Abstract
This study was conducted to determine the effects of dietary addition of α-glyceryl monolaurate (α-GML) on growth performance, immune function, volatile fatty acids production and cecal microbiota in broiler chickens. A total of 480 1-day-old yellow-feathered broilers were randomly assigned in equal numbers to 4 dietary treatments: basal diet (NCO) or supplementations with 30 mg/kg bacitracin (ANT), 500 mg/kg α-GML, or 1,000 mg/kg α-GML (GML2). And, each treatment contained 8 replicates with 15 chickens per replicate. After supplementation with α-GML, the total BW gain and average daily weight gain of broilers increased significantly (P < 0.05) compared with the broilers on the NCO diet. Moreover, compared with the NCO group, higher levels of immune globulin M and immune globulin Y were observed in both GML groups and the ANT group. Concentrations of acetate, propionate, butyrate, valerate, and isovalerate in GML2 were significantly higher (P < 0.05) than those in the NCO group on day 28. However, acetate, propionate, valerate, and isovalerate concentrations were reduced to significantly (P < 0.05) lower than those in the NCO group on day 56. The abundance and diversity of microbiota were found to be improved in broilers that were supplemented with GML, using operational taxonomic unit and diversity analyses. Furthermore, the GML treatments increased favorable microbiota, particularly acid-producing bacteria, on day 28 and, also, reduced opportunistic pathogens, such as Alistipes tidjanibacter and Bacteroides dorei by day 56. These results suggest that α-GML supplementation modulates cecal microbiota and broiler immunity and improves volatile fatty acid levels during the early growth stages of broilers.
Key words: α-glyceryl monolaurate lactate, growth performance, immune globulin, volatile fatty acid, intestinal microbiota
Introduction
Antibiotics, which are used widely in the poultry industry, reduce the incidence of disease, improve growth rates and feeding efficiency, and relieve stress caused by poor breeding environments (Millet and Maertens, 2011; Allen et al., 2013). However, their overuse in animal feed has been recognized as one of the leading causes of the rapid spread of antimicrobial resistance (Barton, 2000; Xiong et al., 2018). Although there are some researchers having claimed that the overlap of antibiotics between human and chicken is very limited (AMI, 2014), any countries including those in the European Union have completely prohibited the use of antibiotics as growth promoters in animal feed (Lee et al., 2014). In China, antibiotics have been banned as growth promoter from feed in the year 2020 (Echemi, 2020). Prohibition of antibiotics encourages the development of alternatives, including plant extracts, probiotics, and organic acids (Allen et al., 2013; Stanton, 2013).
Glycerol monolaurate (GML), a medium-chain fatty acid ester, is found in coconut fat, palmetto, and human milk (Zhang et al., 2016a, Zhang et al., 2016b; Schlievert et al., 2019). Furthermore, it can be formed from the combination of lauric acid (C12) and glycerol (Langone et al., 2002). Glycerol monolaurate has been approved as a food-safe emulsifier and is commonly considered to be a nontoxic compound (Zhang et al., 2009, Zhang et al., 2016a, Zhang et al., 2016b; Zhang et al., 2009; Mo et al., 2019). Moreover, GML shows strong antipathogenic properties (e.g., against bacteria, viruses, and fungi), which has led to its wide use in the food industry (Li et al., 2009; Mueller and Schlievert, 2015; Seleem et al., 2016). Research has shown that GML could protect against Escherichia coli, Staphylococcus aureus, and Bacillus subtilis, and so, it is used in food preservation (Zhang et al., 2009; Yu et al., 2017). In vitro studies have shown that GML inhibits the production of beta-lactamase, toxic shock toxin-1, and other Staphylococcus exoproteins, and then, the inhibition of S. aureus growth was realized (Holland et al., 1994; Projan et al., 1994). It also reduces the production of Candida albicans biofilms and some bacterial biofilms (Hess et al., 2015; Lopes et al., 2016).
Recent studies have shown that GML can be used as a feed additive to improve animal growth performance and health (Fortuoso et al., 2019; Zhao et al., 2019). Fortuoso et al. (2019) found that GML treatment may improve growth performance, reduce numbers of parasite oocysts and E. coli, and augment the production of globulins, while leaving meat quality unaltered. Moreover, Zhao et al. (2019) showed that dietary GML improved egg quality. These studies have demonstrated the potent antimicrobial properties of GML in chicken diets. However, little is known about the effect of dietary α-GML (one of the major configuration compounds obtained through industrial synthesis) supplementation on immune function and gut health in broilers. This study aims to evaluate the effects of α-GML on growth performance, immune function, volatile fatty acid (VFA) production, and cecal microbiota composition in broiler chickens.
Materials and methods
Animals and Dietary Treatments
A total of 480 male 1-day-old Chinese yellow-feathered broiler chickens with an initial BW of 35.42 ± 0.37 g were randomly assigned in equal numbers into 4 dietary treatment groups as follows: 1) NCO group, a basal diet; 2) ANT group, a basal diet with 30 mg/kg bacitracin; 3) GML1 group, a basal diet with 500 mg/kg α-GML; and 4) GML2 group, a basal diet with 1,000 mg/kg α-GML. Each treatment group contained 8 replicates with 15 chickens were assigned randomly per replicate. Each replicate was assigned a cage randomly that had raised wire floors and contained a self-feeder and waterer to provide ad libitum access to feed and water. The experiment was lasted for 56 d including 2 phases. The basal diet in 2 phases (1–28 d and 28–56 d) was formulated to meet the specifications for broiler chickens suggested by the NRC (NRC, 1994) and Nutrient Requirements of Yellow-Feathered Broiler (NY/T 33, 2004, China) and contained no antibiotics (Table 1). The dosages of bacitracin and α-GML determintion were from our previous experiment (data not publishe) and the production were provided by Vegamax Biotechnology Co., Ltd. (Anji, China). And, the diet provided was in a powdered form. The temperature of the room was maintained at 32°C to 34°C for the first wk and then reduced by 3°C to 5°C per week to reach a final temperature of 26°C. The lighting program provided was 23L:1D until day 7 and then 18L:6D until day 56 age. The experiment was approved by the Animal Care and Use Committee of the Zhejiang Agriculture and Forestry University.
Table 1.
Composition and nutrient levels of the basal diet.
| Items | Ages (day) |
|
|---|---|---|
| 1–28 | 28–56 | |
| Ingredients(air-dry basis,%) | ||
| Corn | 53 | 53 |
| Soybean meal | 24.5 | 16 |
| Extruded soybean | 5 | 3 |
| Corn Distillers Dried Grains with Solubles | 8 | 8 |
| Rice bran | 8 | |
| Corn gluten | 2 | |
| Soybean oil | 1.7 | 4.5 |
| Limestone | 1.3 | 1.5 |
| Fermented soybean meal | 2.5 | |
| Premix12 | 4 | 4 |
| Total | 100.00 | 100.00 |
| Nutrient levels | ||
| ME (kcal/kg) | 2,916 | 3,090 |
| CP (%) | 20.3 | 17.2 |
| Lysine(%) | 1.19 | 0.96 |
| Methionine + Cysteine(%) | 0.89 | 0.74 |
| Calcium(%) | 0.87 | 0.73 |
| Total phosphorus(%) | 0.6 | 0.57 |
Minimal vitamin levels per kg of food: vitamin A (retinyl acetate), 1,500 IU; cholecalciferol, 200 IU; vitamin E (DL-α-tocopheryl acetate), 10 IU; riboflavin, 3.5 mg; pantothenic acid, 10 mg; niacin, 30 mg; cobalamin, 10 μg; choline chloride, 1,000 mg; biotin, 0.15 mg; folic acid, 0.5 mg; thiamine 1.5 mg; pyridoxine 3.0 mg.
Minimal mineral levels per kg of diet: Fe, 80.00 mg; Cu, 8.00 mg; Mn, 60.00 mg; Zn, 40.00 mg; I, 0.18 mg; Se, 0.15 mg.
Growth Performance
Broilers were weighed individually at the beginning, middle (28 d), and end (56 d) of the experiment. ADG for each cage was calculated.
Sample Collection
On day 28 and day 56, eight broilers with similar weights were taken from each treatment (1 bird selected from each replicates). After weighing, a 10-mL blood sample was collected from a wing vein of each bird. Serum samples were obtained by centrifuging at 3,000 × g for 10 min at 4°C. After blood samples were collected, the broilers were immediately euthanized by cervical dislocation. And, the cecal contents (approximately 2–3 g) were collected after the autopsy in a relatively clean working area, and sterilized instruments and sample collection tubes are used to minimize environmental contamination. All samples were stored at −80°C until analysis. And, to minimize effects of circadian variations on the measured parameters, the samples were collected from 1 bird per treatment by repeating the cycle of NCO, ANT, GML1, and GML2 until the end.
Serum Parameter Analysis
IgA, IgM, and IgY were assayed using chicken-specific immunoturbidimetry kits (Huamei Biological Engineering Research Institute, Wuhan, China) based on the ELISA and measured using a multifunctional microporous plate detector (BioTek Synergy H1).
Volatile Fatty Acid Analysis
Following the assay method of Yang et al. (2019), the concentration of cecal VFA was estimated by Headspace Sampler Gas Chromatography (Agilent Technologies) using a method by Thanh et al. (2009). One gram of cecum content was dissolved in water, and the supernatant was extracted after centrifugation to mix with 25% (m/v, 1:3) phosphorous acid. The concentration of VFA were measured using an Agilent Technologies 7890A Network System (Agilent Technologies) equipped with a 30 m × 0.32 mm × 1.8 μm column (DB-624; Agilent Technologies) and flame ionization detector.
16S rRNA Sequencing of Microflora in Cecum Contents
The microbial community in cecum contents collected from each broiler was analyzed. The Illumina-HiSeq platform (Novogene Bioinformatics Technology Co., Ltd., Beijing, China) was used to detect the V4 region of the 16S rRNA gene. There was a 97% similarity between the taxonomy and Ribosomal Database Project classifier, allocated using Quantitative Insights Into Microbial Ecology and UPARSE software. Clustering and species classification analyses of the operational taxonomic units (OTU) were based on valid data. As per the results of OTU clustering, corresponding species information and species-based abundance distributions can be obtained by using species annotations created for each OTU sequence. In addition, alpha diversity (species richness and evenness) was calculated along with a Venn diagram so as to understand which OTU were common or specific to different groups. We aligned OTU with multiple sequences to construct a phylogenetic tree. MetaStat software was used to do a principal component analysis and an unweighted pair-group method with arithmetic mean cluster tree, which can display the differences in community structure between different groups.
Statistical Analysis
The effect of the α-GML supplementation on the different parameters was analyzed by using 1-way ANOVA that was performed SPSS 22.0 software (SPSS Inc.). Differences were determined using the Tukey-Kramer test. Probability values less than 0.05 were considered statistically significant. Figures were prepared using GraphPad Prism, version 7.0 (Graphpad Software Inc.).
Result
Growth Performance
The effects of α-GML on the growth performance of broilers are shown in Table 2. The BW of broiler chickens in the GML1 and GML2 groups were increased (P < 0.05) compared with those of the NCO group on day 28 and day 56 but were not different from those in the ANT group. Moreover, the broilers in GML1 and GML2 had significantly higher (P < 0.05) ADG than those in the NCO group both on day 28 and day 56. However, BW and ADG in the GML1 treatment were not found to be different from those in GML2.
Table 2.
Effects of dietary supplementation of α-GML of growth performance in broilers.
| Items | Treatments1 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| NCO | ANT | GML1 | GML2 | |||
| BW, g | ||||||
| 1 d | 35.53 | 35.49 | 35.46 | 35.23 | 0.08 | 0.506 |
| 28 d | 472.50b | 512.68a | 521.79a | 508.61a | 6.20 | 0.001 |
| 56 d | 1,490.00b | 1,540.98a | 1,559.98a | 1,540.23a | 6.17 | 0.001 |
| ADG, (g/day) | ||||||
| 1–28 d | 15.61b | 17.04a | 17.37a | 16.91a | 0.16 | 0.001 |
| 29–56 d | 36.34 | 36.73 | 37.08 | 36.84 | 0.15 | 0.42 |
| 1–56 d | 25.97b | 26.88a | 27.22a | 26.88a | 0.11 | 0.001 |
a,bMean with different superscripts in the same row differ significantly (P < 0.05).
Abbreviations: ANT, basal diet supplemented with 30 mg/kg bacitracin; GLM, glycerol monolaurate; GML1, basal diet supplemented with 500 mg/kg α-GML; GML2, basal diet supplemented with 1,000 mg/kg α-GML; NCO, basal diet provided as control.
The mean represent results from 8 replicate cages per treatment.
Ig in Serum
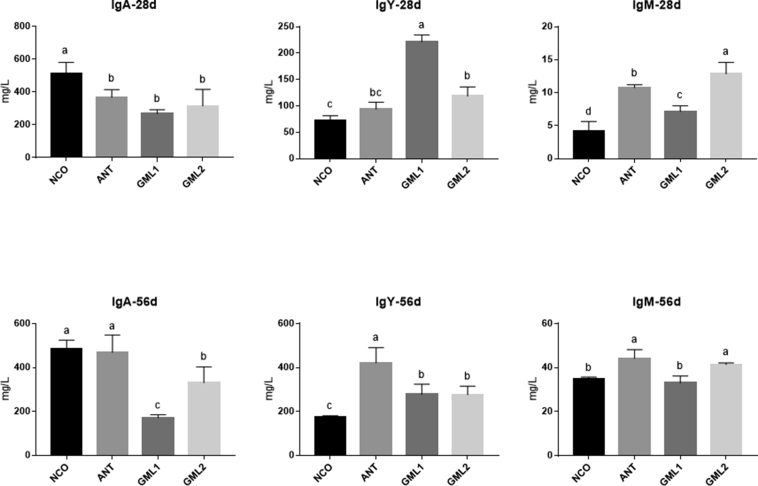
Serum samples were used to analyze the key Ig on day of 28 and 56 (Figure 1). Results show that compared with the NCO group, broilers fed with α-GML (GML1 and GML2) exhibited higher (P < 0.05) levels of IgM and IgY and lower (P < 0.05) IgA levels on day 28. And, at day 56, compared with the NCO and ANT groups, we observed a decrease (P < 0.05) in IgA levels in groups GML1 and GML2. Levels of IgY were higher (P < 0.05) in both GML1 and GML2 groups than that in the NCO group. In addition, IgM levels increased (P = 0.002) in the GML2 group compared with the NCO group but no significant differences between the GML1 and NCO (P = 0.337) (Figure 1).
Figure 1.
Levels of Ig in the serum of broiler chickens given experimental diets at day 28 and day 56. Abbreviations: ANT, basal diet supplemented with 30 mg/kg bacitracin; GML1, basal diet supplemented with 500 mg/kg α-GML; GML2, basal diet supplemented with 1,000 mg/kg α-GML; NCO, basal diet provided as the control. Bars represent mean ± SD (n = 8). Different lowercase letters (a, b) above bars represent significantly different means (P < 0.05).
Volatile Fatty Acid Levels in Cecal Contents
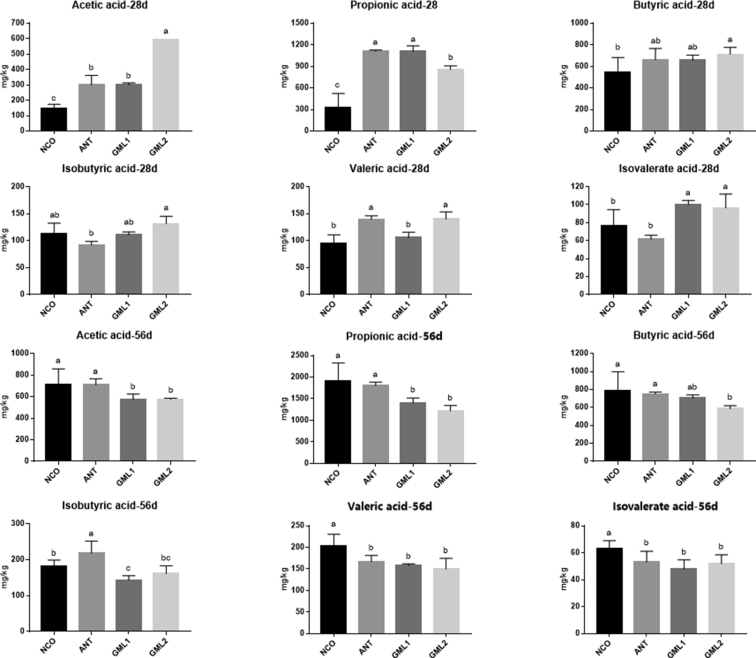
Levels of VFA (acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid) in cecum contents are presented in Figure 2. Results showed that, on day 28, concentrations of acetic acid, propionic acid, butyric acid, valeric acid, and isovaleric acid in GML2 broilers' cecal contents were higher (P < 0.05) than NCO. Furthermore, compared with the ANT group, acetic acid, isobutyric acid, and isovaleric acid levels in the GML2 group also were higher (P < 0.05) on day 28. Compared with the NCO group, α-GML in feed tended to cause a reduction in VFA, but no difference (P > 0.05) was detected in either the GML1 or GML2 groups on day 56. In addition, there was no difference (P = 0.254) in butyric acid levels in the GML1 group compared with the NCO or ANT groups at day 56.
Figure 2.
Levels of volatile fatty acid in cecum contents of broiler chickens diets at day 28 and day 56. Abbreviations: ANT, basal diet supplemented with 30 mg/kg bacitracin; GML1, basal diet supplemented with 500 mg/kg α-GML; GML2, basal diet supplemented with 1,000 mg/kg α-GML; NCO, basal diet provided as the control. Bars represent mean ± SD (n = 8). Different lowercase letters (a, b) above bars represent significantly different means (P < 0.05).
Cecal Microbiota
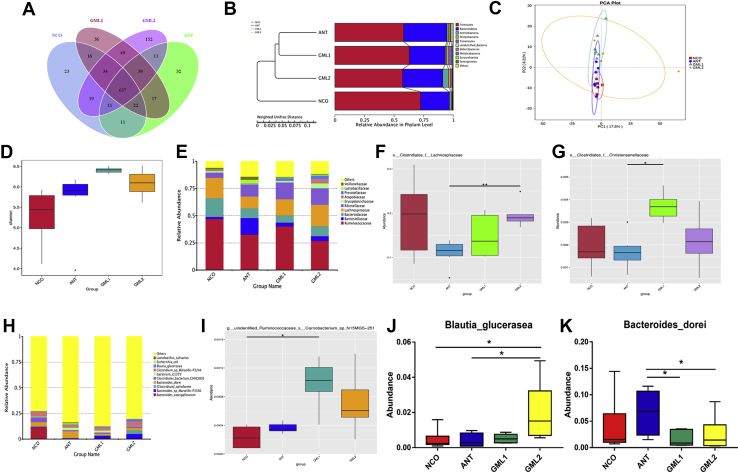
The abundance and diversity of cecal microbiota in each treatment are summarized in Figure 3 (day 28) and Figure 4 (day 56). The Venn diagram shows that a total of 617 OTU are shared among the 4 treatment groups. In addition, there are unique OTU in the GML1 (number: 56) and GML2 (number: 152) groups, and these are more numerous than those in the NCO and ANT groups on day 28 (Figure 3A). The unweighted pair-group method with arithmetic mean cluster tree revealed that the dominant bacterial phyla in broiler cecal contents in our study on day 28 were Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Tenericutes. The principal component analysis results indicate that the microflora of GML1 and GML2 broilers were different from the NCO group (Figure 3C). Alpha diversity (Shannon index) was higher in the GML groups, especially in the GML1 group, than in the NCO or ANT groups (Figure 3D).
Figure 3.
The abundance and diversity of microbial community in cecal contents of broilers at day 28. (A) The Venn diagram summarizing the numbers of common and unique OTU in the microflora community in cecal contents of broilers. (B) The UPGMA Cluster Tree displaying the relative abundances of predominant bacteria at the species level in each group (unweighted UniFrac distance). (C) The principle component analysis (PCA) plot about the cecal microflora. (D) The Shannon index reflecting species diversity within and between groups. (E, H) The top 10 relative abundance of microflora community (level family and level species) indicate by histogram. (F, G, I–K) The some bacteria with significant differences between groups (level family, level species) indicate by histogram. Abbreviations: ANT, basal diet supplemented with 30 mg/kg bacitracin; GML1, basal diet supplemented with 500 mg/kg α-GML; GML2, basal diet supplemented with 1,000 mg/kg α-GML; NCO, basal diet provided as the control; OTU, operational taxonomic unit; UPGMA, unweighted pair-group method with arithmetic mean. Bars represent mean ± SD (n = 6). ∗Means different (P < 0.05), ∗∗means significant difference (P < 0.01).
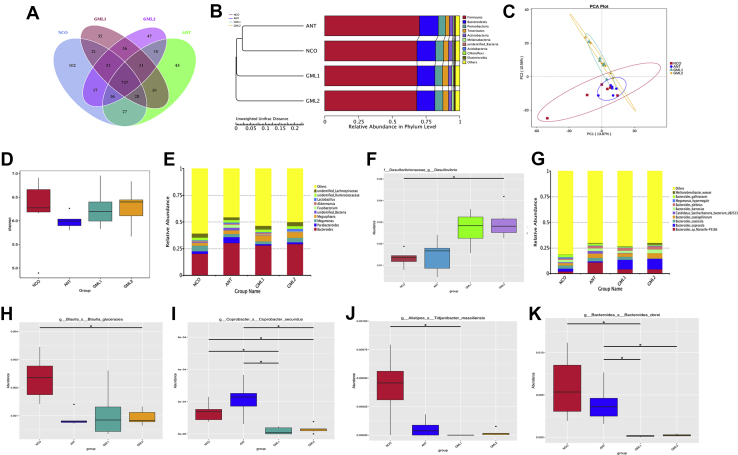
Figure 4.
The abundance and diversity of microbial community in cecal contents on broilers at day 56. (A) The Venn diagram summarizing the numbers of common and unique OTU in the microflora community in cecal contents of broilers. (B) The UPGMA Cluster Tree displaying the relative abundances of predominant bacteria at the species level in each group (unweighted UniFrac distance). (C) The principle component analysis (PCA) plot about the cecal microflora. (D) The Shannon index reflecting species diversity within and between groups. (E, G) The top 10 relative abundance of microflora community (level genes and level species) indicate by histogram. (F, H–K) The some bacterias with significant differences between groups (level genes, level species) indicate by histogram. Abbreviations: ANT, basal diet supplemented with 30 mg/kg bacitracin; GML1, basal diet supplemented with 500 mg/kg α-GML; GML2, basal diet supplemented with 1,000 mg/kg α-GML; NCO, basal diet provided as the control; OTU, operational taxonomic unit; UPGMA, unweighted pair-group method with arithmetic mean. Bars represent mean ± SD (n = 6). ∗Means different (P < 0.05), ∗∗means significant difference (P < 0.01).
The dominant bacterial families in the cecal contents of broilers in our study were Ruminococcaceae, Barnesiellaceae, Bacteroidaceae, Lachnospiraceae, and Rikenellaceae, as shown by taxonomic classification of the microbial compositions on day 28 (Figure 3E). In addition, the relative abundances of Lachnospiraceae in the GML1 group and Christensenellaceae in the GML2 group were higher (P < 0.05 and P < 0.05, respectively) than those in the ANT group (Figures 3F and 3G). At the species level, we observed that Bacteroides caecigallinarum, Bacteroides sp., Clostridium spiroforme, Bacteroides dorei, and Clostridiales bacterium were the dominant strains in all samples (Figure 3H). Moreover, we found that the acid-producing bacteria Ruminococcaceae carnobacterium was more abundant in cecal contents from the GML1 group (P < 0.05) than the NCO group, while higher (P < 0.05) abundances of Blautia glucerasea were observed in the GML2 group than in the NCO and ANT groups. In addition, we also found that compared with the ANT group, there was a reduction (P < 0.05) in the harmful bacteria B. dorei in the GML1 and GML2 groups (Figures 3I–3K).
By day 56, the cecal microflora composition of broilers in our experimental treatments had changed. Figure 4A shows that there were 727 shared OTU between all groups. In addition, there were fewer unique OTU in the GML1 (number: 55) and GML2 (number: 47) groups than in the NCO group. The total number of OTU in the GML1 (979) and GML2 (975) groups were higher than that in the ANT (879) group on day 56. In addition, the dominant bacterial phyla were similar to those observed on day 28 (Figure 4B). The principal component analysis results indicated that the microflora of the GML1 group was similar to that of GML2 but very different from the NCO and ANT groups (Figure 4C). The Shannon index was higher in both the GML1 and GML2 groups than in the ANT group (Figure 4D). As shown in Figures 4E–F, genes from Bacteroides, Parabacteroides, Megamonas, Megasphaera, and an unidentified bacterium were dominant. After the addition of α-GML, the abundance of Desulfovibrio in broilers increased (P < 0.05) compared with those in the NCO group. At the species level, Bacteroides sp., Bacteroides coprocola, Bacteroides caecicola, Bacteroides caecigallinarum, and Candidatus saccharibacteria were the dominant species in all samples (Figure 4G). The abundances of acid-producing bacteria B. glucerasea and Coprobacter secundus were higher (P < 0.05) in the NCO group than in the GML groups (Figures 4H and 4I). Abundances of pathogenic bacteria, Alistipes tidjanibacter (P < 0.05), and B. dorei (P < 0.05) were lower in the GML1 group than in the NCO group (Figures 4J and 4K).
Discussion
The treatment of livestock with GML has received increased attention in the last few years. Similar to the findings in our study, research by Snoeck et al. (2011) and Fortuoso et al. (2019) have shown that GML has the potential to be used as a growth promoter for it can improve the weight gain and reduce the feed conversion, as well as for the improvement of immune function by augment the production of important molecules, such as globulins. We found that supplementation with 500 mg/kg or 1,000 mg/kg α-GML significantly increased broiler BW and ADG than the basal diet. Our results may be explained by findings from previous studies that found that the improvement of growth performance was related to the antibacterial effects of α-GML. This chemical compound has the ability to reduce the abundance of pathogenic bacteria and interferes with parasite multiplication in the intestine (Mueller and Schlievert, 2015; Fortuoso et al., 2019).
Ig (IgY, IgA, and IgM) are essential in an animal's immune response against pathogens. As an important part of the immune system, IgG (IgY), IgA, and IgM, which display antibody activities and bind specifically to an antigen, are produced by B cells that have differentiated into plasma cells (Sharma, 1999). IgM is the first Ig produced during a primary infection and can be transported through polarized mucosal tissues (Schroeder and Cavacini, 2010). Thus, it provides the first layer of protection against invading pathogens (Choi and Baumgarth, 2008; Justel et al., 2013). In addition, IgY is the major antibody in chickens and is present at high concentrations in serum and egg yolk (Thirumalai et al., 2019; Zhang et al., 2017).
And, it has been shown that specific IgY has significant inhibitory effects on bacterial growth and resisted parasite infection (Gujral et al., 2017; Qin et al., 2016; Li et al., 2015). Moreover, additional supplement with IgY can improve the immunity (Hussein et al., 2020). Fortuoso et al. (2019) found that when chicken feed is supplemented with GML, the levels of globulins and total proteins in their serum significantly increased. Our study demonstrates that adding α-GML increases IgM and IgY levels in broilers compared with broilers in the NCO group. IgA plays an important role in preventing the invasion of pathogens, such as bacteria and viruses, through a variety of antibody-mediated innate effector cell mechanisms (Pabst, 2012; Davis et al., 2020). IgA levels increase when the body is under stress or subject to bacterial infection, and so on (Pabst, 2012; Bunker and Bendelac, 2018; Wilmore et al., 2018), and changes in IgA levels were associated with intestinal flora (Macpherson et al., 2018; Beller et al., 2020; Suzuki, 2020). Previous research has shown that GML can inhibit bacteria growth and regulates intestinal flora to relieve stress (Fortuoso et al., 2019; Zhao et al., 2019). In our experiment, the decrease in IgA levels may be connected with it. However, research on the effects of α-GML on Ig is limited, so this mechanism warrants further investigation.
Intestinal microbiota have a variety of important functions in organisms, such as immune regulation and metabolic regulation (Sittipo et al., 2018; Michaudel and Sokol, 2020). As a complex ecosystem with a dynamic diversity of species, the composition of the intestinal microbiota gets altered based on the diet and over time (Tilocca et al., 2017). For example, the abundance and presence or absence of OTU contributes to the diversity of cecal microbiota (Zhang et al., 2016a). This is closely correlated with intestinal health. Consistent with the findings of Mo et al. (2019), that GML may affect gut microbial structure, we observed higher alpha and beta diversities of microbial species as well as more unique OTU in the GML1 and GML2 groups. This suggests that the structure of intestinal flora has improved and, thus, intestinal health. In addition, many studies have reported the antibacterial effects of GML. In our study, at the species level, A. tidjanibacter and B. dorei, which can induce intestinal inflammation and have long been considered bacterial pathogens (Moschen et al., 2016; Parker et al., 2020), were observed to significantly decrease in the GML groups. This may have resulted in the reduction of IgA (after supplementation with α-GML) and helps clarify the underlying mechanism by which GML mediates immune function enhancement. Furthermore, compositional and functional changes in gut microbiota results in changes in VFA production (Sergeant et al., 2014; Janssens et al., 2017).
Various studies have shown that VFA provide nutrients for the regeneration and repair of intestinal epithelial cells, decrease intestinal pH, and inhibit the growth of certain harmful microorganisms (Zheng et al., 2017; Che et al., 2019). For example, butyric acid, produced by Clostridium butyricum, improves growth performance and immune function and benefits the balance of intestinal microflora in broiler chickens (Zhang et al., 2016a, Zhang et al., 2016b; Detman et al., 2019; Huang et al., 2019; Zhan et al., 2019). Indeed, previous studies on the effects of GML on VFA levels in broiler cecum contents are lacking. Our study found that after α-GML supplementation, VFA levels increased by day 28, but by day 56, they decreased. Previous studies have found that GML can have bacteriostatic effects (Mueller and Schlievert, 2015) and can modulate intestinal microflora (Mo et al., 2019; Zhao et al., 2020). This fact, combined with research by Kleessen et al. (2001) and Lourenco et al. (2019), indicates that changes in VFA levels may be related to changes in gut flora. In addition, our results from the microbial sequencing analysis showed that the relative abundance of acid-producing bacteria increased, for example Lachnospiraceae and Christensenellaceae, which produce butyrate (Quan et al., 2018; Yacoubi et al., 2018). And, it have been reported that the enrichment of Ruminococcaceae and Blautia lead to higher faecal short chain fatty acids, including butyrate, propionate, valerate, and isovalerate (Upadhyaya et al., 2016; Zhou et al., 2017). The increase of R. carnobacterium and B. glucerasea was consistent with our results, where the propionic acid, butyric acid, valeric acid, and isovaleric acid were found trend to increase on day 28. Moreover, in our study, an increase in Desulfovibrio after α-GML supplementation was observed on day 56. Scanlan et al. (2009) found that Desulfovibrio can break down short-chain fatty acids. In addition, we observed that Coprobacter, which was described as a propionic and acetic acid producer (Liu et al., 2019), and Blautia, also considered to be a producer of some short-chain fatty acids (Liu et al., 2015; Upadhyaya et al., 2016; Zhang et al., 2019), were in lower abundance in the GML1 and GML2 groups. This explains the observed reduction in acetic acid, propionic acid, valeric acid, and isovaleric acid in cecum contents in broilers at day 56. Finally, our experimental results indicate that short-term additions of α-GML can improve intestinal flora health, but long-term addition may have adverse effects on intestinal flora and VFA. Further research is still needed on the regulatory mechanisms. Moreover, combined with previous research and ours indicated that α-GML had great potentialities as new additives for broilers.
In conclusion, the results of our study suggest that dietary α-GML supplementation in broiler feed improves BW and ADG and enhances immune responses by adjusting Ig levels, such as those of IgM and IgY. In addition, short-term supplementation of α-GML may increase the production of VFA. These positive effects may be associated with an increased abundance of gut bacterial flora, in particular various acid-producing bacterial groups, and a reduction in B. dorei and A. tidjanibacter, which are known to cause inflammation and disease.
Acknowledgments
Financial support for this research was received from the Zhejiang Provincial Key Research and Development Program (No. 2019C02051) and the National key research and development program intergovernmental international innovation cooperation project (No. 2018YFE0112700). We are grateful to Vegamax Biotechnology Co., Ltd. (Anji, Zhejiang, China) for providing the α-glyceryl monolaurate lactate product.
Disclosures
The authors declare no conflicts of interest.
References
- Allen H.K., Levine U.Y., Looft T., Bandrick M., Casey T.A. Treatment, promotion, commotion: antibiotic alternatives in food-producing animals. Trends Microbiol. 2013;21:114–119. doi: 10.1016/j.tim.2012.11.001. [DOI] [PubMed] [Google Scholar]
- American Meat Institute (AMI) Nor. A. M. I.; Washington, DC: 2014. The Facts about Antibiotics in Livestock & Poultry Production. [Google Scholar]
- Barton M.D. Antibiotic use in animal feed and its impact on human health. Nutr. Res. Rev. 2000;13:279–299. doi: 10.1079/095442200108729106. [DOI] [PubMed] [Google Scholar]
- Beller A., Kruglov A., Durek P., Von-Goetze V., Werner K., Heinz G.A., Ninnemann J., Lehmann K., Maier R., Hoffmann U., Riedel R., Heiking K., Zimmermann J., Siegmund B., Mashreghi M.F., Radbruch A., Chang H.D. Specific microbiota enhances intestinal IgA levels by inducing TGF-β in T follicular helper cells of Peyer's patches in mice. Eur. J. Immunol. 2020;50:783–794. doi: 10.1002/eji.201948474. [DOI] [PubMed] [Google Scholar]
- Bunker J.J., Bendelac A. IgA responses to microbiota. Immunity. 2018;49:211–224. doi: 10.1016/j.immuni.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che D.S., Adams S., Wei C., Gui X.Q., Atiba E.M., Hai J.J. Effects of Astragalus membranaceus fiber on growth performance, nutrient digestibility, microbial composition, VFA production, gut pH, and immunity of weaned pigs. Microbiologyopen. 2019;8:e00712. doi: 10.1002/mbo3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.S., Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J. Exp. Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S.K., Selva K.J., Kent S.J., Chung A.W. Serum IgA Fc effector functions in infectious disease and cancer. Immunol. Cell Biol. 2020;98:276–286. doi: 10.1111/imcb.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detman A., Mielecki D., Chojnacka A., Salamon A., Błaszczyk M.K., Sikora A. Cell factories converting lactate and acetate to butyrate: Clostridium butyricum and microbial communities from dark fermentation bioreactors. Microb. Cell Factori. 2019;18:36. doi: 10.1186/s12934-019-1085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echemi Chemial news. 2020. https://www.echemi.com/cms/110309.html
- Fortuoso B.F., Dos-Reis J.H., Gebert R.R., Barreta M., Griss G.L., Casagrande R.A., de-Cristo T.G., Santiani F., Campigotto G., Rampazzo L., Stefani L.M., Boiago M.M., Lopes L.Q., Santos R.C.V., Baldissera M.D., Zanette R.A., Tomasi T., Da-Silva A.S. Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: impact on health, performance and meat quality. Microb. Pathog. 2019;129:161–167. doi: 10.1016/j.micpath.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Gujral N., Yoo H., Bamdad F., Lee K.Y., Suh J.W., Sunwoo H. A combination of egg yolk IgY and Phosvitin inhibits the growth of Enterotoxigenic Escherichia coli K88 and K99. Curr. Pharm. Biotechnol. 2017;18:400–409. doi: 10.2174/1389201018666170425120036. [DOI] [PubMed] [Google Scholar]
- Hess D.J., Henry S.M.J., Wells C.L. The Natural Surfactant glycerol monolaurate significantly reduces development of Staphylococcus aureus and Enterococcus faecalis biofilms. Surg. Infect (Larchmt) 2015;16:538–542. doi: 10.1089/sur.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland K.T., Taylor D., Farrell A.M. The effect of glycerol monolaurate on growth of, and production of toxic shock syndrome toxin-1 and lipase by, Staphylococcus aureus. J. Antimicrob. Chemother. 1994;33:41–55. doi: 10.1093/jac/33.1.41. [DOI] [PubMed] [Google Scholar]
- Huang T., Peng X.Y., Gao B., Wei Q.L., Rong X., Yuan M.G., Xu Z.H. The effect of Clostridium butyricum on gut microbiota, immune response and intestinal barrier function during the development of Necrotic Enteritis in chickens. Front. Microbiol. 2019;10:2309. doi: 10.3389/fmicb.2019.02309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M.A., Rehan I.F., Rehan A.F., Eleiwa N.Z., Abdel-Rahman M.A.M., Fahmy S.G., Ahmed A.S., Youssef M., Diab H.M., Batiha G.E., Alrashood S.T., Khan H.A., Shanab O., Ahmed E., Hassan H., Elnagar A., Elkelish A., Hesham A.E.L., Maky M.A. Egg yolk IgY: a Novel trend of feed additives to Limit Drugs and to improve poultry meat quality. Front. Vet. Sci. 2020;7:350. doi: 10.3389/fvets.2020.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S., Ciapaite J., Wolters J.C., Riel N.A.V., Nicolay K. An in vivo magnetic resonance spectroscopy study of the effects of caloric and non-caloric sweeteners on liver lipid metabolism in rats. Nut. 2017;9:476. doi: 10.3390/nu9050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justel M., Socias L., Almansa R., Ramírez P., Gallegos M.C., Fernandez V., Gordon M., Andaluz-Ojeda D., Nogales L., Rojo S., Vallés J., Estella A., Loza A., León C., Lopez-Mestanzac C., Blanco J., Berezo J.Á., Rosich S., Cillòniz C., Torres A., Lejarazu R.O., Martin-Loeches I., Bermejo-Martin J.F. IgM levels in plasma predict outcome in severe pandemic influenza. J. Clin. Virol. 2013;58:564–567. doi: 10.1016/j.jcv.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Kleessen B., Hartmann L., Blaut M. Oligofructose and long-chain inulin: influence on the gut microbial ecology of rats associated with a human faecal flora. Br. J. Nutr. 2001;86:291–300. doi: 10.1079/bjn2001403. [DOI] [PubMed] [Google Scholar]
- Langone M.A.P., De A.M.E., Rezende M.J.C., Sant'Anna G.L. Enzymatic synthesis of medium chain monoglycerides in a solvent-free system. Appl. Biochem. Biotechnol. 2002;98-100:987–996. doi: 10.1385/abab:98-100:1-9:987. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Cho S., Paik H.D., Choi C.W., Nam K.T., Hwang S.G., Kim S.K. Investigation on antibacterial and antioxidant activities, phenolic and flavonoid contents of some Thai edible plants as an alternative for antibiotics. Asian-Australas. J. Anim. Sci. 2014;27:1461–1468. doi: 10.5713/ajas.2013.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.S., Estes J.D., Schlievert P.M., Duan L.J., Brosnahan A.J., Southern P.J., Reilly C.S., Peterson M.L., Schultz-Darken N., Brunner K G, Nephew K.R., Pambuccian S., Lifson J.D., Carlis J.V., Haase A.T. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.Y., Wang L.L., Zhen Y.H., Li S.Y., Xu Y.P. Chicken egg yolk antibodies (IgY) as non-antibiotic production enhancers for use in swine production: a review. J Anim Sci Biotechnol. 2015;6:40. doi: 10.1186/s40104-015-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.M., Li E.Y., Sun Z.Y., Fu D.J., Duan G.Q., Jiang M.M., Yu Y., Mei L., Yang P.C., Tang Y.C., Y Zheng P. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019;9:287. doi: 10.1038/s41598-018-36430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li J.Z., Zhang Y.P., Philip A., Shi E., Chi X., Meng J. Influence of glucose fermentation on CO₂ assimilation to acetate in homoacetogen Blautia coccoides GA-1. J. Ind. Microbiol. Biotechnol. 2015;42:1217–1224. doi: 10.1007/s10295-015-1646-1. [DOI] [PubMed] [Google Scholar]
- Lopes L.Q.S., Santos C.G., Vaucher R.A., Raffin R.P., Santos R.C.V. Nanocapsules with glycerol monolaurate: effects on Candida albicans biofilms. Microb. Pathog. 2016;97:119–124. doi: 10.1016/j.micpath.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Lourenco C., Kelly D., Cantillon J., Cauchi M., Yon M.A., Bentley L., Cox R.D., Turner C. Monitoring type 2 diabetes from volatile faecal metabolome in Cushing's syndrome and single Afmid mouse models via a longitudinal study. Sci. Rep. 2019;9:18779. doi: 10.1038/s41598-019-55339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson A.J., Yilmaz B., Limenitakis J.P., Ganal-Vonarburg S.C. IgA function in relation to the intestinal microbiota. Annu. Rev. Immunol. 2018;36:359–381. doi: 10.1146/annurev-immunol-042617-053238. [DOI] [PubMed] [Google Scholar]
- Michaudel C., Sokol H. The gut microbiota at the Service of Immunometabolism. Cell. Metab. 2020;32:1–10. doi: 10.1016/j.cmet.2020.09.004. [DOI] [PubMed] [Google Scholar]
- Millet S., Maertens L. The European ban on antibiotic growth promoters in animal feed: from challenges to opportunities. Vet. J. 2011;187:143–144. doi: 10.1016/j.tvjl.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Mo Q.F., Fu A.K., Deng L.L., Zhao M.J., Li Y., Zhang H., Feng F.Q. High-dose glycerol monolaurate Up-Regulated Beneficial Indigenous microbiota without inducing metabolic Dysfunction and systemic inflammation: new Insights into its antimicrobial potential. Nutrients. 2019;11:1981. doi: 10.3390/nu11091981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschen A.R., Gerner R.R., Wang J., Klepsch V., Adolph T.E., Reider S.J., Hackl H., Pfister A., Schilling J., Moser P.L., Kempster S.L., Swidsinski A., Orth H.D., Weiss G., Baines J.F., Kaser A., Tilg H. Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations. Cell Host Microbe. 2016;19:455–469. doi: 10.1016/j.chom.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Mueller E.A., Schlievert P.M. Non-aqueous glycerol monolaurate gel exhibits antibacterial and anti-biofilm activity against Gram-positive and Gram-negative pathogens. PLoS One. 2015;10:e0120280. doi: 10.1371/journal.pone.0120280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (National Research Council) 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- NY/T 33 . China Agriculture Press; Beijing, China: 2004. Feeding Standard of Chicken. [Google Scholar]
- Pabst O. New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 2012;12:821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- Parker B.J., Wearsch P.A., Veloo A.C.M., Rodriguez-Palacios A. The Genus Alistipes: gut bacteria with Emerging Implications to inflammation, cancer, and Mental health. Front. Immunol. 2020;11:906. doi: 10.3389/fimmu.2020.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projan S.J., Brown-Skrobot S., Schlievert P.M., Vandenesch F., Novick R.P. Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J. Bacteriol. 1994;176:4204–4209. doi: 10.1128/jb.176.14.4204-4209.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M., Tang X.M., Yin G.W., Liu X.Y., Suo J.X., Tao G.R., Ei-Ashram S., Li Y., Suo X. Chicken IgY Fc expressed by Eimeria mitis enhances the immunogenicity of E. mitis. Parasit Vectors. 2016;9:164. doi: 10.1186/s13071-016-1451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan J.P., Cai G.Y., Ye J., Yang M., Ding R.G., Wang X.W., Zheng E.Q., Fu D.S., Li S.Y., Li S.P., Liu D.W., Yang J., Wu Z.F. A global comparison of the microbiome compositions of three gut locations in commercial pigs with extreme feed conversion ratios. Sci. Rep. 2018;8:4536. doi: 10.1038/s41598-018-22692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan P.D., Shanahan F., Marchesi J.R. Culture-independent analysis of desulfovibrios in the human distal colon of healthy, colorectal cancer and polypectomized individuals. Microbiol. Ecol. 2009;69:213–221. doi: 10.1111/j.1574-6941.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- Schlievert P.M., Kilgore S.H., Seo K.S., Leung D.Y.M. Glycerol monolaurate contributes to the antimicrobial and anti-inflammatory activity of human milk. Sci. Rep. 2019;9:14550. doi: 10.1038/s41598-019-51130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H.W., Cavacini L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010;125:s41–s52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleem D., Chen E., Benso B., Pardi V., Murata R.M. In vitro evaluation of antifungal activity of monolaurin against Candida albicans biofilms. PeerJ. 2016;4:e2148. doi: 10.7717/peerj.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant M.J., Constantinidou C., Cogan T.A., Bedford M.R., C W Penn, Pallen M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One. 2014;9:e91941. doi: 10.1371/journal.pone.0091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J.M. Introduction to poultry vaccines and immunity. Adv. Vet. Med. 1999;41:481–494. doi: 10.1016/s0065-3519(99)80036-6. [DOI] [PubMed] [Google Scholar]
- Sittipo P., Lobionda S., Lee Y.K., Maynard C.L. Intestinal microbiota and the immune system in metabolic diseases. J. Microbiol. 2018;56:154–162. doi: 10.1007/s12275-018-7548-y. [DOI] [PubMed] [Google Scholar]
- Snoeck S.D., Wolf P.J.V.D., Swart W., Heiiman E., Ebbinge B. The effect of the application of mono-lauric acid with glycerol mono-laurate in weaned piglets, on the use of antimicrobials in sow herds. 9th International Conference on the Epidemiology and Control of Biological, Chemical and Physical Hazards in Pigs and Pork, Iowa State University, Ames, IA. 2011. pp. 346–348. [Google Scholar]
- Stanton T.B. A call for antibiotic alternatives research. Trends Microbiol. 2013;21:111–113. doi: 10.1016/j.tim.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Diversified IgA-bacteria Interaction in gut Homeostasis. Adv. Exp. Med. Biol. 2020;1254:105–116. doi: 10.1007/978-981-15-3532-1_9. [DOI] [PubMed] [Google Scholar]
- Tilocca B., Burbach K., Heyer C.M.E., Hoelzle L.E., Mosenthin R., Stefanski V., Camarinha-Silva A., Seifert J. Dietary changes in nutritional studies shape the structural and functional composition of the pigs, fecal microbiome-from days to weeks. Microbiome. 2017;5:144. doi: 10.1186/s40168-017-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh N.T., Loh T.C., Foo H.L., Hair-bejo M., Azhar B.K. Effects of feeding metabolite combinations produced by Lactobacillus plantarumon growth performance, faecal microbial population, small intestine villus height and faecal volatile fatty acids in broilers. Poult. Sci. 2009;50:298–306. doi: 10.1080/00071660902873947. [DOI] [PubMed] [Google Scholar]
- Thirumalai D., Visaga A.S., Vieira-Pires R.S., Zhang X.M., Sekaran S., Krishnan U. Chicken egg yolk antibody (IgY) as diagnostics and therapeutics in parasitic infections - a review. Int. J. Biol. Macromol. 2019;136:755–763. doi: 10.1016/j.ijbiomac.2019.06.118. [DOI] [PubMed] [Google Scholar]
- Upadhyaya B., McCormack L., Fardin-Kia A.R., Juenemann R., Nichenametla S., Clapper J., Specker B., Dey M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 2016;6:28797. doi: 10.1038/srep28797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore J.R., Gaudette B.T., Gomez A.D., Hashemi T., Jones D.D., Gardner C.A., Cole S.D., Misic A.M., Beiting D.P., Allman D. Commensal Microbes induce serum IgA responses that protect against Polymicrobial Sepsis. Cell Host Microbe. 2018;23:302–311. doi: 10.1016/j.chom.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W.G., Sun Y.X., Zeng Z.L. Antimicrobial use and antimicrobial resistance in food animals. Environ. Sci. Pollut. Res. 2018;25:18377–18384. doi: 10.1007/s11356-018-1852-2. [DOI] [PubMed] [Google Scholar]
- Yacoubi N., Saulnier L., Bonnin E., Devillard E., Eeckhaut V., Rhayat L., Ducatelle R., Van I.F. Short-chain arabinoxylans prepared from enzymatically treated wheat grain exert prebiotic effects during the broiler starter period. Poult. Sci. 2018;97:412–424. doi: 10.3382/ps/pex297. [DOI] [PubMed] [Google Scholar]
- Yang C.M., Zhang L.L., Cao G.T., Feng J., Yue M., Xu Y.L., Dai B., Han Q.J., Guo X.Q. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, faecal volatile fatty acids and microflora community in weaned piglets. J. Anim. Sci. 2019;97:133–143. doi: 10.1093/jas/sky426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D.W., Jiang Q.X., Xu Y.S., Xia W.S. The shelf life extension of refrigerated grass carp (Ctenopharyngodon idellus) fillets by chitosan coating combined with glycerol monolaurate. Int. J. Biol. Macromol. 2017;101:448–454. doi: 10.1016/j.ijbiomac.2017.03.038. [DOI] [PubMed] [Google Scholar]
- Zhan H.Q., Dong X.Y., Li L.L., Zheng Y.X., Gong Y.J., Zou X.T. Effects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult. Sci. 2019;98:896–903. doi: 10.3382/ps/pey436. [DOI] [PubMed] [Google Scholar]
- Zhang X.Y., Calvert R.A., Sutton B.J., Doré K.A. IgY: a key isotype in antibody evolution. Biol. Rev. Camb Philos. Soc. 2017;92:2144–2156. doi: 10.1111/brv.12325. [DOI] [PubMed] [Google Scholar]
- Zhang M.S., Sandouk A., Houtman J.C.D. Glycerol Monolaurate (GML) inhibits human T cell signaling and function by disrupting lipid dynamics. Sci. Rep. 2016;6:30225. doi: 10.1038/srep30225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Shi M.X., Ji J.F., Hu X.S., Chen F. Gut microbiota determines the prevention effects of Luffa cylindrica (L.) Roem supplementation against obesity and associated metabolic disorders induced by high-fat diet. FASEB. J. 2019;33:10339–10352. doi: 10.1096/fj.201900488R. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wei H.W., Cui Y.N., Zhao G.Q., Feng F.Q. Antibacterial interactions of monolaurin with commonly used antimicrobials and food components. J. Food Sci. 2009;74:m418–m421. doi: 10.1111/j.1750-3841.2009.01300.x. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang L.L., Zhan X.A., Zeng X.F., Zhou L., Cao G.T., Chen A.G., Yang C.M. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016;7:3. doi: 10.1186/s40104-016-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M.J., Cai H.Y., Liu M.Y., Deng L.L., Li Y., Zhang H., Feng F.Q. Effects of dietary glycerol monolaurate on productive performance, egg quality, serum biochemical indices, and intestinal morphology of laying hens. J. Zhejiang Univ. Sci. B. 2019;20:877–890. doi: 10.1631/jzus.B1800530. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao M.J., Jiang Z.L., Cai H.Y., Li Y., Mo Q.F., Deng L.L., Zhong H., Liu T., Zhang H., Kang J.X., Feng F.Q. Modulation of the gut microbiota during high-dose glycerol monolaurate-mediated Amelioration of obesity in mice fed a high-fat diet. mBio. 2020;11:e00190-20. doi: 10.1128/mBio.00190-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Kelly C.J., Battista K.D., Schaefer R., Lanis J.M., Alexeev E.E., Wang R.X., Onyiah J.C., Kominsky D.J., Colgan S.P. Microbial-derived butyrate Promotes epithelial barrier function through IL-10 Receptor-Dependent Repression of Claudin-2. J. Immunol. 2017;199:2976–2984. doi: 10.4049/jimmunol.1700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Pan Q., Xin F.Z., Zhang R.N., He C.X., Chen G.Y., Liu C., Chen Y.W., Fan J.G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017;23:60–75. doi: 10.3748/wjg.v23.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]