Abstract
The yolk sac (YS) consists of the yolk, which supplies nutrients, and the YS tissue, which surrounds the yolk and provides essential metabolic functions for the developing embryo. The YS tissue is derived from the midgut of the embryo and consists of a layer of endodermal epithelial cells (EEC) in contact with the yolk contents, a mesodermal layer that contains the vascular system and an outer ectodermal layer. The YS tissue is a multifunctional organ that provides essential functions such as host immunity, nutrient uptake, carbohydrate and lipid metabolism, and erythropoiesis. The YS tissue plays a role in immunity by the transport of maternal antibodies in the yolk to the embryonic circulation that feeds the developing embryo. In addition, the YS tissue expresses high mRNA levels of the host defense peptide, avian β-defensin 10 during mid embryogenesis. Owing to its origin, the YS EEC share some functional properties with intestinal epithelial cells such as expression of transporters for amino acids, peptides, monosaccharides, fatty acids, and minerals. The YS tissue stores glycogen and expresses enzymes for glycogen synthesis and breakdown and glucogenesis. This carbohydrate metabolism may play an important role in the hatching process. The mesodermal layer of the YS tissue is the site for erythropoiesis and provides erythrocytes before the maturation of the bone marrow. Other functions of the YS tissue involve synthesis of plasma proteins, lipid transport and cholesterol metabolism, and synthesis of thyroxine. Thus, the YS is an essential organ for the growth, development, and health of the developing embryo. This review will provide an overview of the studies that have investigated the functionalities of the YS tissue at the cellular and molecular levels with a focus on chickens.
Key words: chicken, yolk sac tissue, multifunctional organ
Introduction

The avian embryo relies solely on nutrients derived from the yolk, albumen, and shell to support its growth and development. For this review, we will use the term yolk sac (YS) as the yolk contents plus the YS tissue, which surrounds the yolk. The YS tissue is often called the YS membrane but it is not just a membrane but is a multifunctional tissue that plays an important role in nutrient uptake, performs multiple metabolic functionalities, and is the first line of defense against pathogens in the yolk and thus it is essential to the health of the embryo. Because many organs of the developing embryo have not yet matured, the YS tissue serves as a vital organ for these diverse functions. For example, maturation of the intestine and liver starts around 15 d -, of the thyroid around 10 d and of the bone marrow for erythropoiesis around 14 d of incubation (Freeman and Vince, 1974). The YS tissue acts as the bone marrow for synthesis of blood cells, as the intestine for digestion and transport of nutrients and lipids, as the liver for production of plasma carrier proteins and carbohydrate metabolism, as the thyroid for regulating metabolism and as the immune system for the transfer of antibodies from the hen and production of antimicrobial peptides.
Understanding the functional development of the YS tissue is key to understanding uptake of nutrients from the yolk for growth of the chicken embryo. The functional properties of the YS tissue develop from early to late embryogenesis. Starting around embryonic (E) day 19, the YS is withdrawn into the abdominal cavity and the process is completed around 14 h before hatch (Freeman and Vince, 1974). During these last days of incubation, the YS tissue undergoes a process of degradation before internalization into the body cavity of the chick. Although the chick derives nutrients mainly as protein and carbohydrates from feed after hatch, the chick can still utilize the lipids stored in the residual yolk even as the YS tissue is degrading. In addition to this natural degradation of the YS tissue, any disruption in absorption of the YS content may lead to deficiency of necessary nutrients and maternal antibodies that can result in early chick mortality and poor chick quality. For a comprehensive analysis of the effects of broiler breeder, egg size, egg storage, and incubation temperature on yolk utilization, see the recent review by van der Wagt et al. (2020).
Enhancing our understanding of the cellular and molecular mechanisms that regulate YS function is important for the growth, development, and health of the embryonic chick and overall gut health of the hatched chick. This review will focus on the development and the multifunctional properties of the chicken YS tissue. Although the focus will be on the chicken YS tissue, examples from quail, pigeon, and turkey will be included for comparison when available.
Formation of the yolk and yolk Sac
Yolk Formation and Composition and Uptake by the Embryo
During follicular development, there is rapid growth of a single oocyte to form the primary follicle in the ovary (Johnson, 2015). During this time, lipids, mainly in the form of lipoproteins, are synthesized by the liver and secreted into the plasma of the hen. The lipoproteins are transported from the plasma to the follicles to form the yolk by a receptor-mediated, endocytotic mechanism (Schneider, 2016). Later during formation of the egg as it moves down the oviduct, egg white, and the shell are deposited around the yolk.
Breeder age and egg size affect egg composition (see review by van der Wagt et al., 2020). Larger eggs from hens of the same age flock have a greater percentage of albumen than yolk compared with smaller eggs (Vieira and Moran, 1998). As breeder hens age, egg weight increases due to a larger increment of yolk compared with albumen (O'Sullivan et al., 1991; Peebles et al., 2000; Nangsuay et al., 2011; Traldi et al., 2011). Eggs from 30-wk old breeder hens contained 71% albumen and 29% yolk, whereas eggs from 50-wk old breeder hens had 66% albumen and 34% yolk. The yolk from 30-wk old breeder hens consisted of 55% water, 24% fat, and 18% protein, whereas yolk from 50-wk old breeder hens contained 53% water, 27% fat, and 16% protein (Yadgary et al., 2010). Thus, eggs from older flocks contain more nutrients because they have a greater percentage of nutrient-rich yolk.
The yolk is the main source of lipids and the main source of energy (via fatty acid oxidation) during chick embryonic development. The four major fatty acids present in the yolk before incubation are palmitic (16:0), stearic (18:0), oleic (18:1n-9), and linoleic (18:2n-6) acid. The percentage of these fatty acids change with age of the breeder flock (Sahan et al., 2014). Less than 10% of the yolk fat is absorbed by the chick embryo by E13, whereas from E15 to E21, there was rapid uptake of yolk fat (Yadgary et al., 2010). The long-chain polyunsaturated fatty acid docosahexaenoic acid (22:6) was rapidly utilized from the YS as compared with all other fatty acids between E15 and E19 (Maldjian et al., 1995; Yadgary et al., 2014). Docosahexaenoic acid plays a crucial role in the development of the brain and retina (Anderson et al., 1990), which may explain its rapid uptake to support an almost doubling of brain weight from E15 to E19 (Romanoff, 1960).
The most abundant lipoprotein in yolk is very-low-density lipoprotein (VLDL), which makes up approximately 66% of the yolk dry matter, whereas cholesterol makes up approximately 5.2% of total yolk lipids. Acyl-coenzyme A: cholesterol acyltransferase (ACAT) catalyzes the esterification of cholesterol with a long-chain fatty acid to facilitate lipid utilization. The concentration of esterified cholesterol increased in the YS tissue from E13 to E21, which allowed packaging in VLDL and transport to the fetal liver (Noble, 1986). Acyl-coenzyme A: cholesterol acyltransferase activity increased 3-fold from E9 to E16 in the YS tissue of chick embryos (Shand et al., 1993) and 3-fold from E13 to E22 in the YS of turkey embryos (Ding and Lilburn, 2000). The observed increase in esterified cholesterol corresponds with an in increase in ACAT activity.
Proteomic analyses of the chicken egg yolk plasma have been conducted. Zhu et al. (2020) compared the egg yolk protein composition of fertilized eggs incubated at 0, 10, and 18 d. There was high abundance of vitellogenins at day 10, which may play a role in lipid localization and lipid transportation. Other differentially expressed proteins at day 10 and 18 were also mainly involved in lipid transport and lipid localization. Rehault-Godbert et al. (2014) conducted a proteomic analysis of the chicken egg yolk plasma in fertilized compared with unfertilized eggs after 12 d of incubation. A number of proteins (e.g., retinol binding protein 4 and transthyretin) decreased in the yolk of fertilized eggs due to utilization by the developing embryo, whereas other proteins increased in fertilized eggs (e.g., α-fetoprotein), which may be from synthesis and secretion by the YS tissue (Slade and Milne, 1977).
The age of the breeder flock influences egg size, the ratio of yolk to albumen, and the percentage of fatty acids in the yolk. During the last 7 d of incubation, there is rapid utilization of the yolk fatty acids, especially docosahexaenoic acid. Recent proteomic analyses have provided insight into the effects of time of incubation and fertilization on protein content of the yolk.
Structure and Development of the Yolk Sac Tissue
The YS tissue is the first extraembryonic membrane that advances from the embryonic gut starting around E2 and gradually forms a membrane that encloses the yolk content (Romanoff, 1960; Patten, 1971). The YS tissue consists of 3 cell layers that are laid down in an orderly progression over the next 3 to 4 d. First, ectodermal cells from the embryo rapidly spread over the surface of the yolk. Next, endodermal cells spread between the yolk and the ectodermal cells and form an epithelial cell layer in contact with the yolk, which plays an important role in nutrient uptake. Finally, mesodermal cells migrate between the ectodermal and endodermal layers, which is the site for erythropoiesis (Mobbs and McMillan, 1979; Sheng, 2010; Bauer et al., 2013; Clement et al., 2017). The YS tissue can be morphologically separated into the area vitellina, which does not contain the mesodermal layer and thus no blood vessels, and the area vasculosa, which contains the mesodermal layer, blood cells, and blood vessels. From E2 to E6, there is rapid expansion of the surface area of the area vasculosa and area vitellina. After E6, the surface area of the area vasculosa continues to increase until E12, whereas the surface area of the area vitellina declines (Romanoff, 1960). The area and weight of the YS tissue reached a peak around E15 and E17 and then decreased toward hatch (Yadgary et al., 2013).
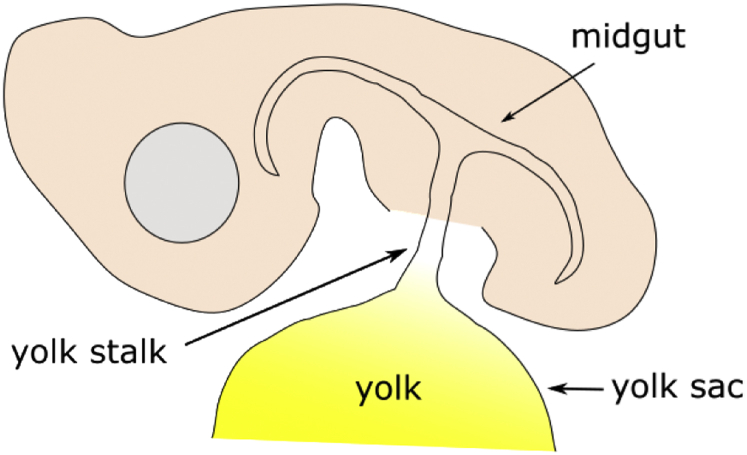
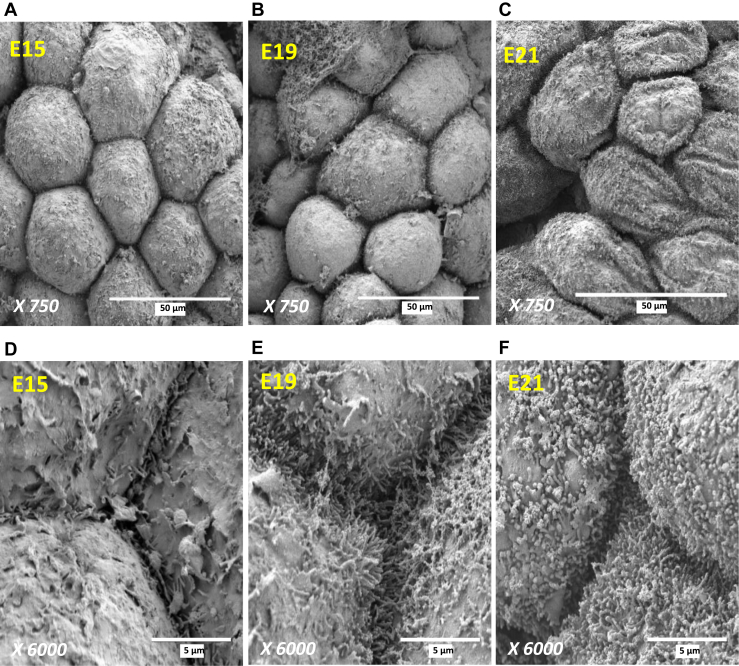
Because the YS tissue develops from the midgut of the intestine (Figure 1), it is not surprising that the YS tissue and intestine share some morphological features (Patten, 1971). The YS tissue contains villus-like structures consisting of a layer of endodermal epithelial cells (EEC) with blood vessels in the center. A scanning electron micrograph of the YS at E15 shows villus-like structures lined with EEC projecting into the yolk (Figure 2). A cross section of a YS villus shows the EEC surrounding a central blood vessel (Figure 3). The YS EEC are multifunctional cells that play roles in both absorption of nutrients and secretion of enzymes and hormones, in contrast to the different specialized cells lining the intestinal villi that play a role in absorption (enterocytes) or secretion (enteroendocrine and goblet cells). On the surface of the YS EEC are microvilli that increase the surface area for maximal interaction with the yolk (Mobbs and McMillan, 1979). This is similar morphologically and functionally to the microvilli on the epithelial cells of the intestinal villi. A scanning electron micrograph shows that at E15, the surface area of EEC is large and microvilli structures have begun to form (Figure 4). At E19, EEC begin to decrease in size while microvilli can be observed mainly on the edges of the cell surface, whereas at E21 EEC are smaller in size and the microvilli cover the entire cell surface. Measurements of 30 EEC per age show that the average apical surface perimeter decreased by 18.2% from E15 to E21 (Dayan, 2019). This decrease in YS surface area likely results in a reduction in nutrient absorption and prepares the YS for internalization into the abdominal cavity of the chick.
Figure 1.
Schematic diagram of a chick embryo at approximately 6 d of incubation showing the yolk sac connected to the midgut through the yolk stalk.
Figure 2.
Scanning electron micrographs of the chicken yolk sac at embryonic day 15. (A) Yolk sac (YS) villus-like structures are seen projecting into the yolk, X40 magnification. (B) Magnified image of YS villus endodermal epithelial cells (EEC), X750 magnification (Dayan, 2019).
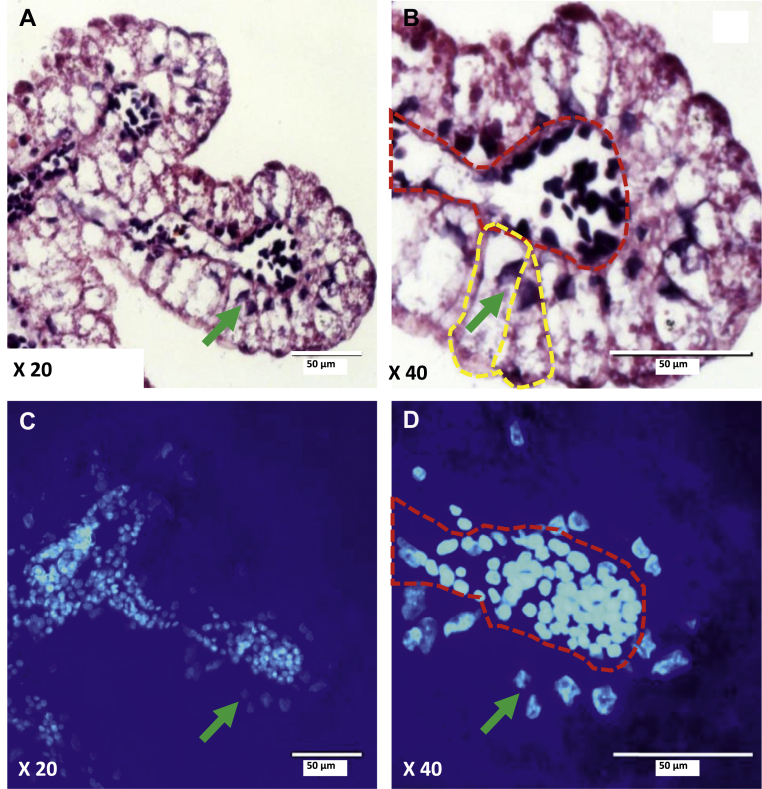
Figure 3.
Cross section of chicken yolk sac villus observed by light and fluorescent microscopy. (A, B) Sections of the yolk sac (YS) at embryonic day 10 stained with hematoxylin and eosin at X20 and X40 magnification, respectively. (C, D) Sections stained with 4′,6-diamidino-2-phenylindole (DAPI). The YS villus comprises endodermal epithelial cells (EEC) (B, yellow frames) that surround a central blood vessel (B and D, red frames). The nuclei of EEC (green arrows) are located at the basolateral part of the cell (Dayan, 2019).
Figure 4.
Scanning electron micrographs of the morphology of chicken yolk sac endodermal epithelial cells (EEC). (A, D) At E15, the surface areas of endodermal epithelial cells (EEC) are large, with microvilli structures beginning to form. (B, E) At E19, EEC begin to decrease in size while microvilli appear at the periphery of the cell surface. (C, F) At E21, EEC are smaller and microvilli cover the entire cell surface (Dayan, 2019).
Like the small intestine, the YS tissue forms a barrier that prevents intercellular transport of large molecules. Mobbs and McMillan (1981) showed that the apical junctions between YS EEC prevented the intercellular passage of tracers such as horseradish peroxidase, ferritin, and latex spheres. The YS expressed mRNA for the tight junction proteins occludin, claudin-1, zona occluden-1, and junctional adhesion molecule-2 (Jia and Wong, unpublished). These results suggest that like the small intestine (Awad et al., 2017) there is a complex of tight junction proteins between YS EEC that regulates the flow of macromolecules and serves as a physical barrier to any pathogens.
At the end of the incubation period from E15 and E17 to E21, YS weight and YS absorptive area declined and the YS tissue began to degrade as the EEC senesced (Yadgary et al., 2013). The EEC changed from a distinct columnar shape to a less-ordered structure, with degraded apical membranes resulting in spillage of the cellular contents into the yolk and reduction in nutrient absorption. During this time, there was downregulation of genes associated with cytoskeleton cell structure and intermediate keratin filaments (Yadgary et al., 2014). The authors speculated that this may be the result of apoptosis, although this has not been experimentally tested.
Preincubation and early embryonic incubation temperature affected development of the YS tissue and uptake of yolk. Lin et al. (2017) examined the effect of preincubation at 23.9°C or 29.4°C in combination with incubation at 37.5°C or 38.1°C from E0 to E5. The 29.4°C preincubation temperature decreased YS weight at E15 and decreased YS vasculature at E6. It is unknown why a higher preincubation temperature had a negative effect on YS weight and YS vasculature. Incubation at 38.1°C from E0 to E5 increased YS vasculature at E7 compared with 37.5°C.
Suboptimal incubation temperature can affect yolk utilization. Nangsuay et al. (2016) showed that incubation at 38.9°C reduced yolk utilization compared with 37.8°C. Dayan et al. (2020) showed that at 37.8°C broiler embryos had a 71.7% decrease in their residual yolk percentage from E15 to E21, whereas embryos incubated at 36.6°C and 39.3°C showed a lower decrease of 63.8 and 37.2%, respectively. This reduced yolk absorption and utilization of egg nutrients by the embryo likely led to lower quality embryos, hatchlings, and growing chickens (Joseph et al., 2006; Sahan et al., 2014; Hamidu et al., 2018).
The YS tissue is derived from the midgut of the developing embryo and consists of 3 cell layers: endoderm, mesoderm, and ectoderm. The endodermal layer is in close contact with the yolk and contains villus-like structures that increase surface area. Endodermal epithelial cells are connected by a barrier that is likely similar to the tight junctions between intestinal epithelial cells. Suboptimal preincubation and incubation temperatures can affect development of the YS tissue and the utilization of yolk. At the end of incubation, the YS tissue begins to degrade as it is internalized into the abdominal cavity of the chick. Owing to its multicellular and multifunctional properties, the YS tissue can also serve as a valuable model for studying cell differentiation.
Functional roles of the yolk Sac
Although oviparous and viviparous species are very different, the YS tissue provides similar multiple functional roles while the organs of the embryo are developing and maturing. A recent review about source and function of the YS during embryogenesis of primates highlights the pivotal role of the YS as a multifunctional hub for hematopoiesis, germ cell development and nutritional supply (Ross and Boroviak, 2020). Cindrova-Davies et al. (2017) used RNA-seq to compare the YS of human, mouse, and chicken and found that despite the differences in mammalian and avian development there is a conservation of function of the YS. Sheng and Foley (2012) similarly identified conservation of function in the YS endoderm of chickens and mammals. In chickens, individual gene and transcriptome analyses of the YS tissue have been performed to examine temporal changes. Nakazawa et al. (2011) used DNA microarrays to profile developmental changes in the chicken YS tissue from E2 to E4, whereas Yadgary et al. (2014) used serial analysis of gene expression to examine gene expression at later times (E13–E21).
Role of the Yolk Sac in Host Immunity
The egg is naturally protected against pathogens by structural barriers and innate immune components (reviewed in Hincke et al., 2019). The albumen contains 2 major antimicrobial proteins, ovotransferrin, and lysozyme. Ovotransferrin is an iron-binding glycoprotein that acts as an antimicrobial by sequestering iron necessary for growth of microorganisms and transfers iron to the embryo (Wu and Acero-Lopez, 2012). Lysozyme makes up 3.5% of total egg white protein and lyses the cell wall of some gram-negative bacteria. In addition to its direct action on the bacterial cell wall, enzymatic hydrolysis of lysozyme generates antimicrobial peptides active against both gram-negative and gram-positive bacteria (Carrillo and Ramos, 2018). The yolk also contains its own defense against pathogens. During egg formation, antibodies are transferred from the hen to the egg yolk and provide passive immunity for the developing embryo (Kovacs-Nolan and Mine, 2012). Little is known about the role that the YS tissue may play in providing immune protection for the developing embryo; however, Zhang and Wong (2019) have shown that the YS tissue expressed high mRNA levels of avian β-defensin 10 during mid embryogenesis. The avian β-defensins (AvBD) are short cationic peptides (<100 amino acids) that possess antimicrobial activity (Cuperus et al., 2013; Zhang and Sunkara, 2014). The combination of antimicrobial peptides in the egg white, maternal antibodies in the yolk, and synthesis of AvBD by the YS tissue may constitute a multifaceted defense strategy to counter any pathogens present in the egg.
The developing chick is dependent on maternally transmitted antibodies that are deposited into the yolk for protection against pathogens until it can synthesize its own antibodies (Kaspers et al., 1996; Kovacs-Nolan and Mine, 2012). The YS tissue plays an important role in passive immunity by taking up maternal yolk immunoglobulin Y antibodies starting around E7 with a dramatic increase in uptake from E19 to hatch and by transporting the antibodies intact into the embryonic circulation through a receptor-mediated transcytotic process (e.g., Brierley and Hemmings, 1956; Kowalczyk et al., 1985; West et al., 2004; Tesar et al., 2008).
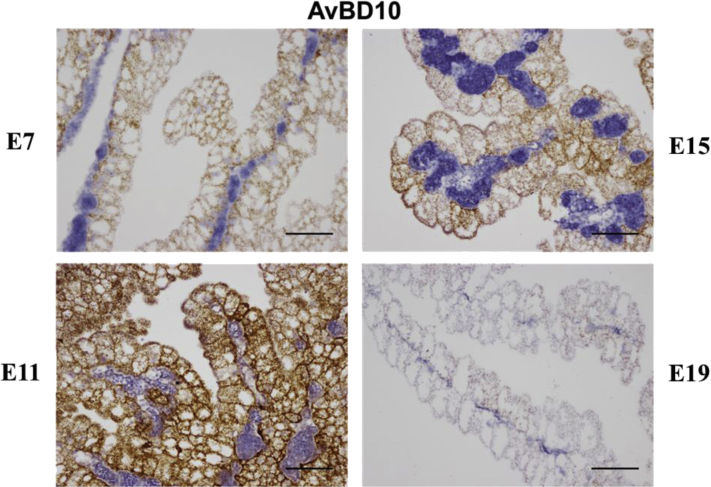
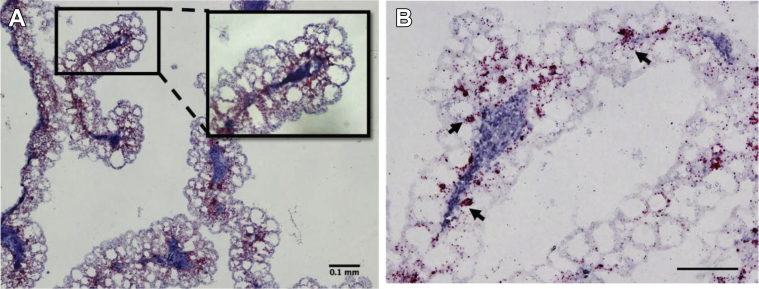
The YS tissue also forms a biological barrier between the embryo and any bacteria present in the yolk. Ding et al. (2017) have hypothesized that the microbiota of the embryo is derived from the maternal hen during the process of egg formation in the oviduct and thus the yolk would be exposed to these bacteria. Expression of the 14 AvBD mRNA (AvBD1 to AvBD14) was examined in the YS tissue from E7 to E19. AvBD1, AvBD2, AvBD7, and AvBD10 mRNA showed high mRNA levels, with AvBD10 the greatest. There was a development-specific expression pattern with low expression at E7, increased expression between E9 and E15, and decreased expression at E19 (Zhang and Wong, 2019). Using in situ hybridization (ISH), AvBD10 mRNA was found to be highly expressed in the YS EEC (Figure 5), whereas AvBD1, AvBD2, and AvBD7 mRNA were expressed in heterophils.
Figure 5.
Developmental expression of the avian β-defensin 10 mRNA in the chicken yolk sac tissue. Avian β-defensin 10 (AvBD10) mRNA was detected in formalin-fixed, paraffin embedded yolk sac tissue at embryonic day 7 (E7), 11 (E11), 15 (E15), and 19 (E19) by RNAscope in situ hybridization and revealed as brown dots (Wang et al., 2012). Tissues were counterstained with hematoxylin. The scale bar represents 0.1 mm (Zhang and Wong, 2019).
Lactotransferrin binds and transports iron, but also possesses antimicrobial activity and is considered as part of the host immune system. Expression of lactotransferrin mRNA in the YS tissue was constant from E13 to E17 and then declined to E21 (Yadgary et al., 2014).
The YS tissue plays an important role in immunity while the immune organs of the embryo are developing. The YS tissue transports maternal antibodies from the yolk to the embryonic blood to provide passive immunity for the embryo. In addition, the YS tissue plays a more direct role by producing antimicrobials such as lactotransferrin and AvBD10. It is possible that AvBD10 synthesized by the YS tissue is secreted into the embryonic circulation to protect the developing embryo.
Role of the Yolk Sac in Nutrient Absorption
Epithelial cells of the YS tissue secrete digestive enzymes and mediate the transport of various nutrients, such as amino acids, carbohydrates, lipids, and minerals using membrane bound transporter proteins. In many cases, the mRNA abundance for these transporters has been assayed by qPCR, which allows quantification but does not allow for the identification of cells expressing these transporters. Because the number of antibodies to these chicken proteins is limited, there are few studies that have examined protein expression. In a few cases, cells expressing the mRNA for these transporters have been identified by ISH.
The expression of selected enzymes and transporters shows development-specific patterns. The abundance of the digestive enzyme aminopeptidase N, the neutral amino acid transporter B0AT, the cationic amino acid transporter CAT1, the peptide transporter PepT1, and the monosaccharide transporter GLUT5 showed greater mRNA abundance during early embryogenesis, which declined toward hatch (Yadgary et al., 2011; Speier et al., 2012). By contrast, the anionic amino acid transporter EAAT3 and the sodium glucose transporter SGLT1 increased toward hatch. Sucrase-isomaltase mRNA did not change from E11 to day of hatch (doh). Using transcriptome analysis, Yadgary et al. (2014) also profiled the expression of many other members of the SLC gene family. There was increased expression of EAAT3 and SGLT1 mRNA and decreased expression of GLUT5, B0AT, and PepT1 mRNA toward hatch. Sucrase-isomaltase activity was detected in the YS of chickens (Matsushita, 1986) and proteases have been purified from the YS tissue of quail (Gerhartz et al., 1997).
Using ISH, expression of PepT1 and SGLT1 mRNA was localized to the EEC of the YS (Figure 6). PepT1 mRNA was barely detectable in cells at E11, expressed strongly at E13, E15, and E17, and declined at doh (Zhang and Wong, 2017). Expression of SGLT1 mRNA in YS EEC was low from E11 to E17, peaked at E19 and then declined at doh (Zhang et al., 2019). For both PepT1 and SGLT1, the ISH results paralleled the gene expression profiles determined by qPCR.
Figure 6.
Expression of peptide transporter PepT1 and sodium glucose transporter SGLT1 mRNA in chicken yolk sac tissue. PepT1 (A) and SGLT1 (B) mRNA were detected in formalin-fixed, paraffin embedded yolk sac (YS) tissue at embryonic days 15 and 19, respectively by RNAscope in situ hybridization and revealed as red dots (Wang et al., 2012). Tissues were counterstained with hematoxylin (Zhang and Wong (2017) and Zhang et al. (2019)).
The differential expression of SGLT1, EAAT3, and PepT1 mRNA may play important roles in the development of the embryo. The increased expression of SGLT1 mRNA during late incubation is likely related to an increased need for glucose for the hatching process and is described further in the section on the role of the YS in carbohydrate metabolism. The similar rise in EAAT3 mRNA at the end of incubation may be important for maintaining YS EEC functionality because EAAT3 transports the anionic amino acid glutamate, which is a major energy source for intestinal EEC (Brosnan and Brosnan, 2013). Because YS and intestinal EEC share functionalities, glutamate may also be a key energy source for YS EEC. For bulk amino acid transport, upregulation of a single peptide transporter such as PepT1, which can transport most of the 400 dipeptides and 8,000 tripeptides (Wong et al., 2017) would be more efficient than upregulation of multiple amino acid transporters that have individual specificities for anionic, neutral, and cationic amino acids. The peak expression of PepT1 mRNA between E13 and E17 would supply the high concentration of amino acids necessary for the rapid growth of the embryo from E12 to hatch (Freeman and Vince, 1974).
The developmental changes in mRNA expression of digestive enzymes and transporters in the YS tissue of pigeons (Columba livia) has also been reported (Dong et al., 2012). The abundance of PepT1 mRNA decreased from E12 to hatch, whereas aminopeptidase N mRNA increased from E12 to E14 and then declined toward hatch. By contrast, SGLT1, GLUT2, and SI mRNA increased from E12 to E16 and then declined at hatch. Egg weight influenced amino acid transporter gene expression in the YS tissue of pigeons. Chen et al. (2016) showed that the abundance of CAT2 and PepT1 mRNA in the YS tissue was greater in heavy compared with light pigeon eggs at E13.
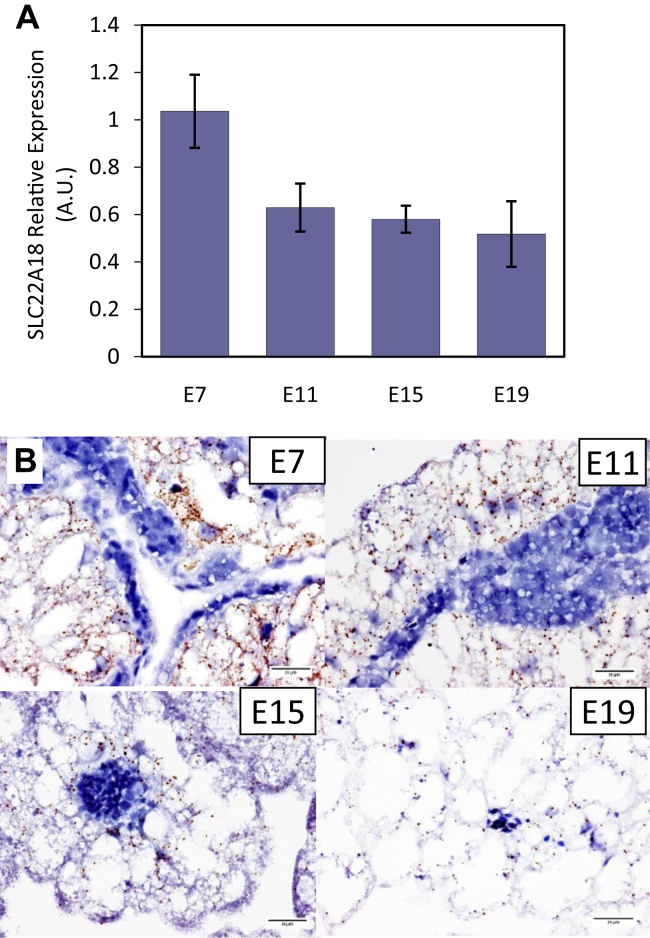
The yolk is a major mineral source for the developing embryo. The yolk mineral composition can be affected by maternal environmental temperatures and maternal diet. Zhu et al. (2017) reported that maternal dietary Zn supplementation with either inorganic or organic Zn increased the Zn content in the yolk and high maternal environmental temperature (32°C) reduced yolk Zn content compared with the control temperature (21°C). Mineral transfer from the yolk to the embryo is mediated by transporters present in the YS tissue. As an example, the transfer of calcium and phosphorus from the yolk to the embryo is essential for embryonic bone development (Li et al., 2014; Torres and Korver, 2018). Analysis of the P, Ca, Fe, Zn, Cu, and Mn levels in yolk and albumen of broiler breeder eggs from 50-week-old breeder hens showed that the yolk is the major origin of Mn (96.5%) and P (94.5%) with only 3.5% of Mn and 5.5% of P originating from the albumen (Yair and Uni, 2011). The percentage of Fe, Ca, Cu, and Zn in the yolk ranged from 87.8 to 62.6%. There was rapid uptake of P, Fe, Zn, Cu, and Mn from the yolk from E11 to E17, after which there was little uptake from the yolk because most of the yolk mineral content had been depleted by E17. By contrast, the uptake of Ca was constant from E11 to hatch, which may reflect the continuous need for Ca for bone growth of the embryo. Consistent with these observed changes in yolk mineral content, Yadgary et al. (2011) showed that the major sodium phosphate transporter NPT2b mRNA increased from E11 to E20 and E21. The calcium transporter TRPV6 mRNA initially decreased from E11 to E13, but then increased from E13 to E19. Expression of the zinc transporter ZnT-1 (SLC22A18), which is also responsible for the efflux of Zn out of enterocytes (Tako et al., 2005), declined from E7 to E19 in YS epithelial cells (Figure 7). This decline may be due to the almost zero level of Zn in the yolk as most of the Zn was absorbed from the yolk before E17 (Yair and Uni, 2011).
Figure 7.
Developmental expression of zinc transporter ZnT1 (SLC22A18) mRNA in chicken yolk sac tissue. (A) Real-time qPCR relative expression of SLC22A18 in the yolk sac (YS) tissue at embryonic day 7 (E7), 11 (E11), 15 (E15), and 19 (E19). Letters indicate significant differences by nonparametric Wilcoxon each = pair test (P < 0.05). (B) SLC22A18 mRNA was detected in formalin-fixed, paraffin embedded YS tissue at E7, E11, E15, and E19 by RNAscope in situ hybridization and revealed as brown dots (Wang et al., 2012). Tissues were counterstained with hematoxylin. Images are magnified 400x. Scale bars indicate 25 μm (Reicher and Zhang, unpublished).
The YS tissue acts like the intestine for the uptake of nutrients, which are essential for the growth and development of the embryo. The expression profiles of a number of digestive enzymes and transporters for amino acids, peptides, monosaccharides, lipids, minerals, and other molecules have been reported. Expression of many of these enzymes and transporters in the YS tissue declines at hatch, when the intestine takes over the role of nutrient uptake from feed.
Role of the Yolk Sac in Carbohydrate Metabolism
The observed increase in glucose content in the yolk near the end of embryogenesis is partially attributed to expression of genes involved in glycogenolysis or glucogenesis in the EEC. At the time of set, the yolk contained 50 mg of glucose but no glycogen (Yadgary and Uni, 2012). The concentration of glucose in the YS increased from 2 mg/g on E11 to 6 mg/g on E19, whereas the glucose concentration in the YS tissue increased slightly from 8 mg/g on E11 to 9 mg/g on E19. During this same time (E11–E19), the concentration of glycogen in the YS increased from 2 mg/g to 25 mg/g, whereas the glycogen concentration in the YS tissue increased from 10 mg/g to 25 mg/g. At E19, liver glucose concentration was 12 mg/g and liver glycogen concentration was 20 mg/g. Because at E19, the weight of the YS tissue was 10-fold that of the liver, the YS tissue stored greater than 12-fold as much glycogen as the liver. Between E19 and E21, glycogen concentration decreased in the YS tissue. The expression of mRNA encoding glycogen synthase, which is involved in glycogen synthesis, and glycogen phosphorylase, which is involved in glycogen breakdown, were examined in the YS tissue. Glycogen synthase mRNA increased from E11 to E21, whereas glycogen phosphorylase mRNA increased from E11 to E19 and then declined to E21. This combination would be expected to lead to an increase in glycogen levels in the YS tissue. The mRNA encoding the glucogenic enzymes fructose 1,6-bisphosphatase, phosphoenol pyruvate carboxykinase, and glucose 6-phosphatase were also examined in the YS tissue in this study. Although fructose 1,6-bisphosphatase mRNA showed a decrease and phosphoenol pyruvate carboxykinase and glucose 6-phosphatase mRNA were relatively unchanged from E11 to E15, the expression of these genes supported the role of the YS tissue in glucogenesis.
The YS tissue provides essential carbohydrates during the late incubation phase. Because the weight of the YS tissue is more than 10 times that of the embryonic liver, the YS tissue plays the major role in glycogen storage, synthesis, and breakdown. Glucose is also synthesized in the YS tissue by glucogenic enzymes and stored as glycogen. As hatch approaches, the glycogen-derived glucose is released, which may be important for the embryo during the hatching process.
Role of the Yolk Sac in Lipid Metabolism
Because the yolk contains a high concentration of lipids, the YS tissue plays an important role in lipid absorption and metabolism. Lipids are absorbed by a receptor mediated endocytotic process (Schneider, 2016). Approximately half of the lipid content of the yolk was absorbed between E13 and E17 by both endocytotic and receptor-mediated uptake mechanisms, which resulted in lipid accumulation in the YS tissue (Noble and Cocchi, 1990; Speake et al., 1998). Yoshizaki et al. (2004) have similarly investigated the uptake of lipids into the YS tissue during development of quail embryos.
The cellular and molecular mechanism that regulates the development of nutrient transport competence of EEC has been described by Bauer et al. (2020). This competence is mediated by a paracrine interaction of the EEC with the mesodermal cell layer. Bone morphogenetic proteins 4 and 7 produced by ectodermal and mesodermal cell layers likely initiate a differentiation program of EEC. The bone morphogenetic proteins promote the upregulation of endocytic receptor expression and thereby provide the EEC with the molecular machinery to produce triglyceride-rich lipoprotein particles. During this time, there is enhanced expression of LRP2, amnionless and cubilin, which make up an endocytic complex and genes involved in lipoprotein synthesis and transport (Bauer et al., 2013; Yadgary et al., 2014).
Lipase activity and bile acids were detected in the YS tissue and YS content. Lipase activity in the YS tissue increased 2-fold from E17 to E21. The level of bile acids in the YS tissue were constant from E11 to E21, but the levels increased 5-fold in the YS content from E17 to E21 (Yadgary et al., 2013). These findings suggest that YS lipids are hydrolyzed in the lipolysosomes into free fatty acids and glycerol, which are then reesterified to form triglycerides and phospholipids and assembled into VLDL particles. Powell et al. (2004) had previously shown that yolk lipid is hydrolyzed and re-esterified during transfer across the YS tissue. The presence of bile acids in the YS tissue could serve as an emulsifier of YS lipids to enhance the action of lipases.
Cholesterol in the yolk is rapidly esterified during the uptake of lipids. Shand et al. (1993) demonstrated that increased activity of ACAT, which is also called sterol O-acyltransferase (SOAT) and decreased activity of cholesteryl hydrolase in the YS tissue promoted the conversion of cholesterol to cholesteryl ester. Wang et al. (2017) found that SOAT1 mRNA and protein were regulated by nutrients and hormones through a cAMP-dependent pathway in the YS tissue of quail.
The YS tissue plays an important role in the interconversion of fatty acids by the expression of various lipid modifying enzymes. For example, expression of Δ6-desaturase and Δ9-desaturase activities in the YS tissue resulted in the conversion of linoleic acid to arachidonic acid and stearic acid to oleic acid, respectively (Noble and Shand, 1985; Noble and Cocchi, 1990; Peebles et al., 1999). These desaturases were also used for the biosynthesis of fatty acids 18:1n-9, 20:4n-6, and 22:6n-3 during the transfer of yolk lipids across the YS tissue (Speake and Deans, 2004). Incubation temperature altered the uptake of fatty acids from the yolk. Yalcin et al. (2008) reported that eggs treated at 38.5°C for 6 h per day between E10 and E18 resulted in reduced levels of 18:1n-9 and 18:2n-6, but increased levels of 16:0 in the residual yolk of chicks at hatch. This indicated that there was enhanced uptake of 18:1n-9 and 18:2n-6 and reduced uptake of 16:0 from the yolk in response to higher incubation temperature.
Fatty acid–binding proteins (FABP) play a role in the uptake of long-chain fatty acids. In the YS tissue of chicks, FABP2 mRNA was constant from E13 to E21, whereas FABP5 mRNA was upregulated from E15 to E21. In turkeys, the activity of FABP in the YS tissue was detected as early as E13, with high activity from E19 to E28 (Ding and Lilburn, 2002).
The development of primary epithelial cell cultures will further enhance our understanding of the function of the YS tissue. Donaldson et al. (1990) cultured chick YS EEC that formed a polarized confluent epithelium, made tight junctions, formed apical microvilli, and showed IgG surface binding. Lin et al. (2016) established a YS cell culture from Japanese quail and showed that the EEC accumulated lipid droplets and expressed lipoprotein lipase and SOAT1 mRNA. These cell lines are useful models to study embryonic lipid transport.
The YS tissue not only transports lipids but also plays a role in the metabolism of lipids. The transport of lipids is regulated by endocytotic and receptor mediated mechanisms. The development of lipid transport is mediated by paracrine cross-talk between EEC and the mesodermal layer. The YS tissue expresses lipases and bile acids, which hydrolyze lipids into free fatty acids for synthesis of triglycerides and phospholipids. In addition, the YS tissue expresses mRNAs for the esterification of cholesterol and interconversion of fatty acids by desaturases. Thus, extensive modification of lipids occurs in the YS tissue.
Role of the Yolk Sac in Erythropoiesis
The development of erythropoiesis and the formation of the vascular network in the YS tissue has been covered in a number of reviews (Sheng, 2010; Nagai et al., 2018). The first wave of erythropoiesis produces primitive erythrocytes, whereas the second wave produces definitive erythrocytes. From E1 to E5, the YS is the sole site for primitive erythropoiesis. Starting at E4 to E4.5, the YS generates definitive erythrocytes until about E15, whereas the bone marrow begins erythropoiesis around E12 to E13. Guedes et al. (2014) used histological analysis to demonstrate that the YS tissue was a unique hematopoietic site from E4 to E12, whereas both the YS tissue and liver were sites for hematopoiesis from E13 to E20. By contrast, Yvernogeau and Robin (2017) demonstrated using an in vivo transplantation assay that the YS tissue was not a site for hematopoietic stem cells. In this study, they transplanted aorta-gonad-mesonephros, YS tissue, and allantois from an E3 GFP-expressing embryo into the chorioallantoic membrane of E4 recipients and found that only aorta-gonad-mesonephros and not YS tissue or allantois resulted in the presence of GFP-expressing cells in the blood, spleen, thymus, bursa of Fabricius, and bone marrow 5 months after transplantation. Definitive erythrocytes proliferate in the YS tissue between E13 and E15 and then migrate to the blood circulation from E15 to E19 (Yadgary et al., 2014). Using ISH, Zhang and Wong (2018) identified cells expressing the stem cell marker Lgr5 (leucine-rich repeat containing G protein-coupled receptor 5) that was localized to the vascular endothelial cells lining the blood vessels. These cells may represent a hematopoietic stem cell population.
The YS tissue plays an essential role in both primitive and definitive erythropoiesis before the development of the bone marrow. For the first 5 d of incubation, the YS is the site of primitive erythropoiesis, with a transition to definitive erythropoiesis around E4. Near the end of incubation, there is a shift of the major site of erythropoiesis from the YS tissue to the bone marrow.
Role of the Yolk Sac in Hepatic Function
During embryogenesis, the YS tissue synthesizes plasma proteins. The YS tissue is the major site of α-fetoprotein (AFP) synthesis with smaller, but significant quantities being produced by the liver (Slade and Milne, 1977). Using transcriptome analysis, Yadgary et al. (2014) demonstrated that a number of mRNA typically associated with the liver were expressed in the YS tissue, such as AFP and albumin. Albumin mRNA abundance was stable between E13 and E21, whereas AFP mRNA abundance declined from E15 to E21.
Expression of transthyretin and retinol-binding protein (RBP) mRNA has been detected in the YS tissue (Yadgary et al., 2014). Transthyretin (TTR) is a principal distributor of thyroid hormone (TH) T4, which is also known as thyroxine, whereas RBP binds to retinol. Both TTR and RBP are normally expressed in the liver and secreted into the blood. In the YS tissue, TTR mRNA showed decreased expression from E17 to E21, whereas RBP4 mRNA was stable between E13 and E21. Thus, the YS tissue plays a key role in mediating the transport of thyroxine and retinol, which are necessary for normal embryo development.
In addition to its role in glycogen storage and metabolism, the YS tissue provides many of the functions of the liver. The YS tissue produces the plasma proteins AFP and albumin, TTR for T4 distribution, and RBP. There are likely many other proteins to be identified that are produced by the YS tissue that are normally associated with the liver.
Role of the Yolk Sac in Regulating Metabolism
The thyroid gland plays an important role in regulating body metabolism by synthesizing and secreting various hormones. Expression of key enzymes that modulate activity of TH was examined in the YS tissue of Hy-Line embryos (layer strain), from E4 to E21 (Too et al., 2017). Yolk TH content decreased linearly with development. Expression of mRNA encoding TTR and TH-inactivating iodothyronine deiodinase 3 was detected until the second week of incubation. The TH-activating deiodinase 2 (DIO2) and transporter of thyroxine, SLCO1C1, were expressed during the last week of incubation. This coincided with the marked increase in circulating thyroxine and reduction in YS weight. The iodothyronine deiodinase 1 (DIO1), which can remove iodine from inactive TH, was expressed throughout incubation. Dayan et al. (2020) examined the effect of incubation temperature on temporal expression of TTR, DIO1 and DIO2 mRNA in the YS tissue of Cobb 500 embryos (broiler strain). Transthyretin mRNA abundance in the YS tissue at the control (37.8°C), cold (36.3°C), and hot (39.3°C) incubation temperatures showed the same pattern which was high from E5 to E11 with a gradual decrease toward hatch. The DIO1 mRNA at the control and cold incubation temperatures showed a similar pattern of a slight increase from E5 to E12 followed by a slight decrease toward hatch, whereas at the hot incubation temperature, there was an almost constant level. DIO2 mRNA expression from E5 to E21 at the control temperature showed a quadratic decrease, whereas at the cold and hot temperatures, there was a linear decrease. The results of Dayan et al. (2020) are not the same as those reported by Too et al. (2017) and may represent the difference between broilers and layers, respectively. In addition, Forrest et al. (1990) showed that the mRNA encoding both α and β TH receptors were detected in the YS tissue from E4 to E19. Together, these results demonstrate that the YS tissue plays a role in mediating TH transfer from the yolk to the embryo via the extra-embryonic bloodstream.
Thyroid hormones also play essential roles in regulating the hatching process, such as the time of hatch, retraction of the YS, and development of the hatching muscle, intestine, and lungs (de Groef et al., 2013). The rise in circulating thyroxine levels before hatch coincides with increases in the abundance of the sodium/iodine symporter, thyroid peroxidase, and thyroglobulin mRNA from the thyroid gland (Grommen et al., 2011). It is not clear what percentage of the rise in circulating T4 is from the YS and from the developing thyroid.
The enzyme 20-hydroxysteroid dehydrogenase modulates the potency of glucocorticoids, such as corticosterone. Rao et al. (2009) showed that a low protein diet fed to hens resulted in downregulation of 20-hydroxysteroid dehydrogenase mRNA in the YS tissue of embryos at E14. Thus, the maternal diet can influence gene expression in the YS tissue.
The YS tissue acts like the thyroid in the production of the active TH hormone thyroxine by expressing mRNA for TH-activating and TH-inactivating enzymes. Incubation temperature can modulate expression of these deiodinases and thus levels of thyroxine. Because the embryonic chick is poikilothermic or unable to regulate its own body temperature during incubation, the YS tissue plays an important role by providing thyroxine for regulating metabolism of the chick.
Summary and conclusion
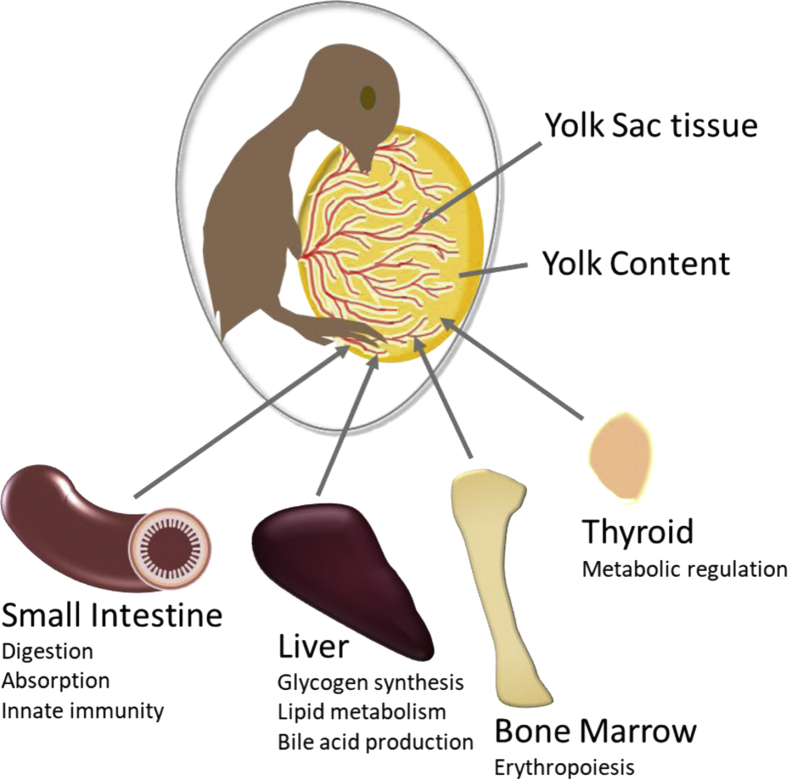
Before the development of fully functional organs, the embryonic chick must rely on the YS tissue to provide all essential metabolic functions for the growth, development, and health of the developing embryo. Because the YS tissue consists of 3 different cell layers and provides these functions, it is not just a membrane but a multifunctional organ. The YS tissue acts as 1) an immune organ by transporting maternal antibodies from the yolk and expressing the host defense peptide AvBD10, 2) the intestine for digestion of proteins and polysaccharides and absorption of amino acids, monosaccharides, lipids, and minerals, 3) the liver for storage of glycogen and expression of enzymes involved in glycogen synthesis and breakdown and glucogenesis, as well as the synthesis of plasma proteins, 4) the bone marrow for both primitive and definitive erythropoiesis, and 5) the thyroid for synthesis of thyroid hormones that regulate metabolism. The multiple functions of the YS tissue are illustrated in Figure 8. The transition of functionality from the YS tissue to the maturing organs of the embryo occurs in a coordinated fashion during incubation.
Figure 8.
A schematic diagram showing the multifunctional properties of the chicken yolk sac tissue. The chicken yolk sac tissue provides the functions of the intestine, liver, bone marrow, and thyroid, while the organs of the embryo are developing and maturing.
Acknowledgments
This project was funded in part by the Binational Agricultural Research and Education (BARD) program, grant US-5074-18CR and the Virginia Agricultural Experiment Station, the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture. The authors also would like to thank Naama Reicher for drawing the illustration in Figure 8.
Disclosures
The authors declare that they have no conflict of interest.
References
- Anderson G.J., Connor W.E., Corliss J.D. Docosahexaenoic acid is the preferred dietary n-3 fatty acid for the development of the brain and retina. Pediat. Res. 1990;27:89–97. doi: 10.1203/00006450-199001000-00023. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Hess C., Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 2017;9:60. doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R., Plieschnig J.A., Finkes T., Riegler B., Hermann M., Schneider W.J. The developing chicken yolk sac acquires nutrient transport competence by an orchestrated differentiation process of its endodermal epithelial cells. J. Biol. Chem. 2013;288:1088–1098. doi: 10.1074/jbc.M112.393090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R., Tondl P., Schneider W.J. A differentiation program induced by bone morphogenetic proteins 4 and 7 in endodermal epithelial cells provides the molecular basis for efficient nutrient transport by the chicken yolk sac. Dev. Dyn. 2020;249:222–236. doi: 10.1002/dvdy.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley J., Hemmings W.A. The selective transport of antibodies from the yolk sac to the circulation of ther chick. Development. 1956;4:34–41. [Google Scholar]
- Brosnan J.T., Brosnan M.E. Glutamate: a truly functional amino acid. Amino Acids. 2013;453:413–418. doi: 10.1007/s00726-012-1280-4. [DOI] [PubMed] [Google Scholar]
- Carrillo W., Ramos M. Identification of antimicrobial peptides of native and heated hydrolysates from hen egg white lysozyme. J. Med. Food. 2018;21:915–926. doi: 10.1089/jmf.2017.0132. [DOI] [PubMed] [Google Scholar]
- Chen M.X., Li X.G., Yan H.C., Wang X.Q., Gao C.Q. Effect of egg weight on composition, embryonic growth, and expression of amino acid transporter genes in yolk sac membranes and small intestines of the domestic pigeon (Columba livia) Poult. Sci. 2016;95:1425–1432. doi: 10.3382/ps/pew044. [DOI] [PubMed] [Google Scholar]
- Cindrova-Davies T., Jauniaux E., Elliot M.G., gong S., Burton G.J., Charnock-Jones D.S. RNA-seq reveals conservation of function among the yolk sacs of human, mouse and chicken. Proc. Natl. Acad. Sci. USA. 2017;114:E4753–E4761. doi: 10.1073/pnas.1702560114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement R., Mauroy B., Cornlissen A.J.M. Tissue growth pressure drives early blood flow in the chicken yolk sac. Dev. Dyn. 2017;246:573–584. doi: 10.1002/dvdy.24516. [DOI] [PubMed] [Google Scholar]
- Cuperus T., Coorens M., van Dijk A., Haagsman H.P. Avian host defense peptides. Dev. Comp. Immunol. 2013;41:352–369. doi: 10.1016/j.dci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Dayan J. The Hebrew University of Jerusalem, Rehovot, Israel; 2019. Chicken Embryos Yolk Sac Tissue Development and Function during Incubation. MS Thesis. [Google Scholar]
- Dayan J., Reicher N., Melkman-Zehavi T., Uni Z. Incubation temperature affects yolk utilization through changes in expression of yolk sac tissue functional genes. Poult. Sci. 2020;99:6128–6138. doi: 10.1016/j.psj.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groef B., Grommen S.V.H., Darras V.M. Hatching the cleidoic egg: the role of thyroid hormones. Front. Endocrinol. 2013;4:63. doi: 10.3389/fendo.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Dai R., Yang L., He C., Xu K., Liu S., Zhao W., Xiao L., Luo L., Zhang Y., Meng H. Inheritance and establishment of gut microbiota in chickens. Front. Microbiol. 2017;8:1967. doi: 10.3389/fmicb.2017.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S.T., Lilburn M.S. The developmental expression of acyl-coenzyme A:cholesterol acyltransferase in the yolk sac membrane, liver, and intestine of developing embryos and posthatch turkeys. Poult. Sci. 2000;79:1460–1464. doi: 10.1093/ps/79.10.1460. [DOI] [PubMed] [Google Scholar]
- Ding S.T., Lilburn M.S. The ontogeny of fatty acid-binding protein in Turkey (Meleagridis gallopavo) intestine and yolk sac membrane during embryonic and early posthatch development. Poult. Sci. 2002;81:1065–1070. doi: 10.1093/ps/81.7.1065. [DOI] [PubMed] [Google Scholar]
- Donaldson J.G., Bogenman E., Roth T.F. Cultured chick yolk sac epithelium: structure and IgG surface binding. Eur. J. Cell Biol. 1990;53:246–254. [PubMed] [Google Scholar]
- Dong X.Y., Wang Y.M., Yuan C., Zhou X.T. The ontogeny of nutrient transporter and digestive enzyme gene xpression in domestic pigeon (Columba livia) intestine and yolk sac membrane during pre- and posthatch development. Poult. Sci. 2012;91:1974–1982. doi: 10.3382/ps.2012-02164. [DOI] [PubMed] [Google Scholar]
- Forrest D., Sjöberg M., Vennström B. Contrasting developmental and tissue-specific expression of alpha and beta thyroid hormone receptor genes. EMBOJ. 1990;9:1519–1528. doi: 10.1002/j.1460-2075.1990.tb08270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B.M., Vince M.A. Chapman and Hall; London: 1974. Development of the Avian Embryo. [Google Scholar]
- Gerhartz B., Auerswald E.A., Mentele R., Fritz H., Machleidt W., Kolb H.J., Wittmann J. Proteolytic enzymes in yolk-sac membrane of quail egg. Purification and enzymatic characterisation. Comp. Biochem. Physiol. Part B. Biochem. Mol. Biol. 1997;118:159–166. doi: 10.1016/s0305-0491(97)00034-5. [DOI] [PubMed] [Google Scholar]
- Grommen S.V., Iwasawa A., Beck V., Darras V.M., De Groef B. Ontogenic expression profiles of thyroid-specific genes in embryonic and hatching chicks. Domest. Anim. Endocrinol. 2011;40:10–18. doi: 10.1016/j.domaniend.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Guedes P.T., de Oliveira B.C.E.P.D., de Abreu Manso P.P., Caputo L.F.G., Cotta-Pereira G., Pelajo-Machado M. Histological analyses demonstrate the temporary contribution of yolk sac, liver, and bone marrow to hematopoiesis during chicken development. PLoS One. 2014;9:e90975. doi: 10.1371/journal.pone.0090975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidu J.A., Torres C.A., Johnson-Dahl M.L., Korver D.R. Physiological response of broiler embryos to different incubator temperature profiles and maternal flock age during incubation. 1. Embryonic metabolism and day-old chick quality. Poult. Sci. 2018;97:2934–2946. doi: 10.3382/ps/pey089. [DOI] [PubMed] [Google Scholar]
- Hincke M.T., Da Silva M., Guyot N., Gautron J., McKee M.D., Guabiraba-Brito R., Réhault-Godbert S. Dynamics of structural barriers and innate immune components during incubation of the avian egg: critical interplay between autonomous embryonic development and maternal anticipation. J. Innate Immunol. 2019;11:111–124. doi: 10.1159/000493719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.L. Reproduction in the female. In: Scanes C.G., editor. Sturkie’s Avian Physiology. 6th ed. Elsevier; London, UK: 2015. pp. 635–665. [Google Scholar]
- Joseph N.S., Lourens A., Moran E.T., Jr. The effects of suboptimal eggshell temperature during incubation on broiler chick quality, live performance, and further processing yield. Poult. Sci. 2006;85:932–938. doi: 10.1093/ps/85.5.932. [DOI] [PubMed] [Google Scholar]
- Kaspers B., Bondl H., Gobel T.W.F. Transfer of IgA from albumen into the yolk sac during embryonic development in the chicken. J. Vet. Med. A. 1996;43:225–231. doi: 10.1111/j.1439-0442.1996.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Kovacs-Nolan J., Mine Y. Egg yolk antibodies for passive immunity. Annu. Rev. Food Sci. Technol. 2012;3:163–182. doi: 10.1146/annurev-food-022811-101137. [DOI] [PubMed] [Google Scholar]
- Kowalczyk K., Daiss J., Halpern J., Roth T.F. Quantitation of maternal-fetal IgG transport in the chicken. Immunology. 1985;54:755–762. [PMC free article] [PubMed] [Google Scholar]
- Li C., Geng F., Huang X., Ma M., Zhang X. Phosvitin phosphorus is involved in chicken embryo bone formation through dephosphorylation. Poult. Sci. 2014;93:3065–3072. doi: 10.3382/ps.2014-04098. [DOI] [PubMed] [Google Scholar]
- Lin H.J., Wang S.H., Pan Y.H., Ding S.–T. Primary endodermal epithelial cell culture from the yolk sac membrane of Japanese quail embryos. J. Vis. Exp. 2016;109:53624. doi: 10.3791/53624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.M., Druyan S., Yahav S., Brake J. Thermal treatments prior to and during the beginning of incubation affects development of the broiler embryo and yolk sac membranes, and live performance and carcass characteristics. Poult. Sci. 2017;96:1939–1947. doi: 10.3382/ps/pew467. [DOI] [PubMed] [Google Scholar]
- Maldjian A., Farkas K., Noble R.C., Cocchi M., Speake B.K. The transfer of docosahexaenoic acid from the yolk to the tissues of the chick embryo. Biochim. Biophys. Acta. 1995;1258:81–89. doi: 10.1016/0005-2760(95)00101-h. [DOI] [PubMed] [Google Scholar]
- Matsushita S. Presence of sucrase in the yolk sac of the chick. J. Exp. Zool. 1986;237:337-346. doi: 10.1002/jez.1402370306. [DOI] [PubMed] [Google Scholar]
- Mobbs I.G., McMillan D.B. Structure of the endodermal epithelium of the chick yolk sac during early stages of development. Am. J. Anat. 1979;155:287–310. doi: 10.1002/aja.1001550302. [DOI] [PubMed] [Google Scholar]
- Mobbs I.G., McMillan D.B. Transport across endodermal cells of the chick yolk sac during early stages of development. Am. J. Anat. 1981;160:285–308. doi: 10.1002/aja.1001600307. [DOI] [PubMed] [Google Scholar]
- Nagai H., Shin M., Weng W., Nakazawa F., Jakt L.M., Alev C., Sheng G. Early hematopoietic and vascular development in the chick. Int. J. Dev. Biol. 2018;62:137–144. doi: 10.1387/ijdb.170291gs. [DOI] [PubMed] [Google Scholar]
- Nakazawa F., Alev C., Jakt L.M., Sheng G. Yolk sac endoderm is the major source of serum proteins and lipids and is involved in the regulation of vascular integrity in early chick development. Dev. Dyn. 2011;240:2002–2010. doi: 10.1002/dvdy.22690. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Meijerhof R., van den Anker I., Heetkamp M.J.W., De Souza Morita V., Kemp B., van den Brand H. Effects of breeder age, strain, and eggshell temperature on nutrient metabolism of broiler embryos. Poult. Sci. 2016;95:1666–1679. doi: 10.3382/ps/pew417. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Ruangpanit Y., Meijerhof R., Attamangkune S. Yolk absorption and embryo development of small and large eggs originating from young and old breeder hens. Poult. Sci. 2011;90:2648–2655. doi: 10.3382/ps.2011-01415. [DOI] [PubMed] [Google Scholar]
- Noble R.C., Shand J.H. Unsaturated fatty acid compositional changes and desaturation during the embryonic development of the chicken. Lipids. 1985;20:278–282. doi: 10.1007/BF02534260. [DOI] [PubMed] [Google Scholar]
- Noble R.C. Lipid metabolism in the chick embryo. Proc. Nutr. Soc. 1986;B45:17–25. doi: 10.1079/pns19860030. [DOI] [PubMed] [Google Scholar]
- Noble R.C., Cocchi M. Lipid metabolism and the neonatal chicken. Prog. Lipid Res. 1990;29:107–140. doi: 10.1016/0163-7827(90)90014-c. [DOI] [PubMed] [Google Scholar]
- O’Sullivan N.P., Dunnington E.A., Siegel P.B. Relationships among age of dam, egg components, embryo lipid transfer, and hatchability of broiler breeder eggs. Poult. Sci. 1991;70:2180–2185. doi: 10.3382/ps.0702180. [DOI] [PubMed] [Google Scholar]
- Patten B.M. 5th ed. McGraw-Hill Inc.; New York, NY: 1971. Early Embryology of the Chick. [Google Scholar]
- Peebles E.D., Lumu I.J., Miller S., Pansky T., Whitmarsh S., Latour M.A., Gerard P.D. Embryo and yolk compositional relationships in broiler hatching eggs during incubation. Poult. Sci. 1999;78:1435–1442. doi: 10.1093/ps/78.10.1435. [DOI] [PubMed] [Google Scholar]
- Peebles E.D., Zumwalt C.D., Doyle S.M., Gerard P.D., Latour M.A., Boyle C.R., Smith T.W. Effects of breeder age and dietary fat source and level on broiler hatching egg characteristics. Poult. Sci. 2000;79:698–704. doi: 10.1093/ps/79.5.698. [DOI] [PubMed] [Google Scholar]
- Powell K.A., Deans E.A., Speake B.K. Fatty acid esterification in the yolk sac membrane of the avian embryo. J. Comp. Physiol. Part B Biochem. Systemic Env. Physiol. 2004;174:163–168. doi: 10.1007/s00360-003-0401-5. [DOI] [PubMed] [Google Scholar]
- Rao K., Xie J., Yang X., Chen L., Grossmann R., Zhao R. Maternal low-protein diet programmes offspring growth in association with alterations in yolk leptin deposition and gene expression in yolk-sac membrane, hypothalamus and muscle of developing Langshan chicken embryos. Br. J. Nutr. 2009;102:848–857. doi: 10.1017/S0007114509276434. [DOI] [PubMed] [Google Scholar]
- Rehault-Godbert S., Mann K., Bourin M., Brionne A., Nys Y. Effect of embryonic development on the chicken egg yolk plasma proteome after 12 days of incubation. Agric. Food Chem. 2014;62:2531–2540. doi: 10.1021/jf404512x. [DOI] [PubMed] [Google Scholar]
- Romanoff A.L. The Macmillan Co.; New York: 1960. The Avian Embryo. [Google Scholar]
- Ross C., Boroviak T.E. Origin and function of the yolk sac in primate embryogenesis. Nat. Commun. 2020;11:3760. doi: 10.1038/s41467-020-17575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahan U., Ipek A., Sozcu A. Yolk sac fatty acid composition, yolk absorption, embryo development, and chick quality during incubation in eggs from young and old broiler breeders. Poult. Sci. 2014;93:2069–2077. doi: 10.3382/ps.2013-03850. [DOI] [PubMed] [Google Scholar]
- Schneider W.J. Lipid transport to avian oocytes and to the developing embryo. J. Biomed. Res. 2016;30:174–180. doi: 10.7555/JBR.30.20150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand J.H., West D.W., McCartney R.J., Noble R.C., Speake B.K. The esterification of cholesterol in the yolk sac membrane of the chick embryo. Lipid. 1993;28:621–625. doi: 10.1007/BF02536056. [DOI] [PubMed] [Google Scholar]
- Sheng G. Primitive and definitive erythropoiesis in the yolk sac: a bird’s eye view. Intl. J. Dev. Biol. 2010;54:1033–1043. doi: 10.1387/ijdb.103105gs. [DOI] [PubMed] [Google Scholar]
- Sheng G., Foley A.C. Diversification and conservation of the extraembryonic tissues in mediating nutrient uptake during amniote development. Ann. N.Y. Acad. Sci. 2012;1271:97–103. doi: 10.1111/j.1749-6632.2012.06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade B., Milne J. Localization and synthesis of alpha-fetoprotein in the chicken. Cell Tissue Res. 1977;180:411–419. doi: 10.1007/BF00227605. [DOI] [PubMed] [Google Scholar]
- Speake B.K., Murray A.M., Noble R.C. Transport and transformations of yolk lipids during development of the avian embryo. Prog. Lipid Res. 1998;37:1–32. doi: 10.1016/s0163-7827(97)00012-x. [DOI] [PubMed] [Google Scholar]
- Speake B.K., Deans E.A. Biosynthesis of oleic, arachidonic and docosahexaenoic acids from their C18 precursors in the yolk sac membrane of the avian embryo. Comp. Biochem. Physiol. Part B, Biochem. Mol. Biol. 2004;138:407–414. doi: 10.1016/j.cbpc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Speier J.S., Yadgary L., Uni Z., Wong E.A. Gene expression of nutrient transporters and digestive enzymes in the yolk sac membrane and small intestine of the developing embryonic chick. Poult. Sci. 2012;91:1941–1949. doi: 10.3382/ps.2011-02092. [DOI] [PubMed] [Google Scholar]
- Tako E., Ferket P.R., Uni Z. Changes in chicken intestinal zinc exporter mRNA expression and small intestinal functionality following intra-amniotic zinc-methionine administration. J. Nutr. Biochem. 2005;16:339–346. doi: 10.1016/j.jnutbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Tesar D.B., Cheung E.J., Bjorkman P.J. The chicken yolk sac IgY receptor, a mammalian mannose receptor family member, transcytoses IgY across polarized epithelial cells. Mol. Biol. Cell. 2008;19:1587–1593. doi: 10.1091/mbc.E07-09-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Too H. C. M. Shibata, M. Yayota, Darras V.M., Iwasawa A. Expression of thyroid hormone regulator genes in the yolk sac membrane of the developing chicken embryo. J. Repro. Dev. 2017;63:463–472. doi: 10.1262/jrd.2017-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres C.A., Korver D.R. Influences of trace mineral nutrition and maternal flock age on broiler embryo bone development. Poult. Sci. 2018;97:2996–3003. doi: 10.3382/ps/pey136. [DOI] [PubMed] [Google Scholar]
- Traldi A.B., Menten J.F.M., Silva C.S., Rizzo P.V., Pereira P.W.Z., Santarosa J. What determines hatchling weight: breeder age or incubated egg weight? Braz. J. Poult. Sci. 2011;13:283–285. [Google Scholar]
- van der Wagt I., de Jong I.C., Mitchell M.A., Molenaar R., van den Brand H. A review on yolk sac utilization in poultry. Poult. Sci. 2020;99:2162–2175. doi: 10.1016/j.psj.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira S.L., Moran E.T., Jr. Broiler chicks hatch from egg weight extremes and diverse breeder strains. J. Appl. Poult. Res. 1998;7:392–402. [Google Scholar]
- Wang F., Flanagan J., Su N., Wang L.C., Bui S., Nielson A., Wu X.Y., Vo H.T., Ma X.J., Luo Y.L. RNAscope a novel in situ RNA analysis platform for formalin-fixed, paraffin embedded tissues. J. Mol. Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.H., Lin H.J., Lin Y.Y., Chen Y.J., Pan Y.H., Tung C.T., Mersmann H.J., Ding S.T. Embryonic cholesterol esterification is regulated by a cyclic AMP-dependent pathway in yolk sac membrane-derived endodermal epithelial cells. PLoS One. 2017;12:e0187560. doi: 10.1371/journal.pone.0187560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A.P., Jr., Herr A.B., Bjorkman P.J. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A2 receptor homolog. Immunity. 2004;20:601–610. doi: 10.1016/s1074-7613(04)00113-x. [DOI] [PubMed] [Google Scholar]
- Wong E., Gilbert E.R., Miska K.B. Nutrient transporter gene expression in poultry, livestock and fish. In: Scanes C.G., Hill R.A., editors. Biology of Domestic Animals. CRC Press, Taylor and Francis; Boca Raton, FL: 2017. [Google Scholar]
- Wu J., Acero-Lopez A. Ovotransferrin: structure, bioactivities and preparation. Food Res. Intl. 2012;46:480–487. [Google Scholar]
- Yadgary L., Cahaner A., Kedar O., Uni Z. Yolk sac nutrient composition and fat uptake in late-term embryos in eggs from young and old broiler breeder hens. Poult. Sci. 2010;89:2441–2452. doi: 10.3382/ps.2010-00681. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Yair R., Uni Z. The chick embryo yolk sac membrane expresses nutrient transporter and digestive enzyme genes. Poult. Sci. 2011;90:410–416. doi: 10.3382/ps.2010-01075. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Uni Z. Yolk sac carbohydrate levels and gene expression of key gluconeogenic and glycogenic enzymes during chick embryonic development. Poult. Sci. 2012;91:444–453. doi: 10.3382/ps.2011-01669. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Kedar O., Adepeju O., Uni Z. Changes in yolk sac membrane absoptive area and fat digestion during chick embryonic development. Poult. Sci. 2013;92:1634–1640. doi: 10.3382/ps.2012-02886. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Wong E.A., Uni Z. Temporal transcriptome analysis of the chicken embryo yolk sac. BMC Genomics. 2014;15:690. doi: 10.1186/1471-2164-15-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yair R., Uni Z. Content and uptake of minerals in the yolk of broiler embryos during incubation and effect of nutrient enrichment. Poult. Sci. 2011;90:1523–1531. doi: 10.3382/ps.2010-01283. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Bagdatlioglu N., Bruggeman V., Babacanoglu E., Uysal I., Buyse J., Decuypere E., Siegel P.B. Acclimation to heat during incubation. 2. Embryo composition and residual egg yolk sac fatty acid profiles in chicks. Poult. Sci. 2008;87:1229–1236. doi: 10.3382/ps.2007-00436. [DOI] [PubMed] [Google Scholar]
- Yoshizaki N., Soga M., Ito Y., Mao K.M., Sultana F., Yonezawa S. Two-step consumption of yolk granules during the devbelopment of qual embryos. Dev. Growth Differ. 2004;46:229–238. doi: 10.1111/j.1440-169X.2004.00740.x. [DOI] [PubMed] [Google Scholar]
- Yvernogeau L., Robin C. Restricted intra-embryonic origin of bona fide hematopoietic stem cells in the chicken. Development. 2017;144:2352–2363. doi: 10.1242/dev.151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Sunkara L.T. Avian antimicrobial host defense peptides: from biology to therapeutic applications. Pharmaceuticals. 2014;7:220–247. doi: 10.3390/ph7030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wong E.A. Spatial transcriptional profile of PepT1 mRNA in the yolk sac and small intestine in broiler chickens. Poult. Sci. 2017;96:2871–2876. doi: 10.3382/ps/pex056. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wong E.A. Identification of cells expressing OLFM4 and LGR5 mRNA by in situ hybridization in the yolk sac and small intestine of embryonic and early posthatch chicks. Poult. Sci. 2018;97:628–633. doi: 10.3382/ps/pex328. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li H., Kidrick J., Wong E.A. Localization of cells expressing SGLT1 mRNA in the yolk sac and small intestine of broilers. Poult. Sci. 2019;98:984–990. doi: 10.3382/ps/pey343. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wong E.A. Expression of avian β-defensin mRNA in the chicken yolk sac. Dev. Comp. Immunol. 2019;95:89–95. doi: 10.1016/j.dci.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Zhu W., Zhang J., He K., Geng Z., Chen X. Proteomic analysis of fertilized egg yolk proteins during embryonic development. Poult. Sci. 2020;99:2775–2784. doi: 10.1016/j.psj.2019.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.W., Li W.X., Lu L., Zhang L.Y., Ji C., Lin X., Liu H.C., Odle J., Luo X.G. Impact of maternal heat stress in conjunction with dietary zinc supplementation on hatchability, embryonic development, and growth performance in offspring broilers. Poult. Sci. 2017;96:2351–2359. doi: 10.3382/ps/pew481. [DOI] [PubMed] [Google Scholar]