Abstract
The primary cause of necrotic enteritis (NE) disease in chickens is the NetB-positive Clostridium perfringens bacterium. Many factors are known to affect the severity of NE in the challenge models of broiler chickens, and one of these factors is the virulence of C. perfringens strain. This study was conducted to evaluate the effect of 2 pathogenic C. perfringens strains in a NE challenge model on gut health and mRNA expression of genes encoding apoptosis, tight junction, immunity, and nutrient transporters in broilers. Day-old Ross-308 male broilers (n = 468) were allocated in a 2 × 3 factorial arrangement of treatments with in-feed antibiotics (no or yes) and challenge (Non, C. perfringens strain NE18, and C. perfringens strain NE36) as the factors. The birds in the challenged groups were inoculated with Eimeria species on day 9 and with a fresh suspension of C. perfringens NE18 or NE36 on day 14 and 15. Sample collection was performed on 2 birds of each pen on day 16. Necrotic enteritis challenge, impaired feed conversion ratio during day 0 to 16 compared with the control group where the effect of the NE36 challenge was more severe than that with NE18 (P < 0.001). The mRNA expression of mucin-2, immunoglobulin-G, occludin (P < 0.001), and tight junction protein-1 (P < 0.05) genes were downregulated in both challenged groups compared with the nonchallenged counterparts. Antibiotic supplementation, on the other hand, increased weight gain, and feed intake in all challenged birds (P < 0.01), but upregulated mucin-5ac and alanine, serine, cysteine, and threonine transporter-1 (P < 0.05) only in the NE18 challenged birds. The challenge with NE36 significantly upregulated caspase-8 and claudin-1 (P < 0.001), but downregulated glucose transporter-2 (P < 0.001) compared with the NE18 challenge. These results suggest that NE challenge is detrimental to the performance of broilers through compromised intestinal health, and different C. perfringens strains can affect the severity of the disease through modulating the expression of intestinal genes encoding proteins responsible for apoptosis, gut integrity, immunity, mucus production, and nutrient transporters.
Key words: necrotic enteritis, Clostridium perfringens, virulence, strains, gene expression, broiler
Introduction
Clostridium perfringens is known to be the causative agent of necrotic enteritis (NE) in chickens. This gram-positive anaerobic bacterium is a resident in the intestinal tract of both humans and livestock and usually does not cause disease (Cooper and Songer, 2010). However, with the removal of in-feed antibiotics from broiler diets, there has been an immense increase in NE incidence in poultry farms in Europe and the Unites States (McDevitt et al., 2006). The cost of NE worldwide has been estimated to be 6 billion dollars annually, which includes not only direct loss due to broiler mortality but also reduced performance and management costs (Wade and Keyburn, 2015). The proliferation of C. perfringens along with one or more predisposing factors in the gut mediates the disease by the production of, mostly, extracellular protein toxins (Craven, 2000).
Recently, numerous developments have been made for understanding the pathogenesis of necrotic enteritis in broilers (Kaldhusdal et al., 2016). In the presence of predisposing factors, the strains that produce the pore-forming NetB toxin can lead to the production of necrotic enteritis, as this toxin is essential for the disease to occur when predisposing factors are present (Rood et al., 2018). Recent reports have shown that the pathogenesis and virulence of C. perfringens are also controlled by other genes or loci (Coursodon et al., 2012; Zhou et al., 2017) and these factors could contribute to the different severity levels of the NE disease.
The importance of the intestinal tract and its critical role in nutrient absorption and immune responses is evident (Lan et al., 2005). The mucosal barrier mechanism in the small intestine serves as the first line of defense and can maintain an essential barrier to microbial invasion and protect the intestinal epithelial cells (Elphick and Mahida, 2005). Mucin proteins, such as mucin-2 (MUC2) and mucin-5ac (MUC5ac) maintain a suitable mucous layer as this layer is continually sloughed off by intestinal movements of microbial-derived factors (Horn et al., 2009). Immunoglobulin proteins such as immunoglobulin-M (IgM) and immunoglobulin-G (IgG) are present in the enterocyte brush border and are delivered to the mucus layer to participate in immune responses and clearance of antigens (Hansen et al., 2005). Furthermore, intestinal absorbing epithelial cells are strongly connected by tight junction (TJ) proteins such as claudin-1 (CLDN1), occludin (OCLN), and tight junction protein-1 (TJP1). The function of these proteins is necessary for controlling permeability of the paracellular pathways (Furuse et al., 2002; Elkouby-Naor and Ben-Yosef, 2010). On the other hand, although apoptosis (cell death) usually occurs during development and aging, it can also happen in defense mechanisms such as cell damage caused by disease or toxic agents (Norbury and Hickson, 2001). It is known that activation of caspase family correlates with the onset of apoptosis and cell death (Cohen, 1997). Brush border enzymes, Na+-dependent neutral amino acid transporters, Na+-dependent neutral/cationic amino acid exchanger and, glucose transporter-2 (GLUT2) in the intestinal epithelium are closely associated with intestinal nutrient absorption capacity (Uldry et al., 2002; Hediger et al., 2004; Fotiadis et al., 2013).
Brush border enzymes include aminopeptidase N (APN) and sucrase-isomaltase (SI); Na+-dependent neutral amino acid transporters include B0AT and alanine, serine, cysteine, and threonine transporter-1 (ASCT1), and Na+-dependent neutral/cationic amino acid exchangers include Y + L amino acid transporter-1 (y+LAT1) and Y + L amino acid transporter-2 (y+LAT2).
The effect of the 2 C. perfringens strains, ERE-NE18 (NE18) and WER-NE36 (NE36), on broiler performance, gut microbiota, and intestinal short-chain fatty acid concentrations have been previously reported. Gharib-Naseri et al. (2019) observed a greater negative impact of NE36 challenge on the performance and microbiota profile of broilers compared with NE18 strain. Wade et al. (2015) reported that these 2 strains may have differences in their ability to bind to specific collagens in the extracellular matrix. The expression level of intestinal genes was examined in the present study to determine the changes generated in the intestinal tissue of broilers infected with NE at molecular level. We hypothesized that NE challenge is detrimental for the chickens on their performance through compromised gut health such as intestinal gut integrity, morphology, and regulation of genes coding–related proteins, which may underlie the mechanism of infection by causative agent C. perfringens together with the predisposing factors. In addition, the strains of C. perfringens used in the challenge may play important role in the severity of the infection.
Materials and methods
The following experimental protocol was approved by the Animal Ethics Committee (authority no.: AEC17-024) of the University of New England, Armidale, NSW, 2351, Australia. The protocol was carried out in accordance with the guidelines specified in the Australian Code for the Care and Use of Animals for Scientific Purposes eighth edition 2013. Briefly, Ross-308 were reared on floor pens with feed and water were provided ad libitum for chickens (Gharib-Naseri et al., 2019). Lighting, relative humidity, and the temperature were set in accordance with Ross-308 strain guidelines (Aviagen, 2014).
Experimental Design and Diets
A total of 468 day-old male Ross-308 chickens were obtained from Baiada hatchery in Tamworth, NSW, Australia. On arrival, all birds were weighed and assigned to 36-floor pens with 13 birds in each pen. This experiment compromised 6 treatment groups and was designed as a 2 × 3 factorial arrangement of treatments with antibiotics (no or yes) and challenge (Non, C. perfringens strain NE18, or C. perfringens strain NE36). The control diet (no additive) and a diet supplemented with the antibiotics salinomycin sodium (72 ppm active, Sacox Huvepharma, Sydney, Australia) and zinc bacitracin (50 ppm active, Albac 150, Pfizer Australia Pty Ltd., Sydney, NSW, Australia). Antibiotics were added in diets from the first day of the experiment. All diets were cold-pelleted (65°C–70°C) and chickens were fed starter diets from day 0 to 10, and grower diets from day 11 until the birds were sampled at day 16.
Necrotic Enteritis Challenge
All chickens in both NE challenge groups underwent a series of inoculations to induce NE challenge. The University of New England NE challenge procedure (Rodgers et al., 2015) was followed to introduce subclinical NE disease. In brief, all challenged birds were orally gavaged with 1 mL/bird field Eimeria strains (Eimeria acervulina 5,000 oocytes/mL, Eimeria maxima 5,000 oocytes/mL, Eimeria brunetti 2,500 oocytes/mL) on day 9; chickens in the nonchallenged groups were inoculated with 1 mL of sterile PBS. Primary poultry isolates of the 2 C. perfringens strains (EHE-NE18 and WER-NE36) were obtained from CSIRO Livestock Industries, Geelong, Australia. The challenge inocula were freshly prepared by growing the bacterial strains separately in 100 mL of sterile thioglycolate (USP alternative, Oxoid, Australia) with added starch (10 g/L) and pancreatic digest of casein (5 g/L); this was incubated overnight at 39°C. Stock cultures of C. perfringens strains were later subcultured in thioglycolate broth followed by cooked meat media (Oxoid, Australia). Fresh inoculums of each strain containing approximately 108 CFU/mL C. perfringens were separately prepared and 1 mL of the inoculums were inoculated to the chickens in accordance with their challenge groups on day 14 and 15. Chickens in the nonchallenged groups were gavaged with sterile thioglycolate medium as a sham treatment.
Sample Collection
On day 16, 2 birds from each pen were randomly selected and euthanized to collect blood for the measurement of fluorescein isothiocyanate-dextran (FITC-d), and jejunal tissue for gene expression assay and histomorphological parameters. Blood collection was performed by electrical stunning the birds and immediately collecting blood from the jugular vein before decapitation. After dissecting chickens, 5 cm of the proximal jejunum tissue was excised. Approximately 2 cm of the tissue was separated, flushed with PBS (4°C) and collected in 2 mL Eppendorf tubes filled with RNA later (Qiagen, Germany) and kept at 4°C for 24 h, and then stored in −20°C until required. The rest section of the sample was flushed with PBS and kept in 10% buffered formalin until required for histology processing.
Detection of Fluorescein Isothiocyanate-Dextran in Serum
Two chosen birds from each pen were inoculated with 1 mL FITC-d (4.17 mg/kg bird, average molecular weight 4,000; Sigma–Aldrich Co., Australia), 2.5 h before euthanization and blood collection as described above (Vicuna et al., 2015). Blood samples were kept in room temperature for approximately 3 h followed by 15-min centrifugation at 1,000 × g to separate red blood cells from serum. The supernatant serum samples were collected and fluorescent levels in serum samples were measured with an excitation wavelength of 485 nm and an emission wavelength of 528 nm on a Synergy HT, Multimode microplate reader (SpectraMax M2e, Molecular Devices, CA) as explained by Kuttappan et al. (2015). Levels of fluorescence in the samples were converted to respective FITC-d microgram per milliliter of serum based on a calculated standard curve obtained from known levels of FITC-d.
Histology
Jejunum sections of sampled birds were fixed in formalin solution for at least 3 d. The fixed tissue samples were processed in an automated tissue processor (Thomas Optical and Scientific Co., Melbourne and Sydney, Australia) and embedded in paraffin (Leica EG 1160; Leica Microsystems, Bensheim, D-64625, Germany). The tissue sections were cut at 5 μm using a microtome (Leitz 1516; Leica Microsystems, Bensheim, D-64625, Germany), mounted on slides with DPX Mountant for histology (Aldrich Chemical Company, Inc., Milwaukee, WI 53255, MO). Staining and analysis of the samples were according to M'Sadeq et al. (2015).
RNA Extraction and cDNA Synthesis
Total RNA from each jejunal sample was extracted using TRIsureTM (Bioline, Sydney, Australia) following the manufacturer's instructions. All RNA samples were purified with the RNeasy Mini Kit (Qiagen, Hilden, Germany). The quantity and purity of the samples were measured with NanoDrop ND-8000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA), and integrity with the Agilent 2100 Bioanalyzer, 6000 Nano kit. (Agilent Technologies, Inc., Waldron, Germany). The samples were accepted as high quality if the value of 260/230 was >2.0, 260/280 value was between 2.0 and 2.2, and the RIN number of each sample was higher than 7. The extracted RNA of each sample was reverse transcribed with the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) as per the manufacturer's instructions. The Rotor-Gene 6000 real-time PCR machine (Corbett, Sydney, Australia) was used to convert the RNA into cDNA. The cDNA was diluted 10 times with nuclease-free water and stored at −20°C until required.
Real-Time Quantitative PCR
The primers used in this study were either sourced from literature or newly designed as shown in Table 1. All primers were analyzed specificity by PCR with a subpopulation of samples and fragments separated on Agilent 2100 Bioanalyzer using Agilent DNA 1000 Kit (Agilent Technologies, Inc., Germany). Quantitative PCR was performed in duplicates using an SYBR Green kit SensiFAST SYBR No-ROX (Bioline, Sydney, Australia) with Rotor-Gene 6000 real-time PCR machine (Corbett Research, Sydney, Australia). The PCR reaction was performed in a volume of 10 μL containing 5 μL of 2 × SensiFAST, 400 mM of each primer and 2 μL of 10 × diluted cDNA template. The relative quantity of mRNA of the target genes were calculated by qBase + version 3.0 (Biogazelle, Zwijnbeke, Belgium) software with YWHAZ and HMBS as reference genes that were optimized from 10 widely used housekeeping genes before the analysis of target genes. The qBase + applied an arithmetic mean method to transform logarithmic Cq value to linear relative quantity using the exponential function for relative quantification of target genes (Vandesompele et al., 2002; Hellemans et al., 2007). The output data were exported to SPSS statistical version 22 (IBM SPSS, UK) statistical software for further analysis.
Table 1.
Sequences of primers used for quantitative real-time PCR.
| Gene | Accession N° | Sequence | Size (pb) | Annealing T° | Reference |
|---|---|---|---|---|---|
| CASP1 | AF031351.1 | F-ACATATACCAGCCACGGGAGA R-CATTGTAGCCCAGCCCTTCT |
141 | 60 | This study |
| CASP2 | NM_001167701.1 | F-CAGCGATACCACCAGGAAGC R- GCTTCCAGACTTCGCCTGTATC |
144 | 60 | This study |
| CASP3 | NM_204725.1 | F-TGGTGGAGGTGGAGGAGC R- GTTTCTCTGTATCTTGAAGCACCA |
110 | 62 | This study |
| CASP6 | NM_204726.1 | F- AAGCCTCTCGGGATGACTACA R- TCACCTCGACATGCCTGAAT |
193 | 60 | This study |
| CASP8 | NM_204592.2 | F-GGAGCTGCTATCGGATCAAT R-GGAGCTGCTCTATCGGATCAAT |
126 | 60 | This study |
| CASP9 | XM_424580.5 | F- GGAATGAGGACGAGCCAGAC R- TGTCTGACACCCGAAGTAGCAT |
198 | 60 | This study |
| BCL2 | NM 205339 | F-CACCTGGATGACCGAGTACC R-GTCCAAGATAAGCGCCAAGA |
191 | 60 | (Zhao et al., 2013) |
| IgG | X07174.1 | F: ATCACGTCAAGGGATGCCCG R: ACCAGGCACCTCAGTTTGG |
118 | 60 | (Zhao et al., 2013) |
| IgM | X01613.1 | F: GCATCAGCGTCACCGAAAGC R: TCCGCACTCCATCCTCTTGC |
98 | 60 | (Zhao et al., 2013) |
| MUC2 | XM 001234581.3 | F- CCCTGGAAGTAGAGGTGACTG R- TGACAAGCCATTGAAGGACA |
143 | 60 | (Fan et al., 2015) |
| MUC5ac | XM 003641322.2 | F- AAGACGGCATTTATTTCTCCAC R- TCATTACCAACAAGCCAGTGA |
244 | 60 | (Fan et al., 2015) |
| OCLN | NM_205128.1 | F- ACGGCAGCACCTACCTCAA R- GGGCGAAGAAGCAGATGAG |
123 | 60 | (Du et al., 2016) |
| TJP1 | XM_413773.4 | F-GGATGTTTATTTGGGCGGC R-GTCACCGTGTGTTGTTCCCAT |
187 | 60 | (Zanu et al., 2020) |
| CLDN1 | NM_001013611.2 | F-CTTCATCATTGCAGGTCTGTCAG R-AAATCTGGTGTTAACGGGTGTG |
103 | 60 | (Zanu et al., 2020) |
| APN | NM_001013611.2 | F-AATACGCGCTCGAGAAAACC R-AGCGGGTACGCCGTGTT |
70 | 60 | (Gilbert et al., 2007) |
| ASCT1 | XM 001232899.4 | F-TTGGCCGGGAAGGAGAAG R-AGACCATAGTTGCCTCATTGAATG |
63 | 60 | (Paris and Wong, 2013) |
| b0,+AT | NM_001199133.1 | F-CAGTAGTGAATTCTCTGAGTGTGAAGCT R-GCAATGATTGCCACAACTACCA |
88 | 60 | (Gilbert et al., 2007) |
| B0AT | XM_419056.5 | F-GTGTTTGGAACCCTAAATACGAGG R-TAGCATAGACCCAGCCAGGA |
72 | 60 | (Kheravii et al., 2018) |
| GLUT2 | NM_207178.1 | F-TGATCGTGGCACTGATGGTT R-CCACCAGGAAGACGGAGATA |
171 | 60 | (Kheravii et al., 2018) |
| LAT1 | KT876067.1 | F-GATTGCAACGGGTGATGTGA R- CCCCACACCCACTTTTGTTT |
70 | 60 | (Gilbert et al., 2007) |
| ATP1A1 | NM_205521.1 | F-GTCAACCCGAGGGATGCTAA R-ACTGCTACAATGGCACCCTG |
179 | 60 | (Kheravii et al., 2018) |
| PepT2 | NM_001319028.1 | F-TGACTGGGCATCGGAACAA R-ACCCGTGTCACCATTTTAACCT |
63 | 60 | (Paris and Wong, 2013) |
| SI | XM_015291762.1 | F-GCTTTAAG↓ATGGGCAAGAGGAAG R- CCACCACCAGGCAAAAGAGG |
65 | 60 | (Kheravii et al., 2018) |
| y+LAT1 | XM_418326.5 | F-TACTGAGGCTGACTGGAGGAA R- ACGACGTACAGCACAAT↓ATCTGG |
227 | 62 | (Kheravii et al., 2018) |
| y+LAT2 | NM_001005832.1 | F-GCCCTGTCAGTAAATCAGACAAGA R-TTCAGTTGCATTGTGTTTTGGTT |
82 | 60 | (Gilbert et al., 2007) |
| HMBS | XM 417846.2 | F: GGCTGGGAGAATCGCATAGG R: TCCTGCAGGGCAGATACCAT |
131 | 60 | (Yin et al., 2011) |
| YWHAZ | NM_001031343.1 | F- TTGCTGCTGGAGATGACAAG R- CTTCTTGATACGCCTGTTG |
61 | 60 | (Bagés et al., 2015) |
The genes used for expression analysis in the jejunal tissue are as listed: caspase-1, 2, 3, 6, 8, and 9 (CASP1, CASP2, CASP3, CASP8, CASP9), B-cell lymphoma-2 (BCL2), IgG and IgM, MUC2 and MUC5ac, occludin (OCLN), TJP1, CLDN1, APN, ASCT1, b0,+amino acid transporter (b0,+AT), neutral amino acid transporter (B0AT), GLUT2, large neutral amino acid transporter-1 (LAT1), ATPase Na+/K+ transporting subunit alpha-1 (ATP1A1), peptide transporter-2 (PepT2), SI, y+LAT1, and y+LAT2.
Statistical Analysis
All the data derived were evaluated for normal distribution before statistical analyses. Data were analyzed in accordance with a 2 × 3 factorial arrangement of treatments, using the general linear model procedure of SPSS 24 package to assess the main effects of challenge and antibiotics, and their interactions. Tukeys' test was used to perform pairwise comparisons between means. Significant values were based on P < 0.05; P values between 0.051 and 0.10 were reported as a tendency of significance.
Results
Growth Performance
Table 2 shows the effect of 2 C. perfringens strains (NE18 and NE36) on the performance of birds from day 0 to16. An antibiotic × challenge interaction was observed for weight gain (P < 0.01) and feed intake (P < 0.01), where antibiotics only increased weight gain and feed intake in challenged birds. As expected, the FCR was impaired in both challenge groups with NE18 and NE36 (P < 0.001). In addition, birds challenged with the NE36 strain had higher FCR than those in the NE18 challenge group. No significant antibiotics × challenge interaction was observed for FCR.
Table 2.
Performance results of broiler chickens under necrotic enteritis (NE) challenge using 2 different strains of Clostridium perfringens (NE18 and NE36), from day 0 to 16.
| Challenge | Antibiotic1 | BW | FI | FCR |
|---|---|---|---|---|
| Non | No | 624a | 730a | 1.170 |
| Yes | 620a | 717a | 1.157 | |
| NE18 | No | 453d | 595b | 1.313 |
| Yes | 525b | 660b | 1.257 | |
| NE36 | No | 440d | 598c | 1.359 |
| Yes | 488c | 634c | 1.301 | |
| Main effects | ||||
| Antibiotic | No | 506 | 641 | 1.281a |
| Yes | 545 | 671 | 1.238b | |
| Challenge | Non | 622 | 724 | 1.163c |
| NE18 | 490 | 628 | 1.285b | |
| NE36 | 646 | 616 | 1.330a | |
| P-value | ||||
| Antibiotic | <0.001 | 0.004 | 0.001 | |
| Challenge | <0.001 | <0.001 | <0.001 | |
| Antibiotic × challenge | 0.002 | 0.008 | 0.225 | |
a,b,cMeans with different letters significantly differ on the basis on Tukeys' multiple tests (P < 0.05).
Abbreviations: BW, body weight gain (g/bird); FI, feed intake (g/bird); FCR, feed conversion ratio; Non, nonchallenged; NE18, C. perfringens strains ERE-NE18, 108 CFU/mL; NE36, C. perfringens strain WER-NE36, 108 CFU/mL.
Antibiotic: salinomycin (72 ppm) and zinc bacitracin (50 ppm).
Gut Integrity and Morphology
As shown in Table 3, gut integrity analysis showed higher FITC-d concentrations (P < 0. 001) in the serum of the birds challenged with NE compared with nonchallenged birds. Furthermore, a significant reduction in gut permeability (P < 0.05) was also observed in birds fed with antibiotics. Results of the villus height, crypt depth, and villus/crypt ratio of the jejunum at day 16 are presented in Table 3. Challenged birds showed lower villus height (P < 0.001), deeper crypt (P < 0.001), and lower villus height/crypt depth ratio (P < 0.001) than nonchallenged birds. No antibiotic × challenge interaction was observed for all the variables described. Figure 1 illustrates the histology of jejunum tissue in chickens.
Table 3.
Effect of necrotic enteritis (NE) challenge using 2 different strains of Clostridium perfringens (NE18 and NE36), on broiler chicken gut permeability and jejunal histomorphological parameters at day 16.
| Main effects | FITC-d | Intestinal histology |
||
|---|---|---|---|---|
| Height | Crypt | Height/crypt | ||
| Antibiotic1 | ||||
| No | 0.353a | 453 | 191 | 3.13 |
| Yes | 0.278b | 472 | 173 | 2.29 |
| Challenge | ||||
| Non | 0.199b | 754a | 121b | 6.28a |
| NE18 | 0.350a | 325b | 196a | 1.70b |
| NE36 | 0.406a | 310b | 228a | 1.41b |
| P-value | ||||
| Antibiotic1 | 0.013 | 0.169 | 0.256 | 0.894 |
| Challenge | 0.001 | 0.001 | 0.001 | 0.001 |
| Antibiotic × challenge | 0.581 | 0.432 | 0.338 | 0.969 |
a,bMeans with different letters significantly differ on the basis on Tukeys' multiple tests (P < 0.05).
Abbreviations: FITC-d, fluorescein isothiocyanate-dextran (ug/mL); Non, nonchallenged; NE18, C. perfringens strains ERE-NE18, 108 CFU/mL; NE36, C. perfringens strain WER-NE36, 108 CFU/mL.
Antibiotic: salinomycin (72 ppm) and zinc bacitracin (50 ppm).
Figure 1.
Effect of 2 different strains of Clostridium perfringens (NE18 and NE36) in a necrotic enteritis (NE) challenge model on jejunum histology at day 16. (A) Nonchallenged—no antibiotic; (B) NE challenged using NE18 strain—no antibiotic; (C) NE challenge using NE36 strain—no antibiotic; (D). Nonchallenged plus in feed antibiotics (salinomycin [72 ppm] and zinc bacitracin [50 ppm]); (E) NE challenged using NE18 strain plus in feed antibiotics (salinomycin [72 ppm] and zinc bacitracin [50 ppm]); and (F) NE challenge using NE36 strain plus in feed antibiotics (salinomycin [72 ppm] and zinc bacitracin [50 ppm]).
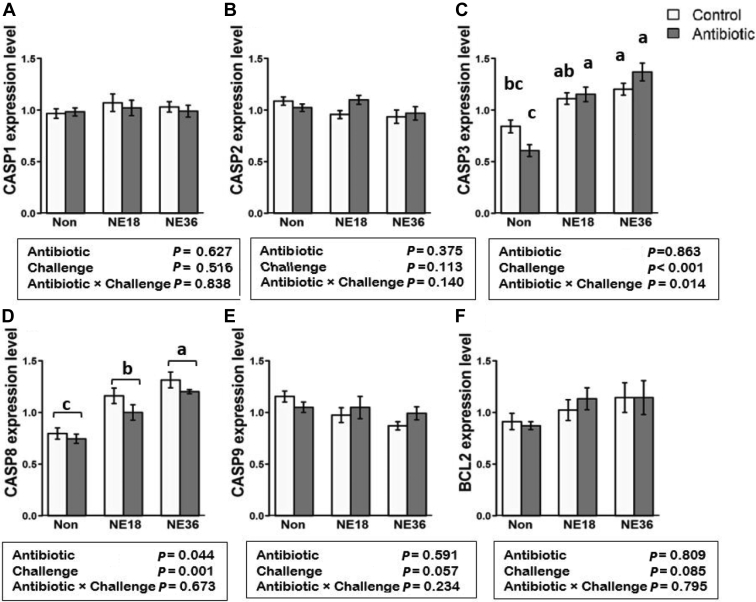
Upregulation of CASP3 and CASP8 by NE Challenge
The mRNA expression of 6 genes related to apoptosis in the jejunum was examined to investigate their responses to the treatments (Figure 2). The genes CASP3 (Figure 2C) and CASP8 (Figure 2D) were upregulated in both challenged groups (P < 0.001) relative to nonchallenged birds, and an antibiotic × challenge interaction was observed in the expression of CASP3 (P < 0.05), where expression of CASP3 was reduced by antibiotics only in nonchallenged birds (Figure 2E). Antibiotics also downregulated the expression of CASP8 (P < 0.05) regardless of the challenge. The NE challenge upregulated CASP8 (P < 0.001) in both NE18 and NE36 groups, whereas birds in the NE36 group had a significantly higher expression of CASP3 and CASP8 (P < 0.001) than birds challenged with NE18. The mRNA expression of CASP1, CASP2, CASP9, and BCL2 were not affected by the C. perfringens strains or antibiotic supplementation.
Figure 2.
mRNA expression of apoptosis related genes in the jejunum tissue of broiler chickens under necrotic enteritis (NE) challenge using 2 different strains of Clostridium perfringens (NE18 and NE36). (A) CASP1, B) CASP2, (C) expression of CASP3 is reduced by antibiotics only in nonchallenged birds. Both challenge groups increased the relative expression of CASP3, (D) increased relative expression of CASP8 in both NE challenged groups. The NE36 challenge showed higher expression than the NE18. Antibiotics reduced the expression of this gene in all groups. (E) CASP9, (F) BCL2. Control: no in-feed antibiotics; Antibiotics: in-fed antibiotics (salinomycin (72 ppm) and zinc bacitracin (50 ppm)); Non: Nonchallenge. a–c bars with different letters significantly differ on the basis on Tukeys' multiple tests (P < 0.05).
Downregulation of OCLN and TJP1 and Upregulation of CLDN1 by NE Challenge
As shown in Figure 3, both challenge groups showed downregulated expression of TJP1 (P < 0.05) and OCLN (P < 0.001) in chickens (Figures 3B and 3C, respectively). CLDN1, on the other hand, was upregulated (P < 0.001) by both NE18 and NE36 strains and birds challenged with NE36 had a significantly higher expression of this gene compared with birds challenged with NE18 strain. A negative correlation (r = −0.564, P < 0.001) between intestinal OCLN expression and blood FITC-d concentrations is shown in Figure 4.
Figure 3.
Relative mRNA expression of tight junction proteins in the jejunum tissue of broilers under necrotic enteritis (NE) challenge using 2 different strains of Clostridium perfringens (NE18 and NE36). (A) Increased expression of CLDN1 in both NE challenge groups, (B, C) downregulation of TJP1 and OCLN in both NE challenge groups. Control: no in-feed antibiotics; Antibiotics: in-fed antibiotics (salinomycin (72 ppm) and zinc bacitracin (50 ppm)); Non: Nonchallenge. a–cbars with different letters significantly differ on the basis on Tukeys' multiple tests (P < 0.05).
Figure 4.
Correlation between FITC-d concentration in serum and OCLN expression in jejunum tissue of broilers chickens. Abbreviations: FITC-d, fluorescein isothiocyanate-dextran; OCLN, occludin.
Downregulation of IgM and IgG, MUC2 and Upregulation of MUC5ac by NE Challenge
The mRNA expression of 2 immunoglobulin genes and 2 mucin genes was also investigated in response to antibiotics and NE challenge is shown in Figure 5. Downregulation of IgG and MUC2 (P < 0.001) and IgM (P < 0.05) were observed in both challenged groups compared with the nonchallenged birds. An antibiotic × challenge interaction was observed for MUC5ac, where the expression of MUC5ac was only upregulated (P < 0.05) in the NE18 group. Furthermore, an antibiotic × challenge interaction was observed for IgM, where the supplementation of antibiotics, only reduced the expression of this gene in the nonchallenged birds (P < 0.05).
Figure 5.
Relative mRNA expression of mucin- and immunoglobulin-related genes in the jejunum tissue of broilers under necrotic enteritis (NE) challenge using 2 different strains of Clostridium perfringens (NE18 and NE36). (A) Both challenged groups downregulated expression of MUC2, (B) expression of MUC5ac was upregulated only in the NE18 challenged birds fed with antibiotic supplementation, (C) both challenged groups downregulated expression of IgG, (D) supplementation of antibiotics only reduced the expression of this gene in the nonchallenged birds. Control: no in-feed antibiotics; Antibiotics: in-fed antibiotics (salinomycin (72 ppm) and zinc bacitracin (50 ppm)); Non: Nonchallenge. a–cbars with different letters significantly differ on the basis on Tukeys' multiple tests (P < 0.05).
Expression of Genes Encoding Digestive Enzymes and Nutrient Transporters
Nutrient transporter gene expression is shown in Figure 6. The genes PepT2 (P < 0.01) and y+LAT2 (P < 0.05) were downregulated in both NE challenge groups. Interestingly, y+LAT1 gene was downregulated by the NE18 challenge (P < 0.001) relative to both nonchallenged and NE36 challenged treatments. An antibiotic × challenge interaction was observed in the expression of b0,+AT (P < 0.05) and B0AT (P < 0.05). The bo,+AT gene was downregulated by antibiotic treatment in only the nonchallenged birds. Challenge with NE18 and NE36 strains, however, indiscriminately downregulated b0,+AT gene regardless of antibiotic treatments, but the extent was different with greater downregulation in the nonantibiotic group than the antibiotic supplemented group. The gene did not show differential expression in response to the NE18 or NE36 challenge. By contrast, antibiotics upregulated the gene B0AT in only the nonchallenged birds, but no changes due to antibiotics were observed in challenged groups. Challenge, however, indiscriminately downregulated B0AT in both groups with or without antibiotic treatments to a greater extent in birds with antibiotic treatment compared with those without. On the other hand, B0AT gene did not show differential expression in both groups of challenged birds.
Figure 6.
Relative mRNA expression of nutrient transporter genes in jejunum tissue of broilers under necrotic enteritis (NE) challenge using 2 different strains of Clostridium perfringens (NE18 and NE36). (A) both challenged groups' downregulated expression of PepT2, (B) bo,+AT gene was downregulated by antibiotic treatment in only the non-challenged birds, (C) antibiotic supplementation upregulated B0AT gene in only the non-challenged birds, (D) Both challenged groups' upregulated expression of LAT2, (E) the NE18 challenge reduced expression of y+LAT1, (F) both challenged groups' downregulated expression of y+LAT2. Control: no in-feed antibiotics; Antibiotics: in-fed antibiotics (salinomycin (72 ppm) and zinc bacitracin (50 ppm)); Non: Non-challenge. a–cbars with different letters significantly differ on the basis on Tukeys' multiple tests (P < 0.05).
Figure 7 illustrates that the expression of APN, ATP1A1, GLUT, and SI (P < 0.001) were downregulated in both challenge groups. Furthermore, GLUT2 expression was lower (P < 0.001) in the NE36 group compared with the NE18 group. Antibiotics upregulated the gene APN (P < 0.05) in all the treatments. In addition, an antibiotic × challenge interaction was observed in the expression of ASCT1 (P < 0.05), where antibiotic supplementation upregulated the expression of this gene in both challenged groups, whereas no changes of ASCT1 expression were observed among the birds without antibiotic treatment. On the other hand, this gene did not show differential expression in response to either of the C. perfringens strains (NE18 and NE36).
Figure 7.
Relative mRNA expression of nutrient transporter genes in the jejunum tissue of broilers under necrotic enteritis (NE) challenge using 2 different strains of Clostridium perfringens (NE18 and NE36). (A) Both challenged groups' downregulated expression of APN. (B) Both challenged groups downregulated expression GLUT2 expression and NE36 group show lower expression of this gene compared with NE18 group, (C) antibiotic supplementation upregulated the expression of ASCT1 gene in both challenged groups, (D-E) both challenged groups' show downregulated expression of ATP1A1 and SI. Control: no in-feed antibiotics; Antibiotics: in-fed antibiotics (salinomycin (72 ppm) and zinc bacitracin (50 ppm)); Non: Nonchallenge. a–cbars with different letters significantly differ on the basis on Tukeys' multiple tests (P < 0.05).
Table 4 illustrates the correlation of jejunum enzyme and nutrient transporters expression levels with broiler weight gain from day 0 to 16. Except for BCL2 and y+LAT1, all genes show significant correlations with weight gain. Very strong positive correlations were observed between weight gain and GLUT2, APN, ATP1A1, or SI (r = > 0.8, P < 0.001) whereas the strongest negative correlation was seen between weight gain and LAT1 (r = −0.617, P < 0.001).
Table 4.
Correlation between the expression levels of enzyme and nutrient transporter genes and weight gain of broilers challenged with 2 models of NE challenge (NE18 and NE36) during day 0–16.
| GLUT2 | APN | ACST1 | BCL2 | b0,+AT | B0AT | LAT1 | ATP1A1 | PepT2 | SI | y+LAT1 | y+LAT2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WG | 0.844∗∗∗ | 0.805∗∗∗ | −0.336∗ | −0.278 | 0.644∗∗∗ | 0.644∗∗∗ | −0.617∗∗∗ | 0.827∗∗∗ | 0.478∗∗ | 0.865∗∗∗ | 0.062 | 0.783∗∗∗ |
Abbreviations: APN, aminopeptidase; ASCT1, alanine, serine, cysteine and threonine transporter-1; ATP1A1, ATPase Na+/K+ transporting subunit alpha-1; BCL2, B-cell lymphoma-2; b0,+AT, b0,+amino acid transporter; B0AT, neutral amino acid transporter; GLUT2, glucose transporter-2; LAT1, large neutral amino acid transporter-1; PepT2, peptide transporter-2; SI, sucrase-isomaltase; WG, weight gain; y+LAT1, Y + L amino acid transporter-1; y+LAT2, Y + L amino acid transporter-2; WG, weight gain.
∗P < 0.05.
∗∗P < 0.01.
∗∗∗P < 0.001.
Discussion
This study investigated responses of NE challenged broilers to antibiotics and 2 C. perfringens strains, NE18 and NE36, and compared their responses to their nonchallenged counterparts and among the challenged birds. A subclinical NE infection is characterized by significant deterioration of bird performance, mild intestinal lesions and no NE-related mortality (Skinner et al., 2010). In the present study, a successful subclinical NE infection was introduced in both challenged groups, as typical signs such as impaired FCR and BW were observed in the challenged birds (Jayaraman et al., 2013; M'Sadeq et al., 2015). Furthermore, we have previously reported higher intestinal lesion score and no significant NE-related mortality in the challenged birds (Gharib-Naseri et al., 2019) which altogether confirm a subclinical NE infection. In the present study, it was observed that, as expected, in-feed antibiotic application alleviated the negative effects of the NE challenge, and the challenge produced by either NE18 and NE36 C. perfringens strain introduced different levels of severity possibly through different effects on intestinal health. The study suggests that the different regulation of genes encoding TJ proteins (CLDN1), apoptosis (CASP8), mucin production (MUC5ac), and intestinal nutrient transporters (GLUT2) compared in the 2 challenge groups may underlie the mechanisms for the severity of infection in birds challenged with NE. The results of this study can lead to the acceptance of our hypothesis that NE challenge can negatively affect chicken performance through compromised intestinal gut integrity, damaged morphology, and regulation of related genes. All these may underlie the mechanism of NE infection produced by the causative agent C. perfringens, together with the predisposing factor Eimeria species applied in the current challenge model. The study also suggest that the strain of C. perfringens with different virulence plays an important role in the severity of NE infection possibly through the modulation of intestinal gene expression responsible for immunity, intestinal integrity, mucus production, apoptosis, and nutrient transporters in the intestine.
The healthy intestinal tract is not only essential for nutrient absorption but also acts as an important barrier against bacterial infection (Balda and Matter, 2008). Therefore, intestinal integrity devised by the TJ are the leading indicators for intestinal epithelium health. Owing to large size of the FITC-d molecules (3–5 kDa), higher concentrations of this substance in the bloodstream indicates damage in the paracellular barrier, caused by Eimeria and C. perfringens challenge (Park et al., 2008b; Latorre et al., 2018). Furthermore, the reduced expression of OCLN and TJP1 in the challenged birds also clearly demonstrates compromised intestinal epithelial TJ by the challenges applied. The strong negative correlation between OCLN expression and FITC-d concentration in the bloodstream is in agreement with the report by Cani et al. (2009) where a negative correlation between OCLN expression and blood FITC-d in mice suffering from inflammation disorders was observed.
Interestingly, CLDN1 is known to be an important protein in the TJ complex, the observation in the present study showed that the significantly increased expression of CLDN1 in challenged groups compared with nonchallenged birds appears opposite to the changes of other TJ proteins. As a transmembrane protein, CLDN1 is a key pore-sealing TJ protein of intestinal epithelium, and it is believed that it may act differently in activated cytokinin and inflammatory situations (Pope et al., 2014). It was believed that CLDN1 has a high affinity to C-terminal domain of C. perfringens enterotoxin (CPE) (Eichner et al., 2017), and the binding of CPE with CLDN1 could form a membrane pore and lead to a massive influx of Ca++, so that necrotic cell death occurs. However, another study reported that because it lacks the ECL-2 sequence favorable for CPE binding CLDN1 is not a receptor for this toxin (Shrestha et al., 2016). Nevertheless, the NE producing C. perfringens strains positive in NetB toxin do not produce CPE (Rood et al., 2018), thus no CPE binding to CLDN1 can be postulated herein. The increased expression of CLDN1 in NE infections has been observed in the present study and reported by other researchers. Bortoluzzi et al. (2019) reported increased expression of CLDN1 in intestinal epithelium of NE challenged broilers. Similar results were also observed by C. perfringens infections alone in chicken intestine (Liu et al., 2012). In humans, on the other hand, it has been reported that CLDN1 is upregulated under disease conditions such as ulcerative colitis and active inflammatory bowel disease (Weber et al., 2008; Devriese et al., 2017; Garcia-Hernandez et al., 2017) and tumor proliferation (Huang et al., 2015; Jian et al., 2015). In a human epithelial cell investigation, Poritz et al. (2011) reported that treating the cells with different doses of tumor necrosis factor alpha caused a significant increase in CLDN1 expression.
As suggested by Singh et al. (2010), CLDN1 could be delocalized from the membrane to the cytoplasm and nucleus where its role becomes signal transduction as opposed to cell adhesion when it is in the membrane. It may be considered that the delocalization of CLDN1 from the membrane to cytoplasm may result in a feedback mechanism in the cell to produce more CLDN1 so that the C. perfringens challenge causes increased expression of CLDN1 as observed in the current study. It seems likely that inflammation can induce the expression of CLDN1. Furthermore, CLDN1 has shown to have antiapoptotic effects in humans (Akasaka et al., 2010) and upregulation of this gene might have a protective impact in the challenged birds with increased apoptosis. Further work should be carried out to investigate whether there are any other possibilities that claudin proteins including CLDN1 are modulated by the C. perfringens infection of chickens, and if so, what function does the protein have under challenge conditions. Overall, the upregulation of CLDN1 by C. perfringens challenge is not necessarily an indication of TJ enhancement as may be intuitively suggested, but it is possible that a disease status of the animals is implied through its function other than as a TJ protein.
Under subclinical necrotic enteritis, intestinal epithelial cells are in a constant mode of inflammation and recovery (Star et al., 2009). This leads to shortened villus and deeper crypts which is the response of intestinal tissue to the inflammation caused by pathogens and their toxins that results in poor nutrient absorption and performance (Xu et al., 2003). In accordance with histological examinations, both NE challenged groups damaged the villus structure, and these results are in agreement with other studies where subclinical NE challenge severely damaged the villis and lamina propria integrity in chicken (Timbermont et al., 2011; Wang et al., 2017). Furthermore, intestinal inflammation can decrease goblet cell numbers, which are the primary sites for mucin gel formation in the intestine (Freitas et al., 2002; Tan et al., 2014; Wu et al., 2018). MUC2 synthesis takes place in the goblet cells, and deficiency of this protein has shown to increase bacterial translocation and intestinal inflammation (Wei et al., 2012). Histological analysis of the jejunum tissue in the present study revealed that NE challenge caused sever shedding in the epithelial layer. It could be then suggested that the downregulation of MUC2 is caused by the intestinal mucosa deterioration, due to the damage caused by NE infections, preventing mucus from renewal and increasing the chance of further infection. Significant damage to the gut structure caused by the NE challenge can also reduce nutrient uptake, which in turn lower immunoglobulin responses, as shown by Konashi et al. (2000). A recent subclinical necrotic enteritis study reported a significant decrease of IgA + B cells in infected chickens (Wang et al., 2017) that supports the findings observed in this study.
Interestingly, MUC5ac was upregulated by the NE36 challenge. In humans, Park et al. (2008a) demonstrated an increase of MUC5ac in patients with colon cancer and Forgue-Lafitte et al. (2007) observed such an increase in patients with ulcerative colitis. This phenomenon was also observed in NE (Forder et al., 2012) and Eimeria-infected (Kitessa et al., 2014) broiler chickens previously. Unlike MUC2 which is widely expressed in the intestine, MUC5ac is highly expressed in the stomach (Van Klinken et al., 1995), and the role of MUC5ac might be different from MUC2 in the intestine thus this may cause a different response to disease challenge. Cell damage and lysis caused by Eimeria sporozoites penetration in epithelium cells and toxins produced by C. perfringens can generate signals that initiate conformational changes in apoptosis proteins (Gao and Kwaik, 2000). Caspases (cysteine-aspartic proteases, cysteine aspartates, or cysteine-dependent aspartate-directed proteases) play a critical role in regulating programmed cell death, including apoptosis, and inflammation (McIlwain et al., 2013). Extrinsic and intrinsic pathways are the 2 main pathways that mediate cell death. The extrinsic pathway is activated by CASP8 which activates executioner caspases (i.e., CASP3, −6, and −7) in their inactive form, that is, procaspase dimer state (Nunes et al., 2005; McIlwain et al., 2013). Caspase-3 is critical for the execution of the apoptotic process in both pathways (Riedl and Shi, 2004). In the present study, elevated CASP8 and CASP3 expression by the challenge suggests an extrinsic activated cell death pathway in the intestine of challenged birds.
Amino acids and energy uptake are also important factors affecting the immune functions and susceptibility to disease in animals (Broer, 2008). Nutrient uptake is mostly regulated by specific transporters across the plasma membrane in the small intestine, and changes in these nutrient transporters may underlie reduced body weight and feed efficiency in NE challenged birds. Na+-dependent neutral/cationic amino acid exchanger such as light chain heteromeric amino acid transporters (LAT1, y+LAT1, y+LAT2), Na+-dependent neutral amino acid transporters such as b0,+AT, B0AT, and ASCT1, and glucose transporters (GLUT1-2) are all the proteins transporting respective nutrients thus their uptake in the epithelium layer. Moreover, most immune responses to pathogens are characterized in decreased appetite and in directing nutrients away from skeletal muscle accretion toward hepatic production and secretion of acute-phase proteins (Humphrey and Klasing, 2004). The addition of antibiotics showed a significant improvement in the performance and gut integrity of challenged birds, and upregulated the expression of ASCT1 in both NE-infected groups. Nonetheless, no other significant effect of antibiotics was observed in the challenged birds. We speculate that the immune system or changes in the microbiota population could have affected the expression of the genes in response to the antibiotic supplementation. Further investigation is needed to understand the relation between the effect of antibiotics and the expression of intestinal genes in NE challenge broilers.
The SI gene is usually found on the surface of intestinal epithelial cells, where it enables the production of the sucrase-isomaltase enzyme and is the key to starch and sugar degradation. This enzyme breaks sucrose and maltose into simple sugars to be absorbed in the intestinal epithelial cells (Diaz-Sotomayor et al., 2013). GLUT2 is present on both basolateral and apical membrane of the intestine, facilitating glucose absorption. It transports monosaccharides from enterocytes into the blood (Kellett et al., 2008). Downregulation of GLUT2 and SI can lead to diminished transport of carbohydrate to the tissues and result in reduced weight gain of the broilers (Su et al., 2014), such as in the case observed in the NE36 group of this study. Aminopeptides such as APN are highly expressed on the brush border of the epithelium and are responsible for final digestion of peptides by N terminus cleavage (Gal-Garber and Uni, 2000). In both challenged groups (NE18 and NE36), the expression of APN was decreased, which may cause reduced nutrient absorption and negatively affect the gut barrier integrity and immune responses (Luan and Xu, 2007). The strong correlations between these genes and the weight gain of chickens may suggest that downregulation of these genes can, at least partially, be responsible for the depleted growth of challenged birds. Furthermore, the downregulation of brush-border transporters, such as b°,+AT and B0AT that regulate free amino acid uptake to the epithelial cells, initiated by the challenge may also show the adverse effects of this infection on the growth and feed efficiency of birds probably by the depleted influx of essential amino acids into epithelial cells which are critical for absorption.
Conclusions
Taken together, our results suggest that both NE challenges produced by the 2 C. perfringens strains, compromised performance, gut integrity and intestinal morphology. The positive effect of antibiotics was evident on the birds; however, owing to the more severe impact of the NE36 challenge on the bird's performance, the effectiveness of antibiotics was lower compared with the NE18 challenge. Further differences were observed in the expression of genes related to TJ, cell death, mucin production, and intestinal transporters between the 2 challenged groups. The mode of action on how C. perfringens produce clinical or subclinical NE has been elucidated as the action of causative toxin NetB (Keyburn et al., 2008). However, the mechanism underlying how these two NE challenges affect the severity of the disease is yet to be illustrated. The differences observed in the expression of intestinal genes in the birds challenged with NE18 and NE36 may provide preliminary evidences on how the hosts respond to the challenge. Further investigation on the genomic factors in the bacteria contributing to these different responses would be appealing for more in-depth study of C. perfringens virulence that leads to different levels of severity of the NE disease.
Acknowledgments
The authors thank Ms. Petrina Young of Eimeria Pty Ltd. for providing Eimeria species. Prof. Robert Moore for providing Clostridium perfringens EHE-NE18 and WER-NE36, and Mr. Jonathan Clay for his help and guidance with the laboratory work.
Disclosures
The authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence the work; there is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the content of this article.
References
- Akasaka H., Sato F., Morohashi S., Wu Y., Liu Y., Kondo J., Odagiri H., Hakamada K., Kijima H. Anti-apoptotic effect of claudin-1 in tamoxifen-treated human breast cancer MCF-7 cells. BMC Cancer. 2010;10:548–561. doi: 10.1186/1471-2407-10-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen . 2014. Ross broiler management manual. Accessed Jun. 2017. http://goldenpoultry.com/wp-content/uploads/2014/09/Ross-Broiler-Handbook-2014i-EN.pdf. [Google Scholar]
- Bagés S., Estany J., Tor M., Pena R. Investigating reference genes for quantitative real-time PCR analysis across four chicken tissues. Gene. 2015;561:82–87. doi: 10.1016/j.gene.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Balda M.S., Matter K. Tight junctions at a glance. J. Cell. Sci. 2008;121:3677–3682. doi: 10.1242/jcs.023887. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C., Vieira B., Lumpkins B., Mathis G., King W., Graugnard D., Dawson K., Applegate T. Can dietary zinc diminish the impact of necrotic enteritis on growth performance of broiler chickens by modulating the intestinal immune-system and microbiota? Poult. Sci. 2019;98:3181–3193. doi: 10.3382/ps/pez045. [DOI] [PubMed] [Google Scholar]
- Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Possemiers S., Van de Wiele T., Guiot Y., Everard A., Rottier O., Geurts L., Naslain D., Neyrinck A.M., Lambert D.M. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G.M. Caspases: the executioners of apoptosis. Biochem. J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K.K., Songer J.G. Virulence of Clostridium perfringens in an experimental model of poultry necrotic enteritis. Vet. Microbiol. 2010;142:323–328. doi: 10.1016/j.vetmic.2009.09.065. [DOI] [PubMed] [Google Scholar]
- Coursodon C., Glock R., Moore K., Cooper K., Songer J. TpeL-producing strains of Clostridium perfringens type A are highly virulent for broiler chicks. Anaerobe. 2012;18:117–121. doi: 10.1016/j.anaerobe.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Craven S. Colonization of the intestinal tract by Clostridium perfringens and fecal shedding in diet-stressed and unstressed broiler chickens. Poult. Sci. 2000;79:843–849. doi: 10.1093/ps/79.6.843. [DOI] [PubMed] [Google Scholar]
- Devriese S., Eeckhaut V., Geirnaert A., Van den Bossche L., Hindryckx P., Van de Wiele T., Van Immerseel F., Ducatelle R., De Vos M., Laukens D. Reduced mucosa-associated Butyricicoccus activity in patients with ulcerative colitis correlates with aberrant claudin-1 expression. J. Crohns Colitis. 2017;11:229–236. doi: 10.1093/ecco-jcc/jjw142. [DOI] [PubMed] [Google Scholar]
- Diaz-Sotomayor M., Quezada-Calvillo R., Avery S.E., Chacko S.K., Yan L.k., Lin A.H.-M., Ao Z.h., Hamaker B.R., Nichols B.L. Maltase-glucoamylase modulates gluconeogenesis and sucrase-isomaltase dominates starch digestion glucogenesis. J. Pediatr. Gastroenterol. Nutr. 2013;57:704–712. doi: 10.1097/MPG.0b013e3182a27438. [DOI] [PubMed] [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., Guo Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016;7:1–10. doi: 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner M., Protze J., Piontek A., Krause G., Piontek J. Targeting and alteration of tight junctions by bacteria and their virulence factors such as Clostridium perfringens enterotoxin. Pflugers Arch. 2017;469:77–90. doi: 10.1007/s00424-016-1902-x. [DOI] [PubMed] [Google Scholar]
- Elkouby-Naor L., Ben-Yosef T. Functions of claudin tight junction proteins and their complex interactions in various physiological systems. Int. Rev. Cell Mol. Bio. 2010;279:1–32. doi: 10.1016/S1937-6448(10)79001-8. [DOI] [PubMed] [Google Scholar]
- Elphick D., Mahida Y. Paneth cells: their role in innate immunity and inflammatory disease. Gut. 2005;54:1802–1809. doi: 10.1136/gut.2005.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Liu S., Liu G., Zhao J., Jiao H., Wang X., Song Z., Lin H. Vitamin A deficiency impairs mucin expression and suppresses the mucosal immune function of the respiratory tract in chicks. PLoS One. 2015;10:1–16. doi: 10.1371/journal.pone.0139131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forder R.E., Nattrass G.S., Geier M.S., Hughes R.J., Hynd P.I. Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult. Sci. 2012;91:1335–1341. doi: 10.3382/ps.2011-02062. [DOI] [PubMed] [Google Scholar]
- Forgue-Lafitte M.E., Fabiani B., Levy P.P., Maurin N., Fléjou J.F., Bara J. Abnormal expression of M1/MUC5AC mucin in distal colon of patients with diverticulitis, ulcerative colitis and cancer. Int. J. Cancer. 2007;121:1543–1549. doi: 10.1002/ijc.22865. [DOI] [PubMed] [Google Scholar]
- Fotiadis D., Kanai Y., Palacín M. The SLC3 and SLC7 families of amino acid transporters. Mol. Aspects Med. 2013;34:139–158. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Freitas M., Axelsson L.-G., Cayuela C., Midtvedt T., Trugnan G. Microbial–host interactions specifically control the glycosylation pattern in intestinal mouse mucosa. Histochem. Cell Biol. 2002;118:149–161. doi: 10.1007/s00418-002-0432-0. [DOI] [PubMed] [Google Scholar]
- Furuse M., Hata M., Furuse K., Yoshida Y., Haratake A., Sugitani Y., Noda T., Kubo A., Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1–deficient mice. J. Cell. Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal-Garber O., Uni Z. Chicken intestinal aminopeptidase: partial sequence of the gene, expression and activity. Poult. Sci. 2000;79:41–45. doi: 10.1093/ps/79.1.41. [DOI] [PubMed] [Google Scholar]
- Gao L., Kwaik Y. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 2000;8:306–313. doi: 10.1016/s0966-842x(00)01784-4. [DOI] [PubMed] [Google Scholar]
- Garcia-Hernandez V., Quiros M., Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann. N. Y. Acad. Sci. 2017;1397:66–79. doi: 10.1111/nyas.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharib-Naseri K., Kheravii S.K., Keerqin C., Morgan N., Swick R., Wu S. Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens. Poult. Sci. 2019;98:6422–6432. doi: 10.3382/ps/pez480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert E., Li H., Emmerson D., Webb K., Wong E. Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poult. Sci. 2007;86:1739–1753. doi: 10.1093/ps/86.8.1739. [DOI] [PubMed] [Google Scholar]
- Hansen G.H., Pedersen E.D., Immerdal L., Niels-Christiansen L.-L., Danielsen E.M. Anti-glycosyl antibodies in lipid rafts of the enterocyte brush border: a possible host defense against pathogens. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:1100–1107. doi: 10.1152/ajpgi.00256.2005. [DOI] [PubMed] [Google Scholar]
- Hediger M.A., Romero M.F., Peng J.B., Rolfs A., Takanaga H., Bruford E.A. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins. Pflügers Archiv. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- Hellemans J., Mortier G., De Paepe A., Speleman F., Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:1–19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn N., Donkin S., Applegate T., Adeola O. Intestinal mucin dynamics: response of broiler chicks and White Pekin ducklings to dietary threonine. Poult. Sci. 2009;88:1906–1914. doi: 10.3382/ps.2009-00009. [DOI] [PubMed] [Google Scholar]
- Huang J., Zhang L., He C., Qu Y., Li J., Zhang J., Du T., Chen X., Yu Y., Liu B. Claudin-1 enhances tumor proliferation and metastasis by regulating cell anoikis in gastric cancer. Oncotarget. 2015;6:1652–1665. doi: 10.18632/oncotarget.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey B., Klasing K. Modulation of nutrient metabolism and homeostasis by the immune system. Worlds Poult. Sci. J. 2004;60:90–100. [Google Scholar]
- Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 2013;92:370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- Jian Y., Chen C., Li B., Tian X. Delocalized Claudin-1 promotes metastasis of human osteosarcoma cells. Biochem. Biophys. Res. Commun. 2015;466:356–361. doi: 10.1016/j.bbrc.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Benestad S.L., Lovland A. Epidemiologic aspects of necrotic enteritis in broiler chickens - disease occurrence and production performance. Avian Pathol. 2016;45:271–274. doi: 10.1080/03079457.2016.1163521. [DOI] [PubMed] [Google Scholar]
- Kellett G.L., Brot-Laroche E., Mace O.J., Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu. Rev. Nutr. 2008;28:35–54. doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- Keyburn A.L., Boyce J.D., Vaz P., Bannam T.L., Ford M.E., Parker D., Di Rubbo A., Rood J.I., Moore R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:1–11. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheravii S.K., Swick R.A., Choct M., Wu S.-B. Upregulation of genes encoding digestive enzymes and nutrient transporters in the digestive system of broiler chickens by dietary supplementation of fiber and inclusion of coarse particle size corn. BMC Genomics. 2018;19:208–222. doi: 10.1186/s12864-018-4592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitessa S.M., Nattrass G.S., Forder R.E., McGrice H.A., Wu S.-B., Hughes R.J. Mucin gene mRNA levels in broilers challenged with Eimeria and/or Clostridium perfringens. Avian Dis. 2014;58:408–414. doi: 10.1637/10757-122313-Reg.1. [DOI] [PubMed] [Google Scholar]
- Konashi S., Takahashi K., Akiba Y. Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Br. J. Nutr. 2000;83:449–456. [PubMed] [Google Scholar]
- Kuttappan V.A., Berghman L.R., Vicuna E.A., Latorre J.D., Menconi A., Wolchok J.D., Wolfenden A.D., Faulkner O.B., Tellez G.I., Hargis B.M., Bielke L.R. Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poult. Sci. 2015;94:1220–1226. doi: 10.3382/ps/pev114. [DOI] [PubMed] [Google Scholar]
- Lan Y., Verstegen M., Tamminga S., Williams B. The role of the commensal gut microbial community in broiler chickens. Worlds Poult. Sci. J. 2005;61:95–104. [Google Scholar]
- Latorre J.D., Adhikari B., Park S.H., Teague K.D., Graham L.E., Mahaffey B.D., Baxter M.F., Hernandez X., Kwon Y.M., Ricke S.C. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front. Vet. Sci. 2018;5:1–11. doi: 10.3389/fvets.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Guo S., Guo Y. Xylanase supplementation to a wheat-based diet alleviated the intestinal mucosal barrier impairment of broiler chickens challenged by Clostridium perfringens. Avian Pathol. 2012;41:291–298. doi: 10.1080/03079457.2012.684089. [DOI] [PubMed] [Google Scholar]
- Luan Y., Xu W. The structure and main functions of aminopeptidase N. Curr. Med. Chem. 2007;14:639–647. doi: 10.2174/092986707780059571. [DOI] [PubMed] [Google Scholar]
- M'Sadeq S.A., Wu S.B., Choct M., Forder R., Swick R.A. Use of yeast cell wall extract as a tool to reduce the impact of necrotic enteritis in broilers. Poult. Sci. 2015;94:898–905. doi: 10.3382/ps/pev035. [DOI] [PubMed] [Google Scholar]
- McDevitt R., Brooker J., Acamovic T., Sparks N. Necrotic enteritis; a continuing challenge for the poultry industry. Worlds Poult. Sci. J. 2006;62:221–247. [Google Scholar]
- McIlwain D.R., Berger T., Mak T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013;5:1–28. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury C.J., Hickson I.D. Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol. 2001;41:367–401. doi: 10.1146/annurev.pharmtox.41.1.367. [DOI] [PubMed] [Google Scholar]
- Nunes V.A., Gozzo A.J., Cruz-Silva I., Juliano M.A., Viel T.A., Godinho R.O., Meirelles F.V., Sampaio M.U., Sampaio C.A., Araujo M.S. Vitamin E prevents cell death induced by mild oxidative stress in chicken skeletal muscle cells. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005;141:225–240. doi: 10.1016/j.cca.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Paris N.E., Wong E.A. Expression of digestive enzymes and nutrient transporters in the intestine of Eimeria maxima-infected chickens. Poult. Sci. 2013;92:1331–1335. doi: 10.3382/ps.2012-02966. [DOI] [PubMed] [Google Scholar]
- Park E.T., Gum J.R., Kakar S., Kwon S.W., Deng G., Kim Y.S. Aberrant expression of SOX2 upregulates MUC5AC gastric foveolar mucin in mucinous cancers of the colorectum and related lesions. Int. J. Cancer. 2008;122:1253–1260. doi: 10.1002/ijc.23225. [DOI] [PubMed] [Google Scholar]
- Park S.S., Lillehoj H.S., Allen P.C., Park D.W., FitzCoy S., Bautista D.A., Lillehoj E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008;52:14–22. doi: 10.1637/7997-041707-Reg. [DOI] [PubMed] [Google Scholar]
- Pope J.L., Ahmad R., Bhat A.A., Washington M.K., Singh A.B., Dhawan P. Claudin-1 overexpression in intestinal epithelial cells enhances susceptibility to adenamatous polyposis coli-mediated colon tumorigenesis. Mol. Cancer. 2014;13:1–13. doi: 10.1186/1476-4598-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poritz L.S., Harris L.R., Kelly A.A., Koltun W.A. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig. Dis. Sci. 2011;56:2802–2809. doi: 10.1007/s10620-011-1688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl S.J., Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- Rodgers N.J., Swick R.A., Geier M.S., Moore R.J., Choct M., Wu S.-B. A Multifactorial Analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015;59:38–45. doi: 10.1637/10774-011614-reg.1. [DOI] [PubMed] [Google Scholar]
- Rood J.I., Adams V., Lacey J., Lyras D., McClane B.A., Melville S.B., Moore R.J., Popoff M.R., Sarker M.R., Songer J.G. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe. 2018;53:5–10. doi: 10.1016/j.anaerobe.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha A., Uzal F.A., McClane B.A. The interaction of Clostridium perfringens enterotoxin with receptor claudins. Anaerobe. 2016;41:18–26. doi: 10.1016/j.anaerobe.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.B., Sharma A., Dhawan P. Claudin family of proteins and cancer: an overview. J. Oncol. 2010;2010:1–11. doi: 10.1155/2010/541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner J.T., Bauer S., Young V., Pauling G., Wilson J. An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Dis. 2010;54:1237–1240. doi: 10.1637/9399-052110-Reg.1. [DOI] [PubMed] [Google Scholar]
- Star L., Bruijn N.d., Rovers M. Dietary beta glucans to fight chronic enteritis. World Poult. 2009;25:14–16. [Google Scholar]
- Su S., Miska K.B., Fetterer R.H., Jenkins M.C., Wong E.A. Expression of digestive enzymes and nutrient transporters in Eimeria acervulina-challenged layers and broilers. Poult. Sci. 2014;93:1217–1226. doi: 10.3382/ps.2013-03807. [DOI] [PubMed] [Google Scholar]
- Tan J., Applegate T.J., Liu S., Guo Y., Eicher S.D. Supplemental dietary L-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens. Br. J. Nutr. 2014;112:1098–1109. doi: 10.1017/S0007114514001846. [DOI] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Uldry M., Ibberson M., Hosokawa M., Thorens B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002;524:199–203. doi: 10.1016/s0014-5793(02)03058-2. [DOI] [PubMed] [Google Scholar]
- Van Klinken B., Dekker J., Buller H., Einerhand A. Mucin gene structure and expression: protection vs. adhesion. Am. J. Physiol. Gastrointest. Liver Physiol. 1995;269:613–627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034.1–research0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicuna E.A., Kuttappan V.A., Galarza-Seeber R., Latorre J.D., Faulkner O.B., Hargis B.M., Tellez G., Bielke L.R. Effect of dexamethasone in feed on intestinal permeability, differential white blood cell counts, and immune organs in broiler chicks. Poult. Sci. 2015;94:2075–2080. doi: 10.3382/ps/pev211. [DOI] [PubMed] [Google Scholar]
- Wade B., Keyburn A. The true cost of necrotic enteritis. World Poult. 2015;31:16–17. [Google Scholar]
- Wade B., Keyburn A.L., Seemann T., Rood J.I., Moore R.J. Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet. Microbiol. 2015;180:299–303. doi: 10.1016/j.vetmic.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Wang H., Ni X., Qing X., Liu L., Lai J., Khalique A., Li G., Pan K., Jing B., Zeng D. Probiotic enhanced intestinal immunity in broilers against subclinical necrotic enteritis. Front. Immunol. 2017;8:1–14. doi: 10.3389/fimmu.2017.01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C.R., Nalle S.C., Tretiakova M., Rubin D.T., Turner J.R. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Invest. 2008;88:1110–1120. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Yang Z., Rey F.E., Ridaura V.K., Davidson N.O., Gordon J.I., Semenkovich C.F. Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell Host & Microbe. 2012;11:140–152. doi: 10.1016/j.chom.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Shao Y., Song B., Zhen W., Wang Z., Guo Y., Shahid M.S., Nie W. Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Sci. Biotechnol. 2018;9:1–14. doi: 10.1186/s40104-017-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Hu C., Xia M., Zhan X., Wang M. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yin R., Liu X., Liu C., Ding Z., Zhang X., Tian F., Liu W., Yu J., Li L., Hrabe de Angelis M., Stoeger T. Systematic selection of housekeeping genes for gene expression normalization in chicken embryo fibroblasts infected with Newcastle disease virus. Biochem. Biophys. Res. Commun. 2011;413:537–540. doi: 10.1016/j.bbrc.2011.08.131. [DOI] [PubMed] [Google Scholar]
- Zanu H., Keerqin C., Kheravii S., Morgan N., Wu S.-B., Bedford M., Swick R. Influence of meat and bone meal, phytase, and antibiotics on broiler chickens challenged with subclinical necrotic enteritis: 2. intestinal permeability, organ weights, hematology, intestinal morphology, and jejunal gene expression. Poult. Sci. 2020;99:2581–2594. doi: 10.1016/j.psj.2019.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F.Q., Zhang Z.W., Yao H.D., Wang L.L., Liu T., Yu X.Y., Li S., Xu S.W. Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res. Vet. Sci. 2013;95:146–155. doi: 10.1016/j.rvsc.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Zhou H., Lepp D., Pei Y., Liu M., Yin X., Ma R., Prescott J.F., Gong J. Influence of pCP1NetB ancillary genes on the virulence of Clostridium perfringens poultry necrotic enteritis strain CP1. Gut Pathog. 2017;9:2–7. doi: 10.1186/s13099-016-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]