Abstract
The objective of this study was to evaluate 2 types of almond hulls (prime hulls and California-type hulls) as alternative feed ingredients for broilers. A total of 560 one-day-old Cobb male chicks were randomly placed to 7 experimental treatments with 8 replicates of 10 birds each. Seven treatments consisted of a corn-soybean meal control diet and diets containing prime hulls or California-type hulls at 3, 6, and 9%. The nitrogen-corrected true metabolizable energy, crude protein, and crude fiber from prime hulls and California-type hulls were 1,624 and 1,514 kcal/kg, 4.8 and 5.0%, and 13.1 and 26.45%, respectively. During 0–19 d of age, the inclusion of the prime hulls at 3 levels had no significant effects on growth performance, but the California-type hulls at 9% increased feed intake (P = 0.02) and feed conversion ratio (P < 0.01), compared with control. The prime hulls at 9% decreased (P < 0.01) ileal dry matter and ileal nitrogen digestibility, and the California-type hulls at 9% only decreased ileal dry matter digestibility, but both prime hulls and California-type hulls at 6% had no effects on ileal dry matter digestibility and nitrogen-corrected apparent metabolizable energy compared to control. In addition, inclusion of prime hulls at 3% decreased (P < 0.01) AMEn compared with control group. There were no significant differences in cecal microbiota diversity at a phylum or genus level among treatments, but 9% inclusion rate of the California-type hulls increased (P < 0.05) the population of certain bacteria in the genus Clostridium and Oscillospira compared with control. In conclusion, as a dietary energy and fiber source, the prime hulls can be used at up to 9% without a negative effect on body weight gain, whereas the California-type hulls can be used up to 6%.
Key words: alternative ingredient, broiler, nutrition, gut microbiota
Introduction
The cost for major traditional feed ingredients is continuously increasing for animal production, whereas utilization of low-cost by-products is not only economical but also sustainable. Almond hull, a byproduct from the almond crop, has become a surplus on the market with the rapid increase in almonds production and consumption (Almond Board of California, 2019). Studies reported that almond hulls as a fibrous resource have a medium nutritive value for ruminants and swine (Homedes et al., 1993; Yalchi and Kargar, 2010; Williams et al., 2018). In broilers, dietary fibers are traditionally recognized as a nutrient diluent with negative effects on nutrient digestion and absorption have been recently gained much more attention to its important roles in immunity and gut health without compromising performance when they are included at a moderate level (Jha and Leterme, 2012; Jiménez-Moreno et al., 2013b; Sadeghi et al., 2015). However, whether almond hulls containing fibers can be used as an alternative ingredient in broilers is unclear.
Aside from dietary fibers, almond hulls are reported to contain an amount of total sugar ranging from 25 to 46%, which has the potential to be an energy source in the broiler diet (Holtman et al., 2015). In addition, almond hulls are rich in antioxidants such as polyphenols, triterpenoids, betulinic acid, oleanolic acid, and ursolic acid with strong antioxidative activities (Esfahlan et al., 2010; Prgomet et al., 2017). The polyphenol compounds from almond hulls have been reported to have a higher in vitro antioxidant capacity compared with vitamin E (Takeoka and Dao, 2003). Previous studies showed almond hulls used in swine diets up to 10% did not compromise the growth performance but reduced body fat (Calvert and Parker, 1985; Homedes et al., 1993). The dietary fiber, fermentable sugars, and antioxidants in almond hulls could be potential valuable nutrients for broilers (Oztürk-Urek et al., 2001; Takeoka and Dao, 2003; Jha and Leterme, 2012). However, there is no nutrient matrix values reported for almond hulls on broilers. Among all the almond species, the prime hulls is from Nonpareil species with lower crude fiber contents, and the California-type hulls are the mixture of hulls and shells produced from California with higher crude fiber content (Almond Board of California, 2019).
Thus, the hypothesis was that nutrients such as dietary fibers, sugars, and antioxidants in almond hulls could be utilized and exert potential benefit beneficial effects on broilers. The objective of this study is to determine the nutrient matrix values for the prime and California-type hulls and evaluate the effect of almond hulls as a feed ingredient on growth performance, nutrient digestibility, and ceca microbiota diversity in broilers.
Materials and methods
The experimental protocol was reviewed and approved by the University of Georgia Institutional Animal Care and Use Committee (A2018 10-010).
Rooster Assay
The precision-fed rooster assay of the nitrogen-corrected true metabolizable energy (TMEn) and true amino acid digestibility for the prime and California-type hulls were determined following the method described by Jones et al. (2018). In short, 20 roosters were fasted for 30 h to empty the gastrointestinal tract and then transferred to individual wire cages. Roosters were precision-fed 35 g of either the prime or the California-type hulls. Excreta were collected for 48 h postfeeding period from individual pans underneath the cages. The almond hulls and excreta were then dried, weighed, and analyzed for crude protein, moisture, and gross energy. The determination of the amino acids followed the same procedure using cecectomized roosters. The final TMEn and amino acids digestibility were obtained by taking the average from 10 samples of each hull species.
Experimental Diets
Dietary treatments consisted of a control diet based on corn-soybean meal; T2-T4 were formulated to contain 3, 6, and 9% of prime hulls; and T5-T7 were formulated to contain 3, 6, and 9% of California-type hulls. The tested almond hull samples were acquired from the almond orchard near Sacramento, CA. All almond hull samples were tested negative on aflatoxins. All diets were formulated as isonitrogenous and isocaloric (Table 1). The 7 treatment diets were mixed individually in a horizontal mixer (Davis Double Ribbon Mixer, Bonner Springs, KS) for 12 min.
Table 1.
Compositions and nutritional levels of diets.
| Item | Control | Prime hull |
California-type hull |
||||
|---|---|---|---|---|---|---|---|
| 3% | 6% | 9% | 3% | 6% | 9% | ||
| Ingredient (%) | |||||||
| Corn | 60.01 | 55.62 | 51.23 | 46.85 | 55.56 | 51.11 | 46.66 |
| SBM -48% | 34.15 | 34.54 | 34.93 | 35.31 | 34.53 | 34.91 | 35.29 |
| Almond hulls | 0 | 3.0 | 6.0 | 9.0 | 3.0 | 6.0 | 9.0 |
| Dical. Phos. | 1.58 | 1.59 | 1.60 | 1.61 | 1.59 | 1.61 | 1.62 |
| Soybean oil | 1.53 | 2.55 | 3.57 | 4.59 | 2.62 | 3.70 | 4.78 |
| Limestone | 1.17 | 1.14 | 1.11 | 1.09 | 1.14 | 1.12 | 1.09 |
| Salt | 0.35 | 0.34 | 0.34 | 0.33 | 0.34 | 0.34 | 0.33 |
| DL-Met | 0.29 | 0.30 | 0.30 | 0.31 | 0.30 | 0.31 | 0.31 |
| Premix1 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 |
| L-Lys | 0.22 | 0.21 | 0.21 | 0.20 | 0.22 | 0.21 | 0.20 |
| L-Thr | 0.07 | 0.07 | 0.08 | 0.07 | 0.07 | 0.07 | 0.07 |
| Cr2O3 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Calculated value (%) | |||||||
| ME (kcal/kg) | 3,010 | 3,010 | 3,010 | 3,010 | 3,010 | 3,010 | 3,010 |
| Crude protein | 21.25 | 21.25 | 21.25 | 21.25 | 21.25 | 21.25 | 21.25 |
| L-Lys | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 |
| DL-Met | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 |
| TSAA | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 |
| L-Thr | 0.86 | 0.86 | 0.86 | 0.86 | 0.86 | 0.86 | 0.86 |
| Ca | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 |
| Nonphytate P | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| Crude fiber | 2.16 | 2.45 | 2.81 | 3.13 | 2.88 | 3.60 | 4.32 |
| Analyzed value (%) | |||||||
| GE (kcal/kg) | 3,965 | 4,007 | 4,044 | 4,118 | 4,056 | 4,123 | 4,156 |
| Crude protein | 20.88 | 21.02 | 21.25 | 20.95 | 20.91 | 20.85 | 21.22 |
| Crude fiber | 2.09 | 2.53 | 2.69 | 3.08 | 2.94 | 3.69 | 4.32 |
Abbreviations: Dical. Phos., dicalcium phosphate; GE, gross energy; ME, metabolizable energy; SBM, soybean meal; TSAA, total sulfur amino acids.
Premix provides per kg of diet: vitamin A, 6,000 IU; vitamin D3, 1,200 IU; vitamin E, 12 IU; vitamin B12, 0.001 mg; riboflavin, 4.8 mg; niacin, 48 mg; d-pantothenic acid, 13 mg; choline chloride, 240 mg; menadione sodium bisulfate, 3.63 mg; folic acid, 6.0 mg; pyridoxine HCl, 5.1 mg; thiamin, 2.4 mg; d-biotin, 0.12 mg; ethoxyquin, 136 mg; manganese, 60 mg; zinc, 50 mg; iron, 30 mg; copper, 5 mg; iodine, 1.5 mg; selenium, 0.5 mg.
Birds Management
A total of 560 one-day-old Cobb 500 male broilers were obtained from a hatchery (Cleveland, GA) and allocated into 7 groups with 8 replicates of 10 birds each in a completely randomized design. Birds were reared in 56 battery cages with 10 birds each in an environmentally controlled room (Petersime Battery Brooder Units) located at the Poultry Research Center at the University of Georgia. The room temperature was set at 34°C in the beginning of the feeding trial, and then gradually adjusted to maintain birds comfortable at 25°C throughout the study. Birds had access to water and feed ad libitum throughout the study. Birds were checked twice a day for general health. Birds and feed were weighed at 0, 7, and 19 d of age to determine body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR).
Sample Collection
At 19 d of age, 5 birds per cage were randomly selected to collect ileal digesta for detecting nutrient digestibility. Fecal samples from each cage were collected from the trays in the last 3 d of the trial for analysis of nitrogen digestibility and AMEn (Adhikari et al., 2020). The fecal and ileal digesta samples were immediately frozen before being dried in an oven dryer (85°C) to a constant weight. Fecal and ileal digesta samples were pooled by the cage, respectively, and ground before analyses. Ceca from one bird per cage in control, or 9% of inclusion of the prime and California-type hulls treatments were collected, snap frozen in liquid nitrogen and later transferred in a freezer at −80°C until further analyses for microbiota.
Chemical Analysis
The proximate analysis, total mineral analysis, and fermentable sugar content including glucose, fructose, and sucrose of 2 almond hull samples, the prime and California-type hulls, were performed by Agricultural and Environmental Services Laboratories in University of Georgia following the method as indicated by AOAC International (1990).
Phytate content in the prime and California-type hulls was measured in accordance with the method by Latta and Eskin (1980), and chromium (III) oxide in the diet and ileal digesta was measured by the method described by Williams et al. (1962). Nitrogen content in feed and digesta was determined using the LECO system as indicated by AOAC International (1990). Gross energy values in feed and digesta were determined using the calorimeter (IKA C1 Compact Bomb Calorimeter, IKA-Werke, Staufen, Germany).
Nutrient digestibility was calculated using the following equation:
| 1 |
where Ci is the concentration of chromium in the diet; Co is the concentration of chromium in the ileal digesta or feces; Ni is the concentration of the nutrient in the diet; No is the concentration of the nutrient in the ileal digesta or feces; all values were expressed as a percentage of dry matter (DM).
AMEn was calculated using the equation described by Sibbald and Slinger (1963) except the nitrogen correction factor was used as 8.22 kcal/g.
DNA Extraction, PCR, and Bioinformatic Analysis
Because 9% inclusion of both hulls provided more fermentable fiber compounds for gut microbiota compared with control, control group and 9% inclusion level of almond hulls were selected in the present study to maximize the determinant of the information matrix for the ANOVA model. The D-optimality criterion was used for selecting the treatments for microbiota analysis (Mandal et al., 2015). DNA extraction of cecal microorganisms was performed using QIAamp Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instruction. The quality of the extracted DNA was ascertained by running 2 μL aliquot on 1% agarose gel electrophoresis. After DNA extraction, all samples were normalized to 10 ng/μL using nuclease-free water. Next, library preparation was performed by amplicon PCR, and amplification was performed using primers targeting 16S rRNA gene. The forward and reverse primer sequences containing illumina overhang adapter and locus-specific sequence for 16S rRNA amplicon were 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′ and 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′, respectively. After the clean-up of the amplicon PCR product, and index PCR was performed for multiplexing run by attaching known Nextera XT dual indices adapter sequence to the target amplicon. Later, the indexed amplicon was outsourced to the University of Georgia Genomics and Bioinformatics Core for sequencing on Illumina MiSeq platform. The paired-end sequencing was performed for 300 bp and the V3-V4 hypervariable region of 16S rRNA gene was targeted.
Demultiplexed sequences received from the core facility was imported into Quantitative Insights Into Microbial Ecology (QIIME version 2.0 release 2019.4, Bolyen et al., 2019) for further processing. Denoising, sequence trimming, and filtering were performed using DADA2 pipeline. The processing for phylogeny and taxonomy was performed using QIIME plugins and a sampling depth of 40,000 sequences per sample was chosen. For taxonomy, a Naïve Bayes classifier pretrained on the Greengenes 13_8 99% OTU was applied. The classified sequences were then analyzed for alpha and beta diversity and the taxonomic composition of the samples was run for relative abundances using taxa bar plot plugin and differential abundance using linear discriminant analysis.
Statistical Analysis
Growth performance and nutrient digestibility results from the prime and California-type hulls with control were analyzed separately via one-way ANOVA for a completely randomized design using the GLM procedure of SAS 9.4. Significant level was set at P < 0.05. Each battery cage was regarded as a statistical unit. Treatment means were further separated using Tukey's range test. A Kruskal-Wallis pairwise test was performed for alpha diversity, and PERMANOVA was used for beta diversity analysis in QIME 2. A histogram was generated for linear discriminant analysis effect size.
Results and discussion
Nutrient Matrix Values of Almond Hulls
Nutrient matrix values of the prime and California-type hulls derived from proximate analysis and rooster assays are presented in Table 2. The prime and California-type hulls contained TMEn at 1,624 and 1,514 kcal/kg, respectively. The prime and California-type hulls had 57.43 and 48.90% of nitrogen-free extract and 18.14 and 15.89% of sugar, respectively. The prime and California-type hulls as fibrous resources contained 13.1 and 26.4% of crude fiber and 4.8 and 5.0% of crude protein, respectively. The prime hulls contained 2,700 ppm of Ca, 900 ppm of P, and 36,300 ppm of K, whereas the California-type hulls had 2,300 ppm of Ca, 800 ppm of P, and 27,600 ppm of K. The remaining minerals and amino acid contents were similar between 2 types of hulls.
Table 2.
Nutrient matrix values of almond hulls (air-dry basis).
| Item | Energy (kcal/kg) |
Proximate analysis and sugar profile (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GE | AMEn | CP | DM | EE | Ash | CF | NFE | Sugar | ||
| Prime | 3,699 | 1,624 | 4.80 | 85.50 | 1.62 | 8.54 | 13.11 | 57.43 | 18.14 | |
| CA | 4,003 | 1,514 | 5.01 | 88.11 | 1.87 | 5.98 | 26.35 | 48.90 | 15.89 | |
| Mineral content (ppm) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca | P | K | Na | Mg | Mn | Fe | Al | Zn | Cu | |
| Prime | 2,700 | 900 | 36,300 | 10 | 0.12 | 8 | 173 | 17 | 8 | 6 |
| CA | 2,300 | 800 | 27,600 | 10 | 0.09 | 9 | 137 | 6 | 6 | 6 |
| Indispensable amino acid content (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arg | His | Ile | Leu | Lys | Met | Phe | Thr | Trp | Val | |
| Prime | 0.13 | 0.07 | 0.12 | 0.20 | 0.15 | 0.04 | 0.13 | 0.13 | <0.02 | 0.17 |
| CA | 0.12 | 0.07 | 0.10 | 0.17 | 0.14 | 0.03 | 0.12 | 0.11 | <0.02 | 0.15 |
Abbreviations: CA, California-type hulls; CF, crude fiber; CP, crude protein; DM, dry matter; EE, ether extract; GE, gross energy; NFE, nitrogen-free extract; Sugar, fermentable sugar; TMEn, nitrogen-corrected true metabolizable energy.
Because the almond hulls were the flesh from the almond fruit, sugars were the primary metabolizable energy source, which was very different from cereals whose metabolizable energy comes from starch. The analyzed sugar contents in the prime and California-type hulls are lower than the previous report in literature with a range of 21.2 to 37.3% by Holtman et al. (2015). The variation in sugar content may come from different almond species and extraction methods used. It must be noticed that the crude fiber content in the almond hulls is correlated to its quality as a feed ingredient because the excessive crude fibers in the hulls are mainly lignin from shells or woody mass of the trees which has little nutrient values for monogastric animals such as poultry and swine due to a lack of endogenous fiber digesting enzymes (Meng and Slominski, 2005; Davy et al., 2016). Crude fiber and pectin are the major antinutrient factors in almond hulls (Diaz-Vargas et al., 2018).
In terms of minerals in the almond hull samples, potassium and calcium were most abundant for the prime and California-type hulls. Compared with cereal or legume, there was no phytic acid presented in the almond hulls because phytic acid mainly existed in kernels as phosphorus storage (Huisman and Tolman, 1992). Both almond hull samples had a low amount of amino acids and all the indispensable amino acids were determined nondigestible based on rooster assays (data not shown). This is in agreement with the studies in ruminants and swine that the nitrogen in almond hulls were not utilized by dairy cows or lambs (Calvert and Parker, 1985; Clutter and Rodiek, 1992; Rad et al., 2016; Williams et al., 2018).
Almond Hulls and Broiler Growth Performance
For the prime hulls, during 0–7 d of age, in comparison with control, inclusion at 3 to 9% had no negative effects on growth performance except 3% which reduced (P = 0.040) FI (Table 3). For the California-type hulls, during 0–7 d, there were no significant differences in growth performance among the treatments; during 0–19 d, 9% treatment increased FI (P = 0.020) and FCR (P = 0.004) compared with control.
Table 3.
Effect of almond hulls on the growth performance of broilers.
| Item | Control | Inclusion rate |
P-value Treatments |
SEM | ||
|---|---|---|---|---|---|---|
| 3% | 6% | 9% | ||||
| Prime hulls | ||||||
| 0–7 d | ||||||
| BWG | 122 | 108 | 115 | 112 | 0.109 | 3.97 |
| FI | 142a | 126b | 141a | 132a,b | 0.040 | 4.19 |
| FCR | 1.169 | 1.173 | 1.184 | 1.236 | 0.170 | 0.02 |
| 0–18 d | ||||||
| BWG | 661 | 632 | 638 | 645 | 0.384 | 11.8 |
| FI | 981 | 1,009 | 1,006 | 988 | 0.694 | 19.3 |
| FCR | 1.486 | 1.599 | 1.579 | 1.535 | 0.061 | 0.03 |
| California-type hulls | ||||||
| 0–7 d | ||||||
| BWG | 122 | 111 | 110 | 114 | 0.145 | 3.81 |
| FI | 142 | 132 | 130 | 137 | 0.140 | 3.83 |
| FCR | 1.169 | 1.188 | 1.188 | 1.202 | 0.490 | 0.01 |
| 0–18 d | ||||||
| BWG | 661 | 618 | 642 | 624 | 0.124 | 12.9 |
| FI | 981b | 972b | 1006a,b | 1057a | 0.020 | 18.9 |
| FCR | 1.486b | 1.577b | 1.573b | 1.697a | 0.004 | 0.03 |
a,bMeans within a row with different superscripts differ at P < 0.05.
Abbreviations: BWG, body weight gain, g/bird; FCR, feed conversion ratio; FI, feed intake, g/bird.
Almond hulls inclusion from 3 to 9% led to an increase in crude fiber in the prime and California-type hulls diets from 2.16 to 3.13% and from 2.88 to 4.32%, respectively. Jiménez-Moreno et al. (2013a,b) reported that 3% of dietary fiber was considered as a moderate level for broilers, and dietary fiber up to 3.6% in broiler diets could stimulate the development of the organs of the gastrointestinal tract in young broilers. In addition, the major fibers in almond hulls are water insoluble including cellulose, hemicellulose, and lignin (Holtman et al., 2015). It has been reported that insoluble fiber could improve nutrient digestion and utilization in broilers (Adedokun et al., 2012); however, high dietary inclusion of fiber could be detrimental for broiler growth (Mateos et al., 2012). Owing to the higher fiber content in 9% California-type hulls, this diet showed more pronounced negative effects on BWG, FI, and FCR than the diets having any levels of prime hulls or lower levels (3 and 6%) of California-type hulls for broilers during 0–19 d of age. Feed conversion ratio in the prime hulls at 6 and 9%, or the California-type hulls at 3 and 6% were not significantly different compared with the control, but the results were numerically not favorable. The numeric reduction in growth performance on higher almond hull levels may lead to a production reduction during the whole production period because our previous study suggested that layers fed CA type hulls at 15% had a lower body weight and fat percentage (Wang et al., 2020.) However, further studies are necessary for determination effect of almond hulls on broiler, particularly for the whole growth period.
The energy from almond hulls is mainly from fermentable sugars including glucose, fructose, and sucrose. Compared with the starch in cereals, sugars have a smaller molecule weight and are more readily absorbed (Aller et al., 2011). The higher fermentable sugar content from 9% inclusion rate of the prime hulls may also partly contribute to the recovery of FCR; however, the beneficial effects might have been mainly from the increasing crude fat level compared with lower inclusion rates of almond hull treatments. The slightly negative effect of the California-type hulls on BWG could be explained by the high fiber diet because fibrous diet regime is associated with weight loss especially on body fat (Mueller-Cunningham et al., 2003); however, the effect of almond hulls and dietary fiber on broiler body compositions needs further studies.
Almond Hulls and Nutrient Digestibility
Inclusion of the prime hulls at 3% decreased (P ≤ 0.001) ileal digestibility of DM and AMEn, and 9% decreased (P ≤ 0.001) DM and ileal protein digestibility. However, 6% had no significant effects on the ileal digestibility of DM and protein, and AMEn (Table 4). For the California-type hulls, ileal DM digestibility was decreased at 9% (P = 0.001) compared with the control.
Table 4.
Effect of almond hulls on the nutrient digestibility of broilers.
| Item | Control | Inclusion rate |
P-value Treatments |
SEM | ||
|---|---|---|---|---|---|---|
| 3% | 6% | 9% | ||||
| Prime hulls | ||||||
| Ileal DM digestibility (%) | 69.8a | 66.2b | 67.1a,b | 62.0c | <0.001 | 0.97 |
| AMEn (kcal/kg) | 2,913a | 2,646b | 2,817a | 2,878a | 0.002 | 46.5 |
| Ileal protein digestibility (%) | 72.7a | 70.5a | 72.1a | 64.4b | 0.001 | 1.32 |
| Total track protein digestibility (%) | 60.7 | 50.8 | 54.6 | 58.5 | 0.068 | 2.49 |
| California-type hulls | ||||||
| Ileal DM digestibility (%) | 69.8a | 65.5b,c | 67.7a,b | 63.3c | 0.001 | 0.95 |
| AMEn (kcal/kg) | 2,914 | 3,045 | 2,819 | 2,808 | 0.061 | 35.6 |
| Ileal protein digestibility (%) | 72.7 | 70.5 | 71.3 | 70.6 | 0.687 | 1.32 |
| Total track protein digestibility (%) | 60.7 | 59.2 | 53.5 | 60.8 | 0.129 | 2.33 |
a,b,cMeans within a row with different superscripts differ at P < 0.05.
Abbreviations: AMEn, nitrogen-corrected apparent metabolizable energy; DM, dry matter.
A lower ileal DM and protein digestibility from 9% almond hulls may be explained by the nondigestible fiber content in almond hulls. It was also reported that ruminants could not utilize the crude protein from almond hulls (Askelson et al., 2014; Rad et al., 2016). Studies on swine and equine also recommended not considering the nitrogen in almond hulls as a protein source (Calvert and Parker, 1985; Clutter and Rodiek, 1992). Another plausible reason for the decrease of crude protein digestibility was the tannins in plants bound to the protein from plant-derived ingredients because almond seed is reported to contain the condensed tannins from 70 to 120 mg/g (Adamczyk et al., 2011; Kahlaoui et al., 2019). However, the total tract protein digestibility was not affected when 9% of prime hulls were used in the diet compared with the control. This result may imply part of bound or undigested protein might be utilized and retained in broilers due to the fermentation process underwent in ceca (Walugembe et al., 2015).
Different from ileal nutrient digestibility, the AMEn was increased as the almond hull inclusion rate increased from 3 to 9%. Because oil in the diet increases the passage time and nutrient digestion (Nitsan et al., 1997), the increasing oil content, when the inclusion rate of almond hulls in the diets was increased to make all diets isocaloric in the present study, could be the major contributor to the increase in AMEn. In addition, the AMEn and total tract protein digestibility of prime hulls at 3% is 400 kcal/kg and 9% lower than CA type hulls, respectively. This difference may be due to the different fiber amount and type in 2 almond hull species. The higher fiber content in CA type hulls is from the almond tree biomass and almond shells, which contain mainly insoluble fiber including lignin and cellulose. California-type hulls used in the present study had 26% crude fiber, which is twice as the amount of crude fiber in the prime hulls. In the regulation of California Department of Agriculture and Food, almond hulls containing more than 15% fiber are required to label as almond hulls and shells. The fibers from shells are mainly lignin and have little nutrient value to birds. The fibers from shells are mainly lignin and have little nutrient value to birds. The insoluble fiber ingredients are chemically inert and have been commonly used as a filler for broiler breeders (Leung et al., 2018) and had no interaction with other nutrients. For prime hulls, the fiber is mainly from almond flesh, which has mainly soluble fiber-like pectin. Inclusion of insoluble fiber at low or moderate level is favorable compared with soluble fiber because of its beneficial effect on broiler performance and nutrient digestibility (Mateos et al., 2012).
In the present result, the excessive lignin from shells in the California-type hulls caused an increase in FI and FCR. The California-type hulls might have diluted available nutrients in this trial, exerting negative effects on broiler FCR and nutrient digestibility. The role of fiber from almond hulls in broiler nutrition is also an interesting topic in the future research because several studies have showed that a moderate dietary fiber had a positive effect on broilers (Mateos et al., 2012; Jiménez-Moreno et al., 2013a; Krás et al., 2013). The future use of almond hulls requires more researches, particularly on the antioxidant and fiber effect.
Almond Hulls and Cecal Microbiota Diversity
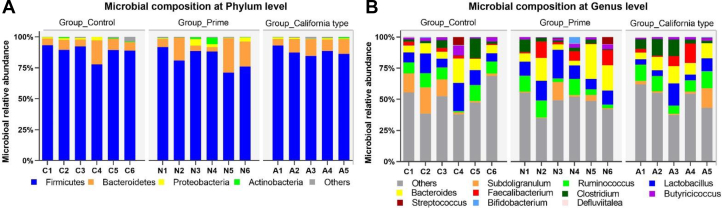
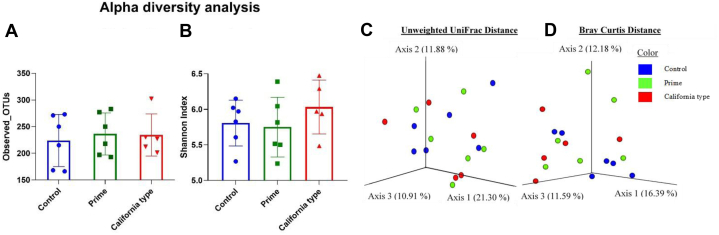
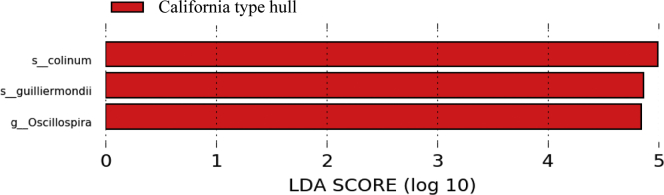
The relative abundance of bacteria among the treatments revealed that Firmicutes and Bacteroidetes were the most abundant phyla, whereas Subdoligranulum, Ruminococcus, Lactobacillus, and Bacteroides were the most abundant genus across the treatments (Figure 1). The statistical significance for alpha (observed OUT and Shannon index) and beta diversity (Unweighted UniFrac and Bray Curtis distance) is presented in Table 5. There was no difference across the treatments for both alpha diversity and beta diversity (Figure 2) for gut microbiota in broilers (Table 5). The linear discriminant analysis (Figure 3) revealed that the California-type hulls group had a higher abundance of Clostridium colinum of family Lachnospiraceae and Oscillospira guilliermondii of family Ruminococcaceae compared with control and the prime hull groups (P < 0.05).
Figure 1.
Stacked bar graph displayed a comparison of microbial relative abundance at phylum (A) level and genus (B) level among treatments in broilers at 19 d of age. Control (C1–C6), 9% prime hulls (N1–N6), 9% California-type hulls (A1–A5).
Table 5.
Statistical significance for alpha and beta diversity analysis of microbiota of broilers.1
| Item | Alpha diversity |
Beta diversity |
|||
|---|---|---|---|---|---|
| Observed OTU | Shannon index | Unweighted UniFrac | Bray Curtis | ||
| Overall P-value | 0.857 | 0.589 | 0.430 | 0.702 | |
| Group 1 | Group 2 | P-value | P-value | P-value | P-value |
| 9% Ca type | Control | 0.855 | 0.465 | 0.636 | 0.391 |
| 9% Ca type | 9% Prime | 0.855 | 0.361 | 0.243 | 0.639 |
| Control | 9% Prime | 0.470 | 0.631 | 0.444 | 0.805 |
Statistical analyses were performed using the Kruskal-Wallis test for alpha diversity and pairwise PERMANOVA for beta diversity analyses.
Figure 2.
Bar chart shows alpha diversity (A: observed OTU and B: Shannon Index) and principal coordinate analysis (PCoA) plot shows beta diversity (C: Unweighted UniFrac and D: Bray Curtis) analysis of treatments at 40,000 reads depth per sample of cecal contents from broilers at 19 d of age fed control, 9% prime hulls, and 9% CA-type hulls.
Figure 3.
Histogram showed linear discriminant analysis (LDA) scores of taxa differentially abundant in microbiota samples of broilers feeding control, 9% prime hulls, and 9% CA-type hulls diet at 19 d of age. Statistical analyses were performed using linear discriminant analysis effect size at P < 0.05.
In general, the treatments did not cause major changes in gut microbial diversity. The population of most normal microbiota was similar across the treatments. Furthermore, a lack of significant beta diversity suggests that selective enrichment of some bacterial species across the treatments is not high. However, previous studies showed that higher fiber contents from alfalfa may contribute to the gut fermentation and microbial diversity (Denayrolles et al., 2007; Dunkley et al., 2007). The different results from the present studies may be due to the fiber sources from almond hulls which are mainly lignin and cellulose. Lignin could covalently link with carbohydrates through ester and ether bonds to form lignin carbohydrate complexes to prevent degradation (Wan et al., 2010).
It was interesting to note that the population of certain bacteria in the genus Clostridium and Oscillospira was differentially abundant in the California-type hulls group compared with the control, particularly for C. colinum. These bacteria are known for causing ulcerative enteritis in broiler, quail, and pigeon (Prescott., 2016). It is possible that increasing proportion of C. colinum is associated with the high fiber content in diets containing 9% CA-type hulls because it has been reported that diets containing fibrous ingredients associated with increasing incidence of disease necrotic enteritis (Choct and Kocher, 2000; Liu et al., 2017). However, another study from our laboratory feeding almond hulls to Eimeria-challenged birds did not further compromise growth performance or cause higher lesion scores compared to the Eimeria-challenged group (Wang et al., 2020). Thus, the increasing proportion of the C. colinum species may not cause any further clinical or subclinical symptoms; however, further studies are necessary to confirm this observation.
Conclusions
Diets containing the prime hulls showed a comparative BWG but a numerically higher FCR than the corn and soybean control diet, whereas broilers fed California-type hulls at 9% compromised both BWG and FCR. In addition, broilers fed the California-type hulls nonetheless showed an abundant C. colinum present in the ceca. Almond hulls could be used at a moderate level in the broiler diet as a dietary energy and fiber source while the inclusion level needs to be further investigated to adjust according to the crude fiber content.
Disclosures
There is no conflict of interest.
References
- Adamczyk B., Adamczyk S., Smolander A., Kitunen V. Tannic acid and Norway spruce condensed tannins can precipitate various organic nitrogen compounds. Soil Biol. Biochem. 2011;43:628–637. [Google Scholar]
- Adhikari R., White D., House J., Kim W.J.P.S. Effects of additional dosage of vitamin D3, vitamin D2, and 25-hydroxyvitamin D3 on calcium and phosphorus utilization, egg quality and bone mineralization in laying hens. Poult. Sci. 2020;99:364–373. doi: 10.3382/ps/pez502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aller E.E., Abete I., Astrup A., Martinez J.A., Baak M.A. Starches, sugars and obesity. Nutrients. 2011;3:341–369. doi: 10.3390/nu3030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond Board of California . Almond Bd. Ca; Sacramento CA: 2019. Almond Almanac. [Google Scholar]
- AOAC . 15th ed. Association of Official Analytical Chemists; Washington, DC: 1990. Official Methods of Analysis. [Google Scholar]
- Adedokun S.A., Ajuwon K.M., Romero L.F., Adeola O. Ileal endogenous amino acid losses: Response of broiler chickens to fiber and Mild Coccidial Vaccine Challenge. Poult. Sci. 2012;91:899–907. doi: 10.3382/ps.2011-01777. [DOI] [PubMed] [Google Scholar]
- Askelson T.E., Campasino A., Lee J.T., Duong T. Evaluation of phytate-degrading Lactobacillus culture administration to broiler chickens. Appl. Environ. Microbiol. 2014;80:943–950. doi: 10.1128/AEM.03155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., Bai Y., Bisanz J.E., Bittinger K., Brejnrod A., Brislawn C.J., Brown C.T., Callahan B.J., Caraballo-Rodríguez A.M., Chase J., Cope E.K., Da Silva R., Diener C., Dorrestein P.C., Douglas G.M., Durall D.M., Duvallet C., Edwardson C.F., Ernst M., Estaki M., Fouquier J., Gauglitz J.M., Gibbons S.M., Gibson D.L., Gonzalez A., Gorlick K., Guo J., Hillmann B., Holmes S., Holste H., Huttenhower C., Huttley G.A., Janssen S., Jarmusch A.K., Jiang L., Kaehler B.D., Kang K.B., Keefe C.R., Keim P., Kelley S.T., Knights D., Koester I., Kosciolek T., Kreps J., Langille M.G.I., Lee J., Ley R., Liu Y.X., Loftfield E., Lozupone C., Maher M., Marotz C., Martin B.D., McDonald D., McIver L.J., Melnik A.V., Metcalf J.L., Morgan S.C., Morton J.T., Naimey A.T., Navas-Molina J.A., Nothias L.F., Orchanian S.B., Pearson T., Peoples S.L., Petras D., Preuss M.L., Pruesse E., Rasmussen L.B., Rivers A., Robeson M.S., 2nd, Rosenthal P., Segata N., Shaffer M., Shiffer A., Sinha R., Song S.J., Spear J.R., Swafford A.D., Thompson L.R., Torres P.J., Trinh P., Tripathi A., Turnbaugh P.J., Ul-Hasan S., van der Hooft J.J.J., Vargas F., Vázquez-Baeza Y., Vogtmann E., von Hippel M., Walters W., Wan Y., Wang M., Warren J., Weber K.C., Williamson C.H.D., Willis A.D., Xu Z.Z., Zaneveld J.R., Zhang Y., Zhu Q., Knight R., Caporaso J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert C., Parker K. Almond hulls produce unexpected results in hog trials. Ca. Agri. 1985;39:14–15. [Google Scholar]
- Choct M., Kocher A. Non-starch carbohydrates: digestion and its secondary effects in monogastrics. Proc. Nutr. Soc. 2000;24:31–38. Nutrition Society of Australia; 1998. [Google Scholar]
- Clutter S., Rodiek A. Feeding value of diets containing almond hulls. J. Equine Vet. Sci. 1992;12:99–102. [Google Scholar]
- Davy J.S., Nader G.A., Stackhouse J.W. University of Calidornia; Daivs: 2016. Drought Tip: Supplemental Feeds for Cattle Operations during Drought. [Google Scholar]
- Denayrolles M., Arturo-Schaan M., Massias B., Bebin K., Elie A., Panheleux-Lebastard M., Urdaci M. Effect of diets with different fibrous contents on broiler gut microflora and short-chain fatty acid (SCFA) production. 16th Euro. Symp. Poult. Nutri. 2007;269:272. [Google Scholar]
- Diaz-Vargas M., Murakami A.E., Pintro P.T.M., Ospina-Rojas I.C., de Souza C.H.P., Eyng C. Dehydrated citrus pulp in broiler diets. Can. J. Anim. Sci. 2018;99:33–40. [Google Scholar]
- Dunkley K., Dunkley C., Njongmeta N., Callaway T., Hume M., Kubena L., Nisbet D., Ricke S. Comparison of in vitro fermentation and molecular microbial profiles of high-fiber feed substrates incubated with chicken cecal inocula. Poult. Sci. 2007;86:801–810. doi: 10.1093/ps/86.5.801. [DOI] [PubMed] [Google Scholar]
- Esfahlan A.J., Jamei R., Esfahlan R.J. The importance of almond (Prunus amygdalus L.) and its by-products. Food Chem. 2010;120:349–360. [Google Scholar]
- Holtman K.M., Offeman R.D., Franqui-Villanueva D., Bayati A.K., Orts W.J. Countercurrent extraction of soluble sugars from almond hulls and Assessment of the Bioenergy potential. J. Agr. Food Chem. 2015;63:2490–2498. doi: 10.1021/jf5048332. [DOI] [PubMed] [Google Scholar]
- Homedes J., Roura E., Keim N., Brown D. Almond hulls in swine diet reduce body fat. Ca. Agr. 1993;47:27–28. [Google Scholar]
- Huisman J., Tolman G.H. Antinutritional Factors in the Plant Proteins of Diets for Non-ruminants. In: Garnsworthy P.C., Haresign W., Cole D.J.A., editors. Recent Advances in Animal Nutrition. Elsevier; New York, NY: 1992. pp. 4–8. [Google Scholar]
- Jha R., Leterme P. Feed ingredients differing in fermentable fibre and indigestible protein content affect fermentation metabolites and faecal nitrogen excretion in growing pigs. Anim. 2012;6:603–611. doi: 10.1017/S1751731111001844. [DOI] [PubMed] [Google Scholar]
- Jiménez-Moreno E., Frikha M., de Coca-Sinova A., García J., Mateos G. Oat hulls and sugar beet pulp in diets for broilers 1. Effects on growth performance and nutrient digestibility. Anim. Feed Sci. Tech. 2013;182:33–43. [Google Scholar]
- Jiménez-Moreno E., Frikha M., de Coca-Sinova A., Lázaro R.P., Mateos G.G. Oat hulls and sugar beet pulp in diets for broilers. 2. Effects on the development of the gastrointestinal tract and on the structure of the jejunal mucosa. Anim. Feed Sci. Tech. 2013;182:44–52. [Google Scholar]
- Jones M.K., Richardson K.E., Starkey C.W., Dale N.M., Davis A.J. Impact of Extended Heat treatment on the amino acid digestibility and TMEn content of a Formaldehyde-treated diet. J. Appl. Poult. Res. 2018;27:550–554. [Google Scholar]
- Kahlaoui M., Borotto D.V.,S., Giovine F., Ben Haj Kbaier H., Bouzouita N., Barbosa Pereira L., Zeppa G. Characterization of polyphenolic compounds extracted from different Varieties of almond hulls (Prunus dulcis L.) Antioxidants. 2019;8:647. doi: 10.3390/antiox8120647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krás R.V., Kessler A.d.M., Ribeiro A.M.L., Henn J., Dos Santos I., Halfen D.P., Bockor L.J. Effect of dietary fiber and genetic strain on the performance and energy balance of broiler chickens. Braz. J. Poult. Sci. 2013;15:15–19. [Google Scholar]
- Latta M., Eskin M. A simple and rapid colorimetric method for phytate determination. J. Agri. Food Chem. 1980;28:1313–1315. [Google Scholar]
- Leung H., Arrazola A., Torrey S., Kiarie E. Utilization of soy hulls, oat hulls, and flax meal fiber in adult broiler breeder hens. Poult. Sci. 2018;97:1368–1372. doi: 10.3382/ps/pex434. [DOI] [PubMed] [Google Scholar]
- Liu N., Wang J.Q., Gu K.T., Deng Q.Q., Wang J.P. Effects of dietary protein levels and multienzyme supplementation on growth performance and markers of gut health of broilers fed a miscellaneous meal based diet. Anim. Feed Sci. Tech. 2017;234:110–117. [Google Scholar]
- Mandal A., Wong W.K., Yu Y. Algorithmic searches for optimal designs. In: Dean A., Morris M., Stufken J., Bingham D., editors. Handbook Design Analysis Experiments. Chapman and Hall/CRC; Boca Raton, FL: 2015. pp. 755–783. [Google Scholar]
- Mateos G.G., Jiménez-Moreno E., Serrano M.P., Lázaro R.P. Poultry Response to high levels of dietary fiber sources Varying in Physical and chemical Characteristics. J. Appl. Poult. Resear. 2012;21:156–174. [Google Scholar]
- Meng X., Slominski B.J.P.S. Nutritive values of corn, soybean meal, canola meal, and peas for broiler chickens as affected by a multicarbohydrase preparation of cell wall degrading enzymes. Poult. Sci. 2005;84:1242–1251. doi: 10.1093/ps/84.8.1242. [DOI] [PubMed] [Google Scholar]
- Mueller-Cunningham W.M., Quintana R., Kasim-Karakas S.E. An ad libitum, very low-fat diet results in weight loss and changes in nutrient intakes in postmenopausal women. J. Am. Diet. Assoc. 2003;103:1600–1606. doi: 10.1016/j.jada.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Nitsan Z., Dvorin A., Zoref Z., Mokady S. Effect of added soyabean oil and dietary energy on metabolisable and net energy of broiler diets. Br. Poult. Sci. 1997;38:101–106. doi: 10.1080/00071669708417948. [DOI] [PubMed] [Google Scholar]
- Oztürk-Urek R., Bozkaya L.A., Tarhan L. The effects of some antioxidant vitamin- and trace element-supplemented diets on activities of SOD, CAT, GSH-Px and LPO levels in chicken tissues. Cell Biochem. Funct. 2001;19:125–132. doi: 10.1002/cbf.905. [DOI] [PubMed] [Google Scholar]
- Prescott J.F., Smyth J.A., Shojadoost B., Vince A. Experimental reproduction of necrotic enteritis in chickens: a review. Avian. Pathol. 2016;45:317–322. doi: 10.1080/03079457.2016.1141345. [DOI] [PubMed] [Google Scholar]
- Prgomet I., Gonçalves B., Domínguez-Perles R., Pascual-Seva N., IRNA Barros A. Valorization challenges to almond residues: Phytochemical composition and functional application. Molecules. 2017;22:1774. doi: 10.3390/molecules22101774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad M.I., Rouzbehan Y., Rezaei J. Effect of dietary replacement of alfalfa with urea-treated almond hulls on intake, growth, digestibility, microbial nitrogen, nitrogen retention, ruminal fermentation, and blood parameters in fattening lambs. J Anim Sci. 2016;94:349–358. doi: 10.2527/jas.2015-9437. [DOI] [PubMed] [Google Scholar]
- Sadeghi A., Toghyani M., Gheisari A. Effect of various fiber types and choice feeding of fiber on performance, gut development, humoral immunity, and fiber preference in broiler chicks. Poult. Sci. 2015;94:2734–2743. doi: 10.3382/ps/pev292. [DOI] [PubMed] [Google Scholar]
- Sibbald I., Slinger S.J. A biological assay for metabolizable energy in poultry feed ingredients together with findings which demonstrate some of the problems associated with the evaluation of fats. Poult. Sci. 1963;42:313–325. [Google Scholar]
- Takeoka G.R., Dao L.T. Antioxidant Constituents of almond [Prunus dulcis (Mill.) D.A. Webb] hulls. J. Agric. Food Chem. 2003;51:496–501. doi: 10.1021/jf020660i. [DOI] [PubMed] [Google Scholar]
- Walugembe M., Hsieh J., Koszewski N., Lamont S., Persia M., Rothschild M.F. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poult. Sci. 2015;94:2351–2359. doi: 10.3382/ps/pev242. [DOI] [PubMed] [Google Scholar]
- Wan C., Li Y. Microbial delignification of corn stover by Ceriporiopsis subvermispora for improving cellulose digestibility. Enzyme Microb. Tech. 2010;47:31–36. [Google Scholar]
- Wang J., Kong F., Kim W.K. Effects of supplementation of almond hulls on growth performance, lesion score and anti-oxidative enzyme activities in Eimeria-challenged broilers. Poult. Sci. Ann. Meet. 2020;106:214. (Abstr.) [Google Scholar]
- Wang J., Kong F., Kim W.K. Effect of almond hull as an alternative ingredient on laying hen performance, egg quality, and body composition. Int. Poult. Sci. Forum. 2020;96:313. (Abstr.) [Google Scholar]
- Williams C.H., David D.J., Iismaa O. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J. Agri. Sci. 1962;59:381–385. [Google Scholar]
- Williams S., Chaves A., Deighton M., Jacobs J., Hannah M., Ribaux B., Morris G., Wales W., Moate P. Influence of feeding supplements of almond hulls and ensiled citrus pulp on the milk production, milk composition, and methane emissions of dairy cows. J. Dairy Sci. 2018;101:2072–2083. doi: 10.3168/jds.2017-13440. [DOI] [PubMed] [Google Scholar]
- Yalchi T., Kargar S. Chemical composition and in situ ruminal degradability of dry matter and neutral detergent fiber from almond hulls. J. Food Agri. Env. 2010;8:781–784. [Google Scholar]