Abstract
The anticoccidial influences of various amounts of Cinnamomum verum powder were compared with that of salinomycin as standard synthetic anticoccidial drug on the anticoccidial indicators and production performance in broilers experimentally exposed to coccidiosis. Broiler chicks at 1 d of age (n = 150) were arbitrarily distributed into 6 groups. Birds from groups 1–3 were received the starter and finisher diets plus 2, 4, and 6 g of cinnamon/kg of the diet, respectively. Birds from group 4 were fed the starter and finisher diets plus 66 mg of salinomycin, group 5 constituted the positive control (PC), with the coccidial challenge, and group 6 constituted the negative control (NC), without the coccidial infection, which were both maintained on diets without any cinnamon. The results showed that BW gain, feed conversion ratio, and production efficiency factor declined considerably (P < 0.05) in the PC compared with the NC. At seventh day postinfection (DPI), the lesion score was significantly (P < 0.05) lower in the ceca of salinomycin-treated birds than the PC. The anticoccidial index was moderate in the chickens treated with salinomycin and 6 g cinnamon at 7 DPI compared with those in the PC group. In addition, cinnamon- or salinomycin-treated birds exhibited lower oocyst values and higher oocyst reduction rate than those in the PC. We concluded that C. verum at level 6 g cinnamon/kg diet moderately reduced coccidiosis and attempted to improved BW, feed conversion ratio, and production efficiency at 7 DPI compared with the infected groups.
Key words: anticoccidial indicator, broiler, Cinnamomum verum, Eimeria tenella
Introduction
In recent year, the intensive production of poultry has been predisposed to an increase in the incidence of poultry diseases. Coccidiosis is a devastating, deadly, and economically damaging parasitic diseases of birds in the world. It causes poor growth, reduced feed conversion ratios (FCR), and high morbidity and mortality with the spread of infectious oocysts to the surrounding environment, resulting in a loss of millions of US dollars annually for the poultry production (Tanweer et al., 2014; Kadykalo et al., 2018). The prevalence of coccidiosis among infected birds has also become one of the predisposing factors for the appearance of other secondary diseases in susceptible birds, such as infection with Clostridium perfringens. Usually, strict hygiene and biosecurity methods, vaccination, and synthetic coccidiostats are used in poultry ration to prevent and control Emeria infection in the poultry sector (Chand et al., 2016; Kadykalo et al., 2018). The use of coccidiostats and other chemicals, such as amprolium, clazuril, diclazuril, monensin, salinomycin, spiramycin, sulfadimethoxine, and toltrazuril, has resulted in drug resistance in poultry. In addition, the residues of coccidiostats and antimicrobial drugs in poultry products may pose a public health concern (Mund et al., 2017; Kadykalo et al., 2018). Owing to the serious risks associated with the use of coccidiostats, there is a robust feeling that synthetic drugs should be replaced by natural additives (Abudabos et al., 2018; Alhotan and Abudabos, 2019; Al-Quraishy et al., 2020).
The use of phytogenic feed additives as an alternative to infeed medicines is a novel idea for the treatment of coccidiosis, and it has a wide range of benefits, such as safety, lack of grace period, natural origin, little or no residual effects, and cost-effectiveness (Abudabos et al., 2017; Alhotan and Abudabos, 2019; Al-Quraishy et al., 2020; Qasem et al., 2020). Recently, feed additives originating from plants, such as Aloe barbadensis, Cinnamomum verum, Tinospora cordifolia, Bambusa arundinacea, Emblica officinalis, Ferula foetida regel, and Tamarindus indica have provided beneficial results against several common diseases in broiler chickens, such as infection with Escherichia coli, Salmonella, mycotoxins, and C. perfringens (Moryani et al., 2020; Prakash et al., 2020). Kadykalo et al. (2018) reviewed the anticoccidial properties of Artemisia, cloves, curcumin, oregano, Quillaja, Sericea lespedeza, tea tree, and thyme as alternative feed additives and their mode of action resulting in reduced coccidiosis indicated by reduced oocysts counts. Among them, Artemisia, clove, tea tree, and thyme were effective in reducing coccidiosis by the reduced number of oocysts.
We have assumed that plant-source natural feed additives are capable of being used as alternatives to synthetic coccidiosis drugs owing to the effective compounds contained therein. Therefore, we aimed to compare the anticoccidial activity of different amounts of C. verum bark powder with synthetic drugs in broilers chickens challenged with Eimeria tenella. Notably, C. verum is considered a natural product belonging to the Lauraceae family, and it has much of biological activities because it contains beneficial antimicrobial phytochemicals, such as cinnamaldehyde, eugenol, camphor, trans-cinnamyl acetate, and proanthocyanidin, vitamins, and other important compounds (Adarsh et al., 2020).
Materials and methods
The study was approved by the Departmental Board of Studies on Ethics, Methodology and Welfare, King Saud University, Kingdom of Saudi Arabia.
Bird Husbandry and Experimental Design
A total of 150 day-old broiler chicks (Ross 308) from the local hatchery – Al Khumasia Company – were reared in a controlled coccidian-free house until experimentation. The birds were randomly assigned to 6 treatment groups. Each treatment group had 25 birds with 5 replicates per group and 5 birds per replicate. Birds in groups 1, 2, and 3 were infected with E. tenella and received 2, 4, and 6 g of cinnamon/kg of the diet, respectively, and those in group 4 received 66 mg salinomycin/kg diet. Birds in groups 5 and 6 were infected, unmedicated as the PC, and uninfected, unmedicated as the NC, respectively.
A starter diet was provided for day 1–21 and a finisher diet was provided for day 22–28, in accordance with the Ross 308 recommendation guide. The basal dietary ingredients and nutrients are shown in Supplementary Table 1. Feed and water were accessible ad libitum. The C. verum bark used in this study was purchased from a local store in Riyadh, Saud Arabia. The dried bark was ground into a fine powder for chemical analysis. The approximate analysis of C. verum powder was conducted to determine moisture, CP, total crude fiber, gross energy, ash, ether extract, total fiber fraction: acid detergent fiber, and neutral detergent fiber as described by Al-Numair et al. (2007). The dry powder of C. verum was mixed with the basal diet. On day 21, birds in each replicate were weighed and had similar weights and sizes, which were recorded as the initial BW before the parasitic challenge.
Unsporulated oocysts of E. tenella were obtained from the feces of naturally infected chickens on the 7 d postinfection (DPI). These unsporulated oocysts were sporulated, purified, and preserved using a 2.5% potassium dichromate solution at 25°C for 72 h following El-Ashram and Suo (2017). Sporulated oocysts that collected from the cecum of naturally infected Baladi chickens have been kindly provided by our colleague's researchers at the parasitology laboratory at the Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia. The identification of sporulated oocysts was through the observation of lesions and by use of morphologic characteristics and morphometry, as cited by McDougald et al. (1997). The sporulated oocysts were kept at 4°C until use. In addition, sporulated oocysts were passaged and propagated 2 to 3 times in 2-wk-old healthy broiler chickens to confirm the site of lesions and activate parasites before use. Furthermore, the oocytes were collected before our trail started, the same our colleagues researchers confirmed the species of Eimeria isolates by amplification of the internal transcribed spacer 1 partial region and sequences analysis. After the confirmation of the parasite species, oocysts were collected, sporulated, and washed, and the dose was adjusted to 4 × 104/100 μL/bird. At 21 d of age, all the birds, except for group 5, were orally gavaged with a 1 mL suspension of distilled water containing 40,000 E. tenella sporulated oocysts (Lee et al., 2012).
Assessment of Anticoccidial Activity
The anticoccidial effectivity of C. verum powder was assessed using bloody diarrhea, relative BW gain (BWG), survival rate, oocyst output/g excreta, and lesion scores. Clinical symptoms and mortality were recorded every DPI. The anticoccidial index (ACI) of each treatment was calculated as stated by Lan et al. (2016): ACI = (relative ratio of BWG + survival rate) − (lesion scores + oocyst value).
The ACI value was assessed using the method suggested by Morisawa et al. (1977): an ACI >180 was considered to have an excellent anticoccidial impact, 160–180 was considered marked, 140–160 was considered moderate, 120–140 was considered as mild, and an ACI <120 was considered inactive.
On day 4–7 after infection, the birds were examined for bloody diarrhea by enumerating bloody excreta 2 times a day. The degree of bloody diarrhea was assigned to at least 1 of 4 categories, from zero to 3, based on the average of bloody excreta pieces rounded to the closest integer as described by Abbas et al. (2010). Briefly, the 0, 1, 2, and 3° represented 0, 33, 33–66, 66–99% blood in total feces, respectively.
Excreta were collected from all replicates of each group from 4–7 DPI. The oocysts were counted using a hemocytometer following the methodology of Holdsworth et al. (2004). In short, 1 g of each well-mixed feces sample was spread in 10 mL water. Oocysts were counted under an optical microscope at suspension of 10 μL. The results have been expressed as oocysts/g output. The oocyst value and oocyst reduction rate were calculated as cited by Lan et al. (2016).
At 0 and 7 DPI, all the chickens in each replicate were weighed to get the starting and final BW, respectively. The BWG has been calculated as the final BW minus the initial BW.
At day 7 DPI (day 28 of age), 1 bird from each replicate (5 bird/group) was slaughtered by cervical dislocation, and the ceca of each bird was removed and inspected. Lesion scores were ranked as 0 to 4 based on the severity of the ceca. Zero represented the normal position and 4 corresponded to each of the foremost severe and dead birds. The cecum length was also measured and recorded as the mean ± SEM in cm to measure the degree of atrophy.
Performance Measurement
Performance measures of the experimental birds included an increase in BWG and feed consumption (FI). All birds were weighed individually, and the BWG was calculated by subtracting the initial BW (at 0 DPI) from the final BW (at 7 DPI). At the end of the trial, the FI of each replicate was measured by subtracting the residual feed weight from the offered feed weight at the start of the trial. The FCR for each treatment was computed using the data obtained from FI and BW and the formula FCR = feed intake (g)/BWG (g). Mortality was recorded when it occurred. The European production efficiency factor (EPEF) was calculated using the method suggested by Griffin (1979):
Statistical Analysis
The Statistical Analysis System (SAS, 2018) statistically analyzes data collected. The anticoccidial and performance indicators were expressed as mean ± SEM and a 1-way variance analysis was used to determine differences in those parameters between groups. Using the nonparametric Kruskal-Wallis H test, the bloody diarrhea and lesion scores of each treatment were compared. The means of data were separated using Ryan-Einot-Gabriel-Welsch's multiple comparison test for statistical differences. A comparison with P-values <0.05 was considered to be significantly different.
Results
Chemical Analysis
The proximate chemical composition of the C. verum bark that we used in the experiment was 3.97% CP, 21.84% crude fiber, 2.85% ash, 41.04% neutral detergent fiber, 41.04% acid detergent fiber, and its calculated calorie contribution was 4462.64 kcal/kg (Supplementary Table 2).
HPLC
A chromatogram of standard of flavonoids and phenols mixture at 280 nm of C. verum obtained using HPLC is shown in Supplementary Figure 1. In addition, the detected component of the bark extracts of the C. verum was caffeine (700 μg/g) based on HPLC analysis (Supplementary Table 3).
Gas Chromatography-Mass Spectrometry
Gas chromatography-mass spectrometry was used to analyze the bark extracts of C. verum. Twenty-eight different compounds were identified as shown in Supplementary Figure 2 and Supplementary Table 4.
Behavior Changes and Bloody Diarrhea
All infected birds demonstrated clinical symptoms of coccidiosis, including clustering together, ruffled feathers, and depression, except for salinomycin-treated groups. Mortality was absent in all treatments except for 1 bird in the PC treatment. Bloody diarrhea was observed 4–7 DPI with E. tenella in groups 1, 2, 3, and 5 (Table 1). However, no diarrhea has been detected in the groups treated with salinomycin and the unmediated uninfected birds throughout the study.
Table 1.
Comparison of mean OPG output (log10/g excreta), bloody diarrhea, and lesion score calculated in the feces of the different groups of chickens.
| Groups | OPG output1 | Bloody diarrhea | Lesion scores |
|---|---|---|---|
| 1.Cinnamomum verum (2 g/kg) | 6.77a,b | 1.60 | 2.0a,b |
| 2.Cinnamomum verum (4 g/kg) | 6.52a,b | 0.60 | 1.6a,b |
| 3.Cinnamomum verum (6 g/kg) | 6.38b | 1.40 | 1.4a,b |
| 4.Salinomycin | 6.38b | 0.00 | 0.8b,c |
| 5.Infected unmedicated | 7.07a | 2.00 | 2.6a |
| 6.Uninfected unmedicated | 0.00c | 0.00 | 0.0c |
| SEM | 0.465 | 0.258 | 0.189 |
| P-Value | <0.0001 | 0.0861 | <0.0001 |
a–cDifferent letters indicate statistically significant differences (P < 0.05).
Abbreviation: OPG, oocysts per gram.
Mean OPG output was calculated at 7 d after inoculation.
Lesion Scores
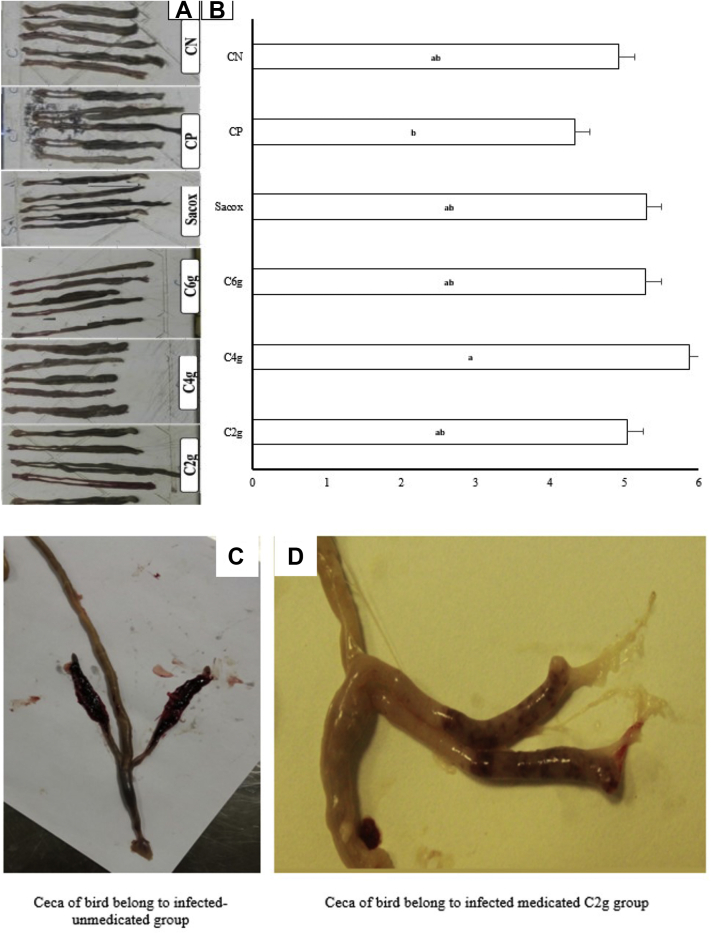
Figures 1A–1D presented the length of the ceca and their pathological lesion scores of experimental groups that graded from normal to severe. The severe pathologic lesions were found in the ceca of the PC, such as shrinking of ceca length (atrophy), wall thickening, erosion, and dark blood clotting. Cecal morphology and shrinking length were significantly (P < 0.05) improved in the medicated group (group 2) compared with the PC. The highest length of the ceca showed in birds that received 4 g CN/kg diet. The ceca in both the NC and the salinomycin groups exhibited typical morphology.
Figure 1.
(A) Cecums collected from each group on the day 7 of the infection. (B) Mean ± SEM (cm) of cecal length to measure the degree of atrophy. a,bThe letters above the columns indicate statistical difference (P < 0.05). Scale bar around 3 cm. (C) Ceca of bird from CP group. (D) Ceca of bird from C2g cinnamon/kg diet group were demonstrated other typical pathologic traits of lesions such as wall thickening, erosion, and dark blood clotting. Abbreviations: C2g, 2 g cinnamon/kg diet; C4g, 4 g cinnamon/kg diet; C6g, 6 g cinnamon/kg diet; CP, basal diet with coccidiosis challenge; CN, basal diet without coccidiosis challenge; Sacox, 66 mg salinomycin/kg diet.
When all the pathologic characteristics were combined, the lesion scores indicated that C. verum powder had significantly (P < 0.05) relieved the undesirable cecal lesions caused by coccidiosis with increasing levels of cinnamon powder (Table 1). The positive impacts of the lower doses of cinnamon powder in groups received 2 and 4 g of cinnamon/kg of diet were not evident (P > 0.05). However, group 3, with the highest dosage of 6 g of cinnamon/kg of diet, showed the least pathologic features, including the lowest lesion score.
All three groups medicated with C. verum powder showed significantly lower lesion scores than the infected unmedicated control (group 5) (P < 0.05).
Oocysts Per Gram Output
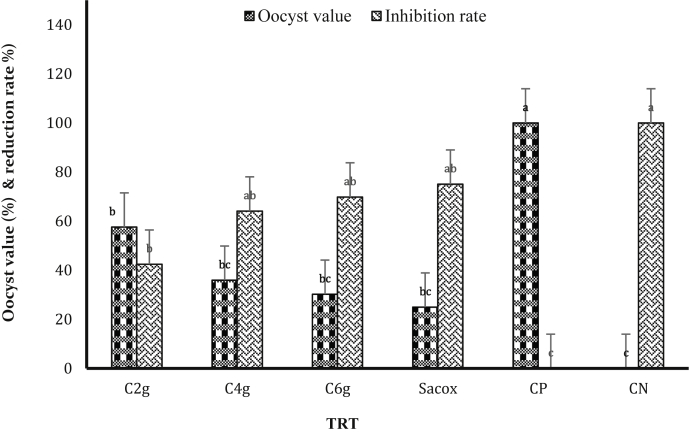
The fecal output of oocysts was not present during the first 4 DPI. Differences in the oocysts/g output between the medicated and nonmedicated chickens were observed from the fifth day onward (Table 1). Oocyst morphology of E. tenella in the infected experimental treatments is shown in Supplementary Figure 3. Although there was no significant difference (P > 0.05) in the lowest dosage groups (group 1 and group 2) compared with the PC group, lower oocyst numbers (P < 0.05) were observed for group 3, which was treated with the higher dosage of 6 g cinnamon/kg diet, and group 4, which was treated with salinomycin (Table 1; Figure 2). Here, excreta from all replicates of each group were collected from 5 to 7 DPI and observed under a microscope; E. tenella oocyst shedding starts at around fifth DPI and reach peak around 6–7 DPI, after that, the shedding reduced.
Figure 2.
Effect of treatments and infection with Eimeria tenella on oocyst value and oocyst reduction rate at 7 d after inoculation. a–c lowercase letters indicate significant differences. Abbreviations: C2g, 2 g cinnamon/kg diet; C4g, 4 g cinnamon/kg diet; C6g, 6 g cinnamon/kg diet; CP, basal diet with coccidiosis challenge; CN, basal diet without coccidiosis challenge; Sacox, 66 mg salinomycin/kg diet.
The inspection of the feces of chicken at 7 DPI showed a high number of log10 of oocysts output reaching 7.07. The number of oocysts significantly decreased (P < 0.05) after treatment of the infected chickens with 6 g of cinnamon/kg of diet and treatment with salinomycin (Table 1). The oocyst reduction rate for groups 1, 2, and 3 was 42.45, 64.09, and 69.81%, respectively (Figure 2). There was a relationship wherein the oocyst output declined with an increase in powder dose.
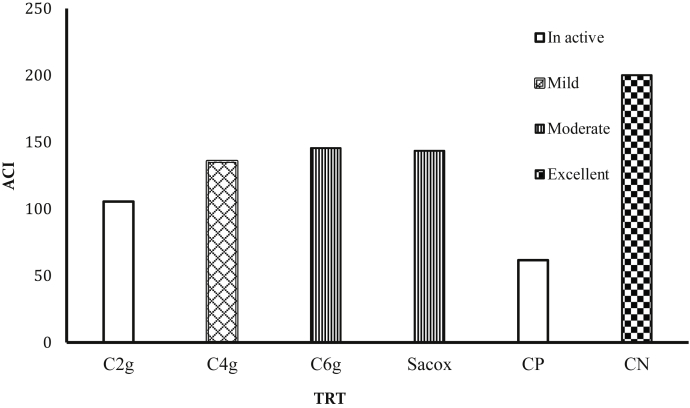
Anticoccidial Index
The PC group and the lowest cinnamon-medicated group (2 g cinnamon/kg diet) showed an inactive anticoccidial effect with the lowest ACI value (Figure 3). The group medicated with the highest concentration of cinnamon and the salinomycin-treated group showed moderate anticoccidial effects with ACI indices of 146 and 143, respectively. The ACI index of 4 g of cinnamon/kg of the diet group was 136, which represents a slight anticoccidial effect. There were no substantial anticoccidial effects among the cinnamon-medicated groups, while the cinnamon-medicated groups had a higher significant anticoccidial effect than the PC group and a lower significant anticoccidial effect than the NC group.
Figure 3.
Effect of experimental treatments and infection with Eimeria tenella on the anticoccidial index (ACI) of chickens at 7 d after inoculation. Abbreviations: C2g, 2 g cinnamon/kg diet; C4g, 4 g cinnamon/kg diet; C6g, 6 g cinnamon/kg diet; CP, basal diet with coccidiosis challenge; CN, basal diet without coccidiosis challenge; Sacox, 66 mg salinomycin/kg diet.
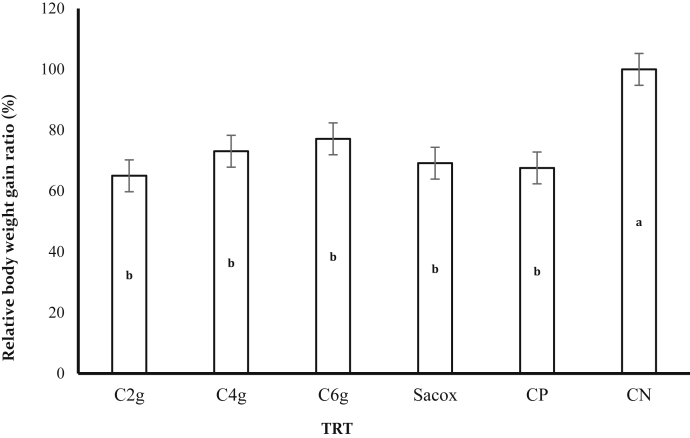
Performance and Production Efficiency
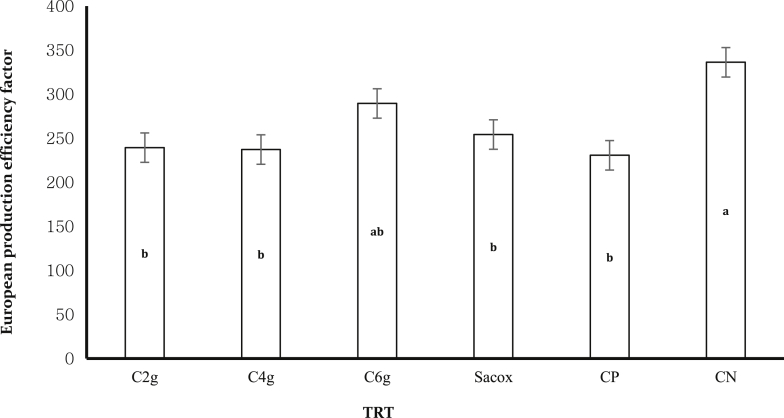
No significant variations were noted in the initial weights of the birds in each treatments (data not shown). The experimental treated groups did not show any significant influence on FI and FCR (P > 0.05) (Table 2). Our data showed that BWG and production efficiency factor (PEF) of treatments 1, 2, 3, and 4 were not significantly different from the PC group (group 5) but had significant reduction (P < 0.05) when compared with the NC control (group 6) (Table 2; Figures 4 and 5). There was no significant difference in the broiler performance between the cinnamon-medicated groups (P > 0.05) at the current dose used.
Table 2.
Live BW, live BW gain (BWG), cumulative feed intake (FI), and feed conversion ratio (FCR) of broiler chickens given experimental diets at 7 d after infection.
| Treatment | BW (kg) | BWG (g) | FI (g) | FCR (g:g) |
|---|---|---|---|---|
| 1 Cinnamomum verum (2 g/kg) | 1.178a,b,1 | 54.28b | 99.53 | 1.84a |
| 2 Cinnamomum verum (4 g/kg) | 1.162a,b | 61.03b | 111.18 | 1.85a |
| 3 Cinnamomum verum (6 g/kg) | 1.228a,b | 64.45b | 102.31 | 1.62a,b |
| 4 Salinomycin | 1.238a,b | 57.75b | 104.35 | 1.81a |
| 5 Infected-unmedicated | 1.120b | 56.45b | 99.35 | 1.81a |
| 6 Uninfected-unmedicated | 1.273a | 83.51a | 117.19 | 1.41b |
| SEM± | 15.879 | 2.419 | 2.265 | 0.050 |
| Probability | 0.043 | 0.001 | 0.131 | 0.055 |
a–cDifferent letters indicate statistically significant differences (P < 0.05).
Mean data was calculated at 7 d after inoculation.
Figure 4.
Effect of experimental treatments and infection with Eimeria tenella for the relative ratio of BW gain of chickens at 7 d after inoculation. Different lowercase letters indicate significant differences. Abbreviations: C2g, 2 g cinnamon/kg diet; C4g, 4 g cinnamon/kg diet; C6g, 6 g cinnamon/kg diet; CP, basal diet with coccidiosis challenge; CN, basal diet without coccidiosis challenge; Sacox, 66 mg salinomycin/kg diet.
Figure 5.
Effect of experimental treatments and infection with Eimeria tenella on the broiler feed conversion ratio at 7 d after inoculation. Different lowercase letters indicate significant differences. Abbreviations: C2g, 2 g cinnamon/kg diet; C4g, 4 g cinnamon/kg diet; C6g, 6 g cinnamon/kg diet; CP, basal diet with coccidiosis challenge; CN, basal diet without coccidiosis challenge; Sacox, 66 mg salinomycin/kg diet.
Noninfected chickens reported a marked increase in BW, BWG, and PEF compared with infected birds. Cinnamon-treated birds, particularly at the 6 g of C. verum/kg of the basal diet, revealed a significant improvement in BW, BWG, and PEF compared with the PC birds and the salinomycin-treated birds. However, this increase was nonsignificant. In summary, our data have shown that C. verum powder performed better than the infected unmedicated control group. However, this influence was not significant at the current levels used.
Discussion
The search for alternatives to synthetic anticoccidial drugs is an important field of study for poultry researchers (Lan et al., 2016; Alhotan and Abudabos, 2019; Al-Quraishy et al., 2020).
This study investigated the efficacy of natural products compared with the standard synthetic anticoccidial product in chickens exposed to the in vivo coccidiosis challenge. Anticoccidial drugs such as salinomycin are an ionophore that have an effect on the chicken coccidia of disrupting ion gradients across the cell membrane of Eimeria parasite. Salinomycin is isolated and originates from natural products called Streptomyces albus, which is a bacterial species of the pseudodisaccharide aminoglycoside salbostatin that is isolated to produce white aerial mycelium and extensively used in coccidiosis control (Behnamifar et al., 2019; Noack et al., 2019). At the outset, we focused on finding an herbal product with anticoccidial properties that could potentially be further developed into a new anticoccidial drug. After viewing a variety of herbs, we focused on C. verum bark. This spice is rich in compounds, including cinnamaldehyde, cinnamate, cinnamic acid, alkaloids, flavonoids, as well as some steroids, fatty acids, peptide, and many essential oils (Rao and Gan, 2014; Adarsh et al., 2020; El-Hack et al., 2020). These vital compounds provide this plant with antioxidant, antimicrobial, antidiabetic, anti-inflammatory, anticancer, lipid-lowering, and cardiovascular disease–lowering properties. Our data confirm that cinnamon powder yields significant anticoccidial properties.
Coccidiosis is a well-known infectious disease. It causes terrible bloody diarrhea in birds by destroying intestinal epithelial cells. Our study observed typical clinical signs of avian coccidiosis, such as paleness, huddling together, ruffled feathers, anorexia, and depression, which are in agreement with observations by Tanweer et al. (2014) and Dubey (2019). The bloody diarrhea of the infected birds was not affected in this trial, although reduced bleeding can protect infected birds against secondary infections, inflammatory response, and toxic substances absorption (Mansoor et al., 2017).
The spread of oocysts in the feces is a risk factor for the prevalence of coccidiosis in intensive farming (Wondimu et al., 2019). We found that C. verum powder significantly (P < 0.0001) reduced the oocysts/g output in the cinnamon-treated groups, suggesting that C. verum powder might play a significant role in controlling large-scale avian coccidiosis epidemics in poultry farms.
The bird cecum is one of the vital digestive organs. When Eimeria destroys the epithelial cells of the cecum, the chicken suffers from malabsorption resulting in poor BWG (Blake and Tomley, 2014; Lan et al., 2016). Supplementation with C. verum powder reduced the burden of the coccidian threat on the macroscopic abnormalities of the ceca. A slight atrophy has been noted in the cinnamon-medicated birds. However, it was more serious in the infected unmedicated control. Results from the observation of cecal lesions and oocyst inhibition showed that cinnamon powder could greatly reduce the coccidiosis burden. The cecal lesion score of birds was reduced most in the synthetic anticoccidial group. The lesion score was reduced in the natural product compared with the PC. However, the value was not significant. The positive effect of cinnamon powder could be owing to the presence of different types of saponins, alkaloids, tannins, and flavonoids, which have antiparasitic, anti-inflammatory, and antioxidant properties (Rao and Gan, 2014; Adarsh et al., 2020; El-Hack et al., 2020). This proposes the in vivo evolution of E. tenella inhibited or retard by the presence of constituents in cinnamon powder. As a result, besides directly targeting the coccidian parasites, the cinnamon might play a crucial role in improving the situations of the diseased birds across its organ-protective properties (Yang et al., 2020).
Synthetic salinomycin used as a standard in the present research showed a moderate anticoccidial impact. Infected birds treated with salinomycin have shown significantly lower signs of coccidiosis, including such bloody diarrhea and oocyst suppression in feces. The anticoccidian effect might partly be attributed to salinomycin becoming a pure compound that is consistent with the study by Behnamifar et al. (2019). Because C. verum powder comprises a wealthy group of phytochemical compounds, it is probable that they will have a cumulative influence on infected birds. To further evaluate the anticoccidial efficacy of C. verum, pure components should be isolated and identified. This will help us to understand the relationships between various individual components and their respective anticoccidial effects and allow better drug development.
Eimeria-infected animals have been shown to exhibit significant weight loss and reduced feed intake (Mehlhorn, 2016), owing to poor nutrient absorption and reduced immune responses resulting in intestinal tissue damage (Chapman, 2014). Our findings agree with weight losses in birds infected with E. tenella compared with the NC. However, we did not find significant differences in feed intake between the experimental groups.
These results indicated that the PC had negative impact on BW, BWG, and PEF. The cinnamon-medicated groups and salinomycin group showed similar effects. Besides, the 6 g of cinnamon/kg of the diet group was somewhat motivating as BW, BWG, and PEF enhanced compared with the PC. In addition, the BW, BWG, and PEF were improved compared with the PC at the other concentrations of cinnamon powder. However, the values were not significantly different.
As expected, performance in the coccidiosis-exposed group that was not treated with any natural or synthetic drugs was most adversely affected. Encouragingly, the performance of the chickens recovered closer to the NC and drug-treated groups. Health benefits of anticoccidial herbal supplementation were attributed to the presence of some bioactive components in cinnamon powder used to improve immunity, antioxidant status, decline bowel inflammation, modulate intestinal microflora, and decrease parasitosis (Vijayan and Thampuran, 2004; Gende et al., 2008; Khaki et al., 2014; El-Hack et al., 2020). Herbal anticoccidial agents maintain the growth of the birds by reducing the destructive effect of the coccidiosis. The harmful effects of coccidiosis were evident in the infected group, and they were ameliorated by the herbal product.
We reach the conclusion that cinnamon powder possesses significant anticoccidial characteristics at the levels tested and can be use in coccidiosis-challenged broiler chickens. Owing to its wide distribution, economical nature, and ease of use, C. verum could serve as a moderate alternative anticoccidial agent and provide a new tactic for the controlling and preventing of poultry coccidiosis.
Acknowledgments
The authors would like to thank Deanship of scientific research for funding and supporting this research through the initiative of DSR Graduate Students Research Support (GSR).
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.11.071.
Disclosures
The authors declare no conflicts of interest.
Supplementary data
References
- Abbas R.Z., Iqbal Z., Khan M.N., Zafar M.A., Zia M.A. Anticoccidial activity of Curcuma longa L. in broilers. Braz. Arch. Biol. Technol. 2010;53:63–67. [Google Scholar]
- Abudabos A.M., Alyemni A.H., Dafalla Y.M., Khan R.U. The effect of phytogenics on growth traits, blood biochemical and intestinal histology in broiler chickens exposed to Clostridium perfringens challenge. J. Appl. Anim. Res. 2018;46:691–695. [Google Scholar]
- Abudabos A.M., Alyemni A.H., Swilam E.O., Al-Ghadi M.A.Q. Comparative anticoccidial effect of some natural products against Eimeria spp. infection on performance traits, intestinal lesion and Occyte number in broiler. Pak. J. Zool. 2017;49:989–1995. [Google Scholar]
- Adarsh A., Chettiyar B., Kanthesh B., Raghu N. Phytochemical Screening and antimicrobial activity of “cinnamon zeylanicum”. IJPRI. 2020;13:22–33. [Google Scholar]
- Al-Numair K.S., Ahmad D., Ahmed S., Al-Assaf A.H. Nutritive value, levels of polyphenols and anti-nutritional factors in Sri Lankan cinnamon (Cinnamomum Zeyalnicum) and Chinese Cinnamon (Cinnamomum Cassia) Res. Bult. King Saud. Univ. 2007;154:5–21. [Google Scholar]
- Al-Quraishy S., Qasem M.A., Al-Shaebi E.M., Murshed M., Mares M.M., Dkhil M.A. Rumex nervosus changed the oxidative status of chicken caecum infected with Eimeria tenella. J. King Saud. Univ. Sci. 2020;32:2207–2211. [Google Scholar]
- Alhotan R.A., Abudabos A. Anticoccidial and antioxidant effects of plants derived polyphenol in broilers exposed to induced coccidiosis. Environ. Sci. Pollut. Res. 2019;26:14194–14199. doi: 10.1007/s11356-019-04615-2. [DOI] [PubMed] [Google Scholar]
- Behnamifar A., Rahimi S., Kiaei M., Fayazi H. Comparison of the effect of probiotic, prebiotic, salinomycin and vaccine in control of coccidiosis in broiler chickens. Iran J. Vet. Res. 2019;20:51–54. [PMC free article] [PubMed] [Google Scholar]
- Blake D.P., Tomley F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014;30:12–19. doi: 10.1016/j.pt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Chand N., Faheem H., Khan R.U., Qureshi M.S., Alhidary I.A., Abudabos A.M. Anticoccidial effect of mananoligosacharide against experimentally induced coccidiosis in broiler. Environ. Sci. Pollut. Res. 2016;23:14414–14421. doi: 10.1007/s11356-016-6600-x. [DOI] [PubMed] [Google Scholar]
- Chapman H. Milestones in avian coccidiosis research: a review. Poult. Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. 1st ed. CRC Press; Boca Raton, FL: 2019. Pages 1–394 in Coccidiosis in Livestock, Poultry, Companion Animals and Humans. [Google Scholar]
- El-Ashram S., Suo X. Electrical cream separator coupled with vacuum filtration for the purification of eimerian oocysts and trichostrongylid eggs. Sci. Rep. 2017;7:1–14. doi: 10.1038/srep43346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hack M.E.A., Alagawany M., Abdel-Moneim A.M.E., Mohammed N.G., Khafaga A.F., Bin-Jumah M., Othman S.I., Allam A.A., Elnesr S.S. Cinnamon (Cinnamomum zeylanicum) oil as a potential alternative to Antibiotics in poultry. Antibiotics. 2020;9:210. doi: 10.3390/antibiotics9050210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gende L.B., Floris I., Fritz R., Eguaras M.J. Antimicrobial activity of cinnamon (Cinnamomum zeylanicum) essential oil and its main components against Paenibacillus larvae from Argentine. Bull. Insectol. 2008;61:1. [Google Scholar]
- Griffin R. The response of cage-reared broiler cockerels to dietary supplements of nitrovin, zinc bacitracin or penicillin used singly or in paired combinations. Br. Poult. Sci. 1979;20:281–287. [Google Scholar]
- Holdsworth P., Conway D.P., McKenzie M.E., Dayton A.D., Chapman H.D., Mathis G.F., Skinner J.T., Mundt H.C., Williams R.B. World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet. Parasitol. 2004;121:189–212. doi: 10.1016/j.vetpar.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kadykalo S., Roberts T., Thompson M., Wilson J., Lang M., Espeisse O. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Agents. 2018;51:304–310. doi: 10.1016/j.ijantimicag.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Khaki A., Khaki A.A., Hajhosseini L., Golzar F.S., Ainehchi N. The anti-oxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr. J. Tradit. Complem. Altern. Med. 2014;11:1–8. doi: 10.4314/ajtcam.v11i4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L., Zuo B., Ding H., Huang Y., Chen X., Du A. Anticoccidial evaluation of a traditional Chinese medicine—Brucea javanica—in broilers. Poult. Sci. 2016;95:811–818. doi: 10.3382/ps/pev441. [DOI] [PubMed] [Google Scholar]
- Lee H.-A., Hong S., Chung Y.-H., Song K.-D., Kim O. Anticoccidial effects of Galla rhois extract on Eimeria tenella-infected chicken. Lab. Anim. Res. 2012;28:193–197. doi: 10.5625/lar.2012.28.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor M., Jamil M., Ali A., Shahzad H., Awan A.A., Gul J. Role of herbal immunomodulators in control of coccidiosis disease. Pak. J. Sci. Ind. Res. Ser. B Biol. Sci. 2017;60:176–182. [Google Scholar]
- McDougald L., Fuller L., Mattiello R. A survey of coccidia on 43 poultry farms in Argentina. Avian Dis. 1997;41:923–929. [PubMed] [Google Scholar]
- Mehlhorn H. 4th ed. Springer-Verlag Berlin Heidelberg; Düsseldorf, Germany: 2016. Encyclopedic Reference of Parasitology. [Google Scholar]
- Morisawa Y., Kataoka M., Kitano N., Matsuzawa T. Studies on anticoccidial agents. 10. Synthesis and anticoccidial activity of 5-nitronicotinamide and its analogs. J. Med. Chem. 1977;20:129–133. doi: 10.1021/jm00211a027. [DOI] [PubMed] [Google Scholar]
- Moryani A.A., Rajput N., Rajput M.N., Shah A.H. Prevalence of common poultry diseases in chicken and influence of different medicinal herbs on the growth of broiler chicken. PAB. 2020;9:1199–1208. [Google Scholar]
- Mund M.D., Khan U.H., Tahir U., Mustafa B.-E.-., Fayyaz A. Antimicrobial drug residues in poultry products and implications on public health: a review. Int. J. Food Prop. 2017;20:1433–1446. [Google Scholar]
- Noack S., Chapman H.D., Selzer P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019;118:2009–2026. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash B., Kumar A., Singh P.P., Songachan L. Antimicrobial and antioxidant properties of phytochemicals: Current status and future perspective. In: Prakash B., editor. Functional and Preservative Properties of Phytochemicals. Elsevier, Academic Press; Cambridge, MA: 2020. pp. 1–45. [Google Scholar]
- Qasem M.A., Dkhil M.A., Al-Shaebi E.M., Murshed M., Mares M., Al-Quraishy S. Rumex nervosus leaf extracts enhance the regulation of goblet cells and the inflammatory response during infection of chickens with Eimeria tenella. J. King Saud Univ. Sci. 2020;32:1818–1823. [Google Scholar]
- Rao P.V., Gan S.H. Cinnamon: a multifaceted medicinal plant. Evid. Based Complement. Altern. Med. 2014;2014:12. doi: 10.1155/2014/642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc . SAS Institute Inc.; Cary, NC: 2018. SAS/OR®15.1 User’s Guide: Mathematical Programming Examples. [Google Scholar]
- Tanweer A.J., Saddique U., Bailey C., Khan R. Antiparasitic effect of wild rue (Peganum harmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitol. Res. 2014;113:2951–2960. doi: 10.1007/s00436-014-3957-y. [DOI] [PubMed] [Google Scholar]
- Vijayan K., Thampuran R.A. Vol. 259. CRC Press; Boca Raton, FL: 2004. Cinnamon and cassia: The Genus Cinnamomum. 11 Pharmacology and Toxicology of Cinnamon and cassia; pp. 259–284. (Books Series: Medicinal and Aromatic Plants - Industrial Profiles). [Google Scholar]
- Wondimu A., Mesfin E., Bayu Y. Prevalence of poultry coccidiosis and associated risk factors in intensive farming system of Gondar town, Ethiopia. Vet. Med. Int. 2019;2019:1–6. doi: 10.1155/2019/5748690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Kennes Y.M., Lepp D., Yin X., Wang Q., Yu H., Yang C., Gong J., Diarra M.S. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult. Sci. 2020;99:936–948. doi: 10.1016/j.psj.2019.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.