 ± 1.26 | 2.86 ± 0.99 | 3.16 ± 1.29 | 1.33 ± 0.81 | ||||
| 40–50 | 2.44 ± 1.01 | 3.38 ± 0.91 | 2.44 ± 1.01 | 1.44 ± 0.51 | ||||
| 50–60 | 4.27 ± 0.90 | 4.70 ± 0.82 | 4.00 ± 1.00 | 2.20 ± 0.63 | ||||
| Erection | 20–30 | 2.40 ± 0.89 | 1.33 ± 0.57 | 3.00 ± 0.70 | 1.00 | <0.001 | 0.001 | 0.862 |
| 30–40 | 2.61 ± 0.77 | 2.93 ± 1.16 | 2.61 ± 0.77 | 1.46 ± 0.63 | ||||
| 40–50 | 2.66 ± 1.00 | 3.33 ± 1.02 | 2.33 ± 0.86 | 1.61 ± 0.50 | ||||
| 50–60 | 4.27 ± 0.64 | 4.40 ± 0.51 | 3.81 ± 0.75 | 2.60 ± 0.96 | ||||
| Orgasm | 20–30 | 3.20 ± 1.43 | 1.33 ± 0.57 | 3.20 ± 1.48 | 1.00 | <0.001 | 0.006 | 0.019 |
| 30–40 | 3.38 ± 0.97 | 2.93 ± 0.96 | 3.33 ± 1.08 | 1.66 ± 0.48 | ||||
| 40–50 | 2.66 ± 0.86 | 3.33 ± 0.97 | 2.33 ± 1.00 | 1.44 ± 0.51 | ||||
| 50–60 | 3.90 ± 0.70 | 3.80 ± 0.63 | 3.54 ± 0.93 | 3.10 ± 0.73 | ||||
| Satisfaction | 20–30 | 2.60 ± 0.89 | 2.33 ± 1.52 | 2.50 ± 1.06 | 2.00 ± 1.73 | <0.001 | 0.009 | 0.006 |
| 30–40 | 2.83 ± 1.09 | 2.80 ± 1.08 | 2.81 ± 1.07 | 1.53 ± 0.83 | ||||
| 40–50 | 2.77 ± 1.20 | 3.33 ± 0.97 | 3.14 ± 1.06 | 1.38 ± 0.50 | ||||
| 50–60 | 3.63 ± 0.50 | 3.30 ± 0.67 | 3.47 ± 0.60 | 2.60 ± 0.88 | ||||
P*: Before and after intervention (Tests of Within-Subjects Effects).
P**: Tests of Between -Group Effects and Within-Subjects Effects (combined test of inter- and intra-group effects).
P***: Time * group * age.
6. Side effects
In HBS-treated group, 5 cases reported mild to moderate headache, one case presented mild back pain, and one case reported muscle cramp. In the placebo group, 2 cases reported low back pain and one case reported heartburn. No serious drug-related adverse complications were observed. There was no significant difference in incidence of adverse events between the two study groups.
7. Discussion
Results obtained from analysis of ASEX data showed that, HBS has a positive effect on sexual experiences of men and significantly improved some aspects of sexual function including desire, arousal, orgasm, erection and satisfaction. In this study, HBS was evaluated clinically as a total preparation. However, the possible mechanism of HBS influence can be discussed by tracing the roles of its constituents.
Sexual arousal initiates a process resulting in release of Nitric Oxide (NO) from nerve terminals and endothelial cells in the corpus cavernosum. NO triggers a cGMP-dependent cascade of events leading to smooth-muscle relaxation in the corpus cavernosum, resulting in increased blood flow and penile erection.21 Phosphodiesterase-5 (PDE5) inhibitor, as an enzyme metabolizes cGMP to counter-act this effect and decrease muscle relaxation. Testosterone, as a hormone influences various aspects of sexual function22,23 and it seems to up-regulate PDE-5 causing longer smooth muscle relaxation in the corpus cavernosum.23 There is some evidence that might explain efficacy of HBS on sexual function. Effect of T. terrestris on sexual and infertility disorders has been shown attributing to the increase in the testosterone levels mediated by steroidal saponins.24,25 Ginsenosides, as the most proposed ingredients of P. ginseng have been found to increase serum testosterone concentration, enhance NO release, and cGMP accumulation in corpus cavernosum in animal and in vitro studies.26,27 Some experimental studies reported an increase in serum levels of testosterone and improvement in sperm analysis parameters by oral taking of Z. officinale extract.28
In this study, HBS was used as nutritional syrup in men. HBS can be introduced as an alternative to other sex drive enhancers with fewer side effects. However, safety of frequent and long term uses needs to be further evaluated.
Therefore, it is suggested to design a clinical trial with a larger sample size and/or in a different geographic and cultural area in order to investigate the effect of HBS on sexual dysfunctions, evaluating the effects of all constituents of HBS and their underlying mechanisms, its possible side effects of prolonged or repeated use, and its efficacy on women in the future works.
8. Conclusion
Results of this study indicated considerable improvement in sexual experience of seemingly healthy men after consuming HBS. This syrup can be introduced as an alternative with fewer side effects.
Sources of funding
This research was financially supported by research grants provided from Taranom Exir Taha Co. (Iran, identification code: 14005104490).
Declaration of competing interest
The authors declare that, there are no known conflicts of interest associated with this publication, and it should be mentioned that, no significant financial support has been received for the present work that could have influenced its outcome.
Acknowledgements
The authors would like to appreciate all the managers and staff of Nuriye Psychiatric Hospital for their attention and cooperation in this project.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Sandroni P. Aphrodisiacs past and present: a historical review. Clin Auton Res. 2001;11(5):303–307. doi: 10.1007/BF02332975. [DOI] [PubMed] [Google Scholar]
- 2.Kruger T.H., Grob C., de Boer C. Placebo and nocebo effects in sexual medicine: an experimental approach. J Sex Marital Ther. 2016;42(8):721–739. doi: 10.1080/0092623X.2015.1113590. [DOI] [PubMed] [Google Scholar]
- 3.Nimrouzi M., Jaladat A.-M., Zarshenas M.M. A panoramic view of medicinal plants traditionally applied for impotence and erectile dysfunction in Persian medicine. J Tradit Complement Med. 2018;10(1):7–12. doi: 10.1016/j.jtcme.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malviya N., Malviya S., Jain S., Vyas S. A review of the potential of medicinal plants in the management and treatment of male sexual dysfunction. Andrologia. Oct 2016;48(8):880–893. doi: 10.1111/and.12677. [DOI] [PubMed] [Google Scholar]
- 5.West E., Krychman M. Natural aphrodisiacs—a review of selected sexual enhancers. Sex Med Rev. 2015;3(4):279–288. doi: 10.1002/smrj.62. [DOI] [PubMed] [Google Scholar]
- 6.De Andrade E., De Mesquita A.A., de Almeida Claro J. Study of the efficacy of Korean Red Ginseng in the treatment of erectile dysfunction. Asian J Androl. 2007;9(2):241–244. doi: 10.1111/j.1745-7262.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi S., Shah A.H., Tariq M., Ageel A.M. Studies on herbal aphrodisiacs used in Arab system of medicine. Am J Chin Med. 1989;17(1-2):57–63. doi: 10.1142/S0192415X89000103. [DOI] [PubMed] [Google Scholar]
- 8.Soltani E., Jangjoo A., Afzal Aghaei M., Dalili A. Effects of preoperative administration of ginger (Zingiber officinale Roscoe) on postoperative nausea and vomiting after laparoscopic cholecystectomy. J Tradit Complement Med. 2017;8(3):387–390. doi: 10.1016/j.jtcme.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aghili Khorasani M.H. Rah-e Kamal Publications; Tehran: 2009. Makhzan al-Advieh. [Google Scholar]
- 10.Vafaei A., Mohammadi S., Fazel A., Soukhtanloo M., Pour A.M., Beheshti F. Effects of carob (Ceratonia siliqua) on sperm quality, testicular structure, testosterone level and oxidative stress in busulfan-induced infertile mice. Pharm Sci. 2018;24(2):104–111. [Google Scholar]
- 11.Mahdiani E., Khadem Haghighian H., Javadi M., Karami A.A., Kavianpour M. Effect of Carob (Ceratonia siliqua L.) oral supplementation on changes of semen parameters, oxidative stress, inflammatory biomarkers and reproductive hormones in infertile men. Sci J Kurdistan Uni Med Sci. 2018;23(3):56–66. [Google Scholar]
- 12.Avicenna . Ehyaol Toras al-Arabi Press; 2010. Qanun fi al Tib [Canon of Medicine] Beiruot. [Google Scholar]
- 13.Afsaneh G.N., Hussein E., Firooz S. Effect of Papaver rhoeas extract on in vitro maturation and developmental competence of immature mouse oocytes. Reprod Med Biol. 2010;9(4):211–215. doi: 10.1007/s12522-010-0059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soulimani R., Younos C., Jarmouni-Idrissi S., Bousta D., Khalouki F., Laila A. Behavioral and pharmaco-toxicological study of Papaver rhoeas L. in mice. J Ethnopharmacol. 2001;74(3):265–274. doi: 10.1016/s0378-8741(00)00383-4. [DOI] [PubMed] [Google Scholar]
- 15.Abdi F., Roozbeh N., Mortazavian A.M. Effects of date palm pollen on fertility: research proposal for a systematic review. BMC Res Notes. 2017;10(1):363. doi: 10.1186/s13104-017-2697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehraban F., Jafari M., Akbartabar Toori M. Effects of date palm pollen (Phoenix dactylifera L.) and Astragalus ovinus on sperm parameters and sex hormones in adult male rats. Iran J reprod Med. 2014;12(10):705–712. [PMC free article] [PubMed] [Google Scholar]
- 17.Raeiszadeh M., Pardakhty A., Sharififar F., Mehrabani M. Phytoniosome: a novel drug delivery for myrtle extract. Iran J Pharm Res (IJPR) 2018;17(3):804. [PMC free article] [PubMed] [Google Scholar]
- 18.Raeiszadeh M., Pardakhty A., Sharififar F. Development, physicochemical characterization, and antimicrobial evaluation of niosomal myrtle essential oil. Res Pharm Sci. 2018;13(3):250. doi: 10.4103/1735-5362.228955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGahuey AJG A., Laukes Cindi A., Moreno Francisco A. The Arizona sexual experience scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26(1):25–40. doi: 10.1080/009262300278623. [DOI] [PubMed] [Google Scholar]
- 20.Raisi F. Arizona sexual experience scale (asex): Persian translation and cultural adaptation: 851. J Sex Med. 2011;8:296–297. [Google Scholar]
- 21.Huang S.A., Lie J.D. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. Pharmacol Ther. 2013;38(7):407–419. [PMC free article] [PubMed] [Google Scholar]
- 22.Isidori A.M., Giannetta E., Gianfrilli D. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol. 2005;63(4):381–394. doi: 10.1111/j.1365-2265.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- 23.Singh S., Ali A., Singh R., Kaur R. Sexual abnormalities in males and their herbal therapeutic aspects. Pharmacologia. 2013;4(4):265–275. [Google Scholar]
- 24.Sanagoo S., Sadeghzadeh Oskouei B., Gassab Abdollahi N., Salehi-Pourmehr H., Hazhir N., Farshbaf-Khalili A. Effect of Tribulus terrestris L. on sperm parameters in men with idiopathic infertility: a systematic review. Complement Ther Med. 2019;42:95–103. doi: 10.1016/j.ctim.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 25.GamalEl Din S.F., Abdel Salam M.A., Mohamed M.S. Tribulus terrestris versus placebo in the treatment of erectile dysfunction and lower urinary tract symptoms in patients with late-onset hypogonadism: a placebo-controlled study. Urologia. 2018;86(2):74–78. doi: 10.1177/0391560318802160. [DOI] [PubMed] [Google Scholar]
- 26.Ying A., Yu Q.T., Guo L. Structural-activity relationship of ginsenosides from steamed ginseng in the treatment of erectile dysfunction. Am J Chin Med. 2018;46(1):137–155. doi: 10.1142/S0192415X18500088. [DOI] [PubMed] [Google Scholar]
- 27.Wang X., Chu S., Qian T., Chen J., Zhang J. Ginsenoside Rg1 improves male copulatory behavior via nitric oxide/cyclic guanosine monophosphate pathway. J Sex Med. 2010;7(2 Pt 1):743–750. doi: 10.1111/j.1743-6109.2009.01482.x. [DOI] [PubMed] [Google Scholar]
- 28.Shalaby M.A., Hamowieh A.R. Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats. Food Chem Toxicol : Int. J. publ. Br. Ind. Biol. Res. Assoc. 2010;48(10):2920–2924. doi: 10.1016/j.fct.2010.07.028. [DOI] [PubMed] [Google Scholar]