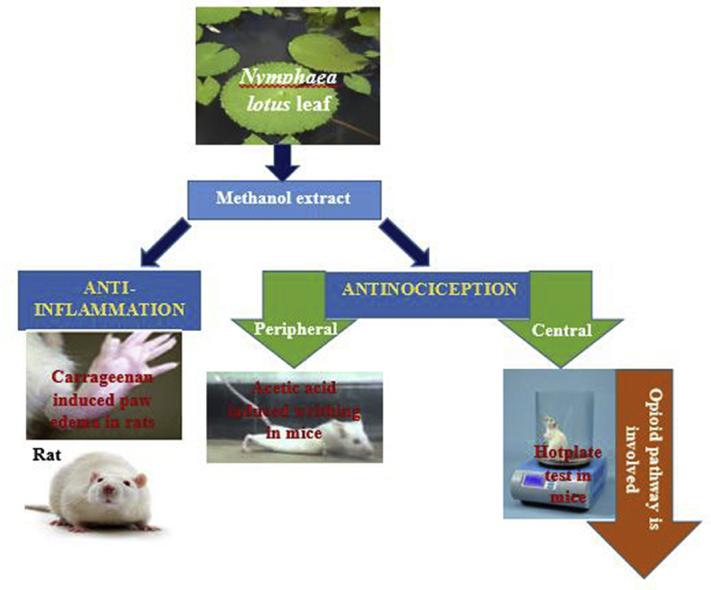
Abstract
The leaf of Nymphaea lotus has been used traditionally for the management of pain and inflammatory diseases. The methanol leaf extract of Nymphaea lotus (NLE) was evaluated for possible anti-nociceptive and anti-inflammatory activities in rats and mice (at the doses of 250, 500 and 1,000 mg/kg) to investigate the existence of scientific basis for the folkloric use of the plant. The standard drugs used were piroxicam (10 mg/kg) and morphine (10 mg/kg). The possible pharmacological mechanism involved in the anti-nociceptive activity was also investigated. The acute toxicity was determined in mice and rats using method of Lorke. The anti-nociceptive activity was evaluated using acetic acid-induced writhing and hot plate tests in mice, while the anti-inflammatory activity was evaluated using carrageenan-induced hind paw edema model in rats. The oral median lethal dose of NLE was found to be greater than 5,000 mg/kg in rats and mice. NLE demonstrated significant and dose-dependent protection against acetic acid induced writhes and increased the reaction time of mice in hot plate test. Pretreatment of the animals with naloxone (2 mg/kg) significantly (p < 0.05) attenuated the anti-nociception elicited by both NLE and morphine. NLE at the doses of 250 and 1,000 mg/kg significantly (p < 0.05) decreased rat paw edema at the 2nd hour in the carrageenan-induced paw edema test. The result of the study revealed that Nymphaea lotus possesses anti-nociceptive activities which may be mediated via the opioidergic system as well as mild anti-inflammatory activities thus providing scientific basis for the use of the plant in the management of pain and inflammatory diseases.
Keywords: Acetic acid, Analgesia, Anti-Inflammation, Nymphaea lotus, Hot plate test, Opioidergic
Graphical abstract
1. Introduction
The use of plants as medicine is an ancient practice common to all societies especially the African society and these practices continue to exist in the developing nations.1 About 80% of Africans rely on traditional plant medicine for their primary health care needs2 of which a major portion involves the use of plant extracts or their active principles.3 It is on these basis that researchers keep on working on medicinal plants in order to produce and/or develop the best medicines for therapeutic use.4
Pain as defined by International Association for the Study of Pain (IASP), is an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.5 It produces significant level of disability affecting economic and societal status.1 Inflammation is a defensive mechanism of the body to remove injurious stimuli and initiate healing process for the tissue, but if it proceeds unchecked, it can lead to onset of certain diseases such as rheumatoid arthritis and atherosclerosis.6 Inflammatory diseases remain one of the world’s major health problems.7 Currently available drugs used for the management of pain and inflammatory diseases are associated with different adverse effects. Moreover, high cost of acquiring the drugs, side effects, toxicities, has limited their use and warrants the screening of potential alternative therapies. The search for pharmacological agent to overcome these shortcomings has become a major goal in pain research. Plants which have been used as medicine over hundreds of years constitute an obvious choice for examination in the current search for therapeutically active drugs.8 Nymphaea lotus Linn is one of such plants. Therefore we evaluated the anti-nociceptive and anti-inflammatory potential of the methanol leaf extract of Nymphaea lotus (Nymphaeaceae) in Wistar rats and Swiss albino mice.
2. Materials and methods
2.1. Collection and identification of the plant
The fresh leaf sample of Nymphaea lotus were collected from Zaria, Kaduna State, Nigeria, in December, 2014. It was identified and authenticated by Mallam Namadi Sanusi, a plant taxonomist at the Herbarium section of the Department of Biological Sciences, Faculty of Sciences, Ahmadu Bello University, Zaria, and voucher specimen number 894 was obtained for future reference.
2.2. Experimental animals
Wistar rats (150–200 g) and Swiss albino mice (18–22 g) of either sex used for the study were obtained from the animal house facility of the Department of Pharmacology and Therapeutics, Faculty of Pharmaceutical Sciences, Ahmadu Bello University, Zaria, Nigeria. They were housed in standard animal cages at room temperature and maintained on a standard diet (Vital feeds, Jos, Nigeria) with water ad libitum. The experiment was carried out in accordance with the criteria outlined in the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health (Publication No. 80-23, revised 1996).
2.3. Drugs and chemicals
Glacial acetic acid (Searle, Essex, England), carrageenan (Sigma-Aldrich, Laborchemikalein GmBH, Germany), piroxicam injection (Pfizer), naloxone hydrochloride (Abcam Plc, Cambrige U.K), morphine sulphate (Martindale Pharmaceuticals, U.K), Dragendorffs reagent, Chips of magnesium metal, Ferric Chloride, Wagner reagent, Sulphuric acid (BDH Ltd, poole, England), Leishman Stain, Agappe Kits (Creatinine PN 51009001, Urea PN 51216001, Sodium PN 510014001 and Potassium PN 51015002), Labcare diagnositic kits, Randox kits (Ref AST AS101) and (Ref ALT AL100) and Dolabo kits (Ref ALP AP307) were used in the study.
2.4. Preparation of the plant extract
The leaf of Nymphaea lotus were cleaned, air dried under shade until crimpy, they were grounded into a coarse powder using mortar and pestle. The powdered leaf weighing 725.83 g was extracted by cold maceration method with 70% methanol for 72 h with occasional shaking. The extract was then filtered and concentrated over a water bath set at 50 °C. The percentage yield was calculated. The extract was then preserved in a labeled air tight container for future use. Stock solutions were freshly prepared with distilled water for each experiment.
2.5. Phytochemical screening
Preliminary phytochemical screening of the methanol leaf extract of Nymphaea lotus (NLE) was carried out using standard method of analysis.9
2.6. Oral acute toxicity studies
The oral acute toxicity studies of methanol leaf extract of Nymphaea lotus (NLE) in mice and rats was conducted using Lorke’s method (1983). The study was carried out in two phases and animals were deprived of food for 12 h prior to administration of the NLE. In phase 1, three groups of three rats/mice per group were treated with 10, 100 and 1000 mg/kg per body weight orally. The treated rats/mice were observed for signs of toxicity or death for the first 3 h and intermittently for 24 h. In phase 2, three rats/mice were orally administered NLE at doses of 1600 mg/kg, 2900 mg/kg and 5000 mg/kg per body weight respectively (based on the result in phase 1). The animals were then observed for signs of toxicity or death for the first 3 h and intermittently for 24 h.
2.7. Anti-nociceptive studies
2.7.1. Acetic acid induced writhes test in mice
The method described by10 was used. Thirty mice were randomly distributed into five groups of six mice each, they were orally administered distilled water (10 ml/kg), NLE (250, 500 and 1,000 mg/kg) and piroxicam (10 mg/kg). Sixty minutes post treatment, acetic acid 0.6% v/v (10 ml/kg) was intraperitoneally administered to each mouse. Five minutes after acetic acid injection, the mice were placed in individual cages and the number of abdominal contractions (writhes) was counted for each mouse for a period of 10 min. For scoring purposes, a writhe was indicated by stretching of the abdomen with simultaneous stretching of at least one hind limb. The formula used for computing percentage inhibition is as follows:
2.7.2. Hot plate test in mice
The method of Eddy11 was used for the study. Thirty mice were randomly divided into five groups of six mice each. The mice were orally administered distilled water (10 ml/kg), NLE (250, 500 and 1,000 mg/kg) and morphine (10 mg/kg). Mice were individually placed on a hot plate (55 ± 1 °C) before drug treatment so that each mouse serves as its own control. Sixty minutes post treatment, each mouse was placed on the hot plate, the time until the mouse either licked the paw, fluttered any of the paws or jumped was taken as reaction time and recorded. A cutoff time of 20s was used to avoid paw tissue damage. The latency was observed and recorded after 30, 60, 120 and 180 min. The percentage protection against thermal pain stimulus (% PATPS) was calculated as follows:
2.8. Test for the involvement of opioid receptors in the anti-nociceptive effect of NLE
The method described by Younos12 was employed. Thirty mice were randomly divided into five groups of six mice each. The mice were administered with the extract thus; group 1: distilled water (10 ml/kg, p.o), group 2: NLE (1,000 mg/kg p.o), group 3: Naloxone (2 mg/kg, i.p) then (after a 10 min interval) NLE (1,000 mg/kg p.o), group 4: morphine (10 mg/kg, p.o), and group 5: Naloxone (2 mg/kg, i.p) then (after a 10 min interval) Morphine (10 mg/kg p.o). The animals were subjected to hotplate test, pain score was observed and recorded as earlier described.
2.9. Anti-inflammatory study
2.9.1. Carrageenan-induced paw edema
The method previously described by Winter13 was used. Thirty rats were randomly divided into five groups of six rats each, they were orally administered distilled water (1 ml/kg), NLE (250, 500 and 1,000 mg/kg) and piroxicam (10 mg/kg). Sixty minutes later acute inflammation was induced by injecting 0.1 ml carrageenan. Carrageenan was prepared as 1% solution in 0.9% NaCl. The paw volume was then measured at 0, 1, 2, 3, 4 and 5 h using digital Vernier caliper to determine the diameter of edema. The difference between the reading at 0 h and different time intervals was taken as the thickness of edema. This was used to calculate the edema index by subtracting the reading at 0 h from the readings at the different time intervals.
2.10. Statistical analysis
Values were expressed as Mean ± Standard Error of the Mean (SEM). The data were analyzed by one way analysis of variance (ANOVA) followed by Dunnett or Bonferroni post hoc test for Multiple Comparison using the graph pad prism (statistical) software. The differences between means were considered significant at p ≤ 0.05.
3. Results
3.1. Acute toxicity study
The mice and rats showed no signs of toxicity and no mortality was recorded during the acute toxicity study. The oral median lethal dose value for mice and rats was estimated to be greater than 5,000 mg/kg.
3.2. Preliminary phytochemical screening
Preliminary phytochemical screening of the methanol leaf extract of Nymphaea lotus (NLE) revealed the presence of cardiac glycosides, saponins, steroids/terpenoids, flavonoids, tannins and alkaloids (Table 1).
Table 1.
Preliminary qualitative phytochemical study of methanol leaf extract of Nymphaea lotus.
| S/N | Constituents | Test | Observation | Inference |
|---|---|---|---|---|
| 1 | Carbohydrates | Keller-Killiani | Pale green colour | Present |
| 2 | Saponins | Frothing | Presence of honey comb froth | Present |
| 3 | Steroids and triterpenes | Lieberman-Buchards | Presence of purple colour | Present |
| 4 | Flavonoids | Shinoda | Presence of red colour | Present |
| Sodium hydrochloride | Presence of yellow colour | Present | ||
| 5 | Tannins | Ferric chloride | Presence of greenish black precipitate | Present |
| 6 | Alkaloids | Dragendoffs | Reddish brown precipitate | Present |
| Wagner | Whitish precipitate | Present | ||
| 7 | Anthraquinones | Bontrager | Bright pink colour | Absent |
3.3. Anti-nociceptive studies
3.3.1. Effect of methanol leaf extract of Nymphaea lotus on acetic acid-induced writhes in mice
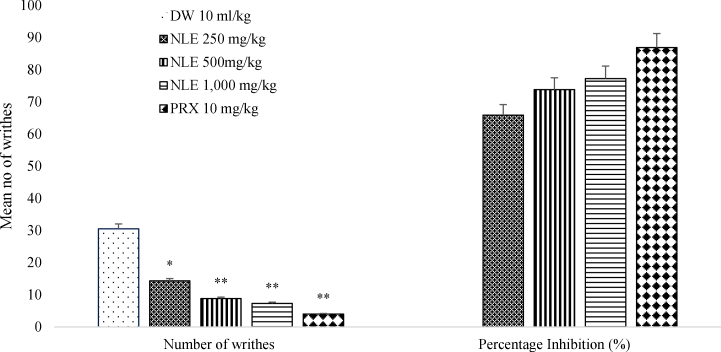
The methanol leaf extract of Nymphaea lotus (NLE) induced a significant and dose-dependent anti-nociceptive effect against acetic acid-induced peritoneal pain. Doses of 250, 500 and 1,000 mg/kg of methanol leaf extract of Nymphaea lotus produced anti-nociceptive effect characterized as a significant reduction (p < 0.01) in the number of writhes (14.33 ± 1.6, 8.83 ± 3.25 and 7.33 ± 1.97 respectively) in the writhing test compared to the negative control (30.50 ± 2.96). The standard drug piroxicam 10 mg/kg also significantly (p < 0.001) reduced the number of writhes to 4.00 ± 1.57. NLE at 250, 500 and 1,000 mg/kg elicited percentage writhes inhibition of 65.85%, 73.77%, and 77.23% respectively; while piroxicam 10 mg/kg elicited 86.88% (Fig. 1).
Fig. 1.
Effect of methanol leaf extract of Nymphaea lotus (NLE) on acetic acid induced writhes test in mice.
Data are expressed as mean ± SEM (n = 6 per group). ∗p < 0.01, ∗∗p < 0.001 significant difference from control. DW = Distilled water, NLE = Methanol leaf extract of Nymphaea lotus, PRX = Piroxicam (10 mg/kg).
3.3.2. Effect of methanol leaf extract of Nymphaea lotus on hot plate test in mice
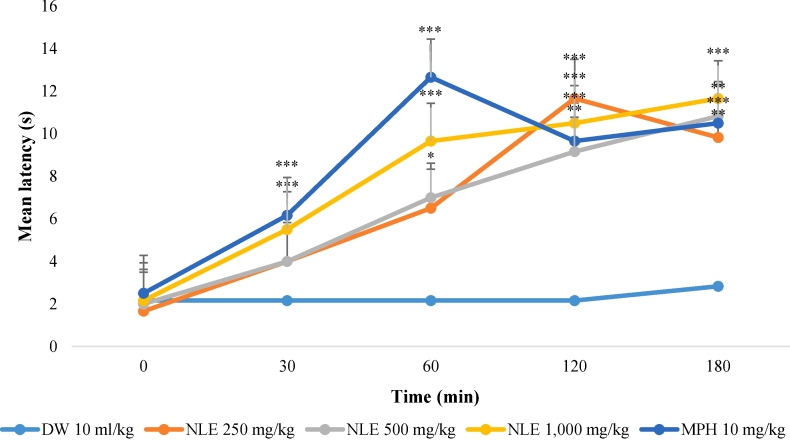
The effects of the methanol leaf extract of Nymphaea lotus (NLE) on mean reaction time in the hot plate test are presented in Fig. 2. The extract at the doses tested; significantly (p < 0.05) increased the latency to response in a dose dependent manner. NLE at 1,000 mg/kg elicited an increase in response latency at all-time points tested. However, at 500 mg/kg NLE exhibited significant increase in latency response at 60 min, 120 min and 180 min. While at 250 mg/kg the effect was only observed at 120 and 180 min. Morphine (10 mg/kg) the positive control showed similar effect as that of 1,000 mg/kg.
Fig. 2.
Effect of methanol leaf extract of Nymphaea lotus (NLE) on hot plate test in mice.
Data are expressed as mean ± SEM time in seconds (n = 6 per group). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 significant difference from negative control (Distilled water 10 ml/kg). DW = Distilled water (10 ml/kg), NLE = Methanol leaf extract of Nymphaea lotus in mg/kg, MPH = Morphine (10 mg/kg).
3.4. Interactive study
3.4.1. Effect of methanol leaf extract of Nymphaea lotus on hot plate test following pre-treatment with naloxone
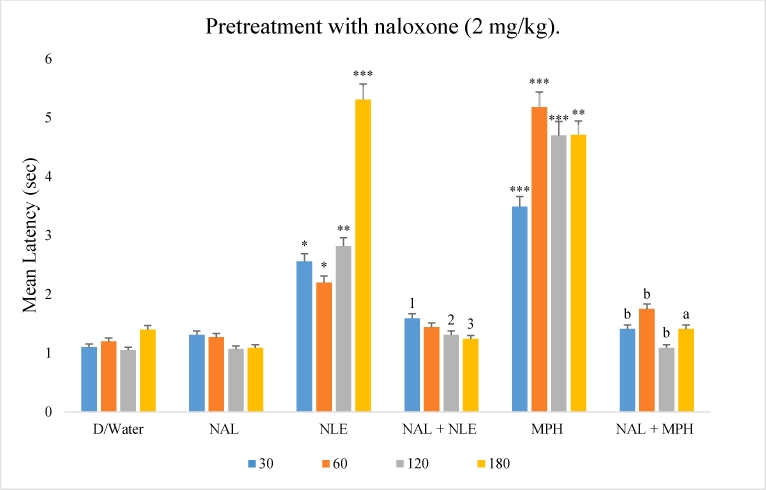
Mice were pretreated with naloxone (2 mg/kg i.p), a non-selective opioid receptor antagonist. The most effective dose of NLE (1,000 mg/kg) was selected for the study. NLE alone elicited increased latency to response to thermal stimulus. Pre-treatment with naloxone reduced (p < 0.05) the anti-nociceptive effect of NLE. The anti-nociceptive effect of morphine was also reversed (p < 0.001) by pretreatment with naloxone (Fig. 3).
Fig. 3.
Effect of methanol leaf extract of Nymphaea lotus (NLE) on hot plate test in mice following pretreatment with naloxone (2 mg/kg).
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 significant difference from negative control (Distilled water, 10 ml/kg), 1p < 0.5, 2p < 0.01, 3p < 0.001 significant difference from NLE group, ap < 0.01, bp < 0.001 significant difference from MPH group. NLE = Methanol leaf extract of Nymphaea lotus (1,000 mg/kg), NAL = Naloxone (2 mg/kg), MPH = Morphine (10 mg/kg).
3.5. Anti-inflammatory study
Effect of methanol leaf extract of Nymphaea lotus on carrageenan induced paw edema in rats.
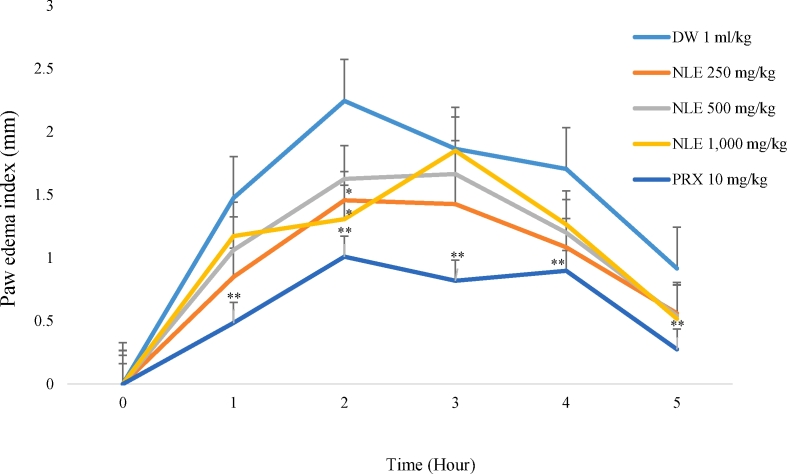
Following the injection of 1% carrageenan into the sub-plantar region of the hind paw of rats in the distilled water group; the diameter of the rat paw increased as edema developed reaching its maximum at the second hour indicating inflammatory activity (Fig. 4). The methanol leaf extract of Nymphaea lotus (250 mg/kg and 1,000 mg/kg) significantly (p < 0.05) decreased paw edema at the 2ndhour with percentage inhibition of 35.8% and 41.73% respectively. Piroxicam the standard drug decreased the edema at all-time points (p < 0.01).
Fig. 4.
Effect of methanol leaf extract of Nymphaea lotus (NLE) on carrageenan-induced rat paw edema.
Data are expressed as mean ± SEM (n = 6 per group). ∗p < 0.05, ∗∗p < 0.01 significant difference from negative control (Distilled water, 1 ml/kg) DW = Distilled water, NLE = Methanol leaf extract of Nymphaea lotus in mg/kg, PRX = Piroxicam (10 mg/kg).
4. Discussion
The oral LD50 value of the methanol leaf extract of Nymphaea lotus (NLE) in both rats and mice were greater than 5,000 mg/kg body weight. This suggests that the extract is practically non-toxic orally, at acute dose levels according to the lethal dose classification of toxic levels of chemicals by Lorke.14
The qualitative phytochemical screening of the methanol leaf extract of Nymphaea lotus revealed the presence of cardiac glycosides, saponins, steroids/terpenoids, flavonoids and alkaloids. These phytochemicals are known to be responsible for several pharmacological activities including the observed anti-nociceptive and anti-inflammatory effects. Anti-nociceptive and anti-inflammatory activities have been observed with phytochemicals such as flavonoids, tannins, alkaloids, saponins Terpenoids and steroids.15, 16, 17, 18 The anti-nociceptive and anti-inflammatory effects observed by methanol leaf extract of Nymphaea lotus was believed to be due to the presence of the several phytochemicals.
The acetic acid induced writhes test is a sensitive method used to evaluate potential anti-nociceptive drugs or compounds that act peripherally such as NSAIDs19,20 and centrally acting analgesics such as morphine.20,21 The injection of acetic acid intraperitoneally produces an abdominal writhing response due to sensitization of chemoreceptors by prostaglandins.22 This model has been associated with increased level of prostaglandins particularly PGE2 and PGEα in peritoneal fluids as well as lipoxygenase products. This enhances inflammatory pain by increasing capillary permeability.23,24 The positive control, piroxicam (10 mg/kg p.o) exhibited significant writhes inhibition in mice, eliciting peripherally acting analgesic effect. Piroxicam reduced the number of writhes induced by acetic acid by inhibiting cyclooxygenase (COX) in peripheral tissues thereby blocking the release and/or synthesis of inflammatory mediators.21 Similarly, oral administration of NLE demonstrated significant and dose dependent attenuation of acetic acid-induced writhes in mice. Thus the anti-nociceptive effect exhibited by the NLE may be due to inhibition of the synthesis and release of prostaglandins and other endogenous substances. The reduction of acetic acid-induced writhes in mice by NLE indicates that the extract may be acting peripherally and/or centrally via the nociceptors.
The hot plate method as described by Eddy11 is the most commonly used thermal nociception model in the evaluation of central analgesic efficacy of drugs or compounds. The hot plate method is one of the most common tests of nociception that is based on a phasic stimulus of higher intensity.25 Pain induced by thermal stimulus of the hot plate is specific for centrally mediated nociception.26 Therefore, the prolongation of reaction latency to pain induced thermally in mice using this model suggests centrally acting anti-nociceptive activity.24 The methanol leaf extract of Nymphaea lotus, at different doses significantly increased reaction time of mice in a dose dependent pattern in the hot plate test. The extract at the dose of 1,000 mg/kg gave the highest percentage protection against thermally induced pain which was comparable to that of morphine (10 mg/kg) the control drug. This demonstrates that NLE possesses anti-nociceptive activity mediated via central mechanism.
To determine if the opioidergic system is involved in the anti-nociceptive activity of NLE, the anti-nociceptive effect of NLE was assessed in the presence of naloxone using the hot plate test. Naloxone is a non-selective opioid receptor antagonist which acts on the mu (μ), kappa (κ) and delta (ɖ) opioid receptors.27 The most effective dose 1,000 mg/kg of NLE in the previous experiment was used for the study. Naloxone significantly inhibited the anti-nociceptive effect of NLE suggesting that activation of the opioidergic system is involved in the anti-nociception produced by NLE. Expectedly, naloxone also inhibited the anti-nociception produced by morphine.
Carrageenan injection into rat paw produces a local acute inflammatory reaction that is a suitable criteria for the evaluation of anti-inflammatory agents.13 The time course of edema development in carrageenan induced paw edema model in rats is generally presented by a biphasic curve.28 The first phase which occurs between 0 and 2.5 h after injection of the phlogistic agent has been attributed to the release of histamines or serotonin.29 The edema volume reaches its maximum approximately 3 h post treatment and then begins to decline. The second phase of the inflammatory reaction is caused by the release of bradykinins, protease, prostaglandins and lysosomes.29,30
Following the injection of 1% carrageenan into the sub-plantar region of the rats’ paw in the negative control group; the diameter of the rats’ paws increased as edema developed reaching its maximum at the second hour indicating inflammatory activity. NLE (250 and 1,000 mg/kg), significantly decreased paw edema at the 2nd hour after injection. However, the 500 mg/kg dose did not show significant reduction in paw edema. NLE failed to significantly inhibit paw edema at the 3rd, 4th and 5th hour, while piroxicam the positive control exhibited significant inhibition of the carrageenan induced inflammation at the 1st, 2nd,3rd,4th and 5th hour.
The extract elicited its anti-inflammatory effect in the early phase. The ability of NLE to inhibit carrageenan-induced paw edema suggests it possess significant effect against acute-inflammation. This further demonstrates the possible central effect of NLE, as all centrally acting agents inhibit this phase of inflammation.31,32
5. Conclusion
The result of the present investigation demonstrates that NLE possesses strong antinociceptive activity in laboratory animals. Furthermore, we show that the activity of the extract may involve opioidergic mechanisms. The extract also exhibited anti-inflammatory properties. Taken together, our findings provide pharmacological rationale for the traditional use of this plant for the treatment of pain and inflammatory conditions. Further studies are required to isolate the possible bioactive constituents responsible for these activities.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Louw Q.A., Morris L.D., Grimmer-Somers K. The prevalence of low back pain in Africa: a systematic review. BMC Muscoskel Disord. 2007;8(1):1. doi: 10.1186/1471-2474-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2005. National Policy on Traditional Medicine and Regulation of Herbal Medicines: Report of a WHO Global Survey. [Google Scholar]
- 3.Farnsworth N.R. Screening plants for new medicines. Biodiversity. 1988;1:83–97. [Google Scholar]
- 4.Usman H., Osuji J.C. Phytochemical and in vitro antimicrobial assay of the leaf extract of Newbouldia laeves. Afr J Tradit, Complementary Altern Med. 2008;4(4):476–480. [PMC free article] [PubMed] [Google Scholar]
- 5.2016. International association for the study of pain.http://www.iasp-pain.org/Taxonomy [Google Scholar]
- 6.Sharma U., Sharma U., Sutar N., Singh A., Shukla D. Anti-inflammatory activity of Cordia dichotoma forst. seeds extracts. Int J Pharm Anal. 2010;2(1):01–04. [Google Scholar]
- 7.Li R.W., Myers S.P., Leach D.N., Lin G.D., Leach G. A cross-cultural study: anti-inflammatory activity of Australian and Chinese plants. J Ethnopharmacol. 2003;85(1):25–32. doi: 10.1016/s0378-8741(02)00336-7. [DOI] [PubMed] [Google Scholar]
- 8.Sampson J., Phillipson J., Bowery N., O’neill M., Houston J., Lewis J. Ethnomedicinally selected plants as sources of potential analgesic compounds: indication of in vitro biological activity in receptor binding assays. Phytother Res. 2000;14(1):24–29. doi: 10.1002/(sici)1099-1573(200002)14:1<24::aid-ptr537>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Evans W.C. fifteenth ed. Bailliere Tindal; London: 2002. Trease and Evans Pharmacology; p. 585. [Google Scholar]
- 10.Koster R., Anderson M., De Beer E.J. Acetic acid-induced analgesic screening. Fedration Proceedings. 1959;18:412–417. [Google Scholar]
- 11.Eddy N.B., Leimbach D. Synthetic analgesics. II. Dithienylbutenyl and dithienylbutylamines. J Pharmacol Exp Therapeut. 1953;107(3):385–393. [PubMed] [Google Scholar]
- 12.Younos C., Rolland A., Fleurentin J., Lanhers M.C., Misslin R., Mortier F. Analgesic and behavioural effects of Morinda citrifolia. Planta Med. 1990;56:430–434. doi: 10.1055/s-2006-961004. [DOI] [PubMed] [Google Scholar]
- 13.Winter C.A., Risley E.A., Nuss G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Exp Biol Med. 1962;111(3):544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 14.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 15.Ahmadiani A., Hosseiny J., Semnanian S. Antinociceptive and anti-inflammatory effects of Elaeagnus angustifolia fruit extract. J Ethnopharmacol. 2000;72(1):287–292. doi: 10.1016/s0378-8741(00)00222-1. [DOI] [PubMed] [Google Scholar]
- 16.Calixto J.B., Beirith A., Ferreira J., Santos A.R.S., Filho V.C., Yunes R.A. Review of antinociceptive plant substances. Phytother Res. 2000;14:401–418. doi: 10.1002/1099-1573(200009)14:6<401::aid-ptr762>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Choi J., Jung H.J., Lee K.T., Park H.J. Antinociceptive and anti-inflammatory effects of the saponin and sapogenins obtained from the stem of Akebia quinata. J Med Food. 2005;8(1):78–85. doi: 10.1089/jmf.2005.8.78. [DOI] [PubMed] [Google Scholar]
- 18.Reanmongkol W., Subhadhirasakul S., Thienmontree S., Thanyapanit K., Kalnaowakul J., Sengsui S. Antinociceptive activity of the alkaloid extract from Kopsia macrophylla leaves in mice. Songklanakarin J Sci Technol. 2005;27(2):509–516. [Google Scholar]
- 19.Mishra D., Ghosh G., Umar P.S., Panda P.K. An Experimental Study of Analgesic actrivities of selective cox-2 inhibitor with conventional NSAIDs. J Pharmaceut Clin Res. 2011;4(1):78–81. [Google Scholar]
- 20.Kakoti B.B., Pradhan P., Borah S., Mahato K., Kumar M. Analgesic and anti-inflammatory activities of the methanolic stem bark extract of Nyetanthes arbortrsitis Linn. BioMed Res Int. 2013 doi: 10.1155/2013/826295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donkor K., Stephen A., Jerry A., Nutifafa T., Nil O.M., Laud K.O. Analgesic and anti-inflammatory activities of Asena, a herbal for treatment of athritis, using rodent models. Med Aromatic Plants Res J. 2013;1(2):20–29. [Google Scholar]
- 22.Sutharson L., Lila K.N., Presanna K.K., Shila E.B., Rajan V.J. Antiinflammatory and antinociceptive activities of methanolic extract of the leaves of Fraxinus floribunda Wallic. Afr J Biotechnol. 2007;6(5):582–585. [Google Scholar]
- 23.Lakshman K., Shivaprasad H., Jaiprakash B., Mohan S. Short communication-anti-inflammatory and antipyretic activities of Hemidesmus indicus root extract. Afr J Tradit, Complementary Altern Med. 2006;3(1):90–94. [Google Scholar]
- 24.Khan H., Saeed M., Khan M.A., Dar A., Khan I. The antinociceptive activity of Polygonatum verticillatum rhizomes in pain models. J Ethnopharmacol. 2010;127(2):521–527. doi: 10.1016/j.jep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Mandegary A., Sayyah M., Heidari M.R. Antinociceptive and anti-inflammatory activity of the seed and root extracts of Ferula gummosa Boiss in mice and rats. Daru. 2004;12(2):58–62. [Google Scholar]
- 26.Parkhouse J., Plaury B.J. BlackWell Co.; Oxford: 1979. Analgesic Drugs; p. 1. Oxford. [Google Scholar]
- 27.Martin W.R. Naloxone. Ann Intern Med. 1976;85:765–768. doi: 10.7326/0003-4819-85-6-765. [DOI] [PubMed] [Google Scholar]
- 28.Vinegar R., Schreiber W., Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Therapeut. 1969;166(1):96–103. [PubMed] [Google Scholar]
- 29.Crunkhorn P., Meacock S. Mediators of the inflammation induced in the rat paw by carrageenin. Br J Pharmacol. 1971;42(3):392–402. doi: 10.1111/j.1476-5381.1971.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiRosa M., Willoughby D.A. Screens for anti-inflammatory drugs. J Pharm Pharmacol. 1971;23:297–298. doi: 10.1111/j.2042-7158.1971.tb08661.x. [DOI] [PubMed] [Google Scholar]
- 31.Asif M. In vivo analgesic and anti-inflammatory effects of Tectona gradlis Linn Stem bark extracts. Malays J Pharmaceut Sci. 2011;9(1):29–43. [Google Scholar]
- 32.Gaertner M., Muker L., Rose J.F. Analgesic triterpines from Sebastiania schottiana roots. Phytomedicine. 1999;6:41–44. doi: 10.1016/S0944-7113(99)80033-6. [DOI] [PubMed] [Google Scholar]