Abstract
The extensive use of antibiotics has, in recent years, caused antimicrobial resistance and multidrug resistance in Escherichia coli to gradually develop into a worldwide problem. These resistant E. coli could be transmitted to humans through animal products and animal feces in the environment, thereby creating a problem for bacterial treatment for humans and animals and resulting in a public health issue. Monitoring the resistance of E. coli throughout the broiler fattening period is therefore of great significance for both the poultry industry and public health. In this longitudinal study, samples were taken from 6 conventional broiler fattening farms in Shandong Province, China, at 3 different times within 1 fattening period. The overall isolation rate of E. coli was 53.04% (375/707). Antibiotic resistance was very common in the E. coli isolated from these farms, and differed for different antibiotics, with ampicillin having the highest rate (92.86%) and cefoxitin the lowest (10.12%). Multidrug resistance was as high as 91.07%. More importantly, both the resistance rate of E. coli to the different drugs and the detection rate of drug resistance genes increased over time. The mobile colistin resistance (mcr-1) gene was detected in 24.40% of the strains, and these strains often carried other drug resistance genes, such as those conferring aminoglycoside, β-lactamase, tetracycline, and sulfonamide resistance. Antimicrobial resistance and drug resistance genes in E. coli were least common in the early fattening stage. The individual detection rates of sul1, sul3, aacC4, aphA3, and mcr-1 were significantly lower (P < 0.05) for the early fattening stage than for the middle and late stages. The rational use of antibiotics, in conjunction with the improvement of the breeding environment during the entire broiler fattening cycle, will be helpful in the development of the poultry industry and the protection of public health.
Key words: Escherichia coli, drug resistance gene, mcr-1, drug sensitivity test
Introduction
Escherichia coli, a widespread pathogenic bacterium in humans and animals, causes a variety of diseases that are difficult to control and results in a large number of deaths in poultry populations, leading to significant economic losses in the poultry industry (Wang et al., 2010; Meguenni et al., 2019; Gao et al., 2020; Kim et al., 2020; Song et al., 2020). In the poultry industry, antibiotics are the most common means used to treat and prevent E. coli infections (Saliu et al., 2017; Roth et al., 2019a,b). However, the excessive use of antibiotics (Kayastha et al., 2020) has led to antimicrobial resistance and multidrug resistance (MDR) in E. coli, making treatment difficult and causing a worldwide public health issue (Kadykalo et al., 2018; Kayastha et al., 2020; Montoro-Dasi et al., 2020). Avian-origin E. coli not only has the potential for zoonotic disease, but also has a close relationship with the drug resistance of human E. coli (Zhuge et al., 2019). E. coli can enter the environment through feces and can be transmitted through animal sheds, soil, air, and even water (Zucker et al., 2000; Duan et al., 2006), while animals used for food production provide another important method for the transmission of E. coli to people (Kadykalo et al., 2018). Studies have suggested that chickens may be the source of antimicrobial-resistant and extraintestinal pathogenic E. coli in humans (Laube et al., 2013; Mellata, 2013; Vounba et al., 2019; Li et al., 2020). The presence of antimicrobial-resistant strains makes the treatment of Enterobacteriaceae infections, including human clinical diarrheal E. coli disease, increasingly difficult and complex (Eltai et al., 2020).
In veterinary use, colistin has ever been administered as a feed additive in many countries such as China, the United States, the European Union, and Japan, to prevent infectious diseases for decades (Poirel et al., 2017). Colistin is considered a last resort drug to treat severe MDR gram-negative bacterial infections in humans; the mobile colistin resistance determinant (mcr-1) has attracted global attention (Liu et al., 2016). The transmission of mcr-1-mediated colistin resistance between animals and humans poses a threat to human health (Yang et al., 2017). Continued surveillance of MDR, including the presence of mcr-1, may help pre-empt the spread of mcr-1 amongst bacterial pathogens (Song et al., 2020). Investigation and monitoring of E. coli resistance, especially mcr-1-mediated resistance, are important when assessing the potential economic and public health implications.
Shandong is a major agricultural province in China where the poultry industry is developing rapidly, with poultry meat production reaching 3.337 million tons in 2019 (https://www.ppxmw.com/news/47926.html). Chicken meat is a potential source of MDR E. coli (Cyoia et al., 2018), and monitoring the resistance of E. coli on broiler farms is therefore of great significance for the prevention and treatment of E. coli disease and protection of public health. However, little information has thus far been available on the characteristics of E. coli resistance throughout the fattening cycle of broilers in Shandong Province. In this longitudinal study, 6 conventional broiler chicken fattening farms, distributed throughout Shandong Province, were selected for long-term investigations. Each broiler flock was investigated 3 times within 1 fattening cycle. The E. coli isolates were tested for resistance to 9 common antibiotics and for the presence of 12 types of drug resistance genes, so as to understand the prevalence of E. coli and the variation in drug resistance during the entire broiler fattening cycle. This will help provide guidance for the prevention and treatment of E. coli and a reference for theoretical research on bacterial resistance.
Materials and methods
Sample Collection
A total of 707 samples were collected from 6 broiler chicken farms located in Tai'an, Liaocheng, Weifang, Linyi, Heze, and Binzhou, respectively, in Shandong Province, China (Figure 1) from May to July 2019. The fattening period for all the examined broilers was approximately 40 d and these were sampled in 3 time periods (Laube et al., 2013); the first sample collection was done at 1 to 2 d of age, the second at 17 to 20 d, and the third at 35 to 40 d. The number of animal houses per farm ranged from 2 to 20, and the size of the animal houses varied from farm to farm (see Supplementary Table 1 for more detailed information). Two same feeding period broiler houses on each farm that harbored more than 2 houses were randomly selected for sampling.
Figure 1.
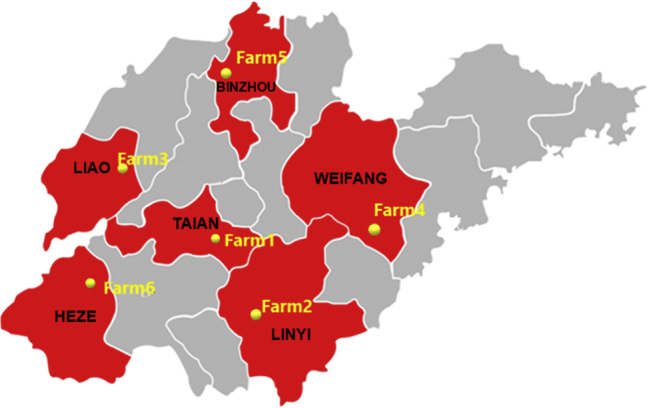
Geographic distribution of the sampling sites across different prefecture-level cities in Shandong, China. A total of 707 samples were collected from 6 broiler fattening farms in the Tai'an (farm 1), Linyi (farm 2), Liaocheng (farm 3), Weifang (farm 4), Binzhou (farm 5), and Heze (farm 6) areas.
During each sampling, 10 chickens were randomly selected from each chicken house and cloacal swab samples were collected using cotton swabs; 3 to 5 fecal samples were also collected from different locations using 2 mL sterile centrifuge tubes (Laube et al., 2013). In this study, 2 water supply devices (nipples) were randomly selected for the collection of 2 drinking water samples in 2 mL sterile centrifuge tubes using disposable sterile straws, and 2 wall-surface samples were collected using cotton swabs. The air samples were prepared as described by Wu et al. (2018); after slight modifications, 2 Luria-Bertani (LB) agar (Solarbio, Beijing, China) plates were placed inside and outside the chicken houses and exposed to air for approximately 15 min to measure the microbial air quality. All cotton swabs were moistened with LB broth (Solarbio) firstly and then sterilized.
Isolation and Identification of E. coli
All samples were stored at 4°C before being processed within 12 h of sampling. Cloacal and environmental swabs were aseptically streaked directly onto MacConkey agar (Hope Bio, Qingdao, China). Fecal samples were initially suspended in 500 μL of aseptic PBS, after which they were diluted 1:100 and 20 μL of the diluent was plated onto MacConkey agar. The water samples were centrifuged at 12,000 rpm for 60 s, and then most of the supernatant was discarded, and the remaining parts were cultured on MacConkey agar. All the MacConkey and LB agar plates were incubated overnight at 37°C. After 3 rounds of purification, putative E. coli isolates were selected based on bacterial colony morphology and confirmed using a microbial mass spectrometer (IVD MALDI Biotyper, Bruker, Bremen, Germany).
Antimicrobial Susceptibility Testing
The Kirby-Bauer disk diffusion method, as described by the Clinical and Laboratory Standards Institute, was used to test the susceptibility of E. coli to 9 commonly used antibiotics (Song et al., 2020), including amikacin (AK), ciprofloxacin, gentamicin, ofloxacin, cefoxitin (FOX), piperacillin, cefotaxime, enoxacin, and ampicillin. The antimicrobial susceptibility testing disks were purchased from Thermo Fisher Scientific, Shanghai, China. The results were interpreted based on the Clinical and Laboratory Standards Institute guidelines (2019). E. coli isolates resistant to more than 3 classes of antimicrobials were defined as MDR isolates.
Detection of Drug Resistance Genes
Bacterial DNA was extracted using a Bacterial Genome Kit (Bioteke, Beijing, China), according to the manufacturer's instructions. The colistin resistance gene (mcr-1) and other genes associated with resistance to aminoglycosides (aadA, aacC2, aphA3, and aacC4), β-lactams (blaCTX-M, blaSHV), tetracyclines (tetA, tetB), and sulfonamides (sul1, sul2, and sul3) were detected using PCR. All the primers and annealing temperatures were slight modifications of those used in previously described procedures (Table 1).
Table 1.
Primer sequences and PCR conditions used to detect antibiotic resistance genes in Escherichia coli isolated from samples collected from broiler chicken farms in Shandong Province, China.
| Gene name | Primer sequences (5′-3′) | Amplicon size (bp) | Annealing temperature (°C) | References |
|---|---|---|---|---|
| aadA | F-GCAGCGCAATGACATTCTTG R-ATCCTTCGGCGCGATTTTG |
282 | 55 | Costa et al., 2008 |
| aacC4 | F-ATGACCTTGCGATGCTCTATGA R-CGAATGCCTGGCGTGTTT |
486 | ||
| aacC2 | F-ACCCTACGAGGAGACTCTGAATG R-CCAAGCATCGGCATCTCATA |
384 | ||
| aphA3 | F-TGACTGGGCACAACAGACAA R-CGGCGATACCGTAAAGCAC |
677 | ||
| CTX-M | F-AGTGAAAGCGAACCGAATC R-CTGTCACCAATGCTTTACC |
365 | 55 | Tian et al., 2011 |
| SHV | F-ATGCGTATATTCGCCTGTG R-CCTCATTCAGTTCCGTTTCC |
502 | ||
| tetA | F-GGCCTCAATTTCCTGACG R-AAGCAGGATGTAGCCTGTGC |
372 | 55 | Guillaume et al., 2000 |
| tetB | F-GAGACGCAATCGAATTCGG R-TTTAGTGGCTATTCTTCCTGCC |
228 | ||
| sul1 | F-GTGACGGTGTTCGGCATTCT R-TCCGAGAAGGTGATTGCGCT |
779 | 58 | Boerlin et al., 2005 |
| sul2 | F-CGGCATCGTCAACATAACCT R-TGTGCGGATGAAGTCAGCTC |
721 | 55 | |
| sul3 | F-GAGCAAGATTTTTGGAATCG R-CTAACCTAGGGCTTTGGA |
790 | 50 | Al Salah et al., 2019 |
| mcr-1 | F-CGGTCAGTCCGTTTGTTC R-CTTGGTCGGTCTGTAGGG |
309 | 55 | Liu et al., 2016 |
Abbreviations: F, forward; mcr-1, mobile colistin resistance; R, reverse.
Data Analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (version 26, IBM Corp., Armonk, NY). The isolation rates, antibiotic resistances, and presence of resistance genes data for the E. coli isolated during the 3 sampling periods were compared using the chi-square test. Differences in the average antibiotic resistance rate and the average detection rate of drug resistance genes among the 3 different stages during 1 fattening cycle were analyzed using one-way ANOVA. P-values less than 0.05 were considered indicative of statistically significant differences.
Results
Prevalence and Distribution of E. coli
Six broiler fattening farms participated in this study, with a total of 707 samples being collected from these 6 farms. The overall isolation rate was 53% (375/707), showing an increasing trend across the 3 different sampling periods, with 51% at 1 to 2 d, 53% at 17 to 20 d, and 55% at 35 to 40 d. However, there was no significant difference in the isolation rate between the 3 stages (P > 0.05).
As is seen in Table 2, the highest detection rate of E. coli was found in the animal source (cloacal swab) samples (82%, 288/353), with average detection levels of 73, 85, and 87% in the animal (cloacal swab) samples collected at the first, second, and third samplings during the course of the fattening period, respectively, demonstrating a significant (P < 0.05) increase in prevalence. The highest detection rate for the environmental samples was for the fecal samples, at 60% (60/100), whereas the lowest was 9% for water samples. And both the detection rates for air and wall samples were 11% or so. A total of 168 strains comprising 24 to 30 per farm were selected for subsequent testing and analysis (Table 3).
Table 2.
Distribution of Escherichia coli-positive samples collected from various locations on broiler chicken farms at 3 stages during a single fattening cycle.
| Sampling time (d) | Detection rates of E. coli isolated from1 |
All | ||||
|---|---|---|---|---|---|---|
| Cloacal swabs | Feces | Wall | Air | Water | ||
| 1–2 | 72.57% (82/113) | 76.67% (23/30) | 18.18% (4/22) | 15.22% (7/46) | 17.39% (4/23) | 51.28% (120/234) |
| 17–20 | 85.00% (102/120) | 56.25% (18/32) | 4.55% (1/22) | 7.14% (3/42) | 5.00% (1/20) | 52.97% (125/236) |
| 35–40 | 86.67% (104/120) | 50.00% (19/38) | 9.09% (2/22) | 11.76% (4/34) | 4.35% (1/23) | 54.85% (130/237) |
| All | 81.59% (288/353) | 60% (60/100) | 10.61% (7/66) | 11.48% (14/122) | 9.09% (6/66) | 53.04% (375/707) |
Numbers in parentheses indicate positive/total.
Table 3.
Isolation rates of Escherichia coli in samples collected from 6 broiler chicken farms at 3 stages during a single fattening cycle, and the distribution among sample types of E. coli isolates selected for testing for antibiotic resistance and MDR rates.
| Sites | Isolation rate of E. coli1 | Distribution of E. coli isolates selected for testing and their MDR rates2 |
||||
|---|---|---|---|---|---|---|
| 1–2 d | 17–20 d | 35–40 d | Sum | MDR rate (%) | ||
| Farm 1 | 57.81% (128) | C (6), F (3), A (1), WL (2) | C (7), F (2), WL (1) | C (6), F (2) | 30 | 90.00a |
| Farm 2 | 50.89% (112) | C (5), F (4), WL (1) | C (6), F (3) | C (6), F (3), A (1), WL (1) | 30 | 96.67a |
| Farm 3 | 43.65% (126) | C (4), F (3), WT (1) | C (6), F (4), A (3) | C (4), F (5) | 30 | 100.00a |
| Farm 4 | 50.82% (122) | C (6), F (1), WT (1) | C (6), F (4), WT (1) | C (6), F (4), A (1) | 30 | 93.33a |
| Farm 5 | 47.97% (123) | C (5), F (4), A (2) | C (5) | C (5), F (1), A (2) | 24 | 62.50b |
| Farm 6 | 70.83% (96) | C (5), F (3), WT (2) | C (5), F (2) | C (5), F (2) | 24 | 100.00a |
| Amount | 53.04% (707) | 59 | 55 | 54 | 168 | 91.07 |
a,bValues that share no common superscript letters differ significantly (P < 0.05).
Abbreviations: A, air; C, cloacal swabs, F, feces; MDR, multidrug resistance; WL, wall; WT, water.
Numbers in parentheses indicate sample numbers associated with the provided percentages.
Numbers in parentheses indicate the number of selected E. coli isolates from each sample type.
Antibiotic Resistance and MDR Profiles
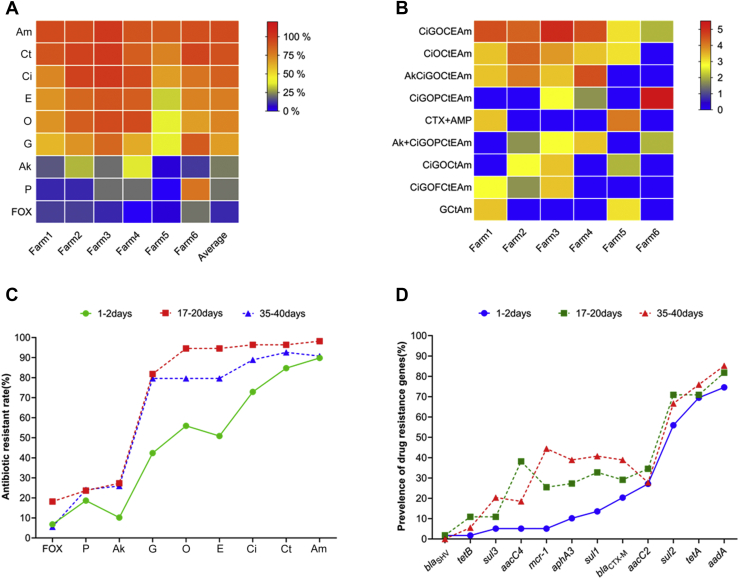
All 168 strains of E. coli showed varying resistance to the 9 antibiotics tested. The lowest rate of resistance was to FOX (10%), while the highest was to ampicillin (93%). With the exception of FOX (10%), AK (21%), and piperacillin (22%), resistance to the antibiotics was high, exceeding 60%. The E. coli isolates from the 6 farms showed similar resistance to the 9 antibiotics, although the resistance rates to all the antibiotics, except FOX (4%), were lower in the Binzhou area (farm 5) than in the other areas tested (Figure 2A).
Figure 2.
Characteristics of drug resistance and drug resistance genes. (A) A heat map showing the distribution of resistance of Escherichia coli isolated from samples from 6 broiler chicken farms to 9 types of antibiotics. The color scale of the individual cells represents the resistance rate, as a percentage. The heat map was constructed by GraphPad Prism 8.0.2 software (GraphPad, San Diego, CA). (B) A heat map showing the distribution of MDR patterns in E. coli isolated from samples from 6 broiler chicken farms. The color scale of the individual cells represents the log2 of the percentage resistance rate. The heat map was constructed by GraphPad Prism 8.0.2 software. (C) Variation in the resistance to 9 types of antibiotics of E. coli isolated from samples from broiler chicken farms at 3 stages during a single fattening cycle. (D) Variation in the rates of detection of a number of resistance genes in E. coli isolated from samples from broiler chicken farms at 3 stages during a single fattening cycle. Abbreviations: Ak, amikacin; Am, ampicillin; Ci, ciprofloxacin; Ct, cefotaxime; E, enoxacin; FOX, cefoxitin; G, gentamicin; MDR, multidrug resistance; O, ofloxacin; P, piperacillin.
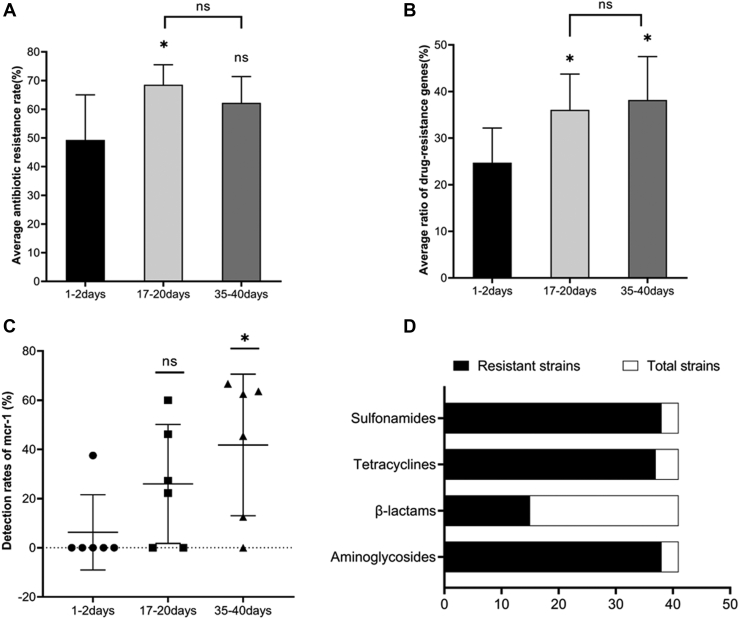
The average drug resistance among the 3 fattening stages (Figure 3A) differed significantly (P = 0.028). During the fattening cycle, the average antibiotic resistance rate increased and then decreased, with the antibiotic resistance rate for the early fattening stage (first sampling) being significantly lower (P = 0.010) than that for the middle (second sampling) stage. The average resistance rate for the terminal stage (third sampling) was slightly higher (P > 0.05) than that for the early stage, but tended to be lower (P > 0.05) than that for the middle stage. As can be seen in Figure 2C, there were highly statistically significant differences in the resistance to the individual antibiotic agents (P < 0.01). However, the antibiotic resistance rate of the E. coli isolates from the first sampling was generally lower than those from the second and the third samplings, with strains isolated during the middle stage having the highest resistance rate, which was consistent with the results illustrated in Figure 3A. In particular, the resistance rates for ciprofloxacin, gentamicin, ofloxacin , AK, and enoxacin were significantly lower in the early stage than in middle and later stages (P < 0.001, P < 0.001, P < 0.001, P = 0.012, and P < 0.001, respectively).
Figure 3.
Detection rates of drug resistance and drug resistance genes at 3 stages. (A) Changes in the average antibiotic resistance rate of Escherichia coli isolated from samples from broiler chicken farms at 3 stages during a single fattening cycle. Statistical significance is indicated by an asterisk (∗P < 0.05, ns: P ≥ 0.05). (B) Changes in the average detection rate of drug resistance genes in E. coli isolated from samples from broiler chicken farms at 3 stages during a single fattening cycle. Statistical significance is indicated by an asterisk (∗P < 0.05, ns: P ≥ 0.05). (C) Changes in the detection rate of mcr-1 gene in E. coli isolated from samples from broiler chicken farms at 3 stages during a single fattening cycle. Statistical significance is indicated by an asterisk (∗P < 0.05, ns: P ≥ 0.05). (D) Antibiotic resistance genes were found to coexist with the mcr-1 gene in E. coli isolated from samples from broiler chicken farms. Abbreviations: mcr-1, mobile colistin resistance; ns, not significant.
A total of 91% (153/168) of the tested E. coli isolates were resistant to at least 3 antibiotics. The lowest MDR rate was 63%, in the Binzhou area (farm 5), whereas the highest was 100%, in the Heze (farm 6) and Liaocheng (farm 3) areas (Table 3). The greatest extent of MDR was for 6 of the 9 tested antibiotics, with this being found in 29% (49/168) of the isolates. The majority of the MDR isolates were resistant to 5 to 7 of the antibiotics, with these accounting for 76% (116/153) of the MDR strains. The difference in the MDR rate among the 3 fattening stages was highly significant (P < 0.01), with the prevalence of MDR from the first sampling being lower than that from the second (P < 0.01) and third (P = 0.021) samplings (Table 4).
Table 4.
MDR rates of 168 Escherichia coli strains isolated from samples collected from broiler chicken farms at 3 stages during a single fattening cycle.
| Age of chickens at samplings (d) | Total number of tested isolates | Number of antimicrobial classes1 |
MDR rate (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| 1–2 | 59 | 0 | 4 | 8 | 9 | 8 | 13 | 8 | 9 | 0 | 79.66a |
| 17–20 | 55 | 0 | 0 | 0 | 1 | 2 | 7 | 19 | 21 | 5 | 100.00b |
| 35–40 | 54 | 1 | 0 | 2 | 3 | 3 | 9 | 22 | 8 | 6 | 94.44b |
a,bValues that share no common superscript letters differ significantly (P < 0.05).
Abbreviation: MDR, multidrug resistance.
Bold numbers represent MDR isolates, as classified as isolates resistant to 3 to 8 of the tested antibiotics.
A total of 40 different antibiotic resistance profiles were detected on the 6 farms, including 16 main drug resistance profiles (more than 3 drug-resistant bacteria) and 9 dominant drug resistance profiles (more than 5 drug-resistant bacteria). The majority of the isolated strains (68%, 114/168) possessed dominant drug resistance profiles (Figure 2B).
Prevalence of Antibiotic Resistance Genes
A number of antibiotic resistance genes were identified among the 168 E. coli isolates, including β-lactam resistance genes (blaTEM and blaCTX-M), aminoglycoside resistance genes (aadA, aacC4, aacC2, and aphA3), tetracycline resistance genes (tetA and tetB), and sulfonamide resistance genes (sul1, sul2, and sul3). The highest detection rate was for aadA (80%), and the lowest for blaSHV (1%). Forty-one of the 168 E. coli isolates (24%) contained the polymyxin resistance gene, mcr-1 (Supplementary Table 3).
As illustrated in Figure 3B, the average detection rate of drug resistance genes tended to increase through the course of the fattening cycle, with the rates recorded for the 3 stages differing significantly (P = 0.026). The average detection rates for the middle and terminal stages were significantly higher than that for the early stage (P = 0.030 and P = 0.012, respectively), but no significant increase was observed from the middle to the terminal fattening stage (P > 0.05). The results for the average detection rate, as illustrated in Figure 3B, reflected the differences in the individual detection rates of sul1, sul3, aacC4, aphA3, and mcr-1, which were significantly lower (P < 0.05) for the early fattening stage than for the middle and late stages (Supplementary Table 3). Especially regarding the mcr-1 gene, most farms (5/6) have zero detection rate for the first time (the early fattening stage) (Figure 3C). According to the sampling time, the detection rates of mcr-1 gene were 6, 26, and 42%, respectively. Overall, 93% (38/41) of the mcr-1-positive isolates contained an aminoglycoside resistance gene, 37% (15/41) contained a β-lactamase resistance gene, 90% (37/41) contained a tetracycline resistance gene, and 93% (38/41) contained a sulfonamide resistance gene (Figure 3D).
Discussion
The broiler farms selected in this study are distributed in 6 different prefecture-level cities in Shandong Province. Cobb, Arbor Acres, and 817 broiler are the main broiler breeds raised in these broiler farms. In this study, the average isolation rate of E. coli from samples collected on broiler farms in Shandong Province was 53%, but this differed among the regions tested. The detection rate for farm 6 was as high as 71%, whereas farm 3 had the lowest rate, at 44% (Table 3). As we all know, good biosecurity procedures are crucial for broiler production (de Castro Burbarelli et al., 2017; Van Limbergen et al., 2018). The biosecurity measures and rearing environments of these broiler farms were different. Among them, farm 6 and farm 1's buildings are relatively old, and the surrounding environments are poor. The traditional breeding model is applied, with chickens standing on the net. The environmental sanitation of farm 1 and field 6 is relatively poor; the air smells of ammonia and the ventilation is not good. It cannot be disinfected every 1 to 2 d. Broiler manure is not cleaned out throughout the breeding cycle. In contrast, farms 2 to 5 were built within the past 3 yr, use chicken cages and automated equipment, and have adopted good environmental sanitation programs. This is especially the case for farm 5, which was only established and put into use in 2019. According to the data, the detection rate of E. coli in the environment was the lowest on farm 5, at 21%, suggesting that the environment and the operating age of the farm may have some influence on the prevalence of bacteria. More importantly, the MDR rate of the E. coli isolated from samples from farm 5 was 63% (Table 3), which was significantly lower than for the other farms (P < 0.01). Furthermore, the E. coli from farm 5 had lower resistance rates for 8 of the antibiotic types tested (Figure 2A). Some studies have reported that animal feces and farm dust were important reservoirs for resistant bacteria and antibiotic resistance genes (Furtula et al., 2010; Chuppava et al., 2019; Luiken et al., 2019, 2020), and that antibiotic-resistant bacteria can spread antibiotic resistance to the environment through animal manure. This suggests that there is an accumulation of resistance in response to the long-term use of antibiotics, leading to a higher prevalence of drug-resistant microbes on older farms than on newer ones; however, other environmental factors cannot be discounted.
E. coli was found to be widely prevalent in the feces samples, with a detection rate of 60% (60/100) (Table 2). This suggests that when animal manure reaches farmlands, it aggravates the antibiotic resistance of soil microorganisms, and that sewage discharged from farms also pollutes rivers. These provide potential routes for the transmission of antibiotic-resistant bacteria from poultry to the environment, and then to humans (Yang et al., 2016; Chuppava et al., 2019). This finding should therefore be taken seriously, as the appropriate treatment and discharge of manure and sewage from farms will be beneficial for the improvement of both the rearing environment and public health. Large amounts of antibiotic residues are released into the environment through urine and feces, and this can induce bacteria to develop antibiotic resistance genes (Zhang et al., 2015). From this perspective, animal excrement is an important indicator for monitoring bacterial resistance, and further research on bacterial resistance in animal feces is needed.
Our data showed that 82% (288/353) of the isolated E. coli came from the chickens (cloacal swab), and even in the 1- to 2-day-old chicks the isolation rate reached 73% (Table 2). It may be that the chicks had already been exposed to E. coli during the hatching stage on the breeding farm, which suggests the vertical transmission of E. coli. Many studies have described the vertical spread of E. coli, with Zhao et al. (2019) reporting that chicks on breeding farms harbored weakly pathogenic E. coli strains, and concluding that the vertical spread of bacteria may occur between chickens belonging to the same species. Dame-Korevaar et al. (2019) found extended-spectrum β-lactamase (ESBL)/plasmid-mediated AmpC-producing E. coli on chicks on their arrival at broiler rearing farms, and suggested that effective bacterial control measures in the hatchery were necessary to reduce the spread of resistant bacteria. Similarly, the data described by Laube et al. (2013) indicated a high occurrence of ESBL/plasmid-mediated AmpC-producing E. coli in 1-day-old broilers, and Poulsen et al. (2017) found that salpingitis causing E. coli were transmitted vertically from parents to chicks, and were responsible for a large number of chick mortalities within the first week of life. The vertical transmission of bacteria to chicks from their parents may be one of the reasons for the high detection rate of E. coli on broilers in this study. It may also be responsible for the high MDR rate found during the early fattening stage, which was in some cases as high as 80% (Table 4), as well as for the high average drug resistance rate and high average detection rate of drug resistance genes during the early fattening stage (Figures 3A and 3B).
One important cause of bacterial resistance to antibiotics is the presence of related resistance genes, and many studies have shown that MDR is associated with bacteria harboring resistance genes (Song et al., 2020). As can be seen in Figures 3A and 3B, the average drug resistance rate and the average detection rate of drug resistance genes both showed increasing trends during a single fattening cycle. This may have been due to the horizontal transmission of drug resistance genes, which can be transferred to animals or to bacteria in the environment via plasmids, transposons, and bacteriophages. Zhuge et al. (2019) studied avian-origin E. coli in eastern China from 2015 to 2017 using multiple-locus sequence typing, and reported that mcr-1 could be transmitted horizontally through avian-origin E. coli. An alternative possibility is that drug-resistant genes are transmitted vertically within a bacterial strain from one generation to the next, using the environment as a medium. However, some scientists believe that the horizontal transmission of drug resistance genes, such as through a contaminant or bacteria, is more important than vertical transmission (Oikarainen et al., 2019). Nevertheless, both transmission pathways may cause the drug resistance rate to increase gradually over time within a fattening cycle. It should also be noted that antibiotic resistance could be aggravated over time through the continuous use of antibiotics. The average drug resistance rate in the late fattening stage was lower than that in the middle stage (Figure 3A), which may have largely been due to the reduction of antibiotic use during the late fattening stage, as required to prevent the occurrence of drug residues in the meat.
Colistin is a last resort for the treatment of infections caused by MDR gram-negative bacteria (Johura et al., 2020), and in recent years, E. coli strains carrying the mcr-1 gene, which confers resistance to colistin, have become widespread in the poultry industry. In this study, the detection rate was very low; in fact, only 1 farm detected the mcr-1 gene at the early fattening stage. The detection rates of mcr-1 increased with time, while the detection rate at last fattening stage increased significantly (P < 0.05). This reminds us that chicks in breeding farms upstream of the industrial chain are clean, and horizontal transmission plays a major role in the pollution of mcr-1 in the fattening farm. Furthermore, mcr-1 tends to coexist with a variety of other resistance genes, such as blaTEM, blaCTX-M, and qnrA (Zhao et al., 2020). In this study, we found that many of the isolates carried multiple antibiotic resistance genes, including ESBL genes, which will probably lead to the emergence of pan-drug-resistant strains. The coexistence of mcr-1 and other antibiotic resistance genes makes the treatment of infections caused by these MDR isolates more difficult (Song et al., 2020). Furthermore, the widespread transmission of MDR E. coli in food animals, especially in broilers, represents an issue of food safety, and poses a major threat to public health.
Conclusions
During the entire broiler fattening cycle, antimicrobial resistance and drug resistance genes in E. coli were least common in the early fattening stage. Antibiotic resistance was common in E. coli present in poultry farms in Shandong Province, most likely as a result of contaminated farm environments and the extensive use of antibiotics. The monitoring and treatment of drug-resistant bacteria in the poultry industry will be a long and difficult task, and one which will require a collaborative effort and should include aspects of chick breeding, the breeding environment, management, and feed additives. The rational use of antibiotics, in conjunction with the improvement of the breeding environment, and determination of other effective antibacterial methods will be helpful in the development of the poultry industry and the protection of public health.
Acknowledgments
This work was supported by the Fund of Shandong Agricultural Major Application Technology Innovation (SD2019XM009), the Key Research and Development Program of Shandong Province (Important Science and Technology Innovation Project, 2019JZZY010735), Collaborative Innovation Program of Shandong Higher Education Institutions (SDE [2017] 11), and the Funds of Shandong “Double Tops” Program (SYL2017YSTD11). We thank Megan North, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript. The study protocol and the poultry studies were approved by the Animal Care and Use Committee of Shandong Agricultural University, Tai'an, China.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.psj.2020.11.064.
Disclosures
No conflict of interest exits in the submission of this manuscript, and the manuscript is approved by all authors for publication.
Supplementary data
References
- Al Salah D.M.M., Laffite A., Pote J. Occurrence of bacterial Markers and antibiotic resistance genes in Sub-Saharan Rivers Receiving animal farm Wastewaters. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-51421-4. 14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerlin P., Travis R., Gyles C.L., Reid-Smith R., Janecko N., Lim H., Nicholson V., McEwen S.A., Friendship R., Archambault M. Antimicrobial resistance and virulence genes of Escherichia coli isolates from swine in Ontario. Appl. Environ. Microbiol. 2005;71:6753–6761. doi: 10.1128/AEM.71.11.6753-6761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuppava B., Keller B., Abd El-Wahab A., Surie C., Visscher C. Resistance reservoirs and multi-drug resistance of commensal Escherichia coli from Excreta and manure isolated in broiler houses with different Flooring designs. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02633. 2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa D., Poeta P., Saenz Y., Coelho A.C., Matos M., Vinue L., Rodrigues J., Torres C. Prevalence of antimicrobial resistance and resistance genes in faecal Escherichia coli isolates recovered from healthy pets. Vet. Microbiol. 2008;127:97–105. doi: 10.1016/j.vetmic.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Cyoia P.S., Koga V.L., Nishio E.K., Houle S., Dozois C.M., de Brito K.C.T., de Brito B.G., Nakazato G., Kobayashi R.K.T. Distribution of ExPEC virulence factors, blaCTX-M, fosA3, and mcr-1 in Escherichia coli isolated from Commercialized chicken Carcasses. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.03254. 3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame-Korevaar A., Fischer E.A.J., van der Goot J., Velkers F., van den Broek J., Veldman K., Ceccarelli D., Mevius D., Stegeman A. Effect of challenge dose of plasmid-mediated extended-spectrum beta-lactamase and AmpC beta-lactamase producing Escherichia coli on time-until-colonization and level of excretion in young broilers. Vet. Microbiol. 2019;239 doi: 10.1016/j.vetmic.2019.108446. 108446. [DOI] [PubMed] [Google Scholar]
- de Castro Burbarelli M.F., do Valle Polycarpo G., Deliberali Lelis K., Granghelli C.A., Carão de Pinho A.C., Ribeiro Almeida Queiroz S., Fernandes A.M., Moro de Souza R.L., Gaglianone Moro M.E., de Andrade Bordin R., de Albuquerque R. Cleaning and disinfection programs against Campylobacter jejuni for broiler chickens: productive performance, microbiological assessment and characterization. Poult. Sci. 2017;96:3188–3198. doi: 10.3382/ps/pex153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H., Chai T., Muller W., Zucker B.A. Concentration of airborne endotoxins and airborne bacteria in Chinese rabbit houses. Berl. Munch. Tierarztl. Wochenschr. 2006;119:40–44. [PubMed] [Google Scholar]
- Eltai N.O., Al Thani A.A., Al Hadidi S.H., Al Ansari K., Yassine H.M. Antibiotic resistance and virulence patterns of pathogenic Escherichia coli strains associated with acute gastroenteritis among children in Qatar. BMC Microbiol. 2020;20:54. doi: 10.1186/s12866-020-01732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtula V., Farrell E.G., Diarrassouba F., Rempel H., Pritchard J., Diarra M.S. Veterinary pharmaceuticals and antibiotic resistance of Escherichia coli isolates in poultry litter from commercial farms and controlled feeding trials. Poult. Sci. 2010;89:180–188. doi: 10.3382/ps.2009-00198. [DOI] [PubMed] [Google Scholar]
- Gao Q., Su S., Li X., Wang H., Liu J., Gao S. Transcriptional analysis of RstA/RstB in avian pathogenic Escherichia coli identifies its role in the regulation of hdeD-mediated virulence and survival in chicken macrophages. Vet. Microbiol. 2020;241 doi: 10.1016/j.vetmic.2019.108555. 108555. [DOI] [PubMed] [Google Scholar]
- Guillaume G., Verbrugge D., Chasseur-Libotte M., Moens W., Collard J. PCR typing of tetracycline resistance determinants (Tet A-E) in Salmonella enterica serotype Hadar and in the microbial community of activated sludges from hospital and urban wastewater treatment facilities in Belgium. FEMS. Microbiol. Ecol. 2000;32:77–85. doi: 10.1111/j.1574-6941.2000.tb00701.x. [DOI] [PubMed] [Google Scholar]
- Johura F.T., Tasnim J., Barman I., Biswas S.R., Jubyda F.T., Sultana M., George C.M., Camilli A., Seed K.D., Ahmed N., Alam M. Colistin-resistant Escherichia coli carrying mcr-1 in food, water, hand rinse, and healthy human gut in Bangladesh. Gut. Pathogens. 2020;12:5. doi: 10.1186/s13099-020-0345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadykalo S.V., Anderson M.E.C., Alsop J.E. Passive surveillance of antimicrobial resistance in Salmonella and Escherichia coli isolates from Ontario livestock, 2007-2015. Can. Vet. J. 2018;59:617–622. [PMC free article] [PubMed] [Google Scholar]
- Kayastha K., Dhungel B., Karki S., Adhikari B., Banjara M.R., Rijal K.R., Ghimire P. Extended-Spectrum beta-lactamase-producing Escherichia coli and Klebsiella species in Pediatric Patients Visiting International Friendship Children's hospital, Kathmandu, Nepal. Infect. Dis. (Auckl). 2020;13 doi: 10.1177/1178633720909798. 1178633720909798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.B., Yoon M.Y., Ha J.S., Seo K.W., Noh E.B., Son S.H., Lee Y.J. Molecular characterization of avian pathogenic Escherichia coli from broiler chickens with colibacillosis. Poult. Sci. 2020;99:1088–1095. doi: 10.1016/j.psj.2019.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube H., Friese A., von Salviati C., Guerra B., Kasbohrer A., Kreienbrock L., Roesler U. Longitudinal monitoring of extended-spectrum-beta-lactamase/AmpC-producing Escherichia coli at German broiler chicken fattening farms. Appl. Environ. Microbiol. 2013;79:4815–4820. doi: 10.1128/AEM.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.Z., Li L., Yu L.P., Liu S., Liu L.J., Wei X.T., Song Y.Y., Liu C., Jiang M.J., Wang F.K. The prevalence of avian-origin mcr-1-positive Escherichia coli with a potential transmission Risk to humans in Tai'an, China. Poult. Sci. 2020;99:5118–5126. doi: 10.1016/j.psj.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., Yu L.F., Gu D., Ren H., Chen X., Lv L., He D., Zhou H., Liang Z., Liu J.H., Shen J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- Luiken R.E.C., Van Gompel L., Munk P., Sarrazin S., Joosten P., Dorado-García A., Borup Hansen R., Knudsen B.E., Bossers A., Wagenaar J.A., Aarestrup F.M., Dewulf J., Mevius D.J., Heederik D.J.J., Smit L.A.M., Schmitt H., consortium E. Associations between antimicrobial use and the faecal resistome on broiler farms from nine European countries. J. Antimicrob. Chemother. 2019;74:2596–2604. doi: 10.1093/jac/dkz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiken R.E.C., Van Gompel L., Bossers A., Munk P., Joosten P., Hansen R.B., Knudsen B.E., García-Cobos S., Dewulf J., Aarestrup F.M., Wagenaar J.A., Smit L.A.M., Mevius D.J., Heederik D.J.J., Schmitt H. Farm dust resistomes and bacterial microbiomes in European poultry and pig farms. Environ. Int. 2020;143 doi: 10.1016/j.envint.2020.105971. 105971. [DOI] [PubMed] [Google Scholar]
- Meguenni N., Chanteloup N., Tourtereau A., Ahmed C.A., Bounar-Kechih S., Schouler C. Virulence and antibiotic resistance profile of avian Escherichia coli strains isolated from colibacillosis lesions in central of Algeria. Vet. World. 2019;12:1840–1848. doi: 10.14202/vetworld.2019.1840-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellata M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013;10:916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoro-Dasi L., Villagra A., Sevilla-Navarro S., Perez-Gracia M.T., Vega S., Marin C. The dynamic of antibiotic resistance in commensal Escherichia coli throughout the growing period in broiler chickens: fast-growing vs. slow-growing breeds. Poult. Sci. 2020;99:1591–1597. doi: 10.1016/j.psj.2019.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikarainen P.E., Pohjola L.K., Pietola E.S., Heikinheimo A. Direct vertical transmission of ESBL/pAmpC-producing Escherichia coli limited in poultry production pyramid. Vet. Microbiol. 2019;231:100–106. doi: 10.1016/j.vetmic.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Poirel L., Jayol A., Nordmann P. Polymyxins: antibacterial Activity, susceptibility testing, and resistance Mechanisms Encoded by plasmids or Chromosomes. Clin. Microbiol. Rev. 2017;30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen L.L., Thofner I., Bisgaard M., Christensen J.P., Olsen R.H., Christensen H. Longitudinal study of transmission of Escherichia coli from broiler breeders to broilers. Vet. Microbiol. 2017;207:13–18. doi: 10.1016/j.vetmic.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Roth N., Hofacre C., Zitz U., Mathis G.F., Moder K., Doupovec B., Berghouse R., Domig K.J. Prevalence of antibiotic-resistant E. coli in broilers challenged with a multi-resistant E. coli strain and received ampicillin, an organic acid-based feed additive or a synbiotic preparation. Poult. Sci. 2019;98:2598–2607. doi: 10.3382/ps/pez004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth N., Käsbohrer A., Mayrhofer S., Zitz U., Hofacre C., Domig K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: a global overview. Poult. Sci. 2019;98:1791–1804. doi: 10.3382/ps/pey539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliu E.M., Vahjen W., Zentek J. Types and prevalence of extended-spectrum beta-lactamase producing Enterobacteriaceae in poultry. Anim. Health Res. Rev. 2017;18:46–57. doi: 10.1017/S1466252317000020. [DOI] [PubMed] [Google Scholar]
- Song Y.Y., Yu L.P., Zhang Y., Dai Y., Wang P., Feng C.L., Liu M.D., Sun S.H., Xie Z.J., Wang F.K. Prevalence and characteristics of multidrug-resistant mcr-1-positive Escherichia coli isolates from broiler chickens in Tai'an, China. Poult. Sci. 2020;99:1117–1123. doi: 10.1016/j.psj.2019.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G.X., Wang H.N., Zhang A.Y., Zhang Y., Yang X., Xu C.W. Detection of resistance to β-lactams and characterization of extended-spectrum lactamases in Escherichia coli isolates from swine. Chin. J. Prevent. Vet. Medic. 2011;33:776–780. (in Chinese) [Google Scholar]
- Van Limbergen T., Dewulf J., Klinkenberg M., Ducatelle R., Gelaude P., Méndez J., Heinola K., Papasolomontos S., Szeleszczuk P., Maes D. Scoring biosecurity in European conventional broiler production. Poult. Sci. 2018;97:74–83. doi: 10.3382/ps/pex296. [DOI] [PubMed] [Google Scholar]
- Vounba P., Arsenault J., Bada-Alambedji R., Fairbrother J.M. Prevalence of antimicrobial resistance and potential pathogenicity, and possible spread of third generation cephalosporin resistance, in Escherichia coli isolated from healthy chicken farms in the region of Dakar, Senegal. PLoS One. 2019;14 doi: 10.1371/journal.pone.0214304. e0214304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.M., Liao X.P., Zhang W.J., Jiang H.X., Sun J., Zhang M.J., He X.F., Lao D.X., Liu Y.H. Prevalence of serogroups, virulence genotypes, antimicrobial resistance, and phylogenetic background of avian pathogenic Escherichia coli in south of China. Foodborne Pathog. Dis. 2010;7:1099–1106. doi: 10.1089/fpd.2010.0542. [DOI] [PubMed] [Google Scholar]
- Wu B., Duan H., Qi Q., Cai Y., Zhong Z., Chai T. Identifying virulence factor genes in E. coli in animal houses and their transmission to outside environments. J. Aerosol Sci. 2018;117:189–199. [Google Scholar]
- Yang Y.Q., Li Y.X., Song T., Yang Y.X., Jiang W., Zhang A.Y., Guo X.Y., Liu B.H., Wang Y.X., Lei C.W., Xiang R., Wang H.N. Colistin resistance gene mcr-1 and its variant in Escherichia coli isolates from chickens in China. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.01204-16. e01204-e01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Zhang H., Guo Y., Tian T. Influence of chicken manure Fertilization on antibiotic-resistant bacteria in soil and the Endophytic bacteria of Pakchoi. Int. J. Environ. Res. Public Health. 2016;13:662. doi: 10.3390/ijerph13070662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.Q., Ying G.G., Pan C.G., Liu Y.S., Zhao J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015;49:6772–6782. doi: 10.1021/acs.est.5b00729. [DOI] [PubMed] [Google Scholar]
- Zhao X., Liu Z., Zhang Y., Yuan X., Hu M., Liu Y. Prevalence and molecular characteristics of avian-origin mcr-1-harboring Escherichia coli in Shandong province, China. Front. Microbiol. 2020;11:255. doi: 10.3389/fmicb.2020.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Wang C.L., Chang S.K., Tsai Y.L., Chou C.H. Characterization of Escherichia coli isolated from day-old chicken Fluff in Taiwanese Hatcheries. Avian Dis. 2019;63:9–16. doi: 10.1637/11935-072318-Reg.1. [DOI] [PubMed] [Google Scholar]
- Zhuge X., Ji Y., Tang F., Sun Y., Jiang M., Hu W., Wu Y., Xue F., Ren J., Zhu W., Dai J. Population structure and antimicrobial resistance traits of avian-origin mcr-1-positive Escherichia coli in Eastern China, 2015 to 2017. Transbound. Emerg. Dis. 2019;66:1920–1929. doi: 10.1111/tbed.13222. [DOI] [PubMed] [Google Scholar]
- Zucker B.A., Trojan S., Muller W. Airborne gram-negative bacterial flora in animal houses. J. Vet. Med. B. Infect. Dis. Vet. Public Health. 2000;47:37–46. doi: 10.1046/j.1439-0450.2000.00308.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.