Abstract
This study was conducted to evaluate the effects of 3 rearing systems (FL: flooring litter rearing, MC: multilayer cage rearing, PN: plastic net rearing) with or without supplemental narasin on growth performance, gastrointestine development and health of broilers. A total of 2,400 one-day-old Ross 308 mixed-sex broilers (1:1 ratio of males and females) were used in a completely randomized design utilizing a 3 × 2 factorial arrangement of treatments, with 12 replicates per treatment. Each replicate for FL, MC, and PN consisted of 34 birds per floor pen, 30 birds per cage, and 36 birds per net pen, respectively, ensuring the same stocking density (12 birds/m2) across the 3 systems. Results showed that lower ADG (average daily gain), ADFI (average daily feed intake), and FCR (feed conversation ratio) observed in the MC group than those of the other 2 systems from 1 to 36 d of age (P < 0.05). Narasin inclusion in the diets decreased ADFI and FCR significantly (P < 0.05). Multilayer cage and PN rearing systems reduced the relative weight of the gizzard significantly (P < 0.05). Compared with FL, MC reduced the relative weight of the duodenum, jejunum, and ileum (P < 0.05). The mRNA expression levels of the ileal IL-1β and IFN-γ in FL were higher than those in PN and MC (P < 0.05). Narasin decreased the ileal mRNA expression of TNF-α (P < 0.05). Different rearing systems changed the ileal microflora structure of broilers. The FL system increased the ileal microbial diversity of broilers and the relative abundance of Actinobacteria. Narasin combined with MC increased the relative abundance of Proteobacteria. In conclusion, birds reared in PN had a higher body weight. The MC birds had poorer intestinal development and health condition, higher abundance of Proteobacteria, but better FCR. The FL rearing appeared to be propitious for gastrointestinal development and health. Narasin inclusion in the diets improved FCR and changed the relative abundance Proteobacteria of broilers.
Key words: rearing system, narasin, growth performance, intestinal microbiota, broilers
Introduction
In China, floor litter system, also known as the deep litter system in other parts of the world, and plastic net rearing are 2 traditional systems for raising broilers. With the development of intensive farming, multilayer cage rearing is becoming widespread, which effectively prevents broilers from having direct contact with their excreta, with one clear benefit where coccidiosis and intestinal diseases are largely eliminated, saving resources and facilitating automated management. Rearing systems are a crucial factor affecting bird comfort, welfare, health, and production efficiency (Willis et al., 2002). Several studies evaluated the effect of different rearing systems on the performance and health of broilers. Thamilvanan et al. (2001) reported that the cage rearing system produces better performance and a higher survival rate than the floor rearing system, whereas Swain et al. (2002) found no significant effect for either the cage or the floor rearing system on live weight gain and feed intake. Santos et al. (2012) revealed that birds raised on floors had better weight gain and FCR than those reared in cages. Contrarily, Mariam et al. (2012) reported that cage rearing improved the growth performance of Cobb broilers. Thus, the literature findings on different rearing systems are equivocal for bird performance. There are numerous underlining issues for the differences. One is the effect of bedding materials on bird health and performance (Choct, 2008) and the other is coccidiosis. Indeed, coccidiosis is a major disease in poultry that causes intestinal lesions, depresses growth, and reduces FCR (Kadykalo et al., 2018). Coccidiostats are usually used to counter the negative effects of coccidiosis in poultry. Narasin, an ionophore coccidiostat, is used to prevent coccidiosis and necrotic enteritis (NE) in broilers (Brennan et al., 2001). Although there were numerous studies either on rearing systems or on coccidiostats for their efficacy in broiler diets, the combination of the 2 in some rearing systems has not extensively examined. This study evaluated the effects of 3 rearing systems on growth performance, gastrointestinal development, and gut microbiota of broilers with or without narasin.
Materials and methods
The study was approved by the Animal Care and Experiment Committee of New Hope Liuhe Corporation. The management and husbandry of the birds strictly followed the Chinese government's regulations on animal welfare. This research on live animals met the guidelines approved by the institutional animal care and use committee.
Experimental Design and Dietary Treatments
A total of 2,400 one-day-old Ross 308 mixed-sex broilers (1:1 ratio of males and females) were used in a completely randomized design utilizing a 3 × 2 factorial arrangement of treatments, with 12 replicates in each treatment. In each replicate for FL, MC, and PN, there are 34 birds per floor pen, 30 birds per cage, and 36 birds per net pen, respectively, ensuring that the stocking density of each rearing system was the same (12.5 birds/m2). Narasin was supplemented at 75 ppm in diets. Table 1 shows the experimental design.
Table 1.
Experimental design.
| Treatment | Systems | Narasin | Birds/pen(cage) |
|---|---|---|---|
| Treatment 1 | Flooring litter rearing (FL) | + | 34 |
| Treatment 2 | Flooring litter rearing (FL) | − | 34 |
| Treatment 3 | Multilayer cage rearing (MC) | + | 30 |
| Treatment 4 | Multilayer cage rearing (MC) | − | 30 |
| Treatment 5 | Plastic net rearing (PN) | + | 36 |
| Treatment 6 | Plastic net rearing (PN) | − | 36 |
Birds were fed crumble-pellet diets from day 1 to 12, and pellet diets from day 13 to 36. Broiler starter (day 1–12), grower (day 13–23), and finisher (day 24–36) diets were formulated to meet Ross 308 strain recommendations (Table 2).
Table 2.
Composition and nutrient levels of basal diets (as is basis, %).
| Items | 1–12 d of age | 13–23 d of age | 24–36 d of age |
|---|---|---|---|
| Ingredients | |||
| Corn | 51.95 | 55.42 | 61.42 |
| Soybean meal | 36.90 | 30.20 | 19.20 |
| Corn DDGS | 4.00 | 6.00 | 8.00 |
| Peanut meal | 2.00 | 3.00 | 4.00 |
| Corn protein powder | - | - | 2.00 |
| Soybean oil | 1.20 | 1.57 | 1.77 |
| CaHPO4 | 1.38 | 1.17 | 0.72 |
| Limestone | 1.23 | 1.09 | 1.09 |
| Premix1 | 0.50 | 0.50 | 0.50 |
| L-Lys·H2SO4 | 0.26 | 0.42 | 0.66 |
| DL-Met | 0.22 | 0.23 | 0.21 |
| L-Thr | 0.06 | 0.10 | 0.13 |
| NaCl | 0.30 | 0.30 | 0.30 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrients levels2 | |||
| Crude protein | 22.52 | 21.00 | 19.50 |
| ME MJ/kg | 10.70 | 11.10 | 11.50 |
| Ca | 0.90 | 0.80 | 0.70 |
| Total P | 0.55 | 0.50 | 0.45 |
Abbreviations: DDGS, distillers dried grains with solubles; ME, metabolizable energy.
The premix provides following per kg diet: I 0.65 mg, Se 0.35 mg, vitamin A 9000 IU, vitamin D3 2000 IU, vitamin E11 IU, vitamin K1.0 mg, vitamin B11.2 mg, vitamin B25.8 mg, niacin 66 mg, pantothenic acid10 mg, vitamin B6 2.6 mg, biotin 0.10 mg, folic acid 0.7 mg, vitamin B12 0.012 mg.
All the values are calculated.
Management and Husbandry
Rice husk was used as a litter material and was uniformly distributed to cover the floor area to a depth of 5 cm in the FL system. The metal frame was covered with a plastic mesh in the net-rearing system to avoid the birds contacting harsh surfaces. Broiler type cage houses of 3 vertical tiers were used in the present study. The brooding temperature was maintained at 33°C for the first day and was gradually decreased by 2°C per week until 21°C and maintained at that level thereafter. During the whole experimental period, chickens had free access to feed and water. Birds were immunized as per commercial practice. The indexes of temperature, humidity, light, and hygiene in the chicken house accord with the hygienic requirements of broilers (GB 14925-1994).
Sample and Data Collection
Growth Performance
Body weights and feed intake (FI) by pen were recorded on day 12, 23, and 36, and mortality was recorded daily. Average weight gain (ADG), average daily feed intake (ADFI), and FCR were calculated for starter, grower, finisher, and overall periods.
Relative Digestive Organ Weights
At 37 d of age, 10 chickens with similar BW were selected from each treatment, weighed, and killed by exsanguinations after CO2 stunning. After an abdominal incision, the length and weight of the proventriculus, gizzard, duodenum, jejunum, and ileum were measured to calculate relative weight of the proventriculus, gizzard, duodenum, jejunum, and ileum.
Intestinal Lesion Score
At 37 d of age, 10 chickens with similar BW were selected from each treatment, weighed, and killed by exsanguinations after CO2 stunning. After an abdominal incision, the small intestine from each bird was opened and scored by 3 independent observers with no reference to treatments. Briefly, lesions were scored using a scale from 0 to 4, in which 0 had normal intestinal appearance, no lesion; 1 had thin walled and friable intestines with small red petechiae (>5); 2 had focal necrotic lesions; 3 had patches of necrosis (1–2 cm long); and 4 had diffused necrosis typical of field cases.
mRNA Expression of Ileal Immune Factors
At 37 d of age, 10 chickens with similar BW were selected from each treatment, weighed, and killed by exsanguinations after CO2 stunning. After an abdominal incision, a middle section of the ileum mucosa was collected for detecting mRNA expression of ileal IL-1β, TNF-α, IL-8, and IFN-γ.
Total RNA was extracted from intestinal segments using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA) following the manufacturer's protocol. The concentration of extracted RNA was measured using a NanoDrop spectrophotometer (ND-1000; NanoDrop Products, Wilmington, DE) at an optical density of 260 nm, and RNA purity was verified by the ratio of absorbance at 260 nm/280 nm. Then, 1 μg of total RNA was used for reverse transcription by a reverse transcription kit (Takara Bio Inc) following the manufacturer's protocol. All the cDNA preparations were stored at −20°C until further use.
Expression levels of the after genes were analyzed by real-time quantitative PCR (RT-PCR): IL-1β, IL-8, TNF-α, IFN-γ, and an endogenous reference gene GAPDH. Gene-specific primer sequences are shown in Table 3. The RT-PCR was performed on the 7500-fluorescence detection system (Applied Biosystems, Foster City, CA) using a commercial SYBR-Green PCR kit (Takara Bio Inc.). In accordance with the manufacturer's protocol, the following PCR conditions were used: 95°C for 30 s, 40 cycles of 95°C for 5 s, and 60°C for 34 s, and followed by the stage of melting curve. At the end of each run, melting curve analysis and subsequent agarose gel electrophoresis of the PCR products were subjected to confirm the amplification specificity. Relative gene expression data were analyzed using the 2−ΔΔCt method as developed by Livak and Schmittgen (2001).
Table 3.
RT-PCR primers and GenBank accession numbers of chicken.
| Target | Primer sequence (5′–3′)a | Accession no. | Product size, bp |
|---|---|---|---|
| IL-1β | F:ACTGGGCATCAAGGGCTA R:GGTAGAAGATGAAGCGGGTC |
NM_204524 | 131 |
| TNF-α | F: GAGCGTTGACTTGGCTGTC R: AAGCAACAACCAGCTATGCAC |
NM_204267 | 64 |
| IL-8 | F: ATGAACGGCAAGCTTGGAGCTG R:TCCAAGCACACCTCTCTTCCATCC |
AJ_009800 | 103 |
| IFN-γ | F: AGCTGACGGTGGACCTATTATT R:GGCTTTGCGCTGGATTC |
Y07922 | 259 |
| GAPDH | F:TGCTGCCCCAGAACATCATCC R: ACGGCAGGTCAGGTCAACAA |
NM_204305.1 | 108 |
Abbreviations: RT-PCR, reverse-transcription polymerase chain reaction; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor; IL-8, interleukin-8; IFN-γ interferon-γ; GAPDH, reduced glyceraldehyde phosphate dehydrogenase.
Intestinal Flora
At day 37, 10 broilers from each treatment were humanely slaughtered, their intestines were excised, and separated by germ free cotton. Ileal digesta was collected and then stored at −80°C after snap freezing with liquid nitrogen for further analysis.
Statistical Analyses
Effects of treatments were analyzed as a 3 × 2 factorial arrangement by two-way analysis of variance. Experimental data were analyzed using the GLM procedures of SAS 9.3 (SAS Inc., Cary, NC). The model included the main effects of rearing system, narasin, and their interaction. Results in the tables were reported as means. When differences among diets were significant, means were separated using Duncan's multiple range test, and significance was set at P < 0.05.
Results
Growth Performance
The bird performance results are shown in Table 4. From day 1 to day 12, PN birds had higher ADG and ADFI, also higher body weight on day 12 than those of the other 2 systems (P < 0.05). FCR of the MC birds was significant lower than that of the PN birds (P < 0.05). However, narasin inclusion reduced ADG, ADFI, and BW (P < 0.05). From day 13 to day 23, the MC birds had lower ADG and ADFI, and lower BW on day 23 than those of the other 2 systems (P < 0.05). Narasin inclusion reduced ADG, ADFI, and BW on day 23 (P < 0.05). From day 24 to day 36, the PN birds had higher ADG and FCR, also BW than those of the CM birds (P < 0.05). Narasin decreased ADFI and FCR (P < 0.05). From day 1 to day 36, the MC birds had lower ADG and ADFI than the FL and PN birds (P < 0.05). There was no significant difference between FL and PN treatments (P > 0.05). Narasin inclusion reduced ADFI and FCR (P < 0.05).
Table 4.
Effects of raising system and narasin on growth performance of broilers.
| System |
Narasin |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|
| FL | CM | PN | + | − | System | Narasin | Interaction | |
| 12dBW/(g) | 441b | 441b | 461a | 443b | 453a | <0.001 | <0.001 | 0.270 |
| 23dBW/(g) | 1,351a | 1,317b | 1,366a | 1,333b | 1,357a | <0.001 | <0.001 | 0.229 |
| 36dBW/(g) | 2,509a | 2,473b | 2,527a | 2,492 | 2,514 | <0.001 | 0.134 | 0.552 |
| 1∼12 d | ||||||||
| ADG/(g/day) | 32.8b | 32.8b | 34.4a | 32.9b | 33.8a | <0.001 | <0.001 | 0.274 |
| FCR | 1.131a,b | 1.121b | 1.136a | 1.133 | 1.126 | 0.029 | 0.142 | 0.587 |
| ADFI/(g/day) | 37.0b | 36.7b | 39.1a | 37.2b | 38.0a | <0.001 | <0.001 | 0.543 |
| Survival rate/(%) | 99.8 | 99.6 | 99.2 | 99.5 | 99.6 | 0.311 | 0.582 | 0.924 |
| 13∼23 d | ||||||||
| ADG/(g/day) | 82.7a | 79.7b | 82.3a | 80.9b | 82.2a | <0.001 | 0.039 | 0.343 |
| FCR | 1.345 | 1.346 | 1.350 | 1.343 | 1.351 | 0.649 | 0.072 | <0.001 |
| ADFI/(g/day) | 111.3a | 107.2b | 111.1a | 108.7b | 111.0a | <0.001 | <0.001 | 0.746 |
| Survival rate/(%) | 99.6 | 99.6 | 98.8 | 99.1 | 99.6 | 0.102 | 0.158 | 0.645 |
| 24∼36 d | ||||||||
| ADG/(g/day) | 89.1 | 88.9 | 89.3 | 89.2 | 89.0 | 0.905 | 0.822 | 0.889 |
| FCR | 1.804a,b | 1.788b | 1.827a | 1.782b | 1.832a | <0.001 | <0.001 | 0.974 |
| ADFI/(g/day) | 160.6a,b | 158.9b | 163.1a | 158.8b | 162a | <0.001 | <0.001 | 0.829 |
| Survival rate/(%) | 99.0 | 99.3 | 98.3 | 99.1 | 98.7 | 0.224 | 0.363 | 0.974 |
| 1∼36 d | ||||||||
| ADG/(g/day) | 68.4a | 67.4b | 68.9a | 67.9 | 68.5 | <0.001 | 0.127 | 0.551 |
| FCR | 1.527b | 1.520b | 1.537a | 1.517b | 1.539a | <0.001 | <0.001 | 0.123 |
| ADFI/(g/day) | 104.4b | 102.4c | 105.9a | 103.0b | 105.4a | <0.001 | <0.001 | 0.727 |
| Survival rate/(%) | 98.4a | 98.5a | 96.4b | 97.7 | 97.9 | <0.001 | 0.731 | 0.841 |
a,bWithin a row, numbers with different superscripts differ statistically at P < 0.05.
Abbreviations: FL, flooring litter rearing; MC, multilayer cage rearing; PN, plastic net rearing; BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio.
1n = 12 replications.
Digestive Organ Development
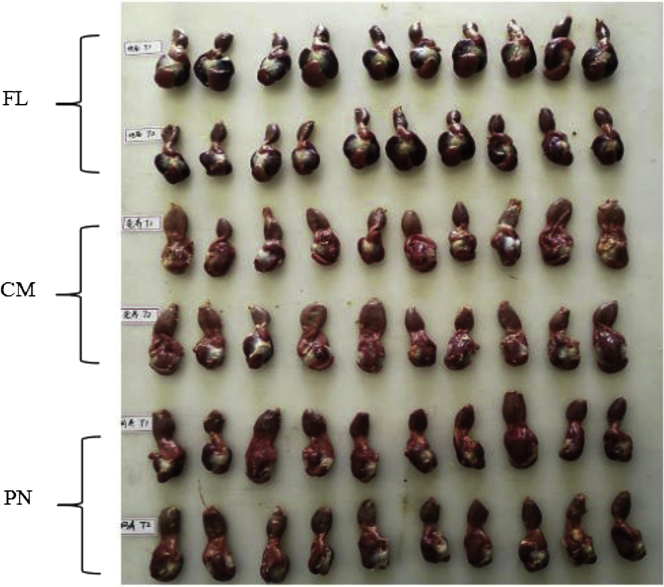
The effect of rearing system and narasin on gizzard and proventriculus development of broilers is shown in Figure 1. The FL birds had brighter and plumper gizzards than the PN and MC birds, whereas their proventriculus and isthmus appeared normal. Both PN and MC reared broilers looked unthrift with small gizzards and swollen proventriculi.
Figure 1.
Development of proventriculus and gizzard. Abbreviations: FL, flooring litter rearing; MC, multilayer cage rearing; PN, plastic net rearing.
The effects of rearing systems and narasin on relative weight of gastrointestinal of broilers are shown in Table 5. Broiler chickens on FL treatment had heavier gizzards than those on MC and PN treatments (P < 0.05). MC significantly reduced the relative weights of the duodenum, jejunum, and ileum compared with the other 2 systems (P < 0.05).
Table 5.
Effects of raising system and narasin on gastrointestine development of broilers.
| Treatment | Relative weight (%) |
Intestine weight length ratio (g/cm) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Gizzard | Proventriculus | Duodenum | Jejunum | Ileum | Duodenum | Jejunum | Ileum | ||
| Main effect | |||||||||
| System | FL | 1.19a | 0.26b | 0.65a | 1.30a | 0.99a | 0.53a | 0.42a | 0.31a |
| MC | 0.93b | 0.39a | 0.48c | 0.98c | 0.74b | 0.47b | 0.34b | 0.27b | |
| PN | 0.87b | 0.34a | 0.57b | 1.10b | 0.94a | 0.51a,b | 0.41a | 0.33a | |
| Narasin | + | 1.00 | 0.33 | 0.55 | 1.12 | 0.89 | 0.48b | 0.38 | 0.30 |
| − | 0.99 | 0.32 | 0.58 | 1.14 | 0.90 | 0.53a | 0.40 | 0.30 | |
| P-value | System | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.026 | <0.001 | <0.001 |
| Narasin | 0.881 | 0.757 | 0.219 | 0.679 | 0.921 | 0.004 | 0.077 | 0.543 | |
| Interaction | 0.240 | 0.724 | 0.118 | 0.031 | 0.697 | 0.210 | 0.419 | 0.879 | |
a,bWithin a row, numbers with different superscripts differ statistically at P < 0.05.
Abbreviations: FL, flooring litter rearing; MC, multilayer cage rearing; PN, plastic net rearing.
n = 10 replications
Intestinal Lesion Score and mRNA Expression of Ileum Immune Factors
The intestinal lesion score and ileal mRNA expression are shown in Table 6. Intestinal lesion scores did not differ among rearing systems (P > 0.05) but cage rearing reduced the expression of IL-1β and IFN-γ in the intestinal tract (P < 0.05). The mRNA expression levels of the ileal IL-1β and IFN-γ in FL birds were higher than those in the PN and MC groups (P < 0.05). Narasin decreased the mRNA expressions of TNF-α in the ileum (P < 0.05). Different rearing systems and narasin inclusion showed a significant interaction in the expression level of ileal IL-1β and IL-8(P < 0.05). FL combined with narasin treatment had the highest expression level of IL-1β, whereas FL without narasin treatment had the highest expression level of IL-8. The MC with narasin treatment had the lowest level of expression of IL-1β.
Table 6.
Effects of raising system and narasin on mRNA expression of ileum immune factors of broilers.
| Treatment | Lesion score | IL-1β | TNF-α | IL-8 | IFN-γ | |
|---|---|---|---|---|---|---|
| Main effect | ||||||
| System | FL | 0.30 | 0.97a | 1.06 | 1.32 | 0.94a |
| MC | 0.32 | 0.71c | 1.06 | 1.43 | 0.45c | |
| PN | 0.30 | 0.84b | 1.16 | 1.39 | 0.79b | |
| Anticoccidial | + | 0.29 | 0.83 | 1.00b | 1.32 | 0.75 |
| drug | − | 0.33 | 0.85 | 1.19a | 1.43 | 0.71 |
| P-value | System | 0.931 | 0.001 | 0.504 | 0.629 | <0.001 |
| Narasin | 0.290 | 0.777 | 0.030 | 0.222 | 0.511 | |
| Interaction | 0.407 | 0.002 | 0.510 | 0.002 | 0.068 | |
a,bWithin a row, numbers with different superscripts differ statistically at P < 0.05.
Abbreviations: FL, flooring litter rearing; MC, multilayer cage rearing; PN, plastic net rearing.
n = 8 replications.
Intestinal Microbiota
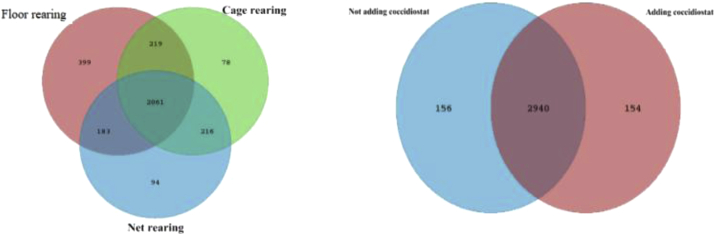
As shown in Figure 2, a total of 3,250 operational taxonomic unit is omitted (OTUs) were identified based on >97% sequencing similarity. Wherein 2,061 OTUs were common in all 3 rearing systems and 2,940 OTUs were common among narasin-included treatments or not. Respectively, 399, 78, and 94 OTUs were exclusive in the FL, NP, and MC groups, whereas 154 and 156 OTUs were exclusive in narasin-included treatments and narasin-free treatments. The specaccum curves and rank abundance curves indicated that a sufficient sequencing coverage was achieved (Figures 3 and 4).
Figure 2.
Venn.
Figure 3.
Species accumulation curves.
Figure 4.
Rank abundance curve.
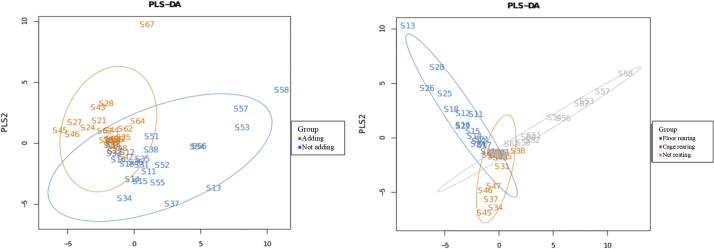
As presented in Table 7, narasin did not affect the alpha diversity of ileal microbiota. Floor rearing numerically increased both the richness index (Chao1 and ACE) and the diversity index (Shannon and Simpson indices). The Shannon index was significantly improved in FL treatments (P < 0.05). Partial least squares discrimination analysis is omitted in Figure 5 indicates that there was differentiation of the microbial community structure among the treatments.
Table 7.
Alpha-diversity of ileal microflora.
| Treatment | Simpson | Chao1 | ACE | Shannon | |
|---|---|---|---|---|---|
| Main effect | |||||
| System | FL | 0.93 | 991.87 | 1,007.09 | 6.53a |
| MC | 0.91 | 945.27 | 961.14 | 5.88a | |
| PN | 0.88 | 968.03 | 911.28 | 5.55b | |
| Narasin | + | 0.91 | 927.53 | 946.50 | 5.96 |
| − | 0.91 | 942.58 | 973.18 | 6.01 | |
| P-value | System | 0.184 | 0.604 | 0.547 | 0.013 |
| Narasin | 0.918 | 0.571 | 0.706 | 0.847 | |
| Interaction | 0.48 | 0.228 | 0.271 | 0.462 | |
a,bWithin a row, numbers with different superscripts differ statistically at P < 0.05.
Abbreviations: FL, flooring litter rearing; MC, multilayer cage rearing; PN. plastic net rearing.
n = 8 replications.
Figure 5.
PLS-DA.
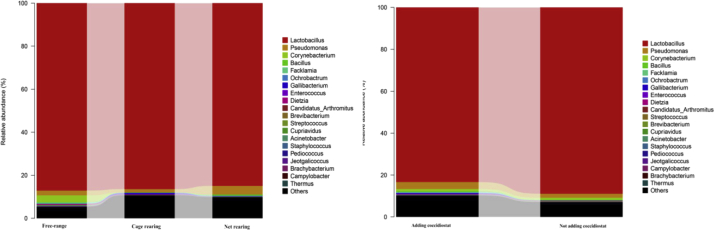
The intestinal microbiota data at the phylum level are shown in Table 8 and Figure 6. At the phylum level, the ileal microbiota was dominated by Firmicutes (65.18∼93.01%), Proteobacteria (3.71∼13.93%), Actinobacteria (0.04∼2.18%), and Cyanobacteria (0.14∼0.50%). FL rearing markedly increased Actinobacteria abundance than other rearing modes (P < 0.05). MC rearing increased Proteobacteria, Thermi, and decreased Bacteroidetes abundances compared with FL and PN systems and also increased Cyanobacteria abundance compared with FL (P < 0.05). Furthermore, narasin increased Proteobacteria abundances compared with the control chicks (P < 0.05).
Table 8.
Microflora structure at phylum level.
| Treatment | Firmicutes | Proteobacteria | Actinomycetes | Cyanobacteria | Bacteroidetes | |
|---|---|---|---|---|---|---|
| Main effect | ||||||
| System | FL | 70.87 | 4.90b | 2.04a | 0.16b | 0.06a |
| MC | 79.09 | 10.18a | 0.20b | 0.45a | 0.02b | |
| PN | 72.72 | 5.89b | 0.06b | 0.31a,b | 0.05a | |
| Narasin | + | 69.11 | 8.97b | 0.75 | 0.37 | 0.05 |
| − | 76.10 | 4.88a | 0.86 | 0.24 | 0.04 | |
| P-value | System | 0.669 | 0.029 | <0.001 | 0.064 | 0.032 |
| Narasin | 0.501 | 0.021 | 0.779 | 0.210 | 0.418 | |
| Interaction | 0.359 | 0.300 | 0.757 | 0.624 | 0.024 | |
a,bWithin a row, numbers with different superscripts differ statistically at P < 0.05.
Abbreviations: FL, flooring litter rearing; MC, multilayer cage rearing; PN, plastic net rearing.
n = 8 replications.
Figure 6.
Effects of rearing condition and narasin on ileal microflora at the phylum level.
As shown in Table 9, and in Figure 7, FL rearing elevated Corynebacterium, Facklamia, Dietzia, Brevibacterium, Staphylococcus abundances than other treatments (P < 0.05). MC rearing markedly increased Bacillus abundance than FL and increased Pseudomonas and Bacillus than PN rearing (P < 0.05). Furthermore, narasin increased Ochrobactrum abundance.
Table 9.
Microflora structure at genus level.
| Treatment | Lactobacillus | Pseudomonas | Corynebacterium | Bacillus | Facklamia | Ochrobactrum | Enterococcus | Dietzia | Brevibacterium | Staphylococcus | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Main effect | |||||||||||
| System | FL | 87.08 | 2.25a,b | 2.09a | 0.09b | 0.37a | 0.06b | 0.07 | 0.32a | 0.11a | 0.08a |
| MC | 84.89 | 3.81a | 0.02b | 0.41a | <0.01b | 0.18a | 0.14 | <0.01b | 0.01b | <0.01b | |
| PN | 90.28 | 1.57b | <0.01b | 0.12b | <0.01b | 0.13a | <0.01 | <0.01b | <0.01b | <0.01b | |
| Narasin | + | 89.01 | 2.76 | 0.67 | 0.22 | 0.16 | 0.16a | 0.10 | 0.07 | 0.05 | 0.03 |
| − | 89.08 | 1.88 | 0.68 | 0.15 | 0.09 | 0.08b | 0.01 | 0.09 | 0.03 | 0.02 | |
| P-value | System | 0.515 | 0.005 | 0.001 | <0.001 | 0.001 | 0.017 | 0.062 | <0.001 | <0.001 | <0.001 |
| Narasin | 0.981 | 0.095 | 0.983 | 0.269 | 0.418 | 0.048 | 0.082 | 0.723 | 0.115 | 0.257 | |
| Interaction | 0.506 | 0.153 | 0.999 | 0.150 | 0.550 | 0.277 | 0.049 | 0.891 | 0.363 | 0.551 | |
a,bWithin a row, numbers with different superscripts differ statistically at P < 0.05.
Abbreviations: FL, flooring litter rearing; MC, multilayer cage rearing; PN, plastic net rearing.
n = 8 replications.
Figure 7.
Effects of rearing condition and narasin on ileal microflora at the genus level.
Table 10 presents the predicted microbial functions at level 1 of the KEGG pathways. Compared with FL and PN systems, MC had significantly more abundance of KEGG pathways affiliated with cellular processes, and less abundance of KEGG pathways belonging to genetic information processing (P < 0.05). PN rearing had significantly less abundance of KEGG pathways affiliated with organismal systems than FL and MC systems. Narasin had larger abundance of KEGG pathways belonging to cellular processes and organismal systems.
Table 10.
Predicted functional changes at level 1.
| Treatment | Cellular processes | Environmental information processing | Genetic information processing | Human diseases | Metabolism | Organismal systems | |
|---|---|---|---|---|---|---|---|
| Main effect | |||||||
| System | FL | 5.00b | 13.87 | 23.68a | 0.85 | 51.06 | 0.44a |
| MC | 5.59a | 14.52 | 22.03b | 0.88 | 51.58 | 0.44a | |
| PN | 5.15b | 14.38 | 23.73a | 0.86 | 50.54 | 0.39b | |
| Narasin | + | 5.37a | 14.36 | 22.73 | 0.89 | 51.13 | 0.44a |
| - | 5.00b | 14.05 | 23.68 | 0.84 | 51.05 | 0.41b | |
| P-value | System | 0.003 | 0.282 | <0.001 | 0.165 | 0.258 | 0.002 |
| Narasin | 0.025 | 0.361 | 0.098 | 0.258 | 0.849 | 0.006 | |
| Interaction | 0.311 | 0.860 | 0.606 | 0.002 | 0.896 | 0.121 | |
a,bWithin a row, numbers with different superscripts differ statistically at P < 0.05.
Abbreviations: FL, flooring litter rearing; MC, multilayer cage rearing; PN, plastic net rearing.
n = 8 replications.
Table 11 shows the top 10 predicted microbial functions at level 2 of the KEGG pathways. PN rearing had less abundance of KEGG pathways affiliated with amino acid metabolism (P < 0.05). MC rearing had less abundance of KEGG pathways affiliated with replication and repair, translation, and nucleotide metabolism and remarkably larger abundances of KEGG pathways belonging to lipid metabolism and xenobiotics biodegradation and metabolism (P < 0.05). FL rearing had less abundance of KEGG pathways affiliated with carbohydrate metabolism compared with other rearing condition and had more abundance of KEGG pathways affiliated with energy metabolism compared with cage feeding (P < 0.05). Narasin markedly decreased the abundance of KEGG pathways affiliated with replication and repair, translation, nucleotide metabolism and increased abundance of KEGG pathways belonging to amino acid metabolism (P < 0.05).
Table 11.
Predicted functional changes at level 2.
| Treatment | Membrane transport | Carbohydrate metabolism | Replication and repair | Amino acid metabolism | Translation | Energy metabolism | Nucleotide metabolism | Lipid metabolism | Xenobiotics biodegradation and metabolism | Metabolism of cofactors and vitamins | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Main effect | |||||||||||
| System | FL | 12.17 | 11.19b | 9.44a | 8.45a | 6.35a | 5.03a | 4.90a | 3.49b | 3.33b | 3.60a |
| MC | 12.71 | 11.98a | 8.67b | 8.35a | 5.73b | 4.88b | 4.39b | 3.75a | 3.89a | 3.09b | |
| PN | 12.73 | 11.97a | 9.32a | 7.76b | 6.22a | 4.97a,b | 4.79a | 3.46b | 3.33b | 3.20b | |
| Narasin | + | 12.53 | 11.50 | 8.93b | 8.43a | 5.92b | 4.97 | 4.58b | 3.62 | 3.56 | 3.35 |
| − | 12.53 | 12.00 | 9.41a | 7.97b | 6.31a | 4.96 | 4.82a | 3.59 | 3.60 | 3.19 | |
| P-value | System | 0.249 | 0.046 | 0.001 | 0.016 | 0.003 | 0.019 | <0.001 | 0.004 | 0.003 | 0.018 |
| Narasin | 0.999 | 0.081 | 0.011 | 0.021 | 0.013 | 0.81 | 0.022 | 0.767 | 0.823 | 0.214 | |
| Interaction | 0.821 | 0.097 | 0.367 | 0.107 | 0.416 | 0.811 | 0.619 | 0.720 | 0.741 | 0.345 | |
a,bWithin a row, numbers with different superscripts differ statistically at P < 0.05.
Abbreviations: FL, flooring litter rearing; MC, multilayer cage rearing; PN, plastic net rearing.
n = 8 replications.
Discussion
Growth Performance
Growth performance is the most direct index for assessing poultry production and can be affected by rearing systems (Chen et al., 2015). Li et al. (2015) reported that cage rearing broilers had poorer growth performance than floor and net rearing broilers in the early phase of production, but cage-raised broilers had the highest feed conversion and slaughter weight at the later phase. Wang et al. (2013) reported that there was no significant difference in body weight, feed intake, mortality rate, and weight gain between the net rearing and floor rearing, whereas FCR of the net reared birds was significantly higher than that of floor reared counterparts. Similarly, Wang et al. (2015) reported that there was no significant difference in the growth performance of broilers among the 3 rearing systems, but FI in the floor rearing system was lower than cage and net rearing systems. The present study showed that the BW of caged-reared broilers was the lowest in all phases, which might be caused by problems associated with the immune function, intestinal health and gut mircoflora. The BW of PN birds was the highest in each stage, with correspondingly a higher FCR and FI, and a lower survival rate. However, FCR of cage-reared broilers was the lowest in the early and late growth phases. Overall, the cage-reared birds had the best FCR. This could be due to the fact that the cages represent a clean environment largely devoid of excessive load of pathogens where the birds do not waste energy on fighting immune challenges and nor do they spend much energy on activity. Similar to cages, net rearing can also prevent broilers from directly contacting with excreta, and more conducive to the growth of broilers than floor rearing, whereas the range of activities for floor broilers is increased, thus increasing energy consumption and the probability of foot pad dermatitis occurring (Bogosavljević et al., 2012; Zhang et al., 2018). Furthermore, net rearing also offers a hygienic environment where the occurrence of coccidiosis can be minimized compared with the floor system. Indeed, we examined the extent of the occurrence of coccidiosis in the 3 systems by addition or absence of narasin, an ionophore coccidiostat widely used in the poultry industry. Narasin is effective in reducing mortality and suppression of growth and feed efficiency associated with NE among broiler chickens challenged with Clostridium perfringens (Brennan et al., 2003; Whelan et al., 2019). Inclusion of narasin in the diet increased BW gain and decreased feed conversion ratio of male broilers with subclinical coccidia challenge (Wang et al., 2018). Narasin is not only used for its anticoccidial effect, but also as a growth promoter in Eimeria-free environments, due to its effect in improving feed conversion efficiency (Waldenstedt et al., 1995). Our study showed that narasin can reduce daily average feed intake of broilers and improve FCR. However, Karimi (2008) showed that under a coccidial and NE-free environment, the prophylactic effect of narasin was insignificant for broiler chicks housed in floor pens using wood shavings as bedding material.
Digestive Organ Development
Our study showed that floor-reared birds had bigger gizzards than their net- and cage-reared counterparts. Broilers raised on floor had directly contact with rice hulls on the ground, consuming an amount of rice hulls that could increase the bulk of the digesta, produce physical dilation of the gizzard walls, and increase the development of the muscular layers and the size of this organ (González-Alvarado et al., 2008). A well-developed gizzard promotes the secretion of digestive enzymes, reduces the rate of proventriculitis, and enhances nutrient digestion. Similarly, Hetland et al. (2003) found that the intake of wood shavings from the litter accounted for 4% of the feed intake, pushing up the gizzard and proventriculus weights of laying hens by 50%.
Studies have shown that the growth rate of gastrointestinal tract of chicks is faster than that of other organs and tissues after hatching (Wittig and Zeitz, 2003). The present study showed that the body weight of caged broilers was lower than that of the other 2 rearing systems. The intestinal tract development followed a similar trend. In addition, cage rearing reduced the relative weight and unit weight of each intestinal tract of broilers. However, the floor-reared broilers ate rice husks and absorbed more crude fiber, which was beneficial to the development and function of the gizzard, leading to improved physical abrasion, and stimulation of the secretion of digestive juice from the proventriculus. The fiber of the kind present in rice hulls belongs to what is known as structural components, which, in an appropriate particle size, plays an important role to stimulate gizzard activity and enhances gut development (González-Alvarado et al., 2008).
In the present study, the addition of narasin had no significant effect on the relative weight of digestive organs in broilers, but significantly reduced the unit weight of the duodenum. Studies have shown that the addition of narasin to the diet reduces the length and relative weight of the duodenum, jejunum, and ileum; the duodenum is the main organ to produce and release digestive enzymes into the broiler gastrointestinal tract, and hence the reduction of the unit weight of duodenum may be caused by the reduction of inflammation (Wang et al., 2018).
Intestinal Lesion Score and Intestinal Immunity
The present study did not detect any significant difference in the lesion score among different rearing systems. However, the use of narasin markedly reduced intestinal damage in broilers, in particular, in caged birds. The intestinal tract is not only the main organ for digestion and absorption, but is also the largest immune organ of broilers. Interleukin plays an important role in the regulation of immune cell differentiation and immune response (Medzhitov et al., 2000; Schroder et al., 2004). There are few reports on the expression of intestinal immune factors in broilers under different rearing systems. Wang et al. (2013) found that the relative expression of proinflammatory factors IL-6 and IFN-γ in jejunal mucosa of broilers in net-rearing and floor rearing systems was significantly lower than that in a low-density free range system, and the immune level of intestinal mucosa of broilers in net rearing was higher. The present study showed that the expression of intestinal mucosal immune factors in broilers was different between net and floor rearing systems, whereas cage rearing significantly reduced the expression of proinflammatory factors IL-1β and IFN-γ in broiler intestinal tract, indicating that the response to intestinal inflammatory factors by cage-reared broilers was not as good as that in floor- or net-reared broilers. In addition, the intestinal lesion score of broilers in cage-reared birds was the worst, which may be related to the low content of immune factors. Similar to our findings, Li (2014) reported that there were lower levels of intestinal mucosal sIgA and IL-2 in broilers raised in cages. Although cage rearing prevents birds from directly contacting with excreta, possibly reducing the potential exposure to pathogenic bacteria, it does not afford the birds any priming effects of microbes for the immune system nor the benefits of the ingestion of litter material that can aid the development of gizzard. The consequence may be poorer disease resistance and less robust birds compared with those reared on floors and in pens.
We also found that the expression level of TNF-α in intestinal mucosa of broiler chickens without narasin was significantly increased, which may be due to the fact that the body is in the stage of inflammatory reaction, and TNF-α produced by monocytes and macrophages is increased to promote cell proliferation and differentiation and repair body injury. Kaldhusdal et al. (2012) reported that narasin supplementation tended to reduce gizzard lesions in broilers (P < 0.10). However, in our case, narasin supplementation did not affect intestinal lesion score, which agrees with the findings of Scheurer et al. (2013).
Intestinal Microbiota
The gastrointestinal tract of broilers has a very complex microflora. Intestinal microflora plays an important role in nutrient digestion and absorption, modulation of the immune system, prevention of diseases, and maintenance of physiological functions (Oakley et al., 2014; Pourabedin et al., 2015). The diversity and composition of the broiler intestinal microflora are regulated by many factors, such as diet, age, antibiotics, genetics, immune response, and pathogen infection (Luo et al., 2017).
In relation to the effect of rearing, it has generally shown that floor rearing usually leads to a richer and more diverse gut microbiota than other systems. For instance, when laying hens are raised on free range settings, they are exposed to a lot of environmental microbes, which enrich their intestinal microflora during pecking litter, scratching and dust-bathing (Wang et al., 2016a; Cui et al., 2017). Wang (2013) reported that birds reared on floors had a much more diverse range of microorganisms in the duodenum, jejunum, and ileum than those raised on nets, although the difference diminished in the ceca. Our results mirrored their findings. Indeed, floor-reared broilers had more unique OTUs which were 411 and 324% higher than that in net and cage reared birds, respectively. The results were also obvious in α-diversity that floor rearing significantly increased the Shannon index as well as numerically increased other α-diversity indicators. It follows that floor rearing can increase the diversity of microorganisms in the intestine, leading to a more diverse intestinal microflora, which could improve the homeostasis of the body, the digestion and absorption of nutrients, and the resistance against pathogens (Wang et al., 2016b).
At a phylum level, the relative abundance of Firmicutes is the highest, with phyla such as Cyanobacteria, Proteobacteria, Actinomycetes having a relatively high abundance. This is consistent with findings in previous studies (Li et al., 2017). Proteobacteria belong to a gram-negative phylum, including many important pathogens such as Salmonella, Vibrio, Helicobacter, as well as some species in the Cyanobacteria, which can produce a variety of neurotoxins leading to diseases (Codd et al., 2005). In our study, the abundance of Proteobacteria and Cyanobacteria in the intestines of floor-reared broilers was reduced. It was probably due to the richness and diversity of the intestinal microflora of floor-reared broilers that may competitively excluded some of the harmful bacteria. This maybe related with more fibers (rice husk) took in floor-reared group, which improved and the microbial diversity in gastrointestinal (Cai et al., 2016). Actinomycetes are also gram-positive bacteria, most of which are saprophytic. In our study, the abundance of Actinomycetes in the floor-reared chicks increased significantly, probably because the birds picked up environmental Actinomycetes from the litter. Other studies (Cui et al., 2017) have also shown an increase in the abundance of Streptomyces belonging to Actinomycetes in the intestine of floor-reared laying hens. The findings suggested that Actinomycetes were major contributors to biological buffering of soils, which can resist the invasion of pathogens (Ningthoujam et al., 2009). Besides, bacteria of Actinomycetes like Streptomyces can produce a variety of antibacterial, antifungal, and antiparasitic substances, which work against harmful bacteria (Watve et al., 2001). In our study, floor and net rearing increased the abundance of ileal Bacteroidetes in broilers. Literature findings indicate that bacteria of Bacteroidetes can hydrolyze a variety of polysaccharides, including cellulose which cannot usually be digested by monogastric animals, and produce organic acids such as propionic acid and succinic acid as the major end-products (Rajilić-Stojanović et al., 2014; Zhang et al., 2017). These organic acids have anti-inflammatory, bacteriostatic, intestinal protection, and many other beneficial effects (De et al., 2016; Jacobson et al., 2018; Fernández-Veledo et al., 2019). In our study, the abundance of ileal Bacteroidetes of cage-reared broilers was reduced, which coincided with a lower level of organic acid production.
At genus level, Corynebacterium, Facklamia, Dietzia, Brevibacterium, and Staphylococcus of FL broilers had higher abundance, most of which belong to Actinomycetes; genus with lower abundance of Pseudomonas and Ochrobactrum, which belong to Proteobacteria. These changes at genus level are in accord with the results at phylum level. Corynebacterium is usually harmless and exists in the host symbiotically. Some species can produce glutamate for the host to utilize (Corynebacterium glutamicum), but some species are pathogens, which could cause diseases such as diphtheria and pseudotuberculosis (Burkovsi, 2008). Facklamia and Dietzia maris in Dietzia have been reported to be pathogens in humans (Koerner et al., 2009; Rahmatiet al., 2017). Brevibacterium could secrete aminopeptidases to hydrolyze protein, leading to improved digestion of dietary protein (Fernández et al., 2000). Staphylococcus is mostly saprophytic and may also enter the intestine if birds have sustained more contact with litter and excreta. But Staphylococcus aureus in Staphylococcus is more pathogenic than other organisms determined in this study. Similar with our findings, Wang et al. (2016a) reported that broilers raised on fresh litter had higher abundance of Corynebacterium, Facklamia, and Staphylococcus compared with those raised on reused litter. In our study, Pseudomonas and Bacillus are more abundant in the intestines of cage-reared broilers. Pseudomonas includes the opportunistic pathogen Pseudomonas aeruginosa. Chicks infected with Pseudomonas show symptoms of diarrhea, ruffled feather, and drooping wings (Shukla and Mishra, 2015). Bacillus includes the probiotic Bacillus subtilis and also includes the pathogenic Bacillus anthracis (Zhao et al., 2017). The present study revealed that the MC and PN chicks had higher abundance of Ochrobactrum in the ileum. Literature reports showed that Ochrobactrum was found in the gut lymphoid tissues and was associated with systemic inflammation (Zhang et al., 2018). Although floor rearing enriches the intestinal flora of the broilers at phylum level, the abundance of many potential pathogens and probiotics generally increase at the genus level. We found that the expression levels of IL-1β and IFN-γ of the ileal mucosa of FL broilers were higher while the expression levels of caged broilers were lower.
Conclusion
Birds reared in different systems experienced different levels of growth performance, gizzard development and gut health. The diversity of the gut microbiota differed between birds raised in the 2 system, so was the expression of some proinflammatory cytokines. Narasin supplementation improved FCR of broilers in general as well as the abundance of Proteobacteria. Further work is required to elucidate the mechanisms by which rearing systems lead to changes in gut microbiota diversity and cytokine expression.
Acknowledgments
This research was funded by the Taishan Industry Leading Talent Project of Shandong (LJNY2015006) and Key Technology Research and Development Program of Shandong (2019JZZY020602).
Disclosures
The authors declared that they have no conflicts of interest to this work.
References
- Bogosavljević-Bošković S., Rakonjac S., Dosković V., Petrovic M. Broiler rearing systems: a review of major fattening results and meat quality traits. Worlds Poult.Sci. 2012;68:217–228. [Google Scholar]
- Brennan J., Bagg R., Barnum D.A., Wilson J., Dick P. Efficacy of narasin in the prevention of necrotic enteritis in broiler chickens. Avian Dis. 2001;45:210–214. [PubMed] [Google Scholar]
- Brennan J., Skinner J., Barnum D.A., Wilson J. The efficacy of bacitracin methylene disalicylate when fed in combination with narasin in the management of necrotic enteritis in broiler chickens. Poul. Sci. 2003;82:360–363. doi: 10.1093/ps/82.3.360. [DOI] [PubMed] [Google Scholar]
- Burkovsi A., editor. Corynebacteria: Genomics and Molecular Biology. Caister Academic Press; Wymondham, United Kingdom: 2008. [Google Scholar]
- Cai Z.M., Wang Z.Y., Yang H.M. Effects of different proportions of rice husk replaced diets on intestinal microflora of geese from 19∼28 days. China Feed. 2016;3:9–12+6. [Google Scholar]
- Chen Y.J., Aorigele C., Yan F., Li Y., Cheng P., Qi Z.L. Effect of production system on welfare traits, growth performance and meat quality of ducks. South Afr. J. Anim. Sci. 2015;45:173–179. [Google Scholar]
- Choct M. Managing gut health through nutrition. Br. Poult. Sci. 2008;50:9–15. doi: 10.1080/00071660802538632. [DOI] [PubMed] [Google Scholar]
- Codd G.A., Morrison L.F., Metcalf J.S. Cyanobacterial toxins: risk management for health protection. Toxicol. Appl. Pharm. 2005;203:264–272. doi: 10.1016/j.taap.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Cui Y.Z., Wang Q.J., Liu S.J., Sun R., Zhou Y.Q., Li Y. Age-related variations in intestinal microflora of free-range and caged hens. Front. Microbiol. 2017;8:1310–1320. doi: 10.3389/fmicb.2017.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De V.F., Kovatcheva-Datchary P., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metabol. 2016;24:151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Fernández J., Mohedano A.F., Gaya P., Medina M., Nuñez M. Purification and properties of two intracellular aminopeptidases produced by Brevibacterium linens SR3. Int. Dairy J. 2000;10:241–248. [Google Scholar]
- Fernández-Veledo S., Vendrell J. Gut microbiota-derived succinate: Friend or foe in human metabolic diseases? Rev. Endocr. Metab. Dis. 2019;20:439–447. doi: 10.1007/s11154-019-09513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alvarado J.M., Jiménez-Moreno E., Valencia D.G., Lázaro R.P., Mateos G.G. Effects of fiber source and heat processing of the cereal on the development and pH of the gastrointestinal tract of broilers fed diets based on corn or rice. Poult. Sci. 2008;87:1779–1795. doi: 10.3382/ps.2008-00070. [DOI] [PubMed] [Google Scholar]
- Hetland H., Svihus B., Krogdahl Å. Effects of oat hulls and wood shavings on digestion in broilers and layers fed diets based on whole or ground wheat. Br. Poul. Sci. 2003;44:275–282. doi: 10.1080/0007166031000124595. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Lam L., Rajendram M., Tamburini F.B., Pham T.H.M., TreurenKali W.V., Pruss M. A gut commensal-produced metabolite mediates colonization resistance to Salmonella infection. Cell Host & Microbe. 2018;24:296–307.e1-307.e7. doi: 10.1016/j.chom.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadykalo S., Roberts T., Thompson M., Espeisse O., Lang M., Wilson J. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Ag. 2018;51:304–310. doi: 10.1016/j.ijantimicag.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Kaldhusdalsupa M.H. Non-soluble fibres and narasin reduce spontaneous gizzard erosion and ulceration in broiler chickens. Avian Patho. 2012;41:227–234. doi: 10.1080/03079457.2012.667559. [DOI] [PubMed] [Google Scholar]
- Karimi A. Effect of narasin and dietary protein source on performance of broiler. J.Bio. Sci. 2008;8:1077–1081. [Google Scholar]
- Koerner R.J., Goodfellow M., Jones A.L. The genus Dietzia: a new home for some known and emerging opportunist pathogens. FEMS Immun. Med. Microbiol. 2009;55:296–305. doi: 10.1111/j.1574-695X.2008.00513.x. [DOI] [PubMed] [Google Scholar]
- Li S.Y. Effects of the scale raises on health and welfare of broilers. China Poul. 2014;36:2–5. [Google Scholar]
- Li J.H., Miao Z.Q., Yang Y., Zhang H., Zhang J.Z., Lu Y.J., Yang Y. Effects of different rearing system and stocking density on growth performance and meat quality of broilers. Chin. J. Anim. Nutr. 2015;27:569–577. [Google Scholar]
- Li Z., Wang W.W., Liu D., Guo Y.M. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS One. 2017;12:e0188634. doi: 10.1371/journal.pone.0188634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo Y., Zhang L., Li H., Smidt H. Different types of dietary fibers Trigger specific Alterations in composition and predicted functions of colonic bacterial Communities in BALB/c Mice. Front. Microbiol. 2017;8:966. doi: 10.3389/fmicb.2017.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariam E.A., Afaf Y.A., Faten K.A., Ragheb G., Mashaly M.M. Production performance of different broiler breeds under different housing systems. Int. Poult. Sci. 2012;11:190–195. [Google Scholar]
- Medzhitov R., Janeway C.J. Advances in immunology: Innate immunity. New Engl. J. Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Ningthoujam D.S., Sanasam S., Tamreihao K., Nimaich S. Antagonistic activities of local actinomycete isolates against rice fungal pathogens. J. Agric. Res. 2009;3:737–742. [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Pourabedin M., Zhao X. Prebiotics and gut microbiota in chickens. FEMS Microbiol. Lett. 2015;362:122. doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- Rahmati E., Martin V., Wong D., Sattler F., Petterson J., Ward P., Butler-Wu S.M., She R.C. Facklamia species as an underrecognized pathogen. Open Forum Infect. Di. 2017;4:1–3. doi: 10.1093/ofid/ofw272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajilić-Stojanović, Vos W.M. De. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014;38:996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos F.B.O., Santos A.A., Oviedo-Rondon E.O., Ferket P.R. Influence of housing system on growth performance and intestinal health of salmonella-challenged broiler chickens. Curr. Res. Poult. Sci. 2012;2:1–10. [Google Scholar]
- Scheurer W., Spring P., Maertens L. Effect of 3 dietary phytogenic products on production performance and coccidiosis in challenged broiler chickens. J. Appl. Poult. Res. 2013;22:591–599. [Google Scholar]
- Schroder K., Hertzog P.J., Ravasi T., David A.H. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Shukla S., Mishra P. Pseudomonas aeruginosa infection in broiler chicks in Jabalpur. J. Med. Internet Res. 2015;6:37–39. [Google Scholar]
- Swain B.K., Sundaram R.N.S., Barbuddhe S.B., Nirmale A.V. Influence of cage and deep litter rearing systems on the performance of broilers. Indian Vet. J. 2002;79:467–469. [Google Scholar]
- Thamilvanan T., Thiagarajan M., Ramesh V., Gnanaraj P.T., Sivakumar T. Performance of broiler chicken under cage and floor systems of management fed differently processed feeds. Indian J. Anim. Sci. 2001;71:985–988. [Google Scholar]
- Waldenstedt L., Elwinger K. Effects of the coccidiostat narasin (monteban(r)) on growth of broiler-chickens in an eimeria-free environment. Archiv. Gefl. 1995;59:61–62. [Google Scholar]
- Wang L.L., Lilburn M., Yu Z.T. Intestinal microbiota of broiler chickens as affected by litter management regimens. Front. Microbiol. 2016;7:593. doi: 10.3389/fmicb.2016.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Henan Agricultural University; Zhengzhou, China: 2013. Effects of Different Feeding Systems and Regulators on Intestinal Microbes and Mucosal Immunity in Broilers. Master's Thesis. [Google Scholar]
- Wang X., Kiess A.S., Peebles E.D., Wamsley K.G.S., Zhai W. Effects of Bacillus subtilis and zinc on the growth performance, internal organ development, and intestinal morphology of male broilers with or without subclinical coccidia challenge. Poul. Sci. 2018;97:3947–3956. doi: 10.3382/ps/pey262. [DOI] [PubMed] [Google Scholar]
- Wang W.W., Li Z., Han Q.Q., Guo Y.M., Zhang B., Romain D. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br. J. Nutri. 2016;116:1878–1888. doi: 10.1017/S0007114516004116. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ru Y.J., Liu G.H., Chang W.H., Zhang S., Yan H.J. Effects of different rearing systems on growth performance, nutrients digestibility, digestive organ weight, carcass traits, and energy utilization in male broiler chickens. Livestock Sci. 2015;176:135–140. [Google Scholar]
- Watve M.G., Tickoo R., Jog M.M., Bhalachandra D.B. How many antibiotics are produced by the genus Streptomyces? Arch. Microbiol. 2001;176:386–390. doi: 10.1007/s002030100345. [DOI] [PubMed] [Google Scholar]
- Whelan R.A., Doranalli K., Rinttila T., Vienola K., Jurgens G., Apajalahti J. The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poult. Sci. 2019;98:3450–3463. doi: 10.3382/ps/pey500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis W.L., Murray C., Talbott C. Campylobacter isolation trends of cage versus floor broiler chickens: a one-year study. Poult. Sci. 2002;81:629–631. doi: 10.1093/ps/81.5.629. [DOI] [PubMed] [Google Scholar]
- Wittig B.M., Zeitz M. The gut as an organ of immunology. Int. J. Colorectal Dis. 2003;18:181–187. doi: 10.1007/s00384-002-0444-1. [DOI] [PubMed] [Google Scholar]
- Zhang B.B., Lv Z.P., Li Z., Wang W.W., Guang L., Guo Y.M. Dietary L-arginine supplementation alleviates the intestinal injury and modulates the gut microbiota in broiler chickens challenged by Clostridium perfringens. Front. Microbiol. 2018;9:1716. doi: 10.3389/fmicb.2018.01716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Razafindrabe R.H.A.K., Chen K.K., Zhao X.H., Yang L., Wang L., Chen X.Y., Jin S.H., Geng Z.Y. Effects of different rearing systems on growth performance, carcass traits, meat quality and serum biochemical parameters of Chaohu ducks. Anim. Sci. 2018;89:672–678. doi: 10.1111/asj.12976. [DOI] [PubMed] [Google Scholar]
- Zhang S.H., Song J.J., Deng Z.X., Chen L., Tian M., Guan W.T. Effects of combined α-galactosidase and xylanase supplementation on nutrient digestibility and growth performance in growing pigs. Arch.Anim. Nutri. 2017;71:441–454. doi: 10.1080/1745039X.2017.1389217. [DOI] [PubMed] [Google Scholar]
- Zhao L.Y., Zhang X., Zuo T., Yu J. The composition of colonic commensal bacteria according to anatomical localization in colorectal cancer. Engineering. 2017;3:90–97. [Google Scholar]