Abstract
A series of studies was conducted to determine the effects of a quillaja and yucca (saponin) combination (QY) product on postvaccination oocyst production, development of coccidial immunity, and final bird performance of broilers administered live coccidiosis vaccines. In all, 3 groups of tests were carried out. Study 1 evaluated the effects of QY (0 and 250 ppm) on oocyst per gram of feces (OPG) following vaccination at day-of-age; OPG were measured from 5 to 12 d postvaccination. Study 2 determined the effects of QY (250 ppm) in the presence of 3 commercial coccidiosis vaccines in floor pens. OPG were measured weekly for birds receiving each vaccine and for each corresponding vaccine group fed QY. To determine whether QY influenced the development of coccidial immunity induced by the 3 vaccines, 5 birds were removed from each pen at 28 d and challenged with pathogenic levels of Eimeria spp. At 6 d post challenge, lesion scores were used to evaluate the effects of QY on immune protection provided by each vaccine. In addition, comparisons of final bird performance were made between birds given each vaccine and their corresponding vaccinates fed QY. Study 3 comprised a meta-analysis of 15 floor pen trials in which 21- and 42-d body weight, feed conversions, and total mortality were compared between coccidiosis-vaccinated broilers and similarly vaccinated broilers fed QY (250 ppm). Results of these experiments indicated that feeding QY to vaccinated broilers did not significantly affect OPG from days 5 through 12 postvaccination (P > 0.05). For each vaccine tested in study 2, OPG values were the highest at 14 and 21 d postvaccination. QY significantly reduced OPG at 14 d postvaccination for 2 of the vaccines tested, and produced a similar effect in 1 vaccine at 21 d postvaccination. The remaining vaccine was not affected by QY in the postvaccination OPG results. Despite these changes in OPG, significant differences in lesion scores following the Eimeria challenge were not observed for any vaccinated groups receiving QY. Irrespective of the vaccine, both interim and final feed conversion values were significantly improved when QY was fed (P < 0.01). Similarly, results of a 15-trial meta-analysis indicated that QY-fed vaccinated broilers had higher body weights, improved feed conversions, and lower mortality than their vaccinated controls. Results show that while QY may induce changes in OPG following vaccination, coccidia-vaccinated broilers fed QY develop immunity equivalent to that of controls and show significant improvements in performance and mortality.
Key words: quillaja and yucca combination, saponin, coccidial vaccination, coccidial immunity, performance
Introduction
Saponins are a broad group of plant-derived compounds that have been found to exert numerous biological effects in animal applications (Cheeke, 2000; Francis et al., 2002). For more than 50 yr saponins have been investigated for their potential application in animal production due to their abilities to reduce pathogen loads, influence nutrient uptake, regulate ammonia production, and affect the growth process (Cheeke, 2000; Francis et al., 2002). Cheeke (2000) suggested that feeding saponins derived from Quillaja saponaria, the Chilean soap bark tree, and Yucca schidigera, a dessert plant of the American southwest, has great potential in poultry production due to these effects. Based on previous work with protozoan parasites, proposals for improved control of Eimeria infections in broilers were suggested (Cheeke, 2000). Recent work has shown that the saponin combination described above displayed anticoccidial activity, and these effects were found to compliment those of existing anticoccidial methods (Bafundo et al., 2020). In fact, a reduction in oocyst cycling and improved bird performance were found when a quillaja and yucca combination (QY) was added to the feed of vaccinated broilers.
Live vaccination of broilers for the control of coccidiosis is now common in the United States, and has become an accepted alternative to preventative anticoccidial medication (Shivaramaiah et al., 2014). However, when live coccidiosis vaccines are used, concerns related to impaired bird performance are often expressed (Chapman et al., 2002; Mathis et al., 2014). Research has shown that vaccine exposure and subsequent coccidial cycling impair nutrient utilization, thereby limiting bird performance (Lee et al., 2011; Arczewska-Wlosek and Swiatkiewicz, 2014). Because initial observations indicated that QY may improve the performance of vaccinated broilers (Bafundo et al. 2020), additional research was suggested to understand the nature and repeatability of these effects. Consequently, the objectives of the studies in this report are related to the QY-associated effects that could potentially influence the anticoccidial and performance responses of vaccinated broilers. Specifically, our studies were designed to: 1) determine the effects of QY (250 ppm) on the viability of the oocysts contained in the vaccinal dose; that is, to determine if QY significantly reduced the number of oocysts produced by broilers spray-vaccinated for coccidiosis at day-of-age, 2) determine the effects of QY (250 ppm) on the ability of vaccinated broilers to develop protective immunity to a significant coccidial challenge at day 28, and 3) determine the effects of QY on performance and mortality in coccidiosis-vaccinated broilers over the course of a 42-d growth cycle. The final objective involved a meta-analysis of 15 floor pen trials comparing vaccinated broilers to similar vaccinates fed QY for a 42-d period.
Materials and methods
All animal welfare and rearing procedures used in this research were approved by the respective Animal Care and Use Committees at Southern Poultry Feed and Research, Inc., Athens, Georgia, and AHPharma, Inc., Hebron, MD.
Products
Testing of the QY used in these trials involved a commercially prepared product (Magni-Phi, Phibro Animal Health Corp., Teaneck, NJ). The product is a combination of 2 natural, plant-based products consisting of the triterpenoid saponins of Q. saponaria and steroidal saponins of Y. schidigera. Quillaja saponins are the major active ingredients of this mixture. Additional details are presented in Bafundo et al. (2020).
The coccidiosis vaccines employed in these trials are commonly used, live vaccines that were administered at day-of-age in accordance with each manufacturer's instructions. Throughout this manuscript, none of these vaccines are identified by their commercial name or manufacturer, but reference to the coccidia they contain, whether virulent (wild type) or attenuated, is made. An additional vaccine is reported to contain a mixture of both virulent and attenuated coccidia and is referred to in this text as a “mixture.” Thus, using the descriptions provided by each manufacturer, the 3 vaccines evaluated in study 2 contained either all attenuated coccidia, all virulent coccidia, or a mixture of both. In study 2, these products are labeled vaccine 1, 2, or 3 so differences among the vaccines were evident. All were specifically designed for use in broiler chickens.
Study 1
Study 1 was conducted to determine the effects of QY administration on oocyst production of male Cobb 500 broilers for 12 d following vaccination. Birds were vaccinated at day 0 and allowed to preen themselves in chick boxes in a lighted area to ensure exposure to the oocysts in the vaccine. Chicks were then randomly assigned to battery cages where they were reared on wire floors, minimizing their reexposure to coccidial oocysts and facilitating collection of fecal material. Diets consisted of a standard, commercial-type corn soy-based broiler starter diet that contained either 0 or 250 ppm QY fed for the duration of the 12-d trial. Treatments were replicated 6 times and each replicate contained 10 broilers. Birds were allowed ad libitum access to feed and water for the duration of the trial. Following days 5, 6, 7, 8, 10, and 12 postvaccination, feces from each pen were evaluated for oocyst per gram of feces (OPG) using the techniques described by Hodgson (1970).
Study 2
Study 2 was designed to determine whether consumption of QY influenced the development of coccidial immunity produced by 3 live coccidiosis vaccines. Thus, 3 commercial vaccines, herein labeled vaccine 1, 2, and 3, respectively, were administered to newly hatched chicks according to each manufacturer's instructions. Following vaccine administration, birds were allowed to preen themselves for 30 min in a well-lighted area to insure vaccine uptake. In all, 6 treatments were utilized: vaccines 1, 2, and 3 were administered to 6 replicate groups of chicks that were then fed diets devoid of QY or any supplemental medication; corresponding to each vaccine were 3 additional treatments of 6 replicates each that were fed the same diets but containing QY 250 ppm. The 6 treatments were arranged in 6 blocks in which each pen contained 52 male Cobb 500 broilers. The pens used in the trial were of equal size so that bird density at the outset of the test was 0.091 m2 (0.85 ft2) per bird. Environmental temperature was monitored twice daily and adjusted to meet the requirements established by the primary breeder. A 22-h lighting schedule was maintained throughout the test period. Diets used in the trial were designed as commercial-type, corn soy-based broiler rations that were formulated to meet or exceed the requirements established by the National Research Council (1994). Starter feeds were provided up to day 18, grower feeds were fed up to day 28, and finisher feeds were administered until day 42. QY (250 ppm) was added to the feed of the intended treatments and provided from days 0 to 42. No antibiotics were used in any treatment at any point in the test.
All birds in the test were reared on used, built-up litter that was derived from previous floor pen tests involving coccidial challenges. Prior to use in the current study, all litter was removed from the pens, mixed together, and redistributed equally among the pens. No supplemental coccidia were added to this bedding material.
Beginning on day 14 and continuing weekly until trial termination, 10 fresh fecal samples per pen were collected and pooled. Following homogenization, aliquots were taken from this material so that OPG for each pen could be determined. The procedures of Hodgson (1970) as modified by Peek and Landman (2003) were used to generate the OPG results. OPG data are presented for each vaccine (vaccine 1, 2, and 3), showing the effects in the absence and presence of QY (250 ppm). Body weight gain and feed conversion values were also determined for each treatment at days 28 and 42 of the trial.
Due to coccidial cycling, immune protection to coccidial challenge increases as birds mature (Danforth, 1998; Jenkins et al., 2017). Thus, by 28 d of age broilers should be refractory to challenge infections (Chapman et al., 2005; Shivaramaiah et al., 2014). To determine whether QY affected the development of coccidial immunity to 3 different vaccines, 5 birds were removed from each pen on day 28 and challenged with a pathogenic dose of Eimeria oocysts. The inoculum used to challenge these birds contained 1 × 105 Eimeria acervulina, 5 × 104 Eimeria maxima, and 7.5 × 104 Eimeria tenella sporulated oocysts. This inoculum produced mean lesion scores of 2.8 in naïve, untreated control birds. The challenged birds were then reared in cages and fed diets devoid of QY or any anticoccidial. Six days post challenge (day 34), all birds were lesion scored for the degree of E. acervulina, E. maxima, and E. tenella infections (Johnson and Reid, 1970). Data are expressed as mean total lesion scores. Analysis of these data involved direct comparisons of each vaccine to its vaccinated counterpart that had been fed QY 250 ppm prior to inoculation. These procedures follow established guidelines used in the evaluation of coccidiosis vaccines (Chapman et al., 2005) and have been applied in similar trials involving coccidiosis vaccination (Mathis et al., 2014).
Study 3
Study 3 consisted of a meta-analysis of performance and mortality results of 15 floor pen trials that were conducted to evaluate the effects of QY (250 ppm) when combined with coccidiosis vaccines. Although each of the 15 trials was designed to address a different objective, common to each of these trials were the following treatments: 1) a control group in which birds were vaccinated for coccidiosis with a live coccidiosis vaccine and fed a standard ration devoid of anticoccidials and QY, and 2) birds vaccinated for coccidiosis as above and then fed QY (250 ppm) for the duration of the 42-d growth period. The individual pen results for these treatments in each of the 15 studies were pooled; comparisons between the 2 treatments were then made.
In each test all broilers were exposed to a live coccidiosis vaccine as previously described, and each vaccine was administered according to the manufacturer's instructions. The vaccines contained either virulent coccidia or a mixture of both virulent and attenuated Eimeria. In each test, treatments were replicated at least 8 times, with some trials utilizing 12 or 16 replications. Replicates contained either 52 or 55 broilers at placement. In all, 164 replications of each treatment contributed to the meta-analysis of the final performance and total mortality. Because all trials did not evaluate interim performance, 120 replications of each treatment were used in the assessment of performance and mortality at day 21. Five of the studies were conducted in Georgia and 10 trials were conducted in Maryland. No antibiotics were used in any of these tests.
The tests were largely conducted with Cobb 500 broilers, although 3 studies utilized Ross 708. Diets used in the trials varied slightly by trial location, but all were corn soy-based rations and designed to meet the requirements of growing broilers established by the National Research Council (1994). Feeding programs and bird density (0.091 m2 or 0.85 ft2 per bird) were approximately the same in all trials. House management procedures followed standard guidelines used across the American broiler industry.
The litter conditions used in each of the individual trials in this analysis were designed to induce an enteric disease challenge. In addition to the use of live coccidiosis vaccines, all birds in these trials were reared on used, built-up litter that was known to contain coccidial oocysts from previously conducted anticoccidial efficacy trials. Procedures used in the Georgia studies followed those described in study 2, above. In the Maryland tests, efforts were made to fortify the pathogenic nature of the contaminated litter that was placed in each pen. To this end, used litter was collected from commercial broiler farms in the Delmarva region of the United States; the farms were known to have had consistent production difficulties with coccidiosis and necrotic enteritis. Further evaluation of this litter confirmed that spores of Clostridium perfringens were indeed present. Samples of this litter were then weighed and distributed equally among the pens in the test facility. The pens in the Maryland tests were also supplemented with additional E. acervulina and E. maxima oocysts. In each trial, 1 × 105 E. acervulina and 3.5 × 104 E. maxima sporulated oocysts per bird were added to the litter of each pen prior to the start of each trial. The challenge was confirmed through scoring of coccidial and clostridial lesions at several points in each trial.
Statistical Analyses
Data in study 1 were analyzed by one-way ANOVA procedures using Statistix 10 software (Analytical Software, Tallahassee, FL). In study 2, ANOVA models using vaccines and QY as variables were used in the evaluation of performance criteria, OPG, and lesion scores. When significant effects were observed for these variables, mean separation using the Least Significant Difference procedure within the Statistix 10 software was employed. In all cases, P < 0.05 was used as an indicator of significance.
Given the strong methodological similarities used in the individual trials contributing to the meta-analysis employed in study 3, a mixed effect model was deemed appropriate for incorporating study variance. The mixed effect model accounted for study variance by treating the observed studies as samples from all possible studies using our study criteria. Models were fit using the R language (R Core Team, 2018) and were analyzed using the lme4 statistical package (Bates et al., 2015).
Results
Results of study 1 are presented in Table 1 and indicate the numbers of oocyst produced following spray vaccination of day-old broilers with a vaccine containing a mixture of virulent and attenuated coccidia. As shown in numerous studies, OPG are maximized at 7 d postvaccination and gradually decrease until about day 10 where OPG become too few to count. Table 1 also demonstrates the effects of QY (250 ppm) administration on oocyst production over this 12-d trial period. Data show that differences in OPG were not significant on days 6, 7, and 8 postvaccination (P > 0.05). A similar response was observed over the course of the 12-d trial.
Table 1.
Influence of QY (250 ppm) on OPG following vaccination with a coccidiosis vaccine1 containing a mixture of virulent and attenuated Eimeria (study 1).
| QY (ppm) | Postvaccination OPG per day |
|||||
|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9–10 | Total OPG Days 5–12 |
|
| 0 | 564 | 2,250 | 6,252 | 4,109 | 0 | 13,175 |
| 250 | 605 | 1,653 | 3,313 | 2,731 | 11 | 8,313 |
Abbreviations: OPG, oocyst per gram of feces; QY, a quillaja and yucca combination.
All birds were spray-vaccinated for coccidiosis at day-of-age and following a period of preening, were reared in wire-floored battery cages for the duration of the test. Feces were collected on the days shown. Statistical differences among treatments were not observed at any time interval (P > 0.05).
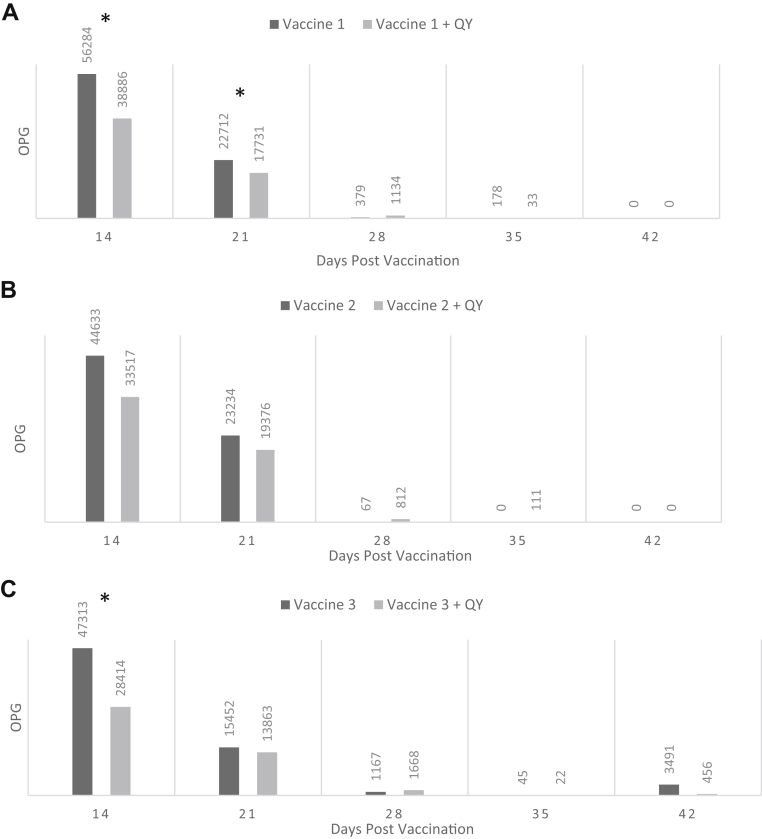
Study 2 was designed to illustrate the effects of QY on OPG over the course of the growth cycle and to determine whether these changes influenced the development of coccidial immunity. Figure 1 shows weekly OPG for each of the 3 vaccines beginning at day 14 and provides comparisons when each of these vaccines was supplemented with QY (250 ppm) in the feed. QY administration significantly reduced OPG values at days 14 and 21 in birds administered vaccine 1. However, significant reductions in OPG by QY were not observed for the remainder of the growth cycle. Similarly, OPG were reduced by QY at day 14 for vaccine 3, but no significant reductions were noted thereafter. QY feeding did not produce significant changes in OPG at any time point for vaccine 2.
Figure 1.
(A–C) Postvaccination OPG values1 for broilers vaccinated with coccidiosis vaccines 1, 2, or 3 in the presence and absence of QY 250 ppm (study 2). 1OPG values represent mean values of 6 replications in which 10 fresh fecal samples per pen were collected at each time point. ∗Comparisons containing an asterisk denote values that are statistically different (P < 0.05). Abbreviations: OPG, oocyst per gram of feces; QY, a quillaja and yucca combination.
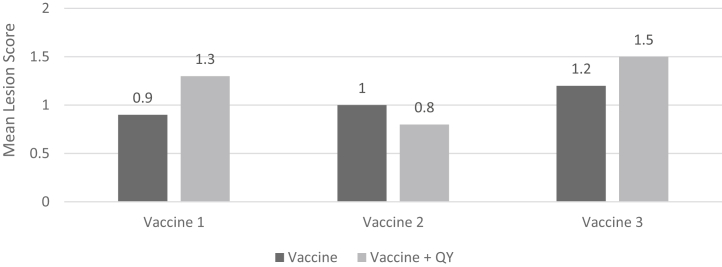
In order to assess the development of coccidial immunity induced by each vaccine and the influence that QY may exert on this process, representative birds from each pen were challenged with pathogenic doses of Eimeria on day 28 of the test. Figure 2 presents the results of this evaluation and shows that mean lesion scores recorded for each vaccine were 0.9, 1.0, and 1.2, respectively, for vaccines 1, 2, and 3. Similarly, vaccinated birds fed QY up to day 28 showed no significant differences in mean lesion scores (1.3, 0.8, and 1.5, respectively) when each QY treatment was compared to its corresponding vaccine control (P > 0.05). Additionally, performance evaluations were made and compared body weight and feed conversion at 2 time points (28 and 42 d) for each vaccine + QY treatment and its corresponding vaccine control. Significant differences in body weight were not observed for any treatment at either time period in this evaluation, and therefore, these data are not shown. However, Table 2 shows that significant improvements in feed conversion were observed at both 28 and 42 d of age when each vaccinated-QY treatment was compared to its corresponding vaccine without QY.
Figure 2.
The effect of coccidiosis vaccination with and without QY (250 ppm) on the development of coccidial immunity produced by 3 commercial vaccines as measured by mean total lesion scores of birds challenged with pathogenic doses of Eimeria1 (study 2). 1Initially, all birds were reared in floor pens. On day 28, 5 birds per replicate were removed from their pens and challenged with 1 × 105 Eimeria acervulina, 5 × 104 Eimeria maxima, and 7.5 × 104 Eimeria tenella sporulated oocysts. Birds were then placed in cages and fed a diet devoid of QY. After 6 d, birds were lesion scored as a measure of their ability to withstand coccidial challenge. Mean lesion score for naïve, untreated controls was 2.8. Within vaccine groups, significant differences between vaccinated birds and vaccinated birds fed QY were not observed (P > 0.05). Abbreviation: QY, a quillaja and yucca combination.
Table 2.
The effect of QY on 28- and 42-d adjusted feed conversion1 of broilers vaccinated with 3 different live coccidiosis vaccines (study 2).
| Treatment | Vaccine 1 |
Vaccine 2 |
Vaccine 3 |
|||
|---|---|---|---|---|---|---|
| FCR |
FCR |
FCR |
||||
| 28 d | 42 d | 28 d | 42 d | 28 d | 42 d | |
| Vaccine | 1.560 ± 0.01a | 1.725 ± 0.01a | 1.595 ± 0.01a | 1.719 ± 0.01a | 1.558 ± 0.01a | 1.724 ± 0.01a |
| Vaccine + QY 250 ppm | 1.547 ± 0.01b | 1.691 ± 0.01b | 1.548 ± 0.01b | 1.679 ± 0.03b | 1.529 ± 0.01b | 1.695 ± 0.02b |
a,bWithin columns, treatment means with different superscripts are statistically different (P < 0.05). Statistical differences among the 3 vaccines were not observed at either time point (P > 0.05). Across all vaccine treatments, the QY effect was significant at both 28 and 42 d (P < 0.0003 and P < 0.0009, respectively).
Abbreviations: FCR, feed conversion ratio; QY, a quillaja and yucca combination.
Values represent the means ± SE of 6 replications per treatment.
Study 3 was carried out to gain a better understanding of performance improvements produced by QY when fed in the presence of coccidiosis vaccines. Under the conditions of enteric disease challenge described above, total mortality was considerably higher than the industry averages. Vaccinated birds fed QY were heavier at 21 and 42 d than their vaccinated controls and showed significant improvements in feed conversion (Table 3). Likewise, 21- and 42-d mortalities were significantly reduced by the addition of QY to these vaccination programs (Table 3).
Table 3.
Interim (21 d) performance, final performance (42 d), and total mortality determined in a meta-analysis of 15 disease-challenged floor-pen trials1 evaluating the effects of QY in birds vaccinated for coccidiosis (study 3).
| Vaccine | Vaccine + QY | Difference | SE of difference | |
|---|---|---|---|---|
| Day 21 | ||||
| BWG (g) | 750 | 789 | 39∗ | 2.93 |
| FCR (g:g) | 1.384 | 1.327 | −0.057∗ | 0.006 |
| Mortality2 | 6.18 | 1.47 | −4.71∗ | 0.331 |
| Day 42 | ||||
| BWG (g) | 2,402 | 2,502 | 100∗ | 7.89 |
| FCR (g:g) | 1.872 | 1.813 | −0.059∗ | 0.005 |
| Mortality3 | 8.13 | 3.59 | −4.54∗ | 0.396 |
∗P < 0.0003.
Abbreviations: BWG, body weight gain; FCR, feed conversion ratio; QY, a quillaja and yucca combination.
Treatment means represent data compiled in the meta-analysis of 120 replications per treatment collected at day 21 and 164 replications per treatment determined at day 42. QY was fed at 250 ppm in all cases.
Percent mortality from all causes through day 21.
Percent mortality from all causes over the course of the grow out.
Discussion
Data from study 1 illustrate that the numbers of oocysts (OPG) produced over the 12 d following spray vaccination of coccidia were not significantly affected by administration of QY in the feed. Results demonstrated that vaccinated birds fed QY are indeed parasitized by vaccinal oocysts and these organisms subsequently completed their life cycles. Data from study 2 show that few differences exist among the 3 commercial vaccines in the pattern of oocyst production (OPG) over the course of a 42-d growth cycle. Each vaccine, whether attenuated, virulent, or a mixture, produced maximal OPG values at the second week postvaccination. These peaks were then followed by a gradual reduction in fecal oocyst numbers for most of the observation points thereafter. These observations agree with those described by Jenkins et al. (2017), who outlined the conditions under which vaccines perform optimally. Overall, vaccinated birds fed QY showed similar patterns of fecal oocyst production with peak OPG values recorded at day 14 postvaccination. However, compared to vaccinated controls, OPG were reduced at 14 and 21 d postvaccination in birds administered QY. Since Chapman et al. (2002) demonstrated that continuous exposure to oocysts in the litter is essential for the development and maintenance of coccidial immunity, differences of this type could be construed as factors that impair the developing immune response to coccidial infection. However, previous work has shown that limitation of coccidial cycling during the late starter and grower periods is associated with performance improvements as birds approach market weight (Mathis et al., 2014). In fact, these findings are consistent with current commercial practices where live coccidiosis vaccines are supported by anticoccidial medication during this phase of production (Mathis et al., 2014). These authors emphasized the importance of using anticoccidial dosages that allow sufficient numbers of coccidia to “leak” through the medication, thereby supporting adequate immune development while reducing the adverse effects of coccidial cycling and improving bird performance. Similar effects occurred with QY in the current study, as OPG were clearly reduced, but not eliminated, for all vaccines at multiple time points in this trial. As in the data reported by Mathis et al. (2014), feed conversion values responded accordingly, as QY significantly improved feed conversion compared to the vaccinated controls at both 28 and 42 d of the test. In addition, these findings agree with an initial evaluation of QY in coccidiosis-vaccinated broilers by showing that a reduction in OPG at 21 and 28 d was associated with significant improvements in final body weights and feed conversions (Bafundo et al., 2020). Thus, reductions in coccidial oocyst numbers (OPG) during the late starter and grower periods can be of benefit, especially if the immune response to coccidial infection is not compromised (Mathis et al., 2014).
Whether in a research or commercial environment, anticoccidial vaccines are evaluated by their ability to induce immunity and protect birds from coccidial challenge (Chapman et al., 2005; Peek and Landman, 2011; Shivaramaiah et al., 2014). Data presented herein illustrate this effect by showing that mean lesion scores for non-vaccinated, challenged controls were 2.8; vaccinated birds challenged with coccidia on day 28 had lesion scores of approximately 1. Because the challenge administered to each bird in this test was severe, mean lesion scores of this nature are indicative of the development of protective immunity in each bird (Shivaramaiah et al., 2014). These data also indicate that QY did not impair this process, as significant differences in lesion scores between each vaccine and its vaccinated counterpart fed QY were not observed. Thus, within the scope of this study, QY reduced coccidial cycling at 14 and 21 d without affecting the level of coccidial immunity provided by each vaccine. Further support for these findings can be derived from feed conversion values recorded at both 28 and 42 d of the trial, where irrespective of the vaccine, broilers fed QY demonstrated significantly improved conversions compared to their corresponding vaccinated controls. When taken together, these data indicate that regardless of vaccine and/or the type of coccidia involved in the vaccinal dose (attenuated, virulent, or a mixture), QY reduced coccidial exposure and improved feed conversion, while maintaining levels of coccidial protection that were equivalent to the vaccine alone.
The procedures used in study 3 were intended to challenge vaccinated broilers with coccidial and bacterial pathogens that increased mortality and impaired performance. In this environment, vaccinated birds fed QY greatly exceeded their vaccinated counterparts in body weight and feed conversion. Significant reductions in total mortality were also recorded. Other than the current commercial usage, these data provide the first comprehensive analysis of performance for QY and reflect many of the same effects reported in a similar study of coccidial vaccinates (Bafundo et al., 2020). As a result, they show the consistency of effects under the conditions of our testing. While a reduction in coccidial cycling (Mathis et al., 2014) may account for some of the differences noted in these trials, it should be clear that the performance differences reported in study 3 exceed those associated with the limitation of coccidial replication. Thus, it is probable that other intestinal changes may be involved. Future research studies that intend to show the effects of QY on intestinal bacterial populations, nutrient uptake and digestibility, and effects on mucosal immunity will hopefully bring about a better understanding of the intestinal effects produced by QY and how they influence bird performance.
Disclosures
There are no conflicts of interest.
References
- Arczewska-Wlosek A., Swiatkiewicz S. Nutrition as a modulatory factor of the efficacy of live coccidiosis vaccines in broiler chickens. World’s Poult. Sci. J. 2014;70:81–92. [Google Scholar]
- Bafundo K.W., Johnson A.B., Mathis G.F. The effects of a combination of Quillaja saponaria and Yucca schidigera on Eimeria spp. in broiler chickens. Avian Dis. 2020;64:300–304. doi: 10.1637/aviandiseases-D-20-00016. [DOI] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. Fitting Linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- Chapman H.D., Cherry T.E., Danforth H.D., Richards G., Shirley M.W., Williams R.B. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int. J. Parasitol. 2002;32:617–629. doi: 10.1016/s0020-7519(01)00362-9. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Roberts B., Shirley M.W., Williams R.B. Guidelines for evaluating the efficacy and safety of live anticoccidial vaccines, and obtaining approval for their use in chickens and turkeys. Avian Pathol. 2005;34:279–290. doi: 10.1080/03079450500178378. [DOI] [PubMed] [Google Scholar]
- Cheeke P.R. Actual and potential application of Yucca schidigera and Quillaja saponaria saponins in human and animal nutrition. J. Anim. Sci. 2000;77:1–10. [Google Scholar]
- Danforth H.D. Use of live oocyst vaccines in the control of avian coccidiosis: experimental studies and field trials. Int. J. Parasitol. 1998;28:1099–1109. doi: 10.1016/s0020-7519(98)00078-2. [DOI] [PubMed] [Google Scholar]
- Francis G., Kerem Z., Makkar H.P.S., Becker K. The biological action of saponins in animal systems: a review. Br. J. Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- Hodgson J.N. Coccidiosis: oocyst counting technique for coccidiostat evaluation. Exp. Parasitol. 1970;28:99–102. doi: 10.1016/0014-4894(70)90073-1. [DOI] [PubMed] [Google Scholar]
- Johnson J.K., Reid W.M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Jenkins M.A., Parker C., Ritter D. Eimeria oocyst concentration and species composition in litter from commercial broiler farms during anticoccidial drug or live Eimeria oocyst vaccine control programs. Avian Dis. 2017;61:214–220. doi: 10.1637/11578-010317-Reg.1. [DOI] [PubMed] [Google Scholar]
- Lee J.T., Eckert N.H., Ameiss K.A., Stevens S.M., Anderson P.N., Anderson S.M., Barry A., McElroy A.P., Danforth H.D., Caldwell D.J. The effect of dietary protein level on performance characteristics of coccidiosis vaccinated and non-vaccinated broilers following mixed-species Eimeria challenge. Poult. Sci. 2011;90:1916–1925. doi: 10.3382/ps.2011-01362. [DOI] [PubMed] [Google Scholar]
- Mathis G.F., Schaeffer J., Cookson K., Dickson J., LaVorgna M., Waldrip D. Effect of lasalocid or salinomycin administration on performance and immunity following coccidia vaccination of commercial broilers. J. Appl. Poult. Res. 2014;23:577–585. [Google Scholar]
- National Research Council . 9th ed. National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Peek H.W., Landman W.J.M. Resistance to anticoccidial drugs of Dutch avian Eimeria spp. field isolates originating from 1996, 1999 and 2001. Avian Pathol. 2003;32:391–401. doi: 10.1080/0307945031000121149. [DOI] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011;31:143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Shivaramaiah C., Barta J.R., Hernandez-Valasco X., Tellez G., Hargis B.M. Coccidiosis: Recent advancements in the immunobiology of Eimeria species, preventative measures, and the importance of vaccination as a control tool against these Apicomplexan parasites. Vet. Med. Res. Rep. 2014;5:23–34. doi: 10.2147/VMRR.S57839. [DOI] [PMC free article] [PubMed] [Google Scholar]