Abstract
Heat stress (HS) is a critical concern to the poultry industry as it affects both productivity and well-being. Various managerial and nutritional strategies have been proposed to mitigate the negative effects of HS in chickens, with plant-based additives showing promise. Recently, we reported the positive effect of a phytogenic feed additive (PFA) on growth performance in HS birds. Owing to the antioxidant nature of these compounds, we sought to further explore the effect of PFA on whole blood circulating chemokines, cytokines, and inflammasomes in HS broilers. Broilers (600 males, 1 d) were randomly assigned to 12 environmental chambers, subjected to 2 environmental conditions (12 h cyclic heat stress, HS, 35°C vs. thermoneutral condition [TN], 24°C) and fed 3 diets (control, PFA-C 250 ppm, PFA-C 400 ppm) in a 2 × 3 factorial design. After 21 d of cyclic HS, blood samples were collected for target gene expression analysis. HS upregulated the expression of superoxide dismutase 1 (SOD1) and downregulated glutathione peroxidase-3 (GPX-3), and there was diet × temperature interaction for SOD2, GPX-1, and GPX-3, where gene expression was increased by PFA-C250 during HS but was unchanged for PFA-C400. Plasma total antioxidant capacity (TAC) and malondialdehyde (MDA) content were increased by HS. Gene expression of interleukin-18 (IL-18) was decreased by HS, without further effect of PFA. HS increased tumor necrosis factor α (TNFα), but this effect was mitigated by PFA-C400. C-C motif chemokine ligands 4 and 20 (CCL4 and CCL20) showed a similar pattern to TNFα, with PFA-C400 ameliorating the negative effect of HS. The nucleotide-binding, leucine-rich repeat and pyrin domain containing 3 (NLRP3) inflammasome was decreased by HS and further lowered by PFA-C400, but the nucleotide-binding oligomerization domain, leucine-rich repeat, and CARD domain containing 3 (NLRC3) and nucleotide-binding, leucine-rich repeat containing X1 (NLRX1) inflammasomes were increased by PFA under TN conditions, with no effects of HS. Heat shock proteins (HSP) and heat shock factors (HSF) were unaffected by PFA or HS. Together these data indicate that gene expression of circulating inflammatory factors are dysregulated during HS, and supplemental dietary PFA may be protective.
Key words: phytogenic feed additive, heat stress, broiler, TNFα, antioxidant
Introduction
Heat stress (HS) is detrimental to all agricultural systems, and poultry production in particular, due in part to the high metabolic activity, body heat production, and decreased thermo-tolerance of modern chicken as a result of genetic selection for rapid growth rate and high efficiency (Settar et al., 1999; Deeb and Cahaner, 2002). Elevated environmental temperatures have significant negative effects on productivity, feed intake, growth performance, meat yield, well-being, and mortality in modern broilers (Lara and Rostagno, 2013; Attia and Hassan, 2017). Because of these challenges, effectively adapting to and managing HS has become an increasing concern to the industry. Maintenance of optimal bird health is key in better withstanding the challenges associated with HS; however, owing to the global heightened concerns on emerging drug-resistant superbugs and the critical need for antibiotic replacement, effective alternatives must be found.
One such alternative to maintain or improve poultry health is the inclusion of plant-based products in the diet. Phytogenic feed additives (PFA) is the term used to describe a wide range of bioactive plant-derived compounds that are incorporated into animal diets as herbs, spices, extracts, and/or essential oils (Windisch et al., 2008). Although their specific effects can differ based on not just the type of plant but also on plant origin, methods of extraction, and dietary formulation, one common characteristic of many PFA is their antioxidant properties (Wang et al., 2008; Settle et al., 2014). However, there is little information regarding their use under HS conditions (Akbarian et al., 2016), and the limited study results differ, depending on the PFA used. As HS induces oxidative stress in poultry and other species (Akbarian et al., 2016), which in turn can lead to oxidative damage and trigger an inflammatory response with increased production of inflammatory cytokines and chemokines (Heled et al., 2013), dietary PFA may help to alleviate these negative effects.
Previously, we reported that the newly developed PFA “Comfort” (PFA-C; Delacon, Steyregg, Austria) had a high total phenolic content and antioxidant activity (Greene et al., 2020). Dietary supplementation with PFA-C decreased body core temperature, increased feed and water intake, and improved BW in heat-stressed broilers (Greene et al., 2020). As PFA can have differing effects based on their composition and the majority of the literature focuses on tissue-specific effects, in the present study, we sought to further explore the effect of the PFA-C supplements on the expression profile of circulating inflammasomes, cytokines, and 11``chemokines in broilers exposed to chronic cyclic HS.
Materials and methods
Animals, Experimental Design, and Sampling
Diet composition, feed intake, water consumption, body weights, and body temperature from this bird trial have been previously reported (Greene et al., 2020). All animal care and procedures were approved by the Institutional Animal Care and Use Committee at the University of Arkansas. Briefly, 600 one-day-old Cobb 500 male chicks were weighed and randomly assigned to 12 environmental chambers. Each chamber was divided into 2 floor pens covered with fresh shavings and equipped with separate feeders and drinkers. Pens were randomly assigned to one of 3 corn-soy–based diets: Control (C), or the same diet supplemented with 2 doses of PFA-C (PFA-C 250 ppm and PFA-C 400 ppm; Delacon, Steyregg, Austria). PFA was added to the diet in the grower (day 12–23) and finisher (day 24–42) phases at an inclusion rate of 1 kg/ton, as recommended by the manufacturer. This PFA consists of encapsulated essential oils, dried herbs and spices, saponins, and bulking and anticaking agents (personal communication with Delacon, Austria). Phenolic content and total antioxidant activity of the 3 diets have been previously reported (Greene et al., 2020). At 8 am on day 21, the temperature was increased to 35°C in 6 of the chambers to induce HS for 12 h, after which it was reduced to 24°C each night. Thermoneutral (TN) chambers were maintained at 24°C. On day 40, blood samples were collected after at least 2 h of HS, mixed with TriZol LS, and stored at −80°C until further analysis.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was extracted from whole blood using Trizol LS reagent (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions, and concentration and quality were determined by using the Take3 microvolume plate and the Synergy HT multimode microplate reader (BioTek, Winooski, VT). RNA was reverse-transcribed using qScript cDNA Synthesis Supermix (Quanta Biosciences, Gaithersburg, MD) and amplified by real-time quantitative PCR (Applied Biosystems 7500 Real Time System) with PowerUp SYBR green master mix (Life Technologies, Carlsbad, CA). Relative expression of the target genes was determined using the 2−ΔΔCT method (Schmittgen and Livak, 2008), with normalization to ribosomal 18S gene expression. Oligonucleotide primer sequences specific for chicken are listed in Table 1. Primer Sequences for HSP27, 60, 70, 90, and HSF1-4 have been previously published (Rajaei-Sharifabadi et al., 2017).
Table 1.
Oligonucleotide real-time qPCR primers.
| Gene | Accession number | Primer sequence | Orientation | Product size, bp |
|---|---|---|---|---|
| IL-6 | NM_204628.1 | GCTTCGACGAGGAGAAATGC | For | 63 |
| GGTAGGTCTGAAAGGCGAACAG | Rev | |||
| IL-18 | NM_204608.1 | TGCAGCTCCAAGGCTTTTAAG | For | 63 |
| CTCAAAGGCCAAGAACATTCCT | Rev | |||
| TNFα | NM_204267.1 | CGTTTGGGAGTGGGCTTTAA | For | 61 |
| GCTGATGGCAGAGGCAGAA | Rev | |||
| CRP | NM_001039564.1 | AAGCTCAGGACAACGAGATCCT | For | 71 |
| TTTCCCCCCCACGTAGAAG | Rev | |||
| NLRP3 | NM_001348947.1 | GTTGGGCAGTTTCACAGGAATAG | For | 63 |
| GCCGCCTGGTCATACAGTGT | Rev | |||
| NLRC5 | NM_001318435.1 | CTCGAAGTAGCCCAGCACATT | For | 80 |
| CATGTCCAGAGGTGTCAGTCTGA | Rev | |||
| NLRC3 | XM_015294675.2 | CTCCAACGCCTCACAAACCT | For | 93 |
| GCCTTTGGTCATTTCCATCTG | Rev | |||
| NLRX1 | XM_004948038.3 | GGCTGAAACGTGGCACAAA | For | 59 |
| GAGTCCAAGCCCAGAAGACAAG | Rev | |||
| CCL4 | NM_204720.1 | CCTGCTGCACCACTTACATAACA | For | 63 |
| TGCTGTAGTGCCTCTGGATGA | Rev | |||
| CCL20 | NM_204438.2 | TGCTGCTTGGAGTGAAAATGC | For | 62 |
| CAGCAGAGAAGCCAAAATCAAA | Rev | |||
| CXCL14 | NM_204712.2 | CCGGCTCGCCATGAAG | For | 54 |
| ATCGCGATGACCAGCAGAA | Rev | |||
| SOD1 | NM_205064.1 | TGGCTTCCATGTGCATGAAT | For | 58 |
| ACGACCTGCGCTGGTACAC | Rev | |||
| SOD2 | NM_204211.1 | GCTGGAGCCCCACATCAGT | For | 61 |
| GGTGGCGTGGTGTTTGCT | Rev | |||
| GPX1 | NM_001277853.2 | TCCCCTGCAACCAATTCG | For | 57 |
| AGCGCAGGATCTCCTCGTT | Rev | |||
| GPX3 | NM_001163232.2 | GGGCGCTGACCATCGAT | For | 59 |
| CATCTTCCCCGCGTACTTTC | Rev | |||
| 18S | AF173612 | TCCCCTCCCGTTACTTGGAT | For | 60 |
| GCGCTCGTCGGCATGTA | Rev |
Plasma Total Antioxidant Capacity and Malondialdehyde Content
Plasma total antioxidant capacity (TAC) and thiobarbituric acid reactive substances (TBARS) were measured with commercially available kits and were performed according to manufacturer's protocol (Cayman Chemical Company, Ann Arbor, MI). Results are reported as mM trolox equivalents (TE) and μM malondialdehyde (MDA), respectively.
Statistical Analysis
Data were analyzed by 2-way ANOVA using GraphPad Prism v. 7.03 (San Diego, CA), with diet (control, PFA250, PFA400) and temperature (TN, HS) as factors using the following model:
where y = the dependent variables, μ = the general mean, αi and βj = diet and temperature effects, (αβ)ij = the interaction between diet and temperature, and εijk = the random error. Bird was the experimental unit. When significant main effects were detected, means were compared using Tukey's multiple range test. Significance was set at α = 0.05. All data are represented as means ± SEM.
Results and dicussion
It is known that genetic selection for rapid growth rate has negatively affected immune competence of modern broilers (Qureshi and Havenstein, 1994). In addition, as elevated global temperature has become a pressing concern, the impact of HS on the inflammatory response in poultry may be a significant contributor to the reductions seen in growth and productivity (Lara and Rostagno, 2013), and understanding this response is necessary to better design targeted treatments or interventions. As a previous study showed that supplementation with a PFA, “Comfort”, partially ameliorated heat stress productivity loss (BW, body core temperature, and so on) (Greene et al., 2020), we sought to further define here its mode of action by determining the expression profile of circulating cytokines, chemokines, and inflammasome genes in whole blood of cyclic heat-stressed broilers.
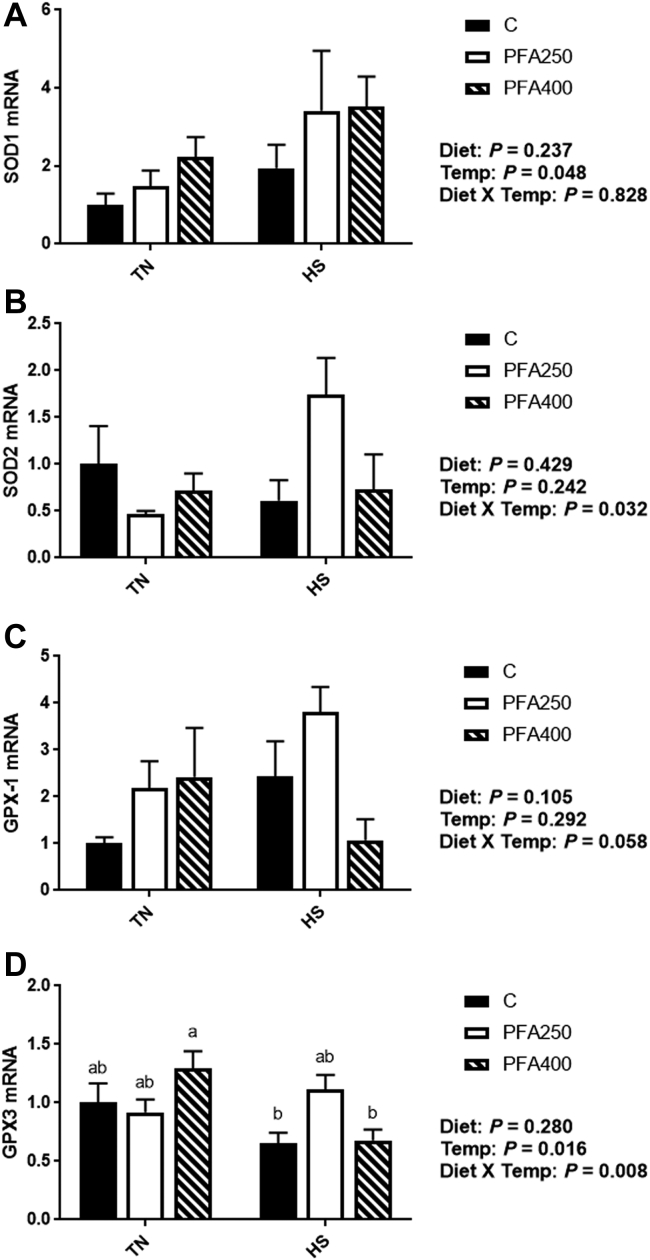
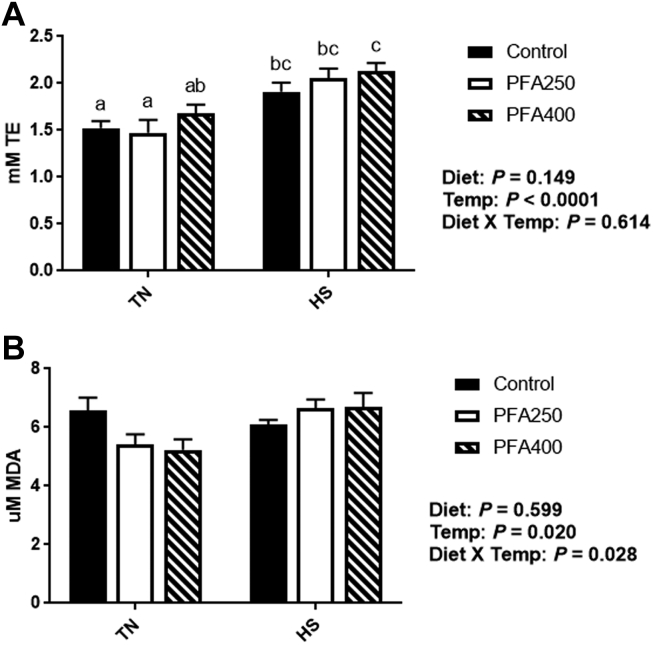
Under normal temperature conditions, the antioxidant systems of chicken are in a state of dynamic equilibrium and can adapt to manage normal challenges. During HS, however, reactive oxygen species are produced in excess of what the system can handle, and oxidative stress occurs (Lin et al., 2006; Azad et al., 2010; Akbarian et al., 2016). The superoxide dismutases (SOD) are key enzymes in the conversion of superoxide free radicals into hydrogen peroxide and molecular oxygen (Fridovich, 1995). Here, we show a main effect of temperature (P < 0.05) on SOD1, the copper- and zinc-dependent SOD, with an increase in gene expression during HS (Figure 1A). There was an interaction between temperature and PFA supplementation on SOD2 gene expression, the manganese-dependent SOD, where it was lower under TN conditions and elevated with HS in the PFA-C250 group, with no changes seen with HS in the PFSA-C400 group (Figure 1B). Once SOD1/2 have converted reactive oxygen species to hydrogen peroxide, glutathione peroxidases (GPX) can complete the reaction to generate water (de Haan et al., 2003). Interaction between temperature and PFA was evident with GPX-1 gene expression, where it was elevated under TN conditions with PFA, but decreased during HS with PFA-C400 (Figure 1C). GPX-3 expression was reduced by HS (Figure 1D), but the effect was mainly seen in the control and PFA-C400 groups. Concurrent with the reduction in GPX, there was an increase in lipid peroxidation products (MDA) in the plasma of HS birds, although this effect was not mitigated by PFA (Figure 2B). The overall effect of temperature on plasma lipid peroxides seems to be driven by the lower levels seen with PFA supplementation in TN conditions, indicating that the PFA may have a beneficial effect, even under nonstressed conditions. This effect may be specific to the PFA composition, as other research has shown increases (Erdoğan et al., 2010), decreases (Jiang et al., 2016; Placha et al., 2019), or no change (Attia et al., 2017a; Attia et al., 2017b) in oxidation products in chicken. Interestingly, it has been shown that HS can lead to the depression of redox systems in birds, but the effect may depend on the duration of the stress (Lin et al., 2000; Lin et al., 2006; Quinteiro-Filho et al., 2010). During acute HS, Lin et al. (2000) found that lipid peroxides were increased in plasma, while SOD1 was unchanged. However, chronic HS exposure increased SOD activity in the liver, while lipid peroxides were decreased (Lin et al., 2000). In the intestine, components of the antioxidant system are upregulated by various dietary PFA in broilers, although the magnitude of these changes, as well as impact on overall growth seem to vary based on the PFA studied, dose, and other stress challenges (Botsoglou et al., 2002; Luna et al., 2010; Paraskeuas et al., 2016; Paraskeuas et al., 2017; Mountzouris et al., 2020).
Figure 1.
Effect of PFA supplementation on circulating antioxidant markers in HS broilers. The relative gene expression of SOD1 (A), SOD2 (B), GPX-1 (C), and GPX-3 (D) was determined by qPCR and analyzed by 2–ΔΔCt method using the C-TN group as a calibrator. Data are presented as mean ± SEM (n = 6-8 birds/group). Main effects of diet, temperature, and the interaction are presented next to each graph. Different letters indicate significant difference at P < 0.05. Abbreviations: GPX, glutathione peroxidase; HS, heat stress; PFA, phytogenic feed additive; SOD, superoxide dismutase; TN, thermoneutral.
Figure 2.
Effect of PFA supplementation on total antioxidant capacity and malondialdehyde in plasma of HS broilers. Total antioxidant capacity (A) and malondialdehyde (B) were measured in plasma. Main effects of diet, temperature, and the interaction are presented next to each graph. Data are presented as mean ± SEM (n = 6-8 birds/group). Different letters indicate significant difference at P < 0.05. Abbreviations: HS, heat stress; PFA, phytogenic feed additive; TE, Trolox Equivalents; TN, thermoneutral.
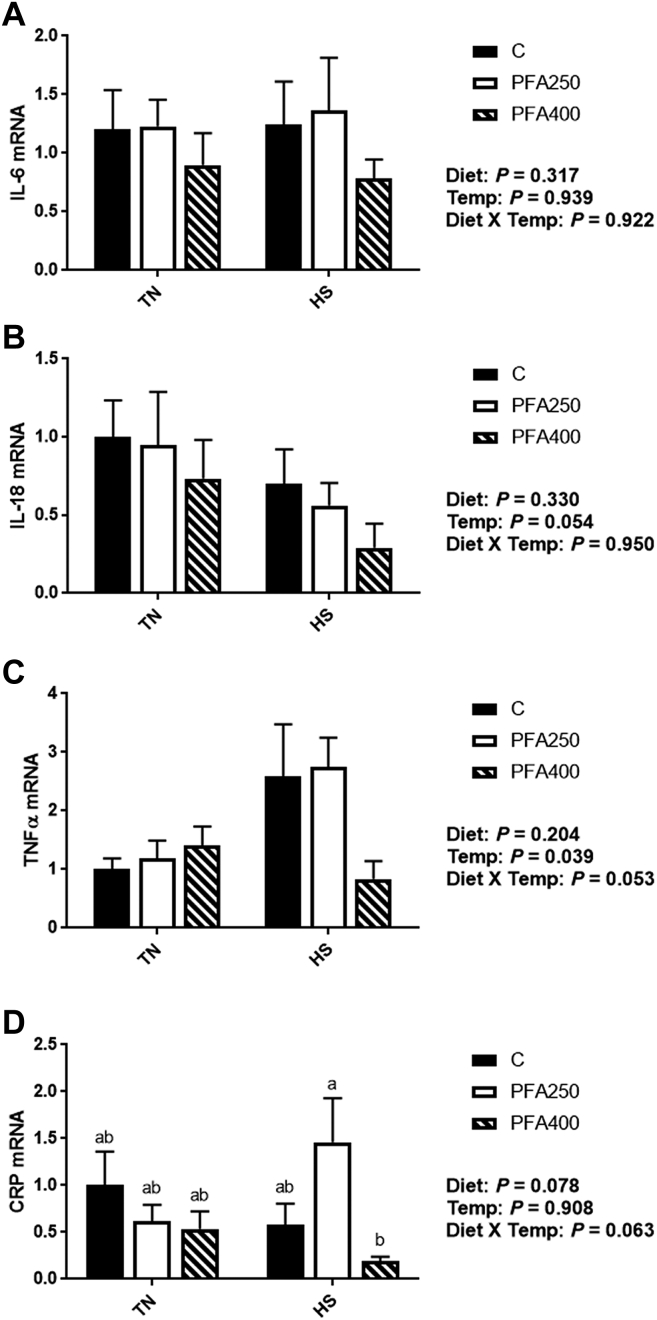
Heat stress has been shown to significantly impact cytokine production (Bouchama and Knochel, 2002; Carroll et al., 2012). Previous research also has shown that PFA may affect the immune system through reductions of proinflammatory cytokines and inflammation (Paraskeuas et al., 2017; Pirgozliev et al., 2019; Shen et al., 2020), although this has been measured primarily in the ceca, intestine, and immune cells. Here, the expression of whole blood interleukin-6 (IL-6) was unaffected by dietary PFA (Figure 3A), but interleukin-18 (IL-18) was decreased by HS, (P = 0.054), primarily driven by larger numerical decreases in the PFA-C treated groups (Figure 3B). Although primarily considered a proinflammatory cytokine, IL-18 expression can differ based on the duration of the stress. Similar to the depression seen here with chronic HS, IL-18 gene expression in chicken lymphocytes was decreased after 1 wk of corticosterone, although it was elevated 3 h after treatment (Shini and Kaiser, 2009). There were no main effects of temperature or diet on C-reactive protein (CRP); however, gene expression was downregulated during HS with PFA-C400 as compared to PFA-C250 (Figure 3D; P < 0.05). Tumor necrosis factor-α (TNFα) gene expression was increased by HS (P < 0.05); however, this increase was not seen in the PFA-C400 treated birds, where levels were similar to that of TN controls (Figure 3C), indicating potential amelioration of the inflammatory response. Although long understood to be critical in inflammation and the immune response of mammals, TNFα has only recently been characterized in chicken (Rohde et al., 2018). In mammals, however, TNFα is increased with cyclic HS in rats (Yun et al., 2012) and due to exertional heat stress in humans (Lu et al., 2004). The PFA carvacrol and thymol have been shown to decrease TNFα in rodents (Cho et al., 2012; Liang et al., 2014), pigs (Zou et al., 2016; Omonijo et al., 2019), and broilers (Hassan and Awad, 2017; Liu et al., 2019). TNFα interacts with the antioxidant system via SOD1, where, in monocytes, TNFα represses SOD1 via JAK/AP-1 (Afonso et al., 2006), and this interaction may help account for the increase seen in SOD1 with PFA-C400 in this study; however, further exploration of this mechanism of action in birds is warranted.
Figure 3.
Effect of PFA supplementation on circulating cytokines markers in HS broilers. The relative gene expression of IL-6 (A), IL-18 (B), TNFα (C), and CRP (D) was determined by qPCR and analyzed by 2–ΔΔCt method using C-TN group as a calibrator. Main effects of diet, temperature, and the interaction are presented next to each graph. Data are presented as mean ± SEM (n = 6-8 birds/group). Different letters indicate significant difference at P < 0.05. Abbreviations: CRP, C-reactive protein; HS, heat stress; IL, interleukin; PFA, phytogenic feed additive; TNFα, tumor necrosis factor alpha; TN, thermoneutral.
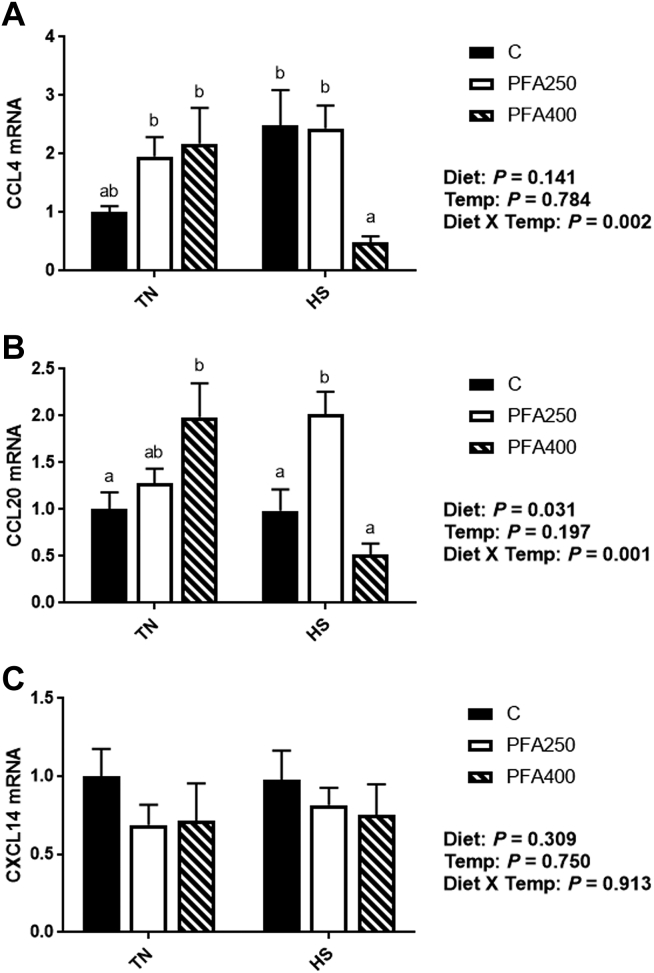
Chemokines are elevated in a wide range of inflammatory diseases, including rheumatoid arthritis and asthma (Charo and Ransohoff, 2006). In chickens, it has been shown that stress can alter the innate cytokine and chemokine responses (Hangalapura et al., 2006; Shini and Kaiser, 2009). TNFα, which responds to HS as shown here and in other research (Nair et al., 2015), stimulates chemokines, which then activate recruitment of dendritic cells and monocytes, leading to inflammation. Here, we show that gene expression of C-C motif chemokine ligands 4 and 20 (CCL4 and CCL20) are decreased by PFA-C400 during HS conditions as compared to the control group (P < 0.05; Figures 4A–4B), which parallels the effects seen in TNFα. There were no changes to the C-X-C motif chemokine ligand 14 (CXCL14; Figure 4C). Saponins, one of the components of the PFA used in this study, may regulate cytokine production via TLR-4, leading to macrophage activation (Naknukool et al., 2011). Conversely, in HD-11 chicken macrophages, addition of an essential oil PFA decreased the expression of CCL4 after lipopolysaccharide challenge (Shen et al., 2020), which is indicative of a decreased inflammatory response.
Figure 4.
Effect of PFA supplementation on circulating antioxidant markers in HS broilers. The relative gene expression of SOD1 (A), SOD2 (B), and GPX-1 (C) was determined by qPCR and analyzed by 2–ΔΔCt method using the C-TN group as a calibrator. Main effects of diet, temperature, and the interaction are presented next to each graph. Data are presented as mean ± SEM (n = 6–8 birds/group). Different letters indicate significant difference at P < 0.05. Abbreviations: CCL, C motif chemokine ligands; CXCL, C-X-C motif chemokine ligand; GPX, glutathione peroxidase; HS, heat stress; PFA, phytogenic feed additive; SOD, superoxide dismutase; TN, thermoneutral; TN, theroneutral.
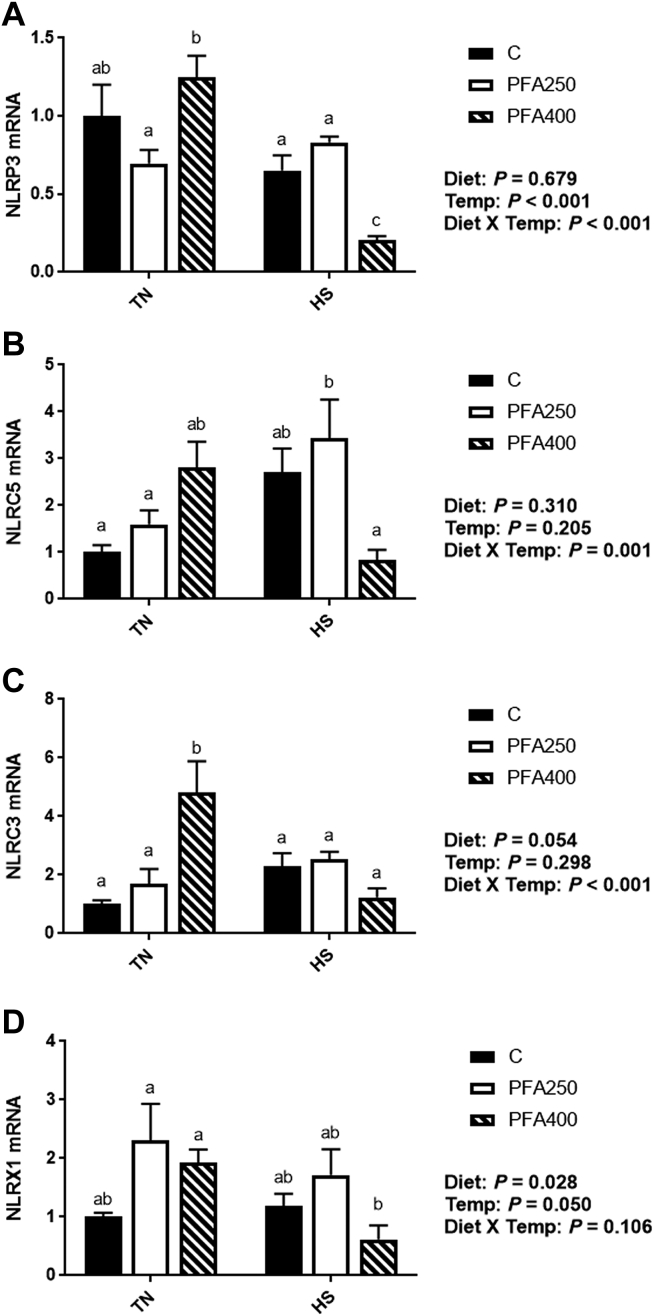
The inflammasome is an intracellular molecular complex that lies at the connection between a cellular stimulus and the inflammatory response. Numerous members of the nucleotide-binding, leucine-rich repeat-containing (NLR) family exist, with some species and tissue specificity, as well as differences in stimuli and activated pathways, and both inflammatory and anti-inflammatory properties (Davis et al., 2011; Lamkanfi and Dixit, 2014). Here, NLRP3, a proinflammatory inflammasome, was decreased (P < 0.05) by HS (Figure 5A), and this effect was enhanced by PFA-C400 in the diet. The NLRC3 inflammasome has been shown to have an anti-inflammatory role through inhibition of the Nuclear Factor Kappa B pathway (Schneider et al., 2012), and in this study, NLRC3 and NLRX1 gene expression showed similar expression patterns, where gene expression was increased by PFA-C during thermoneutral conditions (Figures 5C–5D). It has been suggested that specific phytogenics may also affect inflammation in disease via modulation of Nuclear Factor Kappa B/Mitogen-activated protein kinase pathways (Salminen et al., 2012; Monisha et al., 2016); however, mechanistic study of specific PFA in avian species is lacking. In addition, TNFα regulates transcription of components of the NLRP3 in cryopyrinopathies in mice (McGeough et al., 2017)—an interaction which may help explain the effects seen here.
Figure 5.
Effect of PFA supplementation on circulating antioxidant markers in HS broilers. The relative gene expression of SOD1 (A), SOD2 (B), GPX-1 (C), and GPX-3 (D) was determined by qPCR and analyzed by 2–ΔΔCt method using C-TN group as a calibrator. Main effects of diet, temperature, and the interaction are presented next to each graph. Data are presented as mean ± SEM (n = 6–8 birds/group). Different letters indicate significant difference at P < 0.05. Abbreviations: GPX, glutathione peroxidase; SOD, superoxide dismutase; TN, thermoneutral.
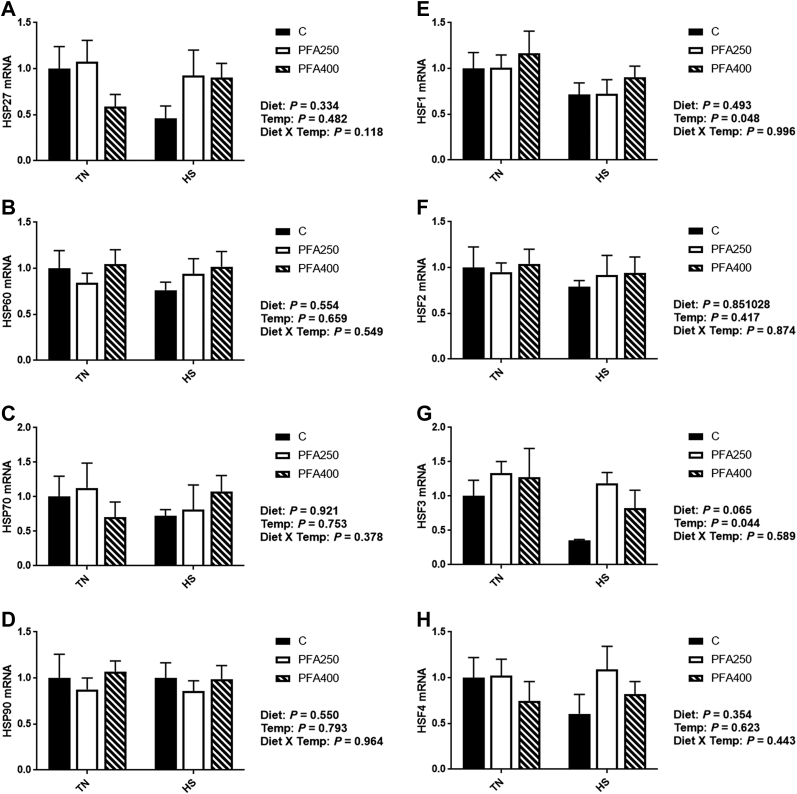
Overall, gene expression of the heat shock proteins (HSP2, 60, 70, and 90) and heat shock factors (HSF1, 2, 3, and 4) were not significantly affected by temperature or PFA in this study (Figures 6A–6H). Although somewhat unexpected, the lack of an effect may be due to acclimation of the birds to the HS condition, a phenomenon known as elastic response. In support of this idea, we (Figure 2A) and others (reviewed in Akbarian et al., 2016) show antioxidant capacity of chronically HS birds to be increased compared to TN conditions, which may reflect the ability of the bird to better manage the increases in reactive oxygen species with HS. In addition, the gene expression of HSP in blood may depend on not only the temperature and duration of HS but also the cell type measured (Oehler et al., 2001). Future studies should examine expression in various cell types in the blood to help determine the key players in the HS response.
Figure 6.
Effect of PFA supplementation on circulating heat shock proteins and heat shock factors in HS broilers. The relative gene expression of HSP27 (A), HSP60 (B), HSP70 (C), HSP90 (D), HSF1 (E), HSF2 (F), HSF3 (G), and HSF4 (H) was determined by qPCR and analyzed by 2–ΔΔCt method using C-TN group as a calibrator. Main effects of diet, temperature, and the interaction are presented next to each graph. Data are presented as mean ± SEM (n = 6–8 birds/group). Different letters indicate significant difference at P < 0.05. Abbreviations: HS, heat stress; PFA, phytogenic feed additive.
Together, our data indicate that dietary PFA-C supplementation can modulate the antioxidant system and inflammatory response in broiler chickens and may account for the alleviation of decreased health and growth performance seen during HS exposure.
Acknowledgments
The authors would like to thank Dr. Hakeem Kadhim and Dr. Barbara De Almeida Mallmann (University of Arkansas, Fayetteville, AR) for their assistance in animal care and sample collection. This study was conducted using a nonrestricted research gift from Delacon (to S.D.). Delacon had no role in conducting the research, generating the data, interpreting the results, or writing the manuscript.
Disclosures
The authors declare no conflicts of interest.
References
- Afonso V., Santos G., Collin P., Khatib A.-M., Mitrovic D.R., Lomri N., Leitman D.C., Lomri A. Tumor necrosis factor-α down-regulates human Cu/Zn superoxide dismutase 1 promoter via JNK/AP-1 signaling pathway. Free Radic. Bio. Med. 2006;41:709–721. doi: 10.1016/j.freeradbiomed.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia Y.A., Al-Harthi M.A., Hassan S.S. Turmeric (Curcuma longa Linn.) as a phytogenic growth promoter alternative for antibiotic and comparable to mannan oligosaccharides for broiler chicks. Rev. Mex. Cienc. Pecu. 2017;8:11–21. [Google Scholar]
- Attia Y.A., Bakhashwain A.A., Bertu N.K. Thyme oil (Thyme vulgaris L.) as a natural growth promoter for broiler chickens reared under hot climate. Ital. J. Anim. Sci. 2017;16:275–282. [Google Scholar]
- Attia Y.A., Hassan S.S. Broiler tolerance to heat stress at various dietary protein/energy levels. Europ. Poult. Sci. 2017;81 [Google Scholar]
- Azad M.A.K., Kikusato M., Sudo S., Amo T., Toyomizu M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp. Biochem. Phys. A. 2010;157:266–271. doi: 10.1016/j.cbpa.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Botsoglou N.A., Christaki E., Fletouris D.J., Florou-Paneri P., Spais A.B. The effect of dietary oregano essential oil on lipid oxidation in raw and cooked chicken during refrigerated storage. Meat Sci. 2002;62:259–265. doi: 10.1016/s0309-1740(01)00256-x. [DOI] [PubMed] [Google Scholar]
- Bouchama A., Knochel J.P. Heat stroke. New Engl. J. Med. 2002;346:1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Carroll J.A., Burdick N.C., Chase C.C., Jr., Coleman S.W., Spiers D.E. Influence of environmental temperature on the physiological, endocrine, and immune responses in livestock exposed to a provocative immune challenge. Domest. Anim. Endocrin. 2012;43:146–153. doi: 10.1016/j.domaniend.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Charo I.F., Ransohoff R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Cho S., Choi Y., Park S., Park T. Carvacrol prevents diet-induced obesity by modulating gene expressions involved in adipogenesis and inflammation in mice fed with high-fat diet. J. Nutr. Biochem. 2012;23:192–201. doi: 10.1016/j.jnutbio.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Davis B.K., Wen H., Ting J.P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan J.B., Crack P.J., Flentjar N., Iannello R.C., Hertzog P.J., Kola I. An imbalance in antioxidant defense affects cellular function: the pathophysiological consequences of a reduction in antioxidant defense in the glutathione peroxidase-1 (Gpx1) knockout mouse. Redox Rep. 2003;8:69–79. doi: 10.1179/135100003125001378. [DOI] [PubMed] [Google Scholar]
- Deeb N., Cahaner A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult. Sci. 2002;81:293–301. doi: 10.1093/ps/81.3.293. [DOI] [PubMed] [Google Scholar]
- Erdoğan Z., Erdoğan S., Aslantaş Ö., Çelik S. Effects of dietary supplementation of synbiotics and phytobiotics on performance, caecal coliform population and some oxidant/antioxidant parameters of broilers. J. Anim. Phys. Anim. Nutr. 2010;94:e40–e48. doi: 10.1111/j.1439-0396.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Greene E.S., Cauble R., Kadhim H., de Almeida Mallmann B., Gu I., Lee S.O., Orlowski S., Dridi S. Protective effects of the phytogenic feed additive “comfort” on growth performance via modulation of hypothalamic feeding- and drinking-related neuropeptides in cyclic heat-stressed broilers. Domest. Anim. Endocrinol. 2020;74 doi: 10.1016/j.domaniend.2020.106487. :106487. [DOI] [PubMed] [Google Scholar]
- Hangalapura B.N., Kaiser M.G., van der Poel J.J., Parmentier H.K., Lamont S.J. Cold stress equally enhances in vivo pro-inflammatory cytokine gene expression in chicken lines divergently selected for antibody responses. Dev. Comp. Immunol. 2006;30:503–511. doi: 10.1016/j.dci.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Hassan F.A.M., Awad A. Impact of thyme powder (Thymus vulgaris L.) supplementation on gene expression profiles of cytokines and economic efficiency of broiler diets. Environ. Sci. Pollut. R. 2017;24:15816–15826. doi: 10.1007/s11356-017-9251-7. [DOI] [PubMed] [Google Scholar]
- Heled Y., Fleischmann C., Epstein Y. Cytokines and their role in hyperthermia and heat stroke. J. Basic Clin. Phys. Pharmacol. 2013;24:85–96. doi: 10.1515/jbcpp-2012-0040. [DOI] [PubMed] [Google Scholar]
- Jiang X.-R., Zhang H.-J., Wang J., Wu S.-G., Yue H.-Y., Lü H.-Y., Cui H., Bontempo V., Qi G.-H. Effect of dried tangerine peel extract supplementation on the growth performance and antioxidant status of broiler chicks. Ital. J. Anim. Sci. 2016;15:642–648. [Google Scholar]
- Lamkanfi M., Dixit Vishva M. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Li F., Fu Y., Cao Y., Song X., Wang T., Wang W., Guo M., Zhou E., Li D., Yang Z., Zhang N. Thymol inhibits LPS-stimulated inflammatory response via down-Regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation. 2014;37:214–222. doi: 10.1007/s10753-013-9732-x. [DOI] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A. 2006;144:11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Lin H., Du R., Zhang Z.Y. Peroxide status in tissues of heat-stressed broilers. Asian-Australas. J. Anim. Sci. 2000;13:1373–1376. [Google Scholar]
- Liu S.D., Song M.H., Yun W., Lee J.H., Kim H.B., Cho J.H. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 2019;98:2026–2033. doi: 10.3382/ps/pey575. [DOI] [PubMed] [Google Scholar]
- Lu K.-C., Wang J.-Y., Lin S.-H., Chu P., Lin Y.-F. Role of circulating cytokines and chemokines in exertional heatstroke. Crit. Care Med. 2004;32:399–403. doi: 10.1097/01.CCM.0000108884.74110.D9. [DOI] [PubMed] [Google Scholar]
- Luna A., Labaque M.C., Zygadlo J.A., Marin R.H. Effects of thymol and carvacrol feed supplementation on lipid oxidation in broiler meat. Poult. Sci. 2010;89:366–370. doi: 10.3382/ps.2009-00130. [DOI] [PubMed] [Google Scholar]
- McGeough M.D., Wree A., Inzaugarat M.E., Haimovich A., Johnson C.D., Peña C.A., Goldbach-Mansky R., Broderick L., Feldstein A.E., Hoffman H.M. TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J. Clin. Invest. 2017;127:4488–4497. doi: 10.1172/JCI90699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monisha J., Padmavathi G., Roy N.K., Deka A., Bordoloi D., Anip A., B Kunnumakkara A. NF-κB blockers gifted by mother nature: Prospectives in cancer cell chemosensitization. Curr. Pharm.Design. 2016;22:4173–4200. doi: 10.2174/1381612822666160609110231. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Paraskeuas V.V., Fegeros K. Priming of intestinal cytoprotective genes and antioxidant capacity by dietary phytogenic inclusion in broilers. Anim. Nutr. 2020;6:305–312. doi: 10.1016/j.aninu.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Arora S., Lim J.Y., Lee L.H., Lim L.H.K. The regulation of TNFα production after heat and endotoxin stimulation is dependent on Annexin-A1 and HSP70. Cell Stress Chaperon. 2015;20:583–593. doi: 10.1007/s12192-015-0580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naknukool S., Horinouchi I., Hatta H. Stimulating macrophage activity in mice and humans by oral administration of Quillaja saponin. Biosci. Biotechnol. Biochem. 2011;75:1889–1893. doi: 10.1271/bbb.110182. [DOI] [PubMed] [Google Scholar]
- Oehler R., Pusch E., Zellner M., Dungel P., Hergovics N., Homoncik M., Eliasen M.M., Brabec M., Roth E. Cell type-specific variations in the induction of Hsp70 in human leukocytes by feverlike whole body hyperthermia. Cell Stress Chaperon. 2001;6:306–315. doi: 10.1379/1466-1268(2001)006<0306:ctsvit>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omonijo F.A., Liu S., Hui Q., Zhang H., Lahaye L., Bodin J.-C., Gong J., Nyachoti M., Yang C. Thymol improves barrier function and attenuates inflammatory responses in porcine intestinal epithelial cells during lipopolysaccharide (LPS)-induced inflammation. J. Agric. Food Chem. 2019;67:615–624. doi: 10.1021/acs.jafc.8b05480. [DOI] [PubMed] [Google Scholar]
- Paraskeuas V., Fegeros K., Palamidi I., Hunger C., Mountzouris K.C. Growth performance, nutrient digestibility, antioxidant capacity, blood biochemical biomarkers and cytokines expression in broiler chickens fed different phytogenic levels. Anim. Nutr. 2017;3:114–120. doi: 10.1016/j.aninu.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskeuas V., Fegeros K., Palamidi I., Theodoropoulos G., Mountzouris K.C. Phytogenic administration and reduction of dietary energy and protein levels affects growth performance, nutrient digestibility and antioxidant status of broilers. J. Poult. Sci. 2016;53:264–273. doi: 10.2141/jpsa.0150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirgozliev V., Mansbridge S.C., Rose S.P., Lillehoj H.S., Bravo D. Immune modulation, growth performance, and nutrient retention in broiler chickens fed a blend of phytogenic feed additives. Poult. Sci. 2019;98:3443–3449. doi: 10.3382/ps/pey472. [DOI] [PubMed] [Google Scholar]
- Placha I., Ocelova V., Chizzola R., Battelli G., Gai F., Bacova K., Faix S. Effect of thymol on the broiler chicken antioxidative defence system after sustained dietary thyme oil application. Br. Poult. Sci. 2019;60:589–596. doi: 10.1080/00071668.2019.1631445. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sá L.R.M., Ferreira A.J.P., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Qureshi M.A., Havenstein G.B. A comparison of the immune performance of a 1991 commercial broiler with a 1957 randombred strain when fed “typical” 1957 and 1991 broiler diets. Poult. Sci. 1994;73:1805–1812. doi: 10.3382/ps.0731805. [DOI] [PubMed] [Google Scholar]
- Rajaei-Sharifabadi H., Ellestad L., Porter T., Donoghue A., Bottje W.G., Dridi S. Noni (Morinda citrifolia) modulates the hypothalamic expression of stress- and metabolic-related genes in broilers exposed to acute heat stress. Front. Genet. 2017;8:192. doi: 10.3389/fgene.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde F., Schusser B., Hron T., Farkašová H., Plachý J., Härtle S., Hejnar J., Elleder D., Kaspers B. Characterization of chicken tumor necrosis factor-α, a long missed cytokine in birds. Front. Immunol. 2018;9:605. doi: 10.3389/fimmu.2018.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Kauppinen A., Kaarniranta K. Phytochemicals suppress nuclear factor-κB signaling: impact on health span and the aging process. Curr. Opin. Clin. Nutr. Metab. Care. 2012;15:23–28. doi: 10.1097/MCO.0b013e32834d3ae7. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schneider M., Zimmermann A.G., Roberts R.A., Zhang L., Swanson K.V., Wen H., Davis B.K., Allen I.C., Holl E.K., Ye Z., Rahman A.H., Conti B.J., Eitas T.K., Koller B.H., Ting J.P.Y. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat. Immunol. 2012;13:823–831. doi: 10.1038/ni.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settar P., Yalçin S., Türkmut L., Ozkan S., Cahanar A. Season by genotype interaction related to broiler growth rate and heat tolerance. Poult. Sci. 1999;78:1353–1358. doi: 10.1093/ps/78.10.1353. [DOI] [PubMed] [Google Scholar]
- Settle T., Leonard S.S., Falkenstein E., Fix N., Van Dyke K., Klandorf H. Effects of a phytogenic feed additive versus an antibiotic feed additive on oxidative stress in broiler chicks and a possible mechanism determined by electron spin resonance. Int. J. Poult. Sci. 2014;13:62–69. doi: 10.3923/ijps.2014.62.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Christensen L.G., Bak S.Y., Christensen N., Kragh K. Immunomodulatory effects of thymol and cinnamaldehyde in chicken cell lines. J. Appl. Anim. Nutr. 2020;8:21–30. [Google Scholar]
- Shini S., Kaiser P. Effects of stress, mimicked by administration of corticosterone in drinking water, on the expression of chicken cytokine and chemokine genes in lymphocytes. Stress. 2009;12:388–399. doi: 10.1080/10253890802526894. [DOI] [PubMed] [Google Scholar]
- Wang L., Piao X.L., Kim S.W., Piao X.S., Shen Y.B., Lee H.S. Effects of Forsythia suspensa extract on growth performance, nutrient digestibility, and antioxidant activities in broiler chickens under high ambient temperature. Poult. Sci. 2008;87:1287–1294. doi: 10.3382/ps.2008-00023. [DOI] [PubMed] [Google Scholar]
- Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008;86:E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Yun S.-H., Moon Y.-S., Sohn S.-H., Jang I.-S. Effects of cyclic heat stress or vitamin C supplementation during cyclic heat stress on HSP70, inflammatory cytokines, and the antioxidant defense system in Sprague Dawley rats. Exper. Anim. 2012;61:543–553. doi: 10.1538/expanim.61.543. [DOI] [PubMed] [Google Scholar]
- Zou Y., Wang J., Peng J., Wei H. Oregano essential oil induces SOD1 and GSH expression through Nrf2 activation and alleviates hydrogen peroxide-induced oxidative damage in IPEC-J2 cells. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/5987183. 5987183. [DOI] [PMC free article] [PubMed] [Google Scholar]