Abstract
Hematophagous invertebrates such as mosquitoes, leeches, mites, ticks, lice and bugs cause various problems for humans. Considering reports on insecticide resistance and requirements for improved environmental and toxicological profiles, there is a continuing need to discover and develop new insecticides and repellents. Ethnobotanical surveys of traditional plant-based repellents provide a direct method of identifying plants for potential use. During five field surveys in Bulang, Jinuo and Lahu villages between August 2018 and July 2019, semi-structured interviews were conducted with 237 informants (151 male, 86 female; mean age 63). Frequency of citation, use value, informant consensus factor and Jaccard index were employed to statistically analyze the collected data. A total of 709 use reports relating to 32 plant species and 71 remedies were collected. Similarities and differences between the three groups, as well as the Dai and Hani of Xishuangbanna, who were studied earlier, were shown through network analysis. These five ethnic groups living in the same area have a common understanding of traditional botanical knowledge against hematophagous invertebrates, but each group also possesses unique knowledge. Recording and protecting this traditional knowledge is potentially useful for protecting this cultural diversity and related biodiversity and can also have important practical applications. In this study, traditional knowledge provided us with many new potential plants for follow-up research for the development of new insecticides and repellents, among which Artemisia indica, Nicotiana tabacum and Clausena excavata are the most promising.
Keywords: Ethnobotanical survey, Insecticide, Repellent, Traditional knowledge
1. Introduction
Hematophagous invertebrates such as mosquitoes, leeches, mites, ticks, lice and bugs cause various problems for humans. They bite people and may cause blisters, necrosis or allergic reactions when they sting (Lavaud et al., 1999). Hematophagous parasites are also a common problem for livestock and are responsible for severe economic losses (Booppha et al., 2010; Campbell and Thomas, 1999). In addition, hematophagous invertebrates are capable of transmitting infectious pathogens between humans and leading to the spread of vector-borne diseases. Every year, globally, there are more than 700,000 deaths from vector-borne diseases, including malaria, dengue fever, Zika fever, yellow fever, chikungunya fever, Chagas disease and Japanese encephalitis (WHO, 2020). Apart from the potential deaths, these diseases can also cause substantial harm and hardship to humans, as many people who survive these infections are left permanently debilitated, disfigured, injured or blind (Chu et al., 2010; Pialoux et al., 2007). The economic burden of vector-borne diseases to both governments and households is significant and has been associated with relatively lower economic development (WHO, 2017).
Humans have used various methods to fight against hematophagous invertebrates. Traditionally, local plants have been used to protect people from stings. For instance, oils have been extracted from plant materials and applied to the body (Roberto et al., 2001). Burning plant materials, including wood and leaves, is also a common method to repel insects (Bockarie et al., 1994; Yang et al., 2006). In addition, toxic solutions are made by adding specific plant materials to water (De Boer et al., 2010). Since the 1940s, synthetic insecticides and repellents have been discovered and widely used. These chemicals have replaced traditional methods in most regions. Two core interventions that prevent and control vector-borne diseases are insecticide-treated nets and indoor residual spraying of insecticides (WHO, 2017). However, after decades of use, more than 550 pest insect species have developed resistance to one or more existing insecticides (Whalon et al., 2008). For example, mosquitoes have developed tolerance to DEET (N, N-diethyl-3-methylbenzamide) (10%) (Brown and Hebert, 1997), a repellent that prevents insect bites for up to 9 h (Tawatsin et al., 2006). In addition, environmental considerations require the continued discovery and development of new insecticides and repellents.
Today, traditional plant-based insect repellents cannot be feasibly used in an urban setting, but they can be used as sources of modern insecticides and repellents. Over several generations, researchers have screened plants that may act as natural repellents and characterized their activities and toxicities. Good examples of effective modern products developed from traditional plants include pyrethrum and neem (Debboun et al., 2006). A small number of people in remote rural areas currently use traditional methods to control mosquitoes and other hematophagous invertebrates due to their low cost (Gakuya et al., 2013; Kweka et al., 2008). At the same time, due to improvements in living standards and the underestimation of the value of this kind of knowledge, traditional knowledge of insect control is rapidly being lost (González et al., 2011).
Three main ethnic groups---the Bulang, Jinuo and Lahu---have been living in the mountainous area of Xishuangbanna for several centuries. Before the 1950s, these ethnic minority communities all lived in the mountainous areas of Xishuangbanna, mainly engaged in swidden agriculture. Because this type of farming is not highly productive, hunting and gathering have also been traditionally important activities for these communities. Through long-term forest life, these communities have gradually accumulated plant utilization knowledge with characteristics specific to their own cultures. Since 1956, with the help of the government, these ethnic groups have improved their living standards, transitioning directly from a primitive society to socialism, and from migratory to sedentary farming.
The aims of this study were as follows: i) to document the traditional insect repellent knowledge of the Bulang, Jinuo and Lahu people in Xishuangbanna; ii) to compare the data collected from these three ethnic groups, as well as ethnobotanical studies of the Dai and Hani people with whom our research group has carried out the same research; and iii) to discuss the potential use of these plants on the basis of ethnobotanical data.
2. Methods
2.1. Study area
Xishuangbanna Dai Autonomous Prefecture is located in southern Yunnan Province, China, bordering Laos and Myanmar in the south and east. This region has a land area of 19,125 km2, with mountainous topography (elevation ranges from 491 m to 2430 m). This area is situated in the tropical monsoon forest zone of Southeast Asia, with a rainy season between May and October and a dry season lasting from November to April (Office of the Government of Xishuangbanna Dai Autonomous Prefecture, 2019). The climate in Xishuangbanna is warm and humid throughout the year, in 2016 the mean monthly temperatures ranged from 14.9 °C to 25.8 °C, according to the meteorological station in Mengla (Station ID: 56969). Rainfall during the wet season accounts for over 80% of the total annual precipitation, while the dry season has a frequent occurrence of heavy fog (Zhao et al., 2013; Zhang et al., 2018). The primary forest vegetation in Xishuangbanna includes seven main types of vegetation: tropical rainforest, tropical seasonal moist forest, tropical monsoon forest, tropical lower montane evergreen broad-leaved forest, tropical palm forest, tropical coniferous forest, and bamboo forest (Zhu et al., 2015). Included as part of the Indo-Burma biodiversity hotspot, Xishuangbanna has a rich diversity of flora and fauna, including 762 species of vertebrates, more than 3000 species of invertebrates and about 4150 species of seed plants (Luo et al., 2016; Myers et al., 2000; Office of the Government of Xishuangbanna Dai Autonomous Prefecture, 2019; Zhu, 2013).
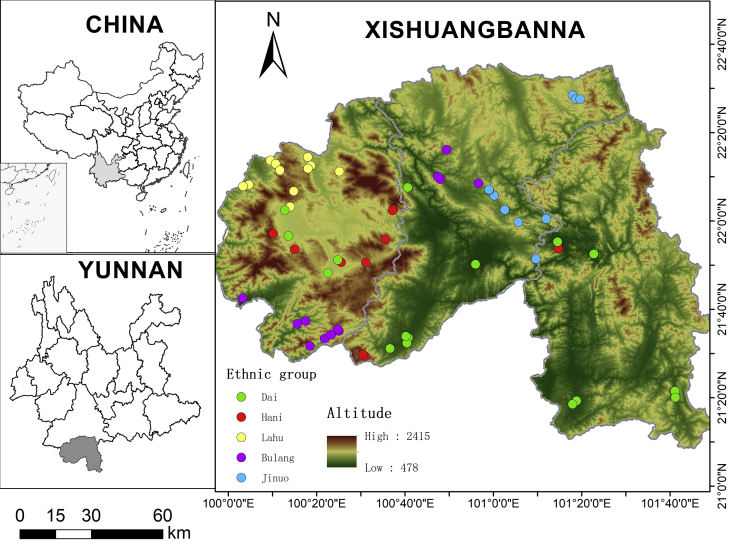
For generations 13 ethnic groups have lived in Xishuangbanna. The Dai people have the largest population, accounting for approximately 33.6% of the population of the whole prefecture. Before liberation, the Dai people ruled other peoples under a feudal system and occupied most basins and valleys of the whole state. The Hani people are the second most populous minority (20.2%), living in the mountainous and semi-mountainous area of the whole state. Bulang (4.98%), Lahu (6.04%) and Jinuo (2.74%) people formerly lived in the hills, but now have partly moved to relatively flatter areas at lower elevations after the establishment of protected areas. In areas above 1000 m, the main cash crops are tea and sugarcane, while those below 1000 m are rubber, rice, and bananas. Based on our previous experiences studying Dai and Hani villages, there are only a small number of people who still retain knowledge of traditional plant-based remedies, most of whom are herbalists. Therefore, we selected 36 villages with herbalists or people who had rich knowledge of medicinal plants in Bulang, Jinuo and Lahu as study sites (as shown in Fig. 1).
Fig. 1.
Location of Xishuangbanna and sites of ethnobotanical surveys of plant-based remedies to control hematophagous invertebrates (including villages of Bulang, Jinuo, and Lahu and previous research sites of Dai and Hani villages).
2.2. Data collection
Ethnobotanical data were collected during five field surveys in Bulang, Jinuo and Lahu villages from August 2018 to July 2019. Meetings with village administrators were first conducted to inform them of the purpose of our work. The village head needed to consent to the research and recommended interviewees such as traditional healers, herbalists and villagers who had sound knowledge of useful plants. We were accompanied by local people as native guides and translators for all our visits. As shown in Table 1, semi-structured interviews were conducted with a total of 237 informants (151 males and 86 females). The ages of the informants ranged from 27 to 98 years old, with an average of 63 years old. We interviewed 45 herbalists, 170 ordinary residents who depend on agriculture, and 12 people with other professions. Questions were asked to obtain detailed information on the plants they use to control hematophagous invertebrates, such as vernacular names, plant parts used, targeted invertebrates, method of preparation, and route of administration. Plants were collected and identified by herbalists with the assistance of researchers during transect walks. The plants were photographed, collected and later identified by researchers. Voucher specimens were deposited in the herbarium of the Kunming Institute of Botany (KUN), China. Plant names and families were defined according to the APG III classification system (APG, 2009).
Table 1.
Gender, age, and occupation of the interviewees in the three ethnic groups.
| Bulang | Jinuo | Lahu | |
|---|---|---|---|
| Gender | |||
| Male | 74 (65.5%) | 42 (66.7%) | 35 (57.4%) |
| Female | 39 (34.5%) | 21 (33.3%) | 26 (42.6%) |
| Age | |||
| <50 | 24 (21.2%) | 10 (16.4%) | 11 (18.0%) |
| 50–59 | 21 (18.6%) | 10 (16.4%) | 11 (18.0%) |
| 60–69 | 36 (31.8%) | 14 (22.2%) | 16 (26.2%) |
| 70–79 | 26 (23.0%) | 16 (25.4%) | 16 (26.2%) |
| >79 | 5 (4.4%) | 13 (20.6%) | 7 (11.5%) |
| Occupation | |||
| Herbalist | 24 (21.2%) | 5 (7.9%) | 16 (26.2%) |
| Farmer | 83 (73.5%) | 55 (87.3%) | 42 (68.9%) |
| Others | 6 (5.3%) | 3 (4.8%) | 3 (4.9%) |
2.3. Data analysis
To statistically analyze the data collected, four different quantitative indices were employed, viz., frequency of citation (FC), use value (UV), informant consensus factor (ICF) and Jaccard index (JI).
For each cited plant species, the number of informants who mentioned usage of the plant was counted as the frequency of citation (FC).
Each plant species mentioned by an interviewee within one use-category (defined according to the invertebrate group target) was counted as a “use report (UR)”. The use value (UV) was calculated to demonstrate the relative importance of species in controlling hematophagous invertebrates (Abe and Ohtani, 2013):
where Ui is the number of UR cited by each informant for a given species, and n refers to the total number of informants. UV is high when there are many use reports for a plant, implying that the plant is important, and low (approach to 0) when there are few reports related to its use.
Many plant species were used to control different hematophagous invertebrates, and these invertebrates were divided into different target categories. The informant consensus factor (ICF) was calculated to measure the agreement between informants about the uses of plants for each category (Heinrich et al., 1998). The ICF was calculated using the following formula:
where Nur refers to the number of use reports in each category and Nt refers to the number of taxa used for a particular category by all informants.
The Jaccard index (JI) was used to compare the similarity of plants used by different ethnic groups (Sivasankari et al., 2014). The JI was calculated using the following formula:
where a is the number of species of ethnic group A, b is the number of species of ethnic group B, and c is the number of species common to both A and B.
NodeXL for Excel was used for network analysis. Ethnic groups and plant taxa were assembled and visualized as a set of nodes (also called vertices), which can be connected (or not) by lines (also called edges), graphically describing interactions between humans and plants. Network analysis has been used for cross-cultural comparative studies of ethnobotany (Ong et al., 2020). In this study, we attempt to use it to address human-plant relations, especially in analyzing variation between different ethnic groups in the same area.
To analyze how traditional insect-repelling plant knowledge varied according to the characteristics of the different informants, we performed an analysis of variance (ANOVA), taking “UR” (number of use reports provided by each informant) as the variable to model and using SPSS 22. software. Similarly, as explanatory variables, we took the three items of personal data requested: “ethnic group”, “gender” and “occupation”. The Duncan analysis method was used for pairwise comparison. The Spearman rank correlation test was employed between the age of informants and UR.
3. Results
3.1. Diversity of traditional botanical remedies against hematophagous invertebrates
Through ethnobotanical surveys, we collected 709 use reports of 32 plant species (71 remedies) that are used by Bulang, Jinuo and Lahu people to prevent or repel hematophagous invertebrates, such as mosquitoes, leeches, chicken mites and lice (Table 2). The taxa of these plants are distributed across 19 families and 30 genera. Among 32 species, the three species with UV higher than 0.40 were Artemisia austroyunnanensis (0.81), Artemisia indica (0.81) and Nicotiana tabacum (0.42). This result indicates the importance of these species in controlling hematophagous invertebrates, regardless of their target categories. The most widely used families are Asteraceae (7 species), Lauraceae (3 species), Rutaceae (3 species), and Solanaceae (3 species). Most species (50%) are herbaceous plants, followed by trees, shrubs and liana (31%, 16% and 3%, respectively). Eight of these plants are cultivated or semi-cultivated: Allium sativum, Artocarpus heterophyllus, Cestrum nocturnum, Cymbopogon citratus, N. tabacum, Prunus persica, Psidium guajava and Toona sinensis. These plants are also used as condiments, fruits, and ornamental plants in the daily life of local people. Most of the other wild plants are easily accessible, except for some woody plants that are rare. Leaves are the most frequently sought plant parts, accounting for 60.6% of cited remedies. Other plant parts used include branches (17.1%), aerial parts (9.2%), barks (5.3%), fruits (3.9%), bulbs (2.6%) and flowers (1.3%).
Table 2.
Overview of the plant species used against hematophagous invertebrates in the Bulang, Jinuo and Lahu villages of Xishuangbanna (China) and references to previous bioactive studies of these or related plant species. FC, frequency of citation. FL, fidelity level. UV, use value.
| Species, family (voucher number) | Most common local name(s)a | Statusb | Habitc | Partsd | Method of application | Target category | FC | UV | Scientific literature |
|---|---|---|---|---|---|---|---|---|---|
| Ageratum conyzoides L., Asteraceae, 19LZN07 | B: nia ge long; ya ming wai | W | H | AP; L | Burned for fumigation; Leaves crushed and applied on skin | Mosquitoes | 13 | 0.05 | Mosquito repellent (Egunyomi et al., 2010; Trongtokit et al., 2005); Pesticide (Bouda et al., 2001; Moreira et al., 2007) |
| Allium sativum L., Amaryllidaceae, 20LZN01 | B: hong huo | C | H | Bu | Crushed and applied on skin | Mosquitoes | 2 | 0.01 | Mosquito repellent (Campbell et al., 2011); Insect repellent and insecticide (Mobki et al., 2014); |
| Crushed and applied on skin | Land leeches | 1 | |||||||
| Artemisia austroyunnanensis Y. Ling & Y.-R. Ling, Asteraceae, 19LZN05 | L: a ka B: bia; se duo yi; a (se) bu lang J: di di; jia kuo jia la; guo fu guo lo |
W | H | Br; L | Burned for fumigation; Leaves crushed and applied on skin; Carrying leaves | Mosquitoes | 145 | 0.81 | Artemisia nilagirica as mosquito larvicide, adulticide, and repellent (Panneerselvam et al., 2012) |
| Leaves crushed and applied on skin | Land leeches | 27 | |||||||
| Put in chicken rings | Chicken mites | 21 | |||||||
| Artemisia indica Willd., Asteraceae, 19LZN06 | L: a ka B: bia; se duo yi; a (se) bu lang J: di di; jia kuo jia la; guo fu guo lo |
W | H | Br; L | Burned for fumigation; Leaves crushed and applied on skin; Carrying leaves; | Mosquitoes | 145 | 0.81 | Artemisia vulgaris as mosquito repellent (Hwang et al., 1985) |
| Leaves crushed and applied on skin | Land leeches | 27 | |||||||
| Put in chicken rings | Chicken mites | 21 | |||||||
| Artocarpus heterophyllus Lam., Moraceae, 19LZN27 | B:ge nong | C | T | Fr | Minced and thrown in the field | Aquatic leeches | 5 | 0.02 | Anthelmintic (Hurtada et al., 2012) |
| Blumea balsamifera (L.) DC., Asteraceae, 19LZN01 | L: xing jin B: se gong gang; gua na |
W | H | Br; L | Burned for fumigation; Leaves crushed and applied on skin | Mosquitoes | 7 | 0.03 | Insecticide (Wang et al., 2012; Chu et al., 2013) |
| Boenninghausenia albiflora (Hook.) Rchb. ex Meisn., Rutaceae, 19LZN14 | B: ki mu o | W | H | L | Burned for fumigation; Placed indoors; Worn on head | Mosquitoes | 8 | 0.06 | Insecticide (Tandon and Mittal, 2018; Khan et al., 2017; Mehmood and Shahzadi, 2014); Ant repellent (Mehmood and Shahzadi, 2014) |
| Placed indoors | Lice | 1 | |||||||
| Leaves crushed and applied on skin | Land leeches | 1 | |||||||
| Put in chicken rings | Chicken mites | 9 | |||||||
| Castanopsis mekongensis A. Camus, Fagaceae, 19LZN42 | B:ga sa | W | T | Ba | Crushed and applied on skin | Land leeches | 3 | 0.01 | – |
| Cestrum nocturnum L., Solanaceae, 19LZN17 | B: da ong ne | C, S | S | Fl | Plant the tree in the yard; Placed indoors; Worn on head | Mosquitoes | 8 | 0.03 | Mosquito repellent and larvicide (El-Sheikh et al., 2016; Patil et al., 2011); Insecticide (Jawale and Dama, 2010) |
| Chromolaena odorata (L.) R.M. King & H. Rob., Asteraceae, 19LZN03 | L: na bo gu B: nia mi hang; nai ge la |
W | H | Br; L | Burned for fumigation | Mosquitoes | 8 | 0.04 | Insecticide (Delobel and Malonga, 1987; Bouda et al., 2001); Mosquito repellent (Gillij et al., 2008; Maharaj et al., 2010; Gade et al., 2017); Mosquito larvicide (Gade et al., 2017) |
| Leaves crushed and applied on skin | Land leeches | 1 | |||||||
| Cinnamomum bejolghota (Buch. -Ham.) Sweet, Lauraceae, 19LZN09 | L: xiang nuo | W | T | Ba | Dried and ground into powder, made into incense for burning | Mosquitoes | 2 | 0.01 | Antihelmintic (Gogoi et al., 2014) |
| Cinnamomum iners Reinw. ex Blume, Lauraceae, 19LZN10 | L: xiang nuo | W | T | Ba | Dried and ground into powder, made into incense for burning | Mosquitoes | 2 | 0.01 |
Cinnamomum osmophloeum as mosquito adulticide and larvicide (Mdoe et al., 2014) Cinnamomum zeylanicum as insecticide (Volpato et al., 2016) |
| Cissus javana DC., Vitaceae, 19LZN34 | B: en dai lai | W | L | L | Put in chicken rings | Chicken mites | 2 | 0.01 | – |
| Clausena excavata Burm. f., Rutaceae, 19LZN13 | L: a xie ci B: guo neng la wai; a kelep J: ya o sha ne |
W | S | L | Burned for fumigation; Crushed and applied on skin; Placed under bed | Mosquitoes | 6 | 0.13 | Mosquito larvicide (Cheng et al., 2009) |
| Placed under bed | Lice | 2 | |||||||
| Put in chicken rings | Chicken mites | 23 | |||||||
| Clerodendrum bungei Steud., Lamiaceae, 19LZN19 | L: a pa nu B: den hen; wa ke le J: bu le ba a pu |
W | S | L | Burned for fumigation | Mosquitoes | 19 | 0.08 | – |
| Put in chicken rings | Chicken mites | 1 | |||||||
| Conoclinium coelestinum (L.) DC., Asteraceae, 19LZN02 | L: gai fang mu B: nia (ya) gong sang J: yi cuo bi ci |
W | H | AP; L | Burned for fumigation; Slightly heated on fire and placed indoors | Mosquitoes | 10 | 0.05 | Acaricide (Nong et al., 2012) |
| Leaves crushed and applied on skin | Land leeches | 2 | |||||||
| Cymbopogon citratus (DC.) Stapf, Poaceae, 19LZN40 | L: zi huo ma | C | H | L | Burned for fumigation | Mosquitoes | 2 | 0.01 | Mosquito repellent (Solomon et al., 2012); Mosquito adulticide (Phasomkusolsil and Soonwera, 2011); Housefly toxicity (Kumar et al., 2013) |
| Datura metel L., Solanaceae, 19LZN18 | B:ke ba | W | H | L | Burned for fumigation | Mosquitoes | 2 | 0.01 | Mosquito larvicide (Murugan et al., 2015; Chakkaravarthy et al., 2011) |
| Elsholtzia blanda (Benth.) Benth., Lamiaceae, 19LZN24 | J: a lie pa xi li; la fe cuo po | W | H | L | Burned for fumigation; Crushed and applied on skin | Mosquitoes | 3 | 0.01 | Insecticide (Lü and He, 2010) |
| Eranthemum shweliense W.W.Sm., Acanthaceae, 19LZN38 | J: xie mu | W | S | L | Crushed and applied on skin | Mosquitoes | 2 | 0.01 | – |
| Laggera pterodonta (DC.) Sch.Bip. ex Oliv., Asteraceae, 19LZN04 | L: su guo ma B: e le bing; de long |
W | H | AP; L | Burned for fumigation; Slightly heated on fire and placed indoors | Mosquitoes | 15 | 0.06 | Mosquito adulticide (Njan-Nloga et al., 2007); Insecticide (Guo et al., 2017) |
| Litsea cubeba (Lour.) Pers., Lauraceae, 19LZN12 | B: da ma lao leng | W | T | L; Fr | Crushed and applied on skin | Mosquitoes | 2 | 0.01 | Mosquito repellent (Noosidum et al., 2008; Trongtokit et al., 2005); Nematicide (Park et al., 2007); Insecticide (Yang et al., 2014) |
| Lobelia seguinii H. Lév. & Vaniot, Campanulaceae, 19LZN39 | L: mi (mu) duo yo | W | H | L | Burned for fumigation | Mosquitoes | 1 | 0.02 | – |
| Put in chicken rings | Chicken mites | 3 | |||||||
| Melicope pteleifolia (Champ. ex Benth.) T.G. Hartley, Rutaceae, 19LZN15 | L: si ci ka | W | S | L | Put in chicken rings | Chicken mites | 2 | 0.01 | Main compound leptol A as mosquito adulticide (Li et al., 2003) |
| Nicotiana tabacum L., Solanaceae, 19LZN16 | L: su B: ga (en) bai; ya; ya qing ai J: ya huo |
C | H | L | Burned for fumigation; Leaves crushed and applied on skin; Leaves soaked in water and sprayed | Mosquitoes | 32 | 0.42 | Insecticide (Akumefula et al., 2014) |
| Leaves crushed and applied on skin; Leaves soaked in water and wiped | Land leeches | 55 | |||||||
| Put in chicken rings | Chicken mites | 12 | |||||||
| Polygonum barbatum L., Polygonaceae,17GY019 | J: she pi | W | H | AP | Put in chicken rings | Chicken mites | 3 | 0.02 | – |
| Put under beds | Lice | 1 | |||||||
| Phyllanthus emblica L., Phyllanthaceae, 19LZN41 | B: xi mi | W | T | Br; L | Fresh branches or dried powdered leaves burned for fumigation; Slightly heated on fire and placed under seats; Placed under bed | Mosquitoes | 6 | 0.02 | Mosquito adulticide (Bagavan et al., 2009); Mosquito larvicide (Bagavan et al., 2009; Jeyasankar et al., 2012); Moth larvicide and anti-feedant (Minj et al., 2017) |
| Pinus yunnanensis Franch., Pinaceae, 19LZN31 | L: tuo sa B: gua gi |
W | T | L; Fr | Burned for fumigation | Mosquitoes | 3 | 0.01 | Pinus longifolia as mosquito repellent and larvicide (Ansari et al., 2005) |
| Prunus persica (L.) Batsch, Rosaceae, 19LZN33 | L: a vi J: se ye; pa kuo |
C | T | L | Leaves crushed and applied on skin | Mosquitoes | 1 | 0.09 | Fly larvicide (Seo and Park, 2012) |
| Put in chicken rings | Chicken mites | 21 | |||||||
| Psidium guajava L., Myrtaceae, 19LZN30 | J: ma gui | C, S | T | L | Put in chicken rings | Chicken mites | 2 | 0.01 | Anthelmintic (Nil et al., 2015) |
| Thalictrum foliolosum DC., Ranunculaceae, 19LZN35 | B:ki mu leng | W | H | L | Burned for fumigation | Mosquitoes | 5 | 0.05 | – |
| Put in chicken rings | Chicken mites | 6 | |||||||
| Toona sinensis (A. Juss.) M. Roem., Meliaceae, 19LZN36 | B: mei yong J: gu si |
C | T | Ba; L | Burned for fumigation | Mosquitoes | 2 | 0.03 | Insect repellent (Chen, 2010); Insecticide (Adfa et al., 2017) |
| Put in chicken rings | Chicken mites | 5 |
B: Bulang; J: Jinuo; L: Lahu.
W: wild; C: cultivated; S: semi-cultivated (cultivated and reverted to wild status, and neglected cultivated plants).
H: herb; T: tree; S: shrub; L: liana.
AP: aerial parts; Ba: bark; Br: branches; Bu: bulbs; Fl: flowers; Fr: fruits; L: leaves.
Mosquitoes are the most important blood-sucking insects because they are ubiquitous in homes, fields and forests. Of the plants collected in this study, 26 species with 41 remedies are used for mosquitoes. The methods for controlling mosquitoes are diverse: direct burning is used for most plants (19 species), followed by smearing (9 species), applying to skin or wearing (8 species), and processing into incense (2 species). On summer evenings, when there are large numbers of mosquitoes, people throw large bundles of plant material into the fire to generate smoke. If there are fewer mosquitoes, then plants are only slightly heated on the fire, hung up, or placed indoors. When farming or traveling, people tie plants to their body or apply plant juice to their skin. In addition, dried bark of Cinnamomum bejolghota and Cinnamomum iners are ground down and processed to incense (Fig. 2). This incense is used in various ritual activities of the Lahu people and was also reported by some informants to be useful in reducing mosquito attacks. Cestrum nocturnum is cultivated by some people as ornamental plants with the belief that its strong scent is able to repel mosquitoes. Artemisia austroyunnanensis and A. indica are the most popular mosquito-repelling plants, with much higher use frequencies than other plants. Local people call these two plants the same name, but they believe the effect of the plant with smaller leaves (A. indica) is better than that of the other (A. austroyunnanensis).
Fig. 2.
The process of ritual incense of Lahu people in Xishuangbanna. The bark of Cinnamomum bejolghota (A) and Cinnamomum iners (B) were dried (C) and ground down (D) into powder. Then, the powder was mixed with hot water (E) and applied on the stick (F). Finally, after drying, the incense can be burned to generate smoke (G).
Plant-based methods for repelling other invertebrates are simpler. Chicken mites are surface parasites that mainly parasitize the feathers and skin of chickens and affect the growth and production of chickens. Clausena excavata, Artemisia austroyunnanensis, A. indica, Prunus persica and 11 other plant species are placed in the chicken rings to repel chicken mites. The Xishuangbanna area is rich in vegetation and abundant in rain. There are land leeches in the fields and forests that cause problems for the local people and their cattle. The fresh leaves of 9 plant species are able to prevent land leech bites when crushed and applied to bare skin. Leaves of N. tabacum soaked in water can be wiped on cow skin with water to help control leeches. Before planting, fruits of A. heterophyllus are minced and thrown in the field, which can reduce leeches in rice fields. In the past, the living and sanitary conditions of the local villagers were terrible. They put Boenninghausenia albiflora, C. excavata or Polygonum barbatum under beds to reduce lice and other ectoparasitic insect attacks.
3.2. Comparative analysis of plant species reported by five ethnic groups
From 2016 to 2019, our research group conducted an ethnobotanical survey of traditional botanical remedies against hematophagous invertebrates in 64 villages of the Bulang, Jinuo, Lahu, Hani and Dai people in Xishuangbanna (see Fig. 1). Each village is inhabited by a single ethnic group. The geographic areas and elevations mainly occupied by the five ethnic groups are different; thus, the elevational range of the study sites varied greatly among the five ethnic groups (Table 3). Hani village has the largest area, while Jinuo has the smallest area. Due to historical and political factors, the Hani people now live in basins, semi-mountains and mountains in a large part of Xishuangbanna. The Jinuo ethnic group lives in a small and relatively concentrated area, namely, the Jinuo Mountain and Buyuan Village Committee. The Dai people live in most valleys and plains throughout the whole state, at lower elevations than the other nearby ethnic groups. The Bulang and Lahu ethnic groups live at various elevations in relatively concentrated areas. When the reported plant species of the ethnic groups were compared, we found that the Hani people contributed 32 species, followed by the Bulang and Dai people with 22 species and 21 species. The Bulang reported the largest number of URs (346), which may have resulted from that group having the largest number of informants. The number of plants and URs collected from the Jinuo people were lower than those collected from other ethnic groups.
Table 3.
Comparison of study sites, informants and collected data from the Bulang, Jinuo, Lahu, Dai and Hani people of Xishuangbanna.
| Ethnic group | Bulang | Jinuo | Lahu | Dai | Hani |
|---|---|---|---|---|---|
| Number of informants | 113 | 63 | 61 | 81 | 91 |
| Number of study sites | 12 | 12 | 12 | 16 | 12 |
| Elevational range of study sites (m) | 633–1358 | 779–1174 | 780–1579 | 536–1202 | 547–1846 |
| Total number of plants collected | 22 | 12 | 17 | 21 | 32 |
| Total number of use reports | 346 | 151 | 232 | 181 | 309 |
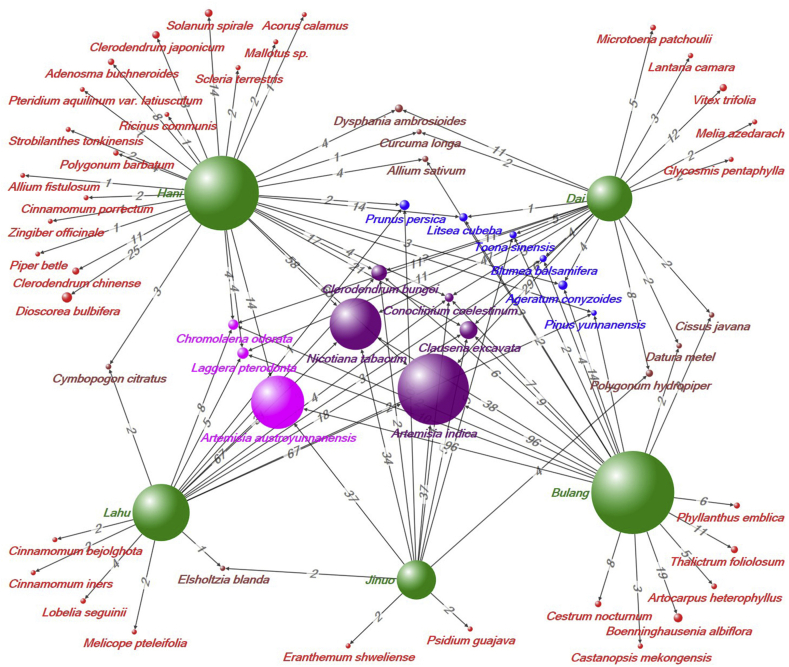
A total of 1219 use reports for 55 plant species were collected from the five ethnic groups (full list is shown on Appendix 1). Of these species, 22 plant species are used by two or more ethnic groups. The network analysis graph (Fig. 3) shows the relationship between plants and ethnic groups. The edge between the plant node and ethnic group node indicates that the plant is utilized by the ethnic group to control hematophagous invertebrates. Five species (Artemisia indica, Clausena excavata, Clerodendrum bungee, Conoclinium coelestinum, and Nicotiana tabacum) are common to all five groups; A. austroyunnanensis, Chromolaena odorata, and Laggera pterodonta are used by four of five groups. Six species are used by three groups, and eight species are used by two groups. These plant nodes generally are larger in size, indicating that they have more use reports recorded. Among them, three plants are obviously used more than other plants, namely, A. indica (294 UR), A. austroyunnanensis (214 UR) and N. tabacum (208 UR). These are the dominant species used, accounting for approximately 59% of the total URs.
Fig. 3.
Ethnic group-plant species network. Plant species are shown linked to the five ethnic groups (green). Plant vertices with purple, fuchsia, blue, brown and red indicate plants reported by 5, 4, 3, 2, and 1 ethnic group(s), respectively. The values along the edges (lines/connections) indicate the number of URs directed to plant species as contributed per ethnic group. The size of the node is converted from the number of URs of the plant.
Apart from similar uses of plants, each ethnic group also used its own unique plant species. “Pendant” plant nodes are those that are only shown as reported from a single ethnic group. Sixteen species (50%) of plants reported by the Hani people were not used by the other ethnic groups. The proportion of specific species for the other four ethnic groups was lower, namely, 26.18% for Bulang, 33.3% for Jino, 23.5% for Lahu, and 23.8% for Dai. The Jaccard index (JI) was calculated to compare plant species reported between any two ethnic groups, and the JI ranged from 18.92 to 43.33 (see Table 4). The highest similarity was between the Bulang and Dai people, while the greatest difference was between the Hani and Jinuo people.
Table 4.
Jaccard index (JI) of plant species used between different ethnic groups of Xishuangbanna.
| Bulang | Jinuo | Lahu | Hani | Dai | |
|---|---|---|---|---|---|
| Bulang | 100.00 | 25.92 | 34.48 | 28.57 | 43.33 |
| Jinuo | 100.00 | 31.82 | 18.92 | 26.92 | |
| Lahu | 100.00 | 28.95 | 26.67 | ||
| Hani | 100.00 | 26.17 | |||
| Dai | 100.00 |
3.3. Targeted hematophagous invertebrates of five ethnic groups
Hematophagous invertebrates contain a large number of animal species. In this study, the main hematophagous invertebrates local people targeted were divided into the following categories: mosquito, chicken mite, land leech, aquatic leech, lice and flea. Categories targeted by different ethnic groups were not exactly the same, and the plants utilized by ethnic groups were also different. Some of the reported plants used by multiple ethnic groups may also have been used to control different categories of invertebrates. For example, Ageratum conyzoides were reported to repel mosquitoes by the Bulang and Dai people, while it was used against land leeches by the Hani people. The heat map (green) in Fig. 4 shows the number of URs for targeted hematophagous invertebrate categories per ethnic group. Mosquito is the most frequently mentioned target category among all five ethnic groups, yielding the largest number of use reports (747 UR). Chicken mites (246 UR) and land leeches (175 UR) were the other two main categories targeted by all five ethnic groups. For the reported plant species (blue heat map), the largest number of taxa were used for mosquitoes by the Bulang, Lahu, Hani and Dai people, while chicken mites were targeted by the Jinuo people. When the blue heat map was compared with the green heat map, not all the trends in these three main categories were mirrored. This may indicate that in the same ethnic group, the importance of category and the richness of knowledge are not necessarily proportional. In the same category, the richness of knowledge of different ethnic groups was compared. In comparison to the other ethnic groups, the Hani people use more plant species for mosquitoes and land leeches, and the Dai people use more plants for chicken mites. In addition, only a small part (4.1%) of the URs were recorded as targeting other categories. The Bulang and Hani people each utilize one species (5 and 25 UR, respectively) against aquatic leeches. Lice is controlled with plants by the Bulang, Jinuo, Dai and Hani people, but only 8 URs were recorded for 7 of the plants collected. Only the Hani people reported 13 URs of 5 plants to prevent flea bites.
Fig. 4.
Heat map for targeted categories, as recorded per ethnic group in Xishuangbanna (B-Bulang, J-Jinuo, L-Lahu, H-Hani, and D-Dai). The blue map shows the number of applicable plant taxa (Nt) recorded per ethnic group, while the green map represents the degree of UR (Nur). Informant consensus factors (ICFs) were calculated for each target category of the five ethnic groups.
With the orange heat map, ICF was calculated to evaluate the variability in the use of plants for each target category. The highest ICF value (1.00) was found for plants the Hani and Bulang people use to control aquatic leeches. For mosquitoes, chicken mites and land leeches, the five ethnic groups all yielded high ICF values (higher than 0.7). These data indicate that each ethnic group has a high level of cultural consensus when selecting plants for these target categories. A low ICF value indicates that the informants disagree on the taxa that should be used within a category. For example, plants used by the Jinuo, Dai and Hani people to control lice were cited by only a few people and were considered to be of low cultural importance.
3.3. Analysis of informant knowledge
Through analysis of variance, the usage of plants in relation to ethnic group, gender and botanical knowledge was determined (Table 5). We found that there were significant differences in the number of URs among the different ethnic groups. The Duncan analysis method was used for pairwise comparisons. The results showed that the average UR of the Dai people was significantly lower than that of Bulang, Lahu and Hani. The average UR of the Lahu and Hani people was also significantly higher than Jinuo people. We divided informants into two groups according to medicinal plant knowledge---herbalists and non-herbalists. The results showed that the average UR of the herbalists was 3.86, which was significantly higher than the average of 2.75 for non-herbalists. Because most herbalists are males, we compared the number of URs provided by non-herbalist male and female informants from the villages. The average UR of male villagers (2.92) was not significantly different than that of female villagers (2.55). A weak positive correlation (r = 0.116; P = 0.04) between the age of the informants (non-herbalists) and the number of URs was observed.
Table 5.
Relationship between UR of informant and ethnic group, occupation, and gender.
| Variable | Number | Average UR | Variance | P (α = 0.05) |
|---|---|---|---|---|
| Ethnic group | ||||
| Bulang | 113 | 3.06ab | 2.18 | 0.00 |
| Jinuo | 63 | 2.40bc | 1.99 | |
| Lahu | 61 | 3.80a | 1.99 | |
| Hani | 91 | 3.38a | 3.00 | |
| Dai | 81 | 2.23c | 2.05 | |
| Medicinal knowledge | ||||
| Herbalist | 83 | 3.86 | 2.42 | 0.00 |
| Non-herbalist | 326 | 2.75 | 2.30 | |
| Gender (informants except for herbalists) | ||||
| Male | 176 | 2.92 | 2.32 | 0.15 |
| Female | 150 | 2.55 | 2.27 | |
Different letters in the upper right corner of the average UR value represent significant difference, P < 0.05.
4. Discussion
Through ethnobotanical surveys, we documented 32 plants that were used by three mountain ethnic groups (Bulang, Jinuo and Lahu) of Xishuangbanna to control hematophagous invertebrates, including mosquitoes, leeches, chicken mites and lice. Local inhabitants did not discern whether the role of the plant was to repel or kill. Even if plants are toxic, it is not always obvious because the plants are usually used in open areas. Most of these plants are cultivated or common wild herbs with a large amount of resources. Thus, they are easily available and accessible to local people, similar to insect-repelling plants in other areas (Innocent et al., 2014; Karunamoorthi et al., 2009; Mavundza et al., 2011). For 21 of the plant species recorded in this study, several bioassays have been performed to test their repellent and/or insecticidal properties (Table 2). The effectiveness of four species traditionally used as insecticides or repellents has been somewhat corroborated by research that has shown that closely related species (from the same genus) have insecticidal or repellent activity. We are unaware of studies that have examined insecticidal or repellent activities of the remaining seven plant species. These untested plants belong to families that have not often garnered attention from researchers who study biological pesticides or repellents. Therefore, ethnobotany may contribute substantially to the discovery of new insecticide or repellent plants.
Including previous surveys of the Dai and Hani people, our research team surveyed a total of five ethnic groups in the Xishuangbanna area. The ethnic groups usually speak their own language and retain their respective cultures, customs and festivals. Villages we visited are often inhabited by a single ethnic group. However, there may also be villages of other ethnic groups nearby, and they can communicate with each other in Mandarin or other national dialects. Network analysis of the relationship between ethnic group and plant showed that 22 of the 55 plant species are used by two or more ethnic groups. There were certain similarities between any two ethnic groups, and the JI ranged from 18.92 to 43.33. The similarity may be due to similar criteria for selecting plants. Most informants believed that plants with strong smells can drive away hematophagous invertebrates, and many plants used by more than one ethnic group were common aromatic plants, including Artemisia indica, Ageratum conyzoides, Chromolaena odorata, Nicotiana tabacum, and Laggera pterodonta. Communication among groups may also be one reason for the use of similar plants among these groups. For example, a Dai informant stated that he learned about the repellent use of Litsea cubeba from the Hani people. The highest similarity among the plants used was between the Bulang and Dai people. This may be because the Bulang and Dai people have the closest cultural exchanges, in large part because the Bulang people can speak the Dai language and share the same religious belief (Hinayana Buddhism) as that of the Dai people. In addition, coincidence may be responsible for the same choice of plants by different ethnic groups.
Apart from similar uses of the plants, each ethnic group also retains its own unique knowledge. These differences in knowledge may be related to their environment. Being in different environments, they have different nearby plant resource banks from which to choose. Artemisia austroyunnanensis is widely used by the Bulang, Jinuo, Lahu and Hani people, but the Dai people do not use it because it is not distributed around Dai villages. The distribution of plants changes with elevation. Therefore, the abundance of plants used by the Hani people may be related to the fact that Hani people live within the widest elevational range, whereas the Jinuo people live within the smallest range. In addition, cultural differences may also be a very important factor. Due to their respective cultural identities, some culturally representative plant uses will not be easily shared. For example, ritual incense made from Cinnamomum bejolghota and C. iners is only used by the Lahu people. The insect-repelling plant Adenosma buchneroides is worn on the head and regarded as a symbol of the Hani people. Many ethnic groups share the custom of hanging plants on door beams, and they think these plants can keep evil spirits out. These plants are usually different and represent their own culture. Some of these plants are also used by an ethnic group only to prevent bites from hematophagous invertebrates, such as Castanopsis mekongensis for the Bulang people, Vitex trifolia for the Dai people, and Acorus calamus for the Hani people.
Mosquitoes, chicken mites and land leeches are the three main categories targeted by all five ethnic groups. The number of URs can reflect the importance of the category in the ethnic group, and the diversity of plant species indicates the richness of the ethnic group's knowledge. Mosquito is the most important target category, yielding the largest number of use reports for all five ethnic groups and the most diverse botanical knowledge for four ethnic groups. Mosquitoes are ubiquitous in homes, fields and forests and may be present year-round in Xishuangbanna. Mosquito bites result in not only discomfort but also risks of contracting many diseases. Xishuangbanna has historically been a high-risk area for malaria, and dengue fever cases have become common in recent years. Public education programs have increased the attention local inhabitants give to mosquito control. Chickens are an important type of poultry that most families raise and are widely eaten and sold for money. Chicken mites seriously affect the health and yield of chickens and therefore are the second most targeted category. The Dai people use more plants for chicken mites than other ethnic groups, while the Hani people use the most plant species against the other categories. Among the plants reported by the Jinuo people, the most part is used for chicken mites. These results may indicate that chickens occupy a more important position in the Dai and Jinuo ethnic groups (Song, 1985). Land leeches not only suck human blood but also negatively affect other mammals, including cattle. However, only a few botanical remedies for the control of lice, fleas and leeches were reported. This may be related to the environments in which local people live. For example, Dai people, who mainly live in forests, report relatively fewer URs and plants related to controlling these pests than do other ethnic groups. Currently, their economic conditions have greatly improved, and more attention has been paid to personal and environmental hygiene. Fleas, lice and other ectoparasites have almost disappeared. With the use of fertilizers and synthetic pesticides, there are few leeches in paddy fields. Changes in economic conditions and production methods have also led to a decline in the level of knowledge about traditional insecticide/repellent plants. Although the Dai people reported more plants, their average knowledge level (UR) was the lowest. This may be because they occupy the center of most towns and are most affected by modern culture.
To screen out the plants with the greatest potential and utilization range, UV, diverse targeted categories and common use among the five ethnic groups were taken into consideration. As a result, Artemisia indica, Nicotiana tabacum, and Clausena excavata were the three plants with the most potential to be developed as insecticides or repellents (Fig. 5). A. indica was the most popular plant that prevented mosquito bites among all five ethnic groups. It has also been widely used to control chicken mites and land leeches. Although many informants reported using A. austroyunnanensis and A. indica for this purpose, they generally believe that the activity of A. indica is better. To date, we have found no reports on the activity of A. indica or A. austroyunnanensis as insect repellent or insecticide. Artemisia vulgaris of the same genus is also an insect-repelling plant traditionally used by people in many regions. It has shown strong mosquito-repelling activity, and its essential oil contains multiple repellent compounds (Hwang et al., 1985). Therefore, A. indica deserves additional and more in-depth research and has the potential to be developed as a broad-spectrum and highly effective insecticide or repellent. N. tabacum is one of the plants that has changed the history of the world. Local people plant it mainly for processing into tobacco. At the same time, it is also used by all ethnic groups to control mosquitoes, chicken mites and land leeches. Smoking was also reported by informants to be useful in preventing insect bites. Tobacco leaves are frequently placed, crushed or soaked in water. N. tabacum extract has been shown to cause 70% mortality in Sitophilus zeamais and Sitophilus oryzea (Akumefula et al., 2014). Nicotine, the main active compound, is one of the best plant-derived pesticides and is widely used in agriculture (Steppuhn et al., 2004). Its activity as a repellent remains to be studied, and it is not known whether other active repellent substances exist in addition to nicotine. C. excavata is commonly used by the five groups to control chicken mites. Four of the five groups also use it to repel mosquitoes. The LC50 values (the concentrations causing 50% larva mortality in 24 h) of its essential oils against fourth-instar larvae of Aedes aegypti and Aedes albopictus were 37.1–40.1 μg/ml and 41.1–41.2 μg/ml, respectively (Cheng et al., 2009). Clausena anisata of the same genus is used traditionally as an insect repellent by various communities in Africa and Asia (Mukandiwa et al., 2016). Laboratory topical application assays indicate that the acetone crude extract (15%) showed 93% repellence and the hexane fraction (7.5%) showed 67% repellence after 3 h (Mukandiwa et al., 2016). Although there are only a few reports of other similar plants, this result may be caused by a variety of factors. This scenario does not mean that the activity of these plants is weak. Therefore, these plants can also be used for activity screening, which is more convenient than the general screening method.
Fig. 5.
Three plants with the most potential to be developed as insecticides or repellents. A, Artemisia indica; B, Nicotiana tabacum; and C, Clausena excavata.
5. Conclusion
The Bulang, Jinuo and Lahu people of Xishuangbanna know how to use specific local plants to protect themselves against hematophagous invertebrates. A total of 709 use reports of 32 plant species were collected from these ethnic groups. The five ethnic groups in the same area have common knowledge about traditional botanical knowledge against hematophagous invertebrates, but they also have maintained their own unique knowledge. These differences mainly are due to the diversity in the environment and culture. The interactive and coexisting relationship between biodiversity and cultural diversity shows that the disappearance of traditional culture has accelerated the loss of biodiversity. Cultural diversity provides not only historical experience and observations for biodiversity protection but also practical value. In this study, our survey of the plants traditionally used by ethnic minority communities to control hematophagous invertebrates has identified several plant species that should be screened for insecticidal and repellent activity, including Artemisia indica, Nicotiana tabacum and Clausena excavata.
Author contributions
Ethnobotanical surveys were conducted by ZL, RF, YG, ZQ, and LW; Specimens were identified by ZL and LW; Data was analyzed by YG, ZL and CW. Manuscript drafted by YG and revised by YW.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgements
We are most grateful to all interviewee from Xishuangbanna for their hospitality and willingness to share their knowledge with us. We are also very grateful to other partners in the field investigation. This study was supported by grant from the National Natural Science Foundation of China [31670337] and Plant Germplasm Resources Innovation Project of Chinese Academy of Sciences [KFJ-BRP-007-002].
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2020.07.009.
Contributor Information
Yi Gou, Email: gouyi@mail.kib.ac.cn.
Zhennan Li, Email: lizhennan@mail.kib.ac.cn.
Ruyan Fan, Email: fanruyan@mail.kib.ac.cn.
Zuchuan Qiu, Email: qiuzuchuan@mail.kib.ac.cn.
Lu Wang, Email: wanglu@mail.kib.ac.cn.
Chen Wang, Email: wangchen@mail.kib.ac.cn.
Yuhua Wang, Email: wangyuhua@mail.kib.ac.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Abe R., Ohtani K. An ethnobotanical study of medicinal plants and traditional therapies on Batan Island, the Philippines. J. Ethnopharmacol. 2013;145:554–565. doi: 10.1016/j.jep.2012.11.029. [DOI] [PubMed] [Google Scholar]
- Adfa M., Kusnanda A., Saputra W.D. Termiticidal activity of Toona sinensis wood vinegar against Coptotermes curvignathus holmgren. Rasayan J. Chem. 2017;10:1088–1093. [Google Scholar]
- Akumefula M.I., Onwusonye J., Osuji C.N. Comparative assessment of the insecticidal potency of tobacco leaves extract (Nicotiana tabacum), black pepper seeds (uziza) extract (Piper guineense) and african pepper seeds (uda) extract (Xylapia aetiopica) Chem. Mater. Res. 2014;6:57–59. [Google Scholar]
- Ansari M.A., Mittal R.K., Razdan R.K., Sreehari U. Larvicidal and mosquito repellent activities of Pine (Pinus longifolia, family: Pinaceae) oil. J. Vector Borne Dis. 2005;42:95–99. [PubMed] [Google Scholar]
- APG An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009;161:105–121. [Google Scholar]
- Bagavan A., Kamaraj C., Elango G. Adulticidal and larvicidal efficacy of some medicinal plant extracts against tick, fluke and mosquitoes. Vet. Parasitol. 2009;166:286–292. doi: 10.1016/j.vetpar.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Bockarie M.J., Service M.W., Barnish G. The effect of woodsmoke on the feeding and resting behaviour of Anopheles gambiae s.s. Acta Trop. 1994;57:337–340. doi: 10.1016/0001-706x(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Booppha B., Eittsayeam S., Pengpat K. Development of bioactive ceramics to control mite and microbial diseases in bee farms. Adv. Mater. Res. 2010;93–94:553–557. [Google Scholar]
- Bouda H., Tapondjou L.A., Fontem D.A. Effect of essential oils from leaves of Ageratum conyzoides, Lantana camara and Chromolaena odorata on the mortality of Sitophilus zeamais (Coleoptera, Curculionidae) J. Stored Prod. Res. 2001;37:103–109. doi: 10.1016/s0022-474x(00)00011-4. [DOI] [PubMed] [Google Scholar]
- Brown M., Hebert A.A. Insect repellents: an overview. J. Am. Acad. Dermatol. 1997;36:243–249. doi: 10.1016/s0190-9622(97)70289-5. [DOI] [PubMed] [Google Scholar]
- Campbell C., Gries R., Khaskin G. Organosulphur constituents in garlic oil elicit antennal and behavioural responses from the yellow fever mosquito. J. Appl. Entomol. 2011;135:374–381. [Google Scholar]
- Campbell J.B., Thomas G.B. University of Nebraska Cooperative Extension; 1999. MP99-40 the Economics and Control of Insects Affecting Beef Cattle in Nebraska (Northern Great Plains) [Google Scholar]
- Chakkaravarthy V., Ambrose T., Vincent S. Bioefficacy of Azadirachta indica (A. Juss) and Datura metel (Linn.) leaves extracts in controlling Culex quinquefasciatus (Diptera: Culicidae) J. Entomol. 2011;8:191–197. [Google Scholar]
- Chen L. Bioactivities of extracts from Toona sinensis against Sitophilus zeamais. J. Anhui Agr. Sci. 2010;1:47–49. [Google Scholar]
- Cheng S.S., Chang H.T., Lin C.Y. Insecticidal activities of leaf and twig essential oils from Clausena excavata against Aedes aegypti and Aedes albopictus larvae. Pest Manag. Sci. 2009;65:339–343. doi: 10.1002/ps.1693. [DOI] [PubMed] [Google Scholar]
- Chu S.S., Du S.S., Liu Z.L. Fumigant compounds from the essential oil of Chinese Blumea balsamifera leaves against the maize weevil (Sitophilus zeamais) J. Chem. 2013;2013 [Google Scholar]
- Chu B.K., Hooper P.J., Bradley M.H. The economic benefits resulting from the first 8 years of the global programme to eliminate lymphatic filariasis (2000-2007) PloS Neglect Trop. Doct. 2010;4:e708. doi: 10.1371/journal.pntd.0000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer H., Vongsombath C., Pålsson K. Botanical repellents and pesticides traditionally used against hematophagous invertebrates in Lao People's Democratic Republic: a comparative study of plants used in 66 villages. J. Med. Entomol. 2010;47:400–414. doi: 10.1603/me09273. [DOI] [PubMed] [Google Scholar]
- Debboun M., Frances S., Strickman D. CRC Press; 2006. Insect Repellents-Principles, Methods, and Uses. [Google Scholar]
- Delobel A., Malonga P. Insecticidal properties of six plant materials against Caryedon serratus (Ol.) (Coleoptera: bruchidae) J. Stored Prod. Res. 1987;23:173–176. [Google Scholar]
- Egunyomi A., Gbadamosi I.T., Idayat A.O. Comparative effectiveness of ethnobotanical mosquito repellents used in Ibadan, Nigeria. J. Appl. Biosci. 2010;36:2383–2388. [Google Scholar]
- El-Sheikh T.M.Y., Al-Fifi Z.I.A., Alabboud M.A. Larvicidal and repellent effect of some Tribulus terrestris L., (Zygophyllaceae) extracts against the dengue fever mosquito, Aedes aegypti (Diptera: Culicidae) J. Saudi Chem. Soc. 2016;20:13–19. [Google Scholar]
- Gade S., Rajamanikyam M., Vadlapudi V. Acetylcholinesterase inhibitory activity of stigmasterol & hexacosanol is responsible for larvicidal and repellent properties of Chromolaena odorata. BBA-Gen. Subjects. 2017;1861:541–550. doi: 10.1016/j.bbagen.2016.11.044. [DOI] [PubMed] [Google Scholar]
- Gakuya D.W., Itonga S.M., Mbaria J.M. Ethnobotanical survey of biopesticides and other medicinal plants traditionally used in Meru central district of Kenya. J. Ethnopharmacol. 2013;145:547–553. doi: 10.1016/j.jep.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Gillij Y.G., Gleiser R.M., Zygadlo J.A. Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour. Technol. 2008;99:2507–2515. doi: 10.1016/j.biortech.2007.04.066. [DOI] [PubMed] [Google Scholar]
- Gogoi B., Kakoti B.B., Bora N.S. In vitro antihelmintic activity of bark extract of Cinnamomum bejolghota (Buch.-Ham.) in Indian adult earthworm (Pheretima posthuma) Asian Pac. J. Trop. Dis. 2014;4:S924–S927. doi: 10.1016/S1995-7645(14)60270-4. [DOI] [PubMed] [Google Scholar]
- González J.A., García-Barriuso M., Gordaliza M. Traditional plant-based remedies to control insect vectors of disease in the Arribes del Duero (western Spain): an ethnobotanical study. J. Ethnopharmacol. 2011;138:595–601. doi: 10.1016/j.jep.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Guo S.-S., Zhang W.-J., You C.-X. Chemical composition of essential oil extracted from Laggera pterodonta and its bioactivities against two stored product insects. J. Food Process. Preserv. 2017;41 [Google Scholar]
- Heinrich M., Ankli A., Frei B. Medicinal plants in Mexico: healers' consensus and cultural importance. Soc. Sci. Med. 1998;47:1859–1871. doi: 10.1016/s0277-9536(98)00181-6. [DOI] [PubMed] [Google Scholar]
- Hurtada J., Divina B., Ducusin R.J. Anthelmintic efficacy of jackfruit (Artocarpus heterophyllus L.) and tamarind (Tamarindus indica L.) leaves decoction against gastrointestinal nematodes of goats. Philipp. J. Vet. Anim. Sci. 2012;38:157–166. [Google Scholar]
- Hwang Y.S., Wu K.H., Kumamoto J. Isolation and identification of mosquito repellents in Artemisia vulgaris. J. Chem. Ecol. 1985;11:1297–1306. doi: 10.1007/BF01024117. [DOI] [PubMed] [Google Scholar]
- Innocent E., Hassanali A., Kisinza W.N. Anti-mosquito plants as an alternative or incremental method for malaria vector control among rural communities of Bagamoyo District, Tanzania. J. Ethnobiol. Ethnomed. 2014;10:56. doi: 10.1186/1746-4269-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawale C., B D. Insecticidal potential of Cestrum sp. (Solanaceae: solanales) against Tribolium castaneum and Tribolium confusum (herbst) (Coleoptera-tenebrionidae) Deccan Curr. Sci. 2010;3:155–161. [Google Scholar]
- Jeyasankar A., Premalatha, Elumalai K. Larvicidal activity of Phyllanthus emblica Linn. (Euphorbiaceae) leaf extracts against important human vector mosquitoes (Diptera: Culicidae) Asian Pac. J. Trop. Dis. 2012;2:S399–S403. doi: 10.1016/S1995-7645(13)60152-2. [DOI] [PubMed] [Google Scholar]
- Karunamoorthi K., Adane M., Fentahun W. Assessment of knowledge and usage custom of traditional insect/mosquito repellent plants in Addis Zemen Town, South Gonder, North Western Ethiopia. J. Ethnopharmacol. 2009;121:49–53. doi: 10.1016/j.jep.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Khan S., Taning C.N.T., Bonneure E. Insecticidal activity of plant-derived extracts against different economically important pest insects. Phytoparasitica. 2017;45:113–124. [Google Scholar]
- Kumar P., Mishra S., Malik A. Housefly (Musca domestica L.) control potential of Cymbopogon citratus Stapf. (Poales: poaceae) essential oil and monoterpenes (citral and 1,8-cineole) Parasitol. Res. 2013;112:69–76. doi: 10.1007/s00436-012-3105-5. [DOI] [PubMed] [Google Scholar]
- Kweka E.J., Mosha F., Lowassa A. Ethnobotanical study of some of mosquito repellent plants in north-eastern Tanzania. Malar. J. 2008;7:152. doi: 10.1186/1475-2875-7-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavaud F., Bouchet F., Mertes P.M. Allergy to the bites of blood-sucking insects: clinical manifestations. Allerg. Immunol. (Paris) 1999;31:311–316. [PubMed] [Google Scholar]
- Li G., Zhu D., Pandey R.K. American Chemical Society; 2003. Phytochemical and Biological Studies on Evodia Lepta, Oriental Foods and Herbs; pp. 247–257. [Google Scholar]
- Luo Z., Tang S., Jiang Z. Conservation of terrestrial vertebrates in a global hotspot of Karst area in Southwestern China. Sci. Rep. 2016;6:25717. doi: 10.1038/srep25717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü J.H., He Y.Q. Fumigant toxicity of Ailanthus altissima Swingle, Atractylodes lancea (Thunb.) DC. and Elsholtzia stauntonii Benth extracts on three major stored-grain insects. Ind. Crop. Prod. 2010;32:681–683. [Google Scholar]
- Maharaj R., Maharaj V., Newmarch M. Evaluation of selected South African ethnomedicinal plants as mosquito repellents against the Anopheles arabiensis mosquito in a rodent model. Malar. J. 2010;9:301. doi: 10.1186/1475-2875-9-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavundza E.J., Maharaj R., Finnie J.F. An ethnobotanical survey of mosquito repellent plants in uMkhanyakude district, KwaZulu-Natal province, South Africa. J. Ethnopharmacol. 2011;137:1516–1520. doi: 10.1016/j.jep.2011.08.040. [DOI] [PubMed] [Google Scholar]
- Mdoe F.P., Cheng S.-S., Msangi S. Activity of Cinnamomum osmophloeum leaf essential oil against Anopheles gambiae s.s. Parasites Vectors. 2014;7:209. doi: 10.1186/1756-3305-7-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood F., Shahzadi P. Insect toxicity and repellent activity of phytochemicals from" flea killer, Boenninghausenia albiflora" against "black garden ant, lasius Niger" of Pakistan. J. Bioanal. Biomed. 2014;6:5. [Google Scholar]
- Minj N., Calistus J., Sri S. Larvicidal and anti-feedant activity of Phyllanthus emblica and Syzygium cumini extracts on the diamondback moth: Plutella xylostella. Int. J. Biol. Res. 2017;2:97–100. [Google Scholar]
- Mobki M., Safavi S.A., Safaralizadeh M.H. Toxicity and repellency of garlic (Allium sativum L.) extract grown in Iran against Tribolium castaneum (Herbst) larvae and adults. Arch. Phytopathol. Plant Protect. 2014;47:59–68. [Google Scholar]
- Moreira M.D., Picanço M.C., Barbosa L.C.d.A. Plant compounds insecticide activity against Coleoptera pests of stored products. Pesqui. Agropecu. Bras. 2007;42:909–915. [Google Scholar]
- Mukandiwa L., Eloff J.N., Naidoo V. Repellent and mosquitocidal effects of leaf extracts of Clausena anisata against the Aedes aegypti mosquito (Diptera: Culicidae) Environ. Sci. Pollut. Res. 2016;23:11257–11266. doi: 10.1007/s11356-016-6318-9. [DOI] [PubMed] [Google Scholar]
- Murugan K., Dinesh D., Kumar P.J. Datura metel-synthesized silver nanoparticles magnify predation of dragonfly nymphs against the malaria vector Anopheles stephensi. Parasitol. Res. 2015;114:4645–4654. doi: 10.1007/s00436-015-4710-x. [DOI] [PubMed] [Google Scholar]
- Myers N., Mittermeier R.A., Mittermeier C.G. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Nil T., Yadav A., Deori K. Anthelmintic effects of Psidium guajava and Lasia spinosa on Hymenolepis diminuta (Cestoda): a scanning electron microscopic study. J. Adv. Microsc. Res. 2015;10:20–23. [Google Scholar]
- Njan-Nloga A., Saotoing P., Tchouankeu J. Effect of essential oils of six local plants used insecticide on adults of Anopheles gambiae, Giles 1902. J. Entomol. 2007;4:444–450. [Google Scholar]
- Nong X., Fang C.-L., Wang J.-H. Acaricidal activity of extract from Eupatorium adenophorum against the Psoroptes cuniculi and Sarcoptes scabiei in vitro. Vet. Parasitol. 2012;187:345–349. doi: 10.1016/j.vetpar.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Noosidum A., Prabaripai A., Chareonviriyaphap T. Excito-repellency properties of essential oils from Melaleuca leucadendron L., Litsea cubeba (Lour.) Persoon, and Litsea salicifolia (Nees) on Aedes aegypti (L.) mosquitoes. J. Vector Ecol. 2008;33:305–313. doi: 10.3376/1081-1710-33.2.305. [DOI] [PubMed] [Google Scholar]
- Office of the Government of Xishuangbanna Dai Autonomous Prefecture . 2019. The Profile of Xishuangbanna Dai Autonomous Prefecture.https://www.xsbn.gov.cn/88.news.detail.dhtml?news_id=34206 [Google Scholar]
- Ong H.G., Kim Y.-D. Medicinal plants for gastrointestinal diseases among the Kuki-Chin ethnolinguistic groups across Bangladesh, India, and Myanmar: a comparative and network analysis study. J. Ethnopharmacol. 2020;251:112415. doi: 10.1016/j.jep.2019.112415. [DOI] [PubMed] [Google Scholar]
- Panneerselvam C., Murugan K., Kovendan K. Mosquito larvicidal, pupicidal, adulticidal, and repellent activity of Artemisia nilagirica (Family: Asteraceae) against Anopheles stephensi and Aedes aegypti. Parasitol. Res. 2012;111:2241–2251. doi: 10.1007/s00436-012-3073-9. [DOI] [PubMed] [Google Scholar]
- Park I.-K., Kim J., Lee S.-G. Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophilus) J. Nematol. 2007;39:275–279. [PMC free article] [PubMed] [Google Scholar]
- Patil C.D., Patil S.V., Salunke B.K. Bioefficacy of Plumbago zeylanica (Plumbaginaceae) and Cestrum nocturnum (Solanaceae) plant extracts against Aedes aegypti (Diptera: culicide) and nontarget fish Poecilia reticulata. Parasitol. Res. 2011;108:1253–1263. doi: 10.1007/s00436-010-2174-6. [DOI] [PubMed] [Google Scholar]
- Phasomkusolsil S., Soonwera M. Efficacy of herbal essential oils as insecticide against Aedes aegypti (linn.), Culex quinquefasciatus (say) and Anopheles dirus (peyton and harrison). SE. Asian J. Trop. Med. Public Health. 2011;42:1083–1092. [PubMed] [Google Scholar]
- Pialoux G., Gaüzère B.-A., Jauréguiberry S. Chikungunya, an epidemic arbovirosis. Lancet Infect. Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- Roberto R., Guido S., Giancarlo M. Could malaria reappear in Italy? Emerg. Inf. Disp. 2001;7:915. doi: 10.3201/eid0706.010601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.-M., Park I.-K. Larvicidal activity of medicinal plant extracts and lignan identified in Phryma leptostachya var. asiatica roots against housefly (Musca domestica L.) Parasitol. Res. 2012;110:1849–1853. doi: 10.1007/s00436-011-2709-5. [DOI] [PubMed] [Google Scholar]
- Sivasankari B., Anandharaj M., Gunasekaran P. An ethnobotanical study of indigenous knowledge on medicinal plants used by the village peoples of Thoppampatti, Dindigul district, Tamilnadu, India. J. Ethnopharmacol. 2014;153:408–423. doi: 10.1016/j.jep.2014.02.040. [DOI] [PubMed] [Google Scholar]
- Solomon B., Gebre-Mariam T., Asres K. Mosquito repellent actions of the essential oils of Cymbopogon citratus, Cymbopogon nardus and Eucalyptus citriodora: evaluation and formulation studies. J. Essent. Oil Bear. Pl. 2012;15:766–773. [Google Scholar]
- Song E. Yunnan People's Publishing House; Kunming: 1985. Minority Religion of China. [Google Scholar]
- Steppuhn A., Gase K., Krock B. Nicotine's defensive function in nature. PLoS Biol. 2004;2:1074–1080. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon S., Mittal A.K. Insecticidal and growth inhibitory activity of essential oils of Boenninghausenia albiflora and Teucrium quadrifarium against Spilarctia obliqua. Biochem. Systemat. Ecol. 2018;81:70–73. [Google Scholar]
- Tawatsin A., Thavara U., Chansang U. Field evaluation of DEET, REPEL CARE®, and three plant-based essential oil repellents against mosquitoes, black flies (Diptera: Simuliidae), and land leeches (Arhynchobdellida: haemadipsidae) in Thailand. J. Am. Mosq. Contr. Assoc. 2006;22:306–313. doi: 10.2987/8756-971X(2006)22[306:FEODRC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Trongtokit Y., Rongsriyam Y., Komalamisra N. Comparative repellency of 38 essential oils against mosquito bites. Phytother Res. 2005;19:303–309. doi: 10.1002/ptr.1637. [DOI] [PubMed] [Google Scholar]
- Volpato A., Baretta D., Zortéa T. Larvicidal and insecticidal effect of Cinnamomum zeylanicum oil (pure and nanostructured) against mealworm (Alphitobius diaperinus) and its possible environmental effects. J. Asia Pac. Entomol. 2016;19:1159–1165. [Google Scholar]
- Wang L., Luo Y., Lu Y. Insecticidal activities of the extracts from 57 southern Chinese herbs against Aleurodicus dispersus. Plant Prot. 2012;38:108–111. [Google Scholar]
- Whalon M., Mota-Sanchez D., Hollingworth R. CAB International; Wallingford: 2008. Analysis of Global Pesticide Resistance in Arthropods. [Google Scholar]
- WHO . 2017. Global Vector Control Response 2017-2030, Geneva. [Google Scholar]
- WHO Vector-borne disease. 2020. https://www.who.int/en/news-room/fact-sheets/detail/vector-borne-diseases
- Yang C.L., Jiang S.H., Xu H.H. Research progresses in plant-originated repellents. Plant Prot. 2006;6:4–9. [Google Scholar]
- Yang K., Wang C.F., You C.X. Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stored product insects. J. Asia Pac. Entomol. 2014;17:459–466. [Google Scholar]
- Zhang J., Song Q.H., Zhang Y.P. Characteristics of fog in Xishuangbanna tropical rainforest and Ailaoshan subtropical evergreen broad-leaved forest. Acta Ecol. Sin. 2018;38:8758–8765. [Google Scholar]
- Zhao J., Zhang Y., Song F. Phenological response of tropical plants to regional climate change in Xishuangbanna, south-western China. J. Trop. Ecol. 2013;29:161–172. [Google Scholar]
- Zhu H. The floras of southern and tropical southeastern Yunnan have been shaped by divergent geological histories. PloS One. 2013;8 doi: 10.1371/journal.pone.0064213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Wang H., Li B. Studies on the forest vegetation of Xishuangbanna. Plant Sci. J. 2015;33:641–726. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.