Abstract
Avian sperm storage tubules (SSTs), which are located in the uterovaginal junction (UVJ) of the oviduct, are primary sperm storage sites after mating or artificial insemination. The mechanism underlying reduced sperm storage efficiency of SSTs which is highly correlated with decreased fertility rates in aged laying breeders remains largely unclear. Here, comparative transcriptomic analysis between the aged and young White Leghorn hens (120 vs. 30 wk) was applied to identify gene expression changes of UVJs containing SSTs. Bioinformatics analysis revealed 567 upregulated and 1998 downregulated differentially expressed genes. Gene ontology analysis was highly enriched in terms of immune system, cell adhesion, and cytoskeleton proteins. Kyoto Encyclopedia of Genes and Genomes analysis revealed 5 significant (P < 0.05) pathways including inositol phosphate and glycerophospholipid metabolism. β-Galactosidase staining of chicken UVJ sections suggested increased cell senescence via aging. Oil Red O staining and immunohistochemistry detection of ADFP both confirmed distribution of lipid droplets in SST cells with increased intensity in aged breeders. The lipid synthesis and metabolism-related genes represented by TFAP2 and PLD1 were differentially expressed in aged laying breeders. The upregulation of IL15 and downregulation of a large number of immune-related genes in aged breeders indicate altered immune homeostasis in UVJs and SSTs. The increased accumulation of lipids, and altered immunity homeostasis, combined with other factors (TJP1, MYL9, AFDN, and RPL13, etc.) are potentially dominant effectors to decrease the sperm storage efficiency and egg fertility in aged laying breeders.
Key words: aging, chicken, sperm storage, breeder, uterovaginal junction
Introduction
The capacity for females to store sperm temporarily in the oviduct varies from days to months across different species (Birkhead and Møller, 2008; Holt and Lloyd, 2010; Matsuzaki and Sasanami, 2017). Birds and some reptiles develop blind-ended sperm storage tubules (SSTs) located in the reproductive system, from where the spermatozoa are preserved for a period of time and later released toward the site of fertilization (Bakst et al., 1994). In avian hens, SSTs are situated at the uterovaginal junction (UVJ) of the oviduct, and are characterized as non-ciliated, single-layered, and columnar epithelium, different from the pseudostratified ciliated epithelial cells in UVJs (Gilbert et al., 1968).
After mating or artificial insemination (AI), a large number of spermatozoa arrive at the UVJ, enter into the SSTs for a short-term or long-term stay, followed with gradual release from SSTs, and travel to the fertilized site in accordance with daily ovarian follicle cycles (Ito et al., 2011). The resident spermatozoa are capable of survival and fertilizing the ovum for days or weeks in hens. Molecules and signals in association with sperm storage in birds have been studied by 2 recently published reports (Holt and Fazeli, 2016; Matsuzaki and Sasanami, 2017). We have previously investigated the impacts of UVJs and SSTs functioning in sperm storage at 2 time points (day 3 and day 9 after AI) and found that HIP1, PDE1C, and calcium-related genes were interesting candidates involved in relatively long-term sperm storage in White Leghorn hens (Yang et al., 2020). These studies were mostly focused on the early or peak egg laying period.
Artificial insemination or natural mating is frequently performed in poultry breeding production. However, the fertility rate is negatively correlated with age in different avian breeders. In Japanese quail, the egg fertility rate is decreased significantly after 55 wk of age (Santos et al., 2013), whereas in broiler and layer breeders, similar age-related fertility reduction is reported in the late egg laying period, which is potentially associated with low efficiency of SSTs in UVJs (Beaumont et al., 1992; Fasenko et al., 1992; Ledur et al., 2000; Gumulka and Kapkowska, 2005).
Aging is regarded to be a consequence of complex interactions among physical, biological, and biochemical processes described in multiple animals as well as in tissues/organs, such as the reproductive tract (luteal, oviduct, and uterine) (Velarde and Menon, 2016; Korovila et al., 2017; Shirasuna and Iwata, 2017). Few studies are conducted to understand the correlation of UVJ tissues with fertility reduction in aged layer breeders. In this study, White Leghorn which is a widely-used model for laying breeder (Atikuzzaman et al., 2015; Huang et al., 2016) is used to investigate the morphological and molecular variations of the sperm storage reservoir(UVJ containing SSTs)within the female reproductive tract via aging. We hypothesize that transcriptomic changes may reveal factors related to fertility decrease. The outcome of this study will extend the understanding of sperm storage reservoirs (UVJ containing SSTs) within the reproduction tract, and provide clues to save fertility decline via aging and make better usage of breeders in poultry breeding stock.
Materials and methods
Sample Collection
All animal experiments involved were carried out following standard procedures (no. 5 proclaim of the Standing Committee of Hubei People's Congress) and approved by the Standing Committee of Hubei People's Congress and the Ethics Committee of Huazhong Agricultural University, China. Single Comb White Leghorn hens aged approximately 30 (n = 5), 65 (n = 5), and 120 (n = 4) week, respectively, were raised individually in stair-step cages in local chicken farms, with standard feeding and management system including light cycling of 16 h light and 8 h dark. All hens were raised up to 3 wk without AI or natural mating before sampling. The hens were euthanized following standard procedures to dissect the oviducts and specify the UVJ tissues containing SSTs under a stereomicroscope. The UVJ tissues of 30 and 120 wk were carefully divided into 3 parts from perpendicular to the ovary-uterus axis, and immediately fixed in 4% paraformaldehyde (PFA) in PBS, embedded in optimal cutting temperature compound, and snap-frozen in liquid nitrogen for RNA isolation, respectively. UVJ tissues of 65 wk were processed for immediate fixation in 4% PFA in PBS, and embedded in optimal cutting temperature compound for further use.
RNA Isolation and cDNA Preparation
Each snap-frozen UVJ sample was homogenized in TRIzol Reagent (Invitrogen, San Diego, CA) to perform RNA isolation and purification according to the manufacturer's instruction. The purified total RNAs of each sample were used for reverse transcription and cDNA synthesis by using PrimeScript RT reagent Kit with gDNA Eraser (Takara, Tianjing, China). The cDNAs of each sample were stored at −20°C for further use.
Transcriptome Sequencing and Data Analysis (120 vs. 30 wk of Age)
Chicken UVJ tissues containing SSTs were selected and subjected to a high-throughput RNA sequencing program (PE150) to compare the aged hens (120 wk) with young hens (30 wk) (n = 4) (Novogene, Beijing, China). More than 6 GB of clean data were obtained for each sequencing library and processed for quality control by FastQC, followed with mapping to chicken reference genome (ftp://ftp.ensembl.org/../pub/release-96/fasta/gallus_gallus/dna/) by hisat2 v2.0.5. HTSeq-count software was used to count the number of reads mapped to each gene. Fragments per kilobase million of each gene were calculated based on the length and read for estimating gene expression level. Differential expression analyses of genes enriched in UVJ samples collected at 120 (n = 4) and 30 wk (n = 5) were performed using the DESeq2 R package (1.16.1) (Bioconductor, Seattle, WA). The resulting P-values were adjusted using the Benjamini and Hochberg's approach for controlling the false discovery rate. Genes with adjusted P-value <0.05 and | log2 (fold change) | > 1 were assigned as differentially expressed transcripts by DESeq2.
Analysis of Differentially Expressed Genes (DEGs) by Quantitative Real-Time PCR (qRT-PCR)
The qRT-PCR primers were designed on Oligo 7.6 (Molecular Biology Insights, Colorado Springs, CO). Primer information has been listed in Supplementary Table 1. Several differentially expressed mRNAs were used for qPCR analysis to verify the gene expression tendency in cDNA samples generated from UVJs of White Leghorn chicken hens aged at about 30 (n = 5) and 120 (n = 4) wk, respectively. The qPCR amplification conditions were as follows: 95°C for 5 min; 40 cycles of 95°C for 10 s and 57.5°C for 30 s; 72°C for 20 s; and 72°C for 5 min. The fold change was calculated by 2−ΔΔCt method followed with significance testing by t test.
Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment, and Protein–Protein Interaction (PPI) Analyses of DEGs
GO enrichment and KEGG pathway analyses were performed by using GO (http://geneontology.org/) and KEGG pathway (https://www.kegg.jp/) databases, respectively. The statistical test and significant level were calculated by the hypergeometric test and Fisher's exact test. False discovery rate correction was also carried out by Benjamini and Hochberg method using the Kobas 3.0 website (http://kobas.cbi.pku.edu.cn/). PPI network analysis of DEGs was carried out by using the STRING website (https://string-db.org/) at the strongest confidence (minimum required interaction score: 0.900), and viewed by using Cytoscape v3.6.1 software (National Institute of General Medical Sciences of the National Institutes of Health, Bethesda, MD).
Histological Study of UVJ Containing SSTs
Paraffin sections of UVJ tissues containing SSTs from hens of 30, 65, and 120 wk of age were prepared according to the standard steps. Briefly, the UVJ tissues containing SSTs were dissected under a stereomicroscope and submerged in running water. The samples were sequentially dehydrated, embedded in paraffin, and processed for sections at 6-μm thickness by a microtome (Leica RM2165, Leica, Wetzlar, Germany). The sections were stained by hematoxylin and eosin and photographed by using a Olympus microscope and imaging system (Olympus BX53, Olympus Corporation, Tokyo, Japan).
Detection of β-Galactosidase Activity in Aged and Young UVJ Tissues Containing SSTs
Frozen sections (10 μm in thickness) from UVJ samples containing SSTs collected at 30, 65, and 120 wk were prepared using a freezing microtome (Leica CM1950, Leica). The sections were stained by using a β-galactosidase staining kit (Solarbio, Beijing, China) according to the manufacturer's instructions and mounted using glycerol. Photographs were taken by the Olympus microscope and imaging system.
Oil Red O Staining of UVJ Containing SSTs
Frozen sections from 30, 65, and 120 wk UVJ samples containing SSTs were prepared with 10-μm thickness. The sections were fixed in 4% PFA for 1 h, briefly rinsed by distilled water and 100% propylene glycol, and stained by 5% Oil Red O for 2 h at 58°C. The sections were then briefly rinsed in 85% propylene glycol and distilled water, stained with hematoxylin for seconds, and mounted using glycerol. Photographs were taken by the Olympus microscope and imaging system.
Immunohistochemistry of ADFP
UVJ frozen sections from 30, 65, and 120 wk samples were prepared with 10-μm thickness. They were stained with standard procedures with the primary antibody ADFP (1:1000) (Proteintech, Wuhan, China) at 4°C for 12 h, followed with secondary antibody incubation for 1 h at room temperature using an immunological kit (Proteintech). Visualization was performed by using DAB (1:50) staining followed by hematoxylin staining for seconds and mounting using glycerol. Photographs were taken by the Olympus microscope and imaging system.
Results
Histological Observation of Aging Process of Chicken UVJ Tissues
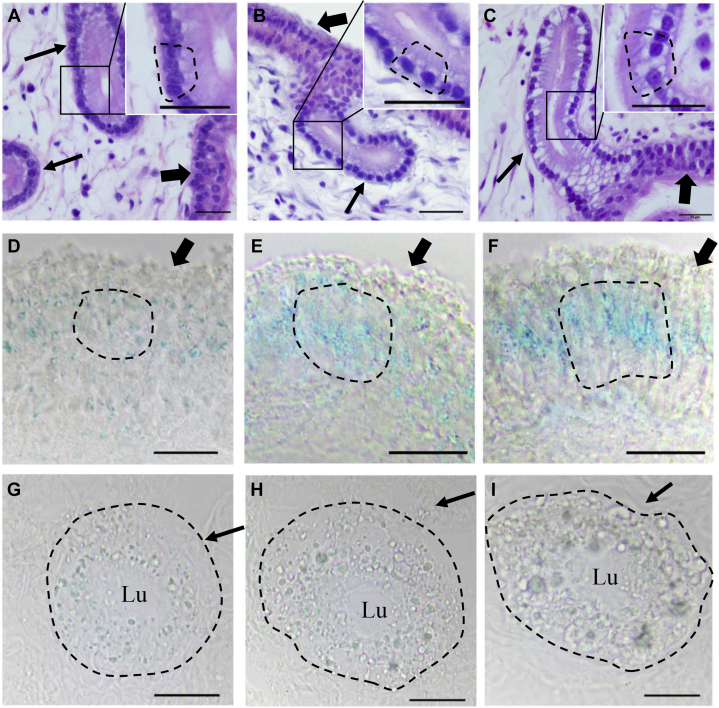
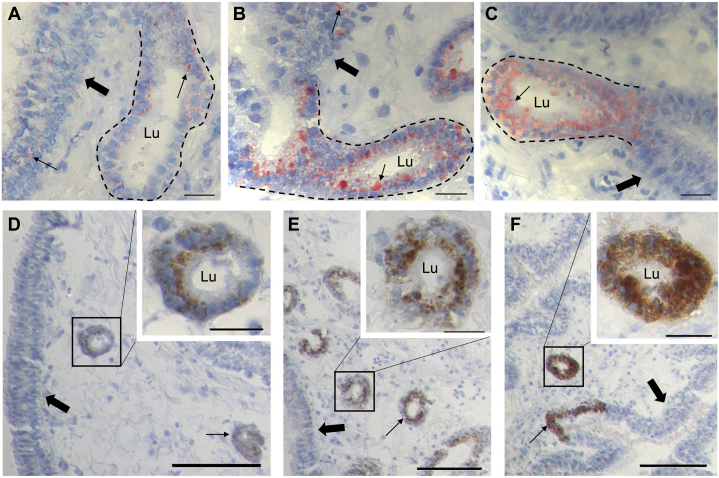
Hematoxylin and eosin-stained tissue sections derived from comparable parts of UVJ samples showed that the epithelial cells of UVJ tissues, which were characterized as pseudostratified ciliated epithelium, displayed no clear differences among the chicken samples at 3 stages (30, 65, and 120 wk of age). It is noteworthy that the cells of SSTs which were dispersed in UVJ tissues and characterized as non-ciliated, single-layered, and columnar epithelium were much different among the samples at 30, 65, and 120 wk of age. The large vesicle which potentially contained lipid droplets was observed to emerge from the perinuclear region of SST cells aged 65 wk and become more obvious at 120 wk (Figures 1A–1C). The UVJ tissue sections stained with cell senescence biomarker β-galactosidase showed weaker signals in the UVJ epithelium at 30 wk, and stronger signals at 65 and 120 wk (Figures 1D–1I). The accumulation of β-galactosidase in the SST epithelium was difficult to distinguish because of the overspread of vesicles (lipid droplet) in samples at 65 and 120 wk of age.
Figure 1.
(A–C) Histological comparison of chicken UVJ folds containing SSTs stained with hematoxylin and eosin among 3 stages (30, 65, and 120 wk of age) (A, B, and C, respectively). The UVJ epithelium was characterized as pseudostratified and ciliated columnar cells (bold arrows), whereas the (tubular or circular) SSTs epithelium was characterized as single layer with columnar cells (narrow arrows). Nucleus was stained with blue. Cytoplasm was stained with red. A large achromatic vesicle (dotted region; the achromatic vesicle cannot be stained either by hematoxylin or eosin) was emerged in the perinuclear region of SST cells, and its size and numbers were significantly increased with aging based on the sections. Scale bar = 20 μm. (D–I) Cell senescence biomarker β-galactosidase staining of 30, 65, and 120 wk of age UVJ containing SSTs sections. The blue color indicated with dotted region represents the β-galactosidase activity. (D–F) β-galactosidase staining of UVJ epithelium (bold arrows) from 30, 65, and 120 wk of age, respectively. The β-galactosidase activity in the UVJ epithelium was increased with aging (dotted region). (G–I) β-galactosidase staining of circular SSTs (dotted region) from 30, 65, and 120 wk of age, respectively. The SST lumen was marked with “Lu.” The staining of β-Galactosidase in SST cells was difficult to distinguish due to the large vesicle (arrow). Scale bar = 20 μm. Abbreviations: SSTs, sperm storage tubules; UVJ, uterovaginal junction.
Transcriptome Data Analysis of UVJs Containing SSTs Between Aged and Young Hens (120 vs. 30 wk)
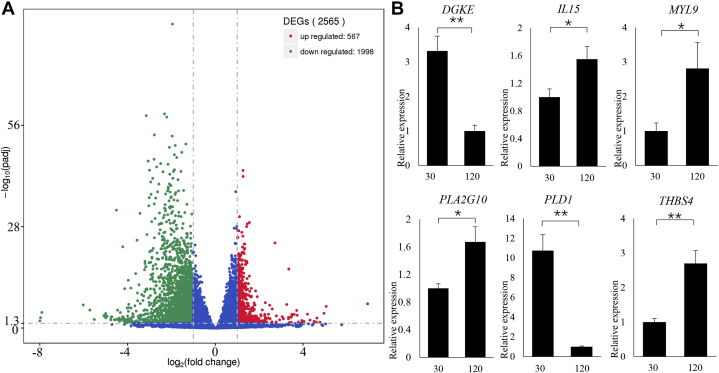
Illumina sequencing of 9 UVJ tissue samples collected from aged (120 wk) and young (30 wk) hens produced about 50 GB raw reads and 203,400,335 clean reads (97% of the raw reads) after quality control. More than 84% of the clean reads were uniquely mapped to a chicken reference genome (Gallus_gallus-6.0). A total of 2,565 genes were identified as DEGs including 567 upregulated and 1998 downregulated transcripts and compared samples aged 120 to 30 wk (120 vs. 30 wk) (Figure 2A and Supplementary Table 2).
Figure 2.
(A) Volcano plots of DEGs enriched in chicken UVJ folds containing SSTs (120 vs. 30 wk of age). The X-axis and Y-axis represented the log2 fold change values and the statistical significance P-values (−log10), respectively. The red and green dots represented the upregulated and downregulated mRNA transcripts with expression exceeding the threshold value within log2 fold change values between 1 and −1, q-value < 0.05. The blue dots represented genes with expression level beyond the stated threshold. (B) qRT-PCR validation of randomly selected DEGs (DGKE, IL15, MYL9, PLA2G10, and THBS4) in chicken UVJ folds (120 vs. 30 wk of age). The number 30 represents UVJ tissues containing SSTs at 30 wk of age (n = 5), whereas 120 represents UVJ tissues containing SSTs at 120 wk of age (n = 4). Error bars represented SEM. “∗” and “∗∗” represented the levels of significant differences at P < 0.05 and P < 0.01, respectively. Abbreviations: DEGs, differentially expressed genes; qRT, quantitative real-time; SSTs, sperm storage tubules.
GO Terms, KEGG Pathway Enrichment, and PPI Analyses of DEGs Between Aged and Young Hens (120 vs. 30 wk)
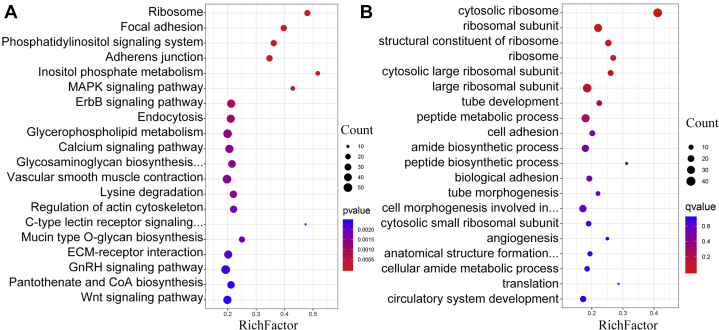
The 2565 DEGs derived from UVJ tissue samples (120 vs. 30 wk) were utilized to perform GO and KEGG analyses. The GO analysis revealed a total of 119 significantly (P < 0.05) enriched terms (Supplementary Table 3) represented by the top 20 terms (Figure 3A). Of those, the most prominent enrichments were immune system development (43 genes enriched), myeloid leukocyte differentiation (13 genes enriched), leukocyte differentiation (24 genes enriched), and regulation of immunoglobulin production (4 genes enriched). Several terms including tube development (44 genes enriched), tube morphogenesis (35 genes enriched), cell adhesion (49 genes enriched), and actin cytoskeleton (21 genes enriched) were also enriched. GO terms that may be highly associated with functional and morphological changes of UVJs containing SSTs between the aged and young chicken hens (120 vs. 30 wk) are shown in Table 1.
Figure 3.
(A) The top 20 enriched GO terms of DEGs comparing the chicken UVJ folds containing SSTs (120 vs. 30 wk of age). (B) The top 20 enriched KEGG pathways of DEGs in UVJ folds containing SSTs (120 vs. 30 wk of age). Abbreviations: Coa, Coenzyme A); DEGs, differentially expressed genes; ECM, Extracellular matrix; ErbB (also named EGFR), epidermal growth factor receptor; GnRH, gonadotrophin releasing hormone; GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; MAPK, mitogen-activated protein kinase; SSTs, sperm storage tubules; UVJ, uterovaginal junction.
Table 1.
Enriched GO terms of DEGs that are potentially related to decreased efficiency of SSTs with aging.
| Term | Count | Gene name |
|---|---|---|
| Immune system development | 34 | ATXN1L, BRAF, CCR7, CD109, CREB1, EP300, FADD, FLVCR1, FNIP1, FOS,1FOXP1, GATA2, GPR68, HDAC4, MITF, PDGFRA, PKNOX1, PREX1, PSEN1, RBM15, RC3H2, RO60, SH3PXD2A, SMAD3, SRC, STON2, SUPT6H, TCF21, TCF3, TCF7, TGFBR2, TRAF6, TREM2, WASF2 |
| Leukocyte differentiation | 24 | BRAF, CCR7, CD109, CREB1, EP300, FADD, FNIP1, FOS, FOXP1, GATA2, GPR68, HDAC4, MITF, PKNOX1, PREX1, PSEN1, RC3H2, SH3PXD2A, SRC, TCF3, TCF7, TGFBR2, TRAF6, TREM2 |
| Myeloid leukocyte differentiation | 13 | CCR7, CD109, CREB1, FADD, FOS, GATA2, GPR68, MITF, PSEN1, SH3PXD2A, SRC, TGFBR2, TRAF6 |
| Regulation of immunoglobulin production | 4 | FOXP1, SUPT6H, TRAF6, XCL1 |
| Cell adhesion | 49 | ADORA2A, ANOS1, BRAF, CCR7, CNTNAP5, COL6A1, CTNNA3, EDIL3, EPHA3, ENSGALG00000003428, EPHA4, FADD, FAT2, FAT4, FLRT2, FLRT3, GPAM, IL6ST, ITGA6, ITGA8, ITGB6, KIF26 B, LAMC1, LPP, LSAMP, MYL9, NDNF, NRP1, NUAK1, PARVA, PEAK1, PLXNB3, PODXL, PREX1, PTPRJ, PTPRU, S100A10, SDC3, SPON1, SRC, TGFBR2, TGFBR3, THBS2, THBS4, TNR, TRAF6, UTRN, VCL, XCL1 |
| Biological adhesion | 49 | ADORA2A, ANOS1, BRAF, CCR7, CNTNAP5, COL6A1, CTNNA3, EDIL3, ENSGALG00000003428, EPHA3, EPHA4, FADD, FAT2, FAT4, FLRT2, FLRT3, GPAM, IL6ST, ITGA6, ITGA8, ITGB6, KIF26 B, LAMC1, LPP, LSAMP, MYL9, NDNF, NRP1, NUAK1, PARVA, PEAK1, PLXNB3, PODXL, PREX1, PTPRJ, PTPRU, S100A10, SDC3, SPON1, SRC, TGFBR2, TGFBR3, THBS2, THBS4, TNR, TRAF6, UTRN, VCL, XCL1 |
| Positive regulation of cell adhesion | 18 | BRAF, CCR7, EDIL3, ENSGALG00000003428, FADD, GPAM, IL6ST, KIF26 B, NDNF, PODXL, PREX1, PTPRJ, S100A10, SDC3, TGFBR2, TRAF6, UTRN, XCL1 |
| Actin binding | 21 | ACTA1, AMOT, FHL2, FSCN1, GAS2L3, HDAC4, MYL9, MYO5A, MYO6, PARD6A, PARVA, PEAK1, PKNOX2, RAB22 A, SEPTIN2, SH3PXD2A, SRC, STK38 L, SYNPO2, TNNC2, VCL |
| Tube development | 44 | AMOT, ATXN1L, COL4A2, CREB1, DAB2IP, EIF2AK3, EP300, EPHA4, ESM1, ETS1, FAT4, FLT1, FLT4, FOXP1, GATA2, GATA6, IPMK, KIF26 B, LUZP1, NDNF, NRP1, PARVA, PCSK5, PDGFRA, PKNOX1, PODXL, PPP1R16 B, PROX1, PSEN1, RBM15, RC3H2, SASH1, SMAD3, TCF21, TCF7, TGFBR2, TGFBR3, THBS2, THBS4, TRAF6, TRIM71, WASF2, WNT7A, WWTR1 |
| Tube morphogenesis | 35 | AMOT, COL4A2, DAB2IP, EIF2AK3, EPHA4, ESM1, ETS1, FAT4, FLT1, FLT4, GATA2, GATA6, IPMK, KIF26 B, LUZP1, NDNF, NRP1, PARVA, PDGFRA, PKNOX1, PODXL, PPP1R16 B, PROX1, PSEN1, RBM15, SASH1, SMAD3, TCF21, TCF7, TGFBR2, THBS2, THBS4, TRAF6, WASF2, WNT7A |
| Cellular response to estradiol stimulus | 4 | NCOA3, NRIP1, SSTR1, SSTR2 |
Abbreviations: DEGs, differentially expressed genes; GO, gene ontology; SSTs, sperm storage tubules.
The bold font in the table represents the upregulated differentially expressed genes; the others are downregulated genes.
KEGG analysis revealed 9 significant (P < 0.05) pathways. Of those, the mitogen-activated protein kinase signaling pathway (39 genes enriched) was the prominent enrichment. Focal adhesion (37 genes enriched) and adherens junction (16 genes enriched), inositol phosphate metabolism (16 genes enriched), and glycerophospholipid metabolism (17 genes enriched) were also enriched. Terms that were potentially relevant to functional and morphological changes and cell metabolism in UVJs containing SSTs (120 vs. 30 wk) are listed in Table 2. The top 20 KEGG terms are presented in Figure 3B.
Table 2.
Differentially expressed genes enriched in KEGG pathways that are potentially related to the decreased efficiency of SSTs with aging.
| Term | Count | Gene name |
|---|---|---|
| Focal adhesion | 37 | ARHGAP35, ARHGAP5, BRAF, COL4A2, COL6A1, DOCK1, ENSGALG00000002389, ENSGALG00000003428, ENSGALG00000007646, ENSGALG00000008141, ENSGALG00000042388, FLNB, FLT1, FLT4, GRB2, IGF1R, ITGA6, ITGA8, ITGA9, ITGB6, ITGB8, LAMC1, MAPK9, MYL9,1PAK3, PAK5, PARVA, PDGFRA, PDGFRB, PIK3R1, PPP1R12 B, SRC, THBS2, THBS4, TNR, VAV2, VCL |
| Adherens junction | 16 | AFDN, CTNNA3, EP300, IGF1R, INSR, PTPN1, PTPRB, PTPRJ, SMAD3, SRC, TCF7, TGFBR2, TJP1, VCL, WASF2, WASF3 |
| Inositol phosphate metabolism | 16 | ALDH6A1, INPP4A, INPP4B, IPMK, ITPK1, ITPKA, MINPP1, MTMR4, PIK3C2A, PIK3CG, PIKFYVE, PIP4K2B, PLCB2, PLCD1, PLCG2, SYNJ1 |
| MAPK signaling pathway | 39 | BRAF, CACNA1C, CACNA2D1, CACNA2D2, DUSP16, DUSP8, ELK4, ENSGALG00000007646, ERBB4, EREG, FLNB, FLT1, FLT4, FOS, GRB2, HSPA2, IGF1R, IL1R1, INSR, MAP2K6, MAP3K1, MAP3K13, MAP3K3, MAP4K4, MAPK8IP3, MAPK9, MECOM, MEF2C, NF1, NGFR, NRK, PDGFRA, PDGFRB, RASGRF2, RPS6KA3, RPS6KA6, TAOK1, TGFBR2, TRAF6 |
| Glycerophospholipid metabolism | 17 | ETNK2, DGKD, DGKQ, DGKE, GPAM, PLD1, LPGAT1, GPD1L, ETNK1, LPIN2, MBOAT2, LPIN1, DGKH, PLA2G10, PLPP4, CDS2, GPAT2 |
Abbreviations: KEGG, Kyoto Encyclopedia of Genes and Genomes; MAPK, mitogen-activated protein kinase; SSTs, sperm storage tubules.
The bold font in the table represents the upregulated differentially expressed genes; the others are downregulated genes.
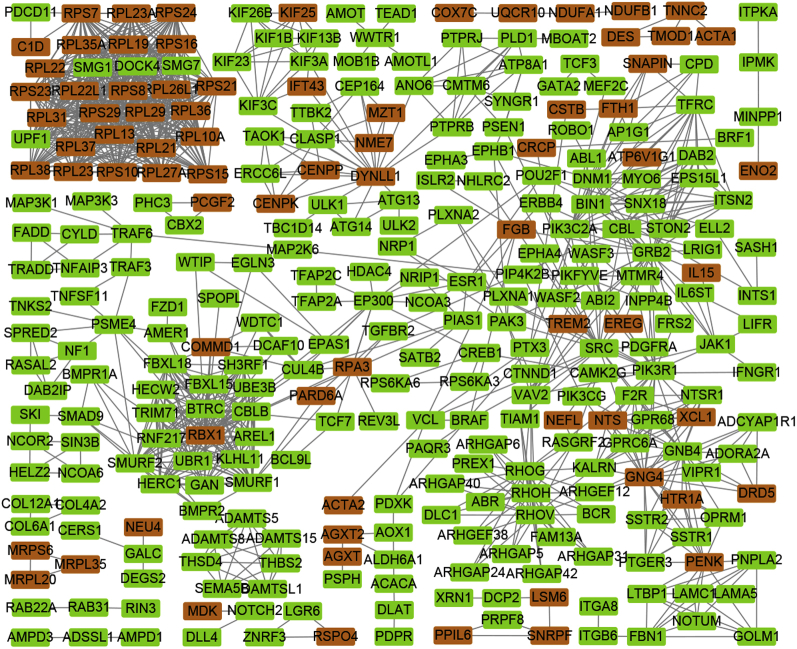
The PPI network established a broad interconnection in cell adhesion proteins, cytoskeleton, and ribosome proteins. The other 2 subnetworks potentially contributing to the cell cytoskeleton including RHOG, RHOH, and RHOV, and immunity response such as JAK1, ILS6T, and interleukin 15 (IL15) were also set up as shown in Figure 4.
Figure 4.
Protein–protein interaction network of DEGs in UVJ containing SSTs (120 vs. 30 wk of age). Network nodes and edges represented proteins and protein–protein associations, respectively. The red and green nodes represented downregulated and upregulated genes, respectively. Abbreviations: DEGs, differentially expressed genes; SSTs, sperm storage tubules; UVJ, uterovaginal junction.
qRT-PCR Validation of Candidate Genes Regulating Age-Dependent Changes of Chicken UVJs
A total of 6 genes (DGKE, IL15, MYL9, PLA2G10, PLD1, and THBS4) were set up for the detailed investigation of gene expression patterns by using the qPCR technique. Validation showed that DGKE and PLD1 genes were downregulated, whereas the IL15, MYL9, PLA2G10, and THBS4 genes were upregulated (120 vs. 30 wk). The expression of these genes in UVJ containing SSTs was consistent with the tendency of the RNA sequencing results (Figure 2B).
Oil Red O Staining and Immunohistochemistry of ADFP
Oil Red O staining of UVJ sections derived from 30, 65, and 120 wk of age showed that almost all lipids were concentrated in SST cells. There were no visible signals detected in the UVJ epithelium except for a few lipids located at the adjacent UVJ epithelial cells. Lipid composition showed increased tendency in SST cells from 30 to 65 wk, and displayed the strongest and fuzzy stains at 120 wk (Figures 5A–5C). Immunohistochemistry of the lipid droplet marker ADFP displayed specific expressions in SST cells, and not in the UVJ epithelium, with weak and strongest expressions at 30 and 120 wk of age, respectively. It showed that lipid droplets were significantly accumulated in SST cells of chicken UVJ tissues with aging (Figures 5D–5F).
Figure 5.
(A–C) Oil Red O staining of UVJ sections at 30, 65, and 120 wk of age, respectively. Tubular SSTs were indicated with dotted lines (simple columnar epithelium). SST lumen was marked as “Lu.” UVJ epithelium is shown with broad arrowhead (pseudostratified epithelium). The nucleus was stained with blue, whereas the lipids were stained with red (thin arrowhead). Most of the lipids were specifically concentrated in the SST cells. A few lipids were located adjacent to UVJ epithelial cells. The large vesicle observed in the perinuclear region of SST cells was stained with red and its size significantly increased with aging compared the sections of 30 and 65 to 120 wk of age. Scale bar = 20 μm. (D–F) Immunohistochemistry of matured lipid droplet marker ADFP in UVJ containing SSTs at 30, 65, and 120 wk of age, respectively. The UVJ epithelial cells are indicated with bold arrows. The SSTs are indicated with narrow arrows or rectangle. SST lumen is indicated with “Lu.” The nucleus was stained with blue, whereas ADFP was stained with brown. ADFP was specifically located in SST cells and its distribution increased with aging, which is consistent with the increasing trend of lipids. Scale bar = 100 μm (general view) and 20 μm (enlarged view). Abbreviations: SSTs, sperm storage tubules; UVJ, uterovaginal junction.
Discussion
Natural mating or AI is essential for female birds to breed the next generation. However, the reduction in fertility rate, laying rate, and egg quality with aging seems inevitable in breeders of layers, broilers, and quails (Beaumont et al., 1992; Ledur et al., 2000; Santos et al., 2013). According to our records, both fertility duration and fertility rate showed negative correlations with aging during the breeding period in laying breeders. White Leghorn hens are well-used breeders for cross breeding, and pure breeding (Campo, 1995; Goto et al., 2011), as well as a good model for fertility-related studies (Atikuzzaman et al., 2015; Huang et al., 2016).
During the reproduction of mammals, the luteal, oviduct, and uterine functions and the pregnancy process are affected by aging (Shirasuna and Iwata, 2017). Aging of the ovary and follicles is regarded as a dominant factor influencing fertility reduction in mammals (Perheentupa and Huhtaniemi, 2009; May-Panloup et al., 2016; Vollenhoven and Hunt, 2018) as well as in egg layers (Holmes et al., 2003). For female birds, such as layers, broilers, quails, and turkeys, the oviduct plays a more specific role in the breeding process. For example, the quality of the albumen and egg shell is mainly related to the function of magnum and uterine tissues, respectively (Herrera et al., 2018; Wistedt et al., 2019). The decreasing efficiency of SSTs in UVJs caused by aging is regarded as another main factor affecting the decline of fertility rate in both breeders of layers and broilers (Beaumont et al., 1992; Fasenko et al., 1992; Gumulka and Kapkowska, 2005). The mechanism underlying the potential impacts of UVJs and SSTs on decreasing fertility rate in aged laying breeders remains largely unexplored. As part of the oviduct, transcriptomic changes of UVJs containing SSTs indicate the changing tendency of the other parts of the oviduct at some point. This study characterized the morphological and molecular changes of UVJs and SSTs in the reproductive system of aged laying breeders represented by White Leghorn hens and will give clues relevant to the fertility decrease via aging.
β-Galactosidase is an important cell senescence biomarker in the epithelium (Dimri et al., 1995). The staining of β-galactosidase confirmed that senescence was observed in the epithelium of both UVJs and SSTs of egg layers aged from 30 to 65 and 120 wk. The signals were much weaker in the UVJ epithelium at 30 wk compared with the stronger signals at 65 and 120 wk. There were no clear differences of the signals observed in UVJs between 65 and 120 wk. This suggests that the aging process is relatively high in the epithelium of UVJs from 65 wk onward.
Accumulation of lipid droplets with increased intensity was also detected by Oil Red O staining in epithelial cells of SSTs via aging from 30 to 65 and 120 wk. The lipid droplets were enveloped by a single phospholipid monolayer containing many different proteins on its surface and hydrophobic neutral lipids which are notably triacylglycerols and sterol esters (Walther and Farese, 2009). Recent studies show that the lipid droplets are functional effectors manipulating energy homeostasis, storing vitamins and signaling precursors, mitigating endoplasmic reticulum oxidative stress, and protein maturation, storage, and turnover (Welte and Gould, 2017). The large perinuclear lipid in SST cells has been reported in chicken, quail, and turkey, and is considered to serve as an intercellular lipid source for resident sperms to maintain structural integrity (Bakst et al., 1994). Further studies demonstrated that lipid droplets in SST cells may be degraded by adipose triglyceride lipase and released into SST lumen to support sperm survival. Additionally, the application of fatty acids such as oleic acid and linoleic acid extends the sperm viability in vitro culture system (Huang et al., 2016). All the above studies suggest that lipids play an important role in the sperm storage process in vivo and in vitro.
ADFP is a membrane-associated protein present in mature lipid droplets and used as a marker for mature lipid droplets (Hodges and Wu, 2010; Marshall et al., 2014). In this study, the immunohistochemistry of ADFP confirmed that lipids and lipid droplets were dynamically accumulated with increased size from 30 to 120 wk of age and displayed the strongest signals in SST cells at 120 wk. The lipid droplets were specifically located in SST cells with increased size via aging. The GO and KEGG analyses enriched from UVJ tissues of aged and young hens revealed a series of genes, represented by ETNK2, PDL1, PLA2G10, MCTP2, and TFAP2, that are potentially related to lipid formation and metabolism. PLA2G10, which was upregulated with aging, is not only proved to function in glycerophospholipid metabolism but is also associated with the release of ω3 polyunsaturated fatty acids and increase of sperm fertility in mice (Murase et al., 2016). PLD1 is functional in promoting lipid droplet formation in NIH 3T3 cells (Andersson et al., 2006). Pex30/MCTP2 is involved in localizing the lipid droplet to endoplasmic reticulum as well as in lipid droplet formation (Wang et al., 2018). TFAP2 is a transcription factor which is regarded as a “master” regulator in lipid droplet biogenesis (Scott et al., 2018). Although the lipid droplets were accumulated in SST cells with aging, these lipid droplet-related genes displayed mostly downregulation, indicating a decreased capability of lipid formation with aging. KEGG pathways including inositol phosphate metabolism and glycerolipid metabolism were significantly enriched comparing the UVJs of aged hens with those of young hens. The lipids serve not only as an energy source but also as an indicator for the aging process such as cell stress and longevity (Goldberg et al., 2009; Welte and Gould, 2017). Genes such as DGKD (diacylglycerol kinase delta), DGKQ (diacylglycerol kinase theta), DGKE (diacylglycerol kinase), and DGKH (diacylglycerol kinase eta) belong to a family of lipid modulating enzymes in producing phosphatidic acid (Nakano, 2015). Glycerol-3-phosphate acyltransferase and glycerol-3-phosphate acyltransferase 2 are important enzymes promoting triacylglycerol biosynthesis (Yu et al., 2017). It is supposed that these lipid-related DEGs may be specifically expressed in SST cells. Furthermore, other factors related to sperm storage such as exosome (Huang et al., 2017) and SST microvilli secretions (Bakst and Bauchan, 2015) also have different lipid compositions (glycerophospholipid and glycerolipid). Transcriptomic changes in lipid synthesis and lipid droplet formation reflect the changing tendency of lipid composition, lipid metabolism, and lipid homeostasis in UVJs and SSTs. The accumulation of lipid droplets in SSTs may be a functional compensation to decrease lipid synthesis and the metabolism process. As lipids are important factors for sperm survival, the observed changes may directly influence sperm survival and efficiency of sperm storage of SSTs in aged laying breeders.
The immune system plays an important role in fertilization, implantation, and maintenance of pregnancy and parturition in female mammals (Abrahams, 2010), as well as in sperm survival in the oviduct of female birds (Zheng et al., 1998; Das et al., 2006). In this study, a series of immune-related genes were identified as DEGs enriched in a comparison of UVJs of aged and young egg layers. FNIP1 plays a crucial role in both invariant natural killer cells and B cell development, survival, and metabolic homeostasis during lymphocyte development (Park et al., 2012; Park et al., 2014). TGF-β signaling is essential for T cell differentiation and function (Gorelik and Flavell, 2000). TGF-β and its receptors (TGF-βR1, TGF-βR2, and TGF-βR3) are reported to function as factors suppressing antisperm immunoreactions during sperm storage in SSTs of chicken hens (Das et al., 2006; Das et al., 2008). In this study, both TGFBR2 and TGFBR3 were shown to downregulate with aging, suggesting a decreased efficiency in the immunosuppression in chicken UVJs via aging.
The immune system is not only correlated with sperm survival in the oviduct of chicken hens, but also changes with aging (Weiskopf et al., 2009). In humans, aging is related to an increase of chronic inflammatory cytokines including CRP, IL-18, TNF-α, and IL-6 in the blood, which is defined as “inflammaging,” a nonspecific state of chronic inflammation (Baylis et al., 2013). In this study, IL15, 3 interleukin receptor genes (IL1R1, IL2RA, and IL17RA), and 1 signal transducer (IL6ST) were shown to be differentially expressed with aging. IL15 is an important pro-inflammatory cytokine to stimulate differentiation and proliferation of T, B, and natural killer cells (Steel et al., 2012). In humans, the circulating IL15 levels are significantly higher in persons with longevity, indicating a higher capacity of defense against infections (Gangemi et al., 2005). Upregulation of IL15 was also observed in aged egg layers indicating a higher pro-inflammatory status in the microenvironment of UVJs, which is disadvantageous to sperm survival. The downregulation of IL1R1, IL2RA, IL17RA, and IL6ST and upregulation of IL15 suggest a novel homeostasis pattern in the immune response of the signal transduction process in aging (Primiani et al., 2014; Pantsulaia et al., 2015; Lazzarini et al., 2019). The varied immune system-related genes in this study are supposed to be consequences of cell senescence, repeated AI, and resistance to infection (Das et al., 2005; Gangemi et al., 2005; Yoshimura and Barua, 2017). Additionally, enrichment of genes such as CCR7, FADD, and PKNOX1(PREP1), in immune system development and differentiation, suggests a maturation process of the immune system and changes of the microenvironment compared older to younger laying breeders (Chung et al., 2007; Forster et al., 2008; Mouasni and Tourneur, 2018). All the above related genes reveal a changing tendency in immune tolerance and immune response, indicating a worsened microenvironment for sperm storage in SSTs of UVJs, which may be another important factor for decreased sperm survival in the oviduct of egg layers.
Studies in the skin, lung, gastrointestinal tract, and kidney reveal that the epithelial barrier is affected by aging accompanied with differentially expressed molecules such as claudin, E-cadherin, and β-catenin (Parrish, 2017). Molecules from the cytoskeleton and extracellular matrix are also affected by aging (D'Souza et al., 2009; Yan et al., 2011; Tanaka et al., 2016). In this study, a series of DEGs were enriched in cell adhesion molecules, cell junction proteins, cytoskeletal proteins, and extracellular matrix proteins in UVJs containing SSTs between aged and young egg laying breeders. Tight junction proteins such as tight junction protein 1 (TJP1), also known as ZO-1, and CLDN5 (claudins 5) are integral membrane proteins and components of tight junction strands, which showed downregulation in aged UVJs containing SSTs, and are consistent with a similar expression pattern in aged kidney, liver, and pancreas (D'Souza et al., 2009). AFDN (adherens junction formation factor) belongs to the adhesion system and plays a key role in pancreatic central lumen morphogenesis and lumen continuity (Azizoglu et al., 2017). MYL9 (myosin light chain protein) is an important myosin regulating gene. Upregulation of MYL9 is related with endothelial morphological changes and permeability in rats via aging (Shehadeh et al., 2011). It indicates that these cell adhesion, junction, and cytoskeleton proteins are changed in both UVJ and SST epithelium with downregulated genes TJP1, CLDN5, and AFDN, and upregulated gene MYL9 affecting the flexibility and lumen expansion process of SSTs, which partially explain why the rate of sperm release from SSTs of older hens was about twice of that observed in younger hens (Brillard, 1993).
Ribosome proteins were significantly enriched in both GO and KEGG analyses. There were 28 ribosomal proteins that showed upregulation in the UVJ tissues of aged hens. Single gene deletion of ribosomal proteins RPL13, RPL16 B, RPL10, RPS6, or RPS18 in yeast strains increases replicative life span (Kaeberlein et al., 2005; Chiocchetti et al., 2007). Reducing the levels of ribosomal proteins (such as RPL19, RPS8, RPS10, and RPS15) and translation-initiation factors in Caenorhabditis elegans also generate a similar phenotype as extended replicative period (Curran and Ruvkun, 2007; Hansen et al., 2007).
It is supposed that the upregulation of these ribosomal proteins is deleterious to the lifespan of the oviduct, which may be also potentially associated with the decreasing tendency of sperm storage efficiency of SSTs in laying breeders via aging.
Conclusion
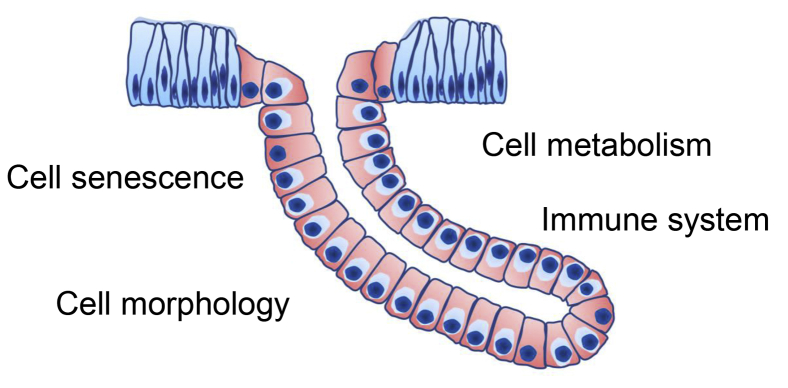
Aging is disadvantageous to sperm storage efficiency of UVJs containing SSTs in laying breeders that is regulated by noteworthy candidate genes such as PLA2G10, PLD1, IL15, MYL9, and TJP1. The variation of cellular lipid components in SSTs and DEGs involved in the immunity system are supposed to be 2 dominant factors responsible for decreasing the efficiency of SSTs. The enriched genes relevant to cell adhesion, cell junction, cytoskeleton, and the extracellular matrix indicate reorganization of both the UVJ epithelium and SST epithelium via aging, which may also potentially influence sperm storage and release after AI. Ribosomal protein-related genes may be also related to the decreased efficiency of SSTs (Figure 6).
Figure 6.
Potential factors regulate decreased sperm storage efficiency of SSTs with aging: (i) cell senescence has negative effects on the function of SSTs; (ii) the metabolic pattern, such as lipid synthesis and metabolism, potentially influences sperm survival in SSTs; (iii) immune homeostasis pattern, including upregulation of proinflammatory factors and downregulation of immunosuppression factors, is important for sperm survival in the oviduct; (iv) changes of cell adhesion molecule, cell junction, and cytoskeleton may influence the SSTs morphology and sperm release process. Abbreviations: SSTs, sperm storage tubules; UVJ, uterovaginal junction.
This study firstly reported the dynamic molecular balance of chicken UVJ folds containing SSTs using the egg layer of White Leghorn hens as a model. The outcome may facilitate further understanding of the decreasing fertility rate in aged laying breeders in poultry and provide clues to save fertility decline via aging and make better use of breeders in poultry breeding stock.
Acknowledgments
We are grateful for the warm support from Beijing Huadu Yukou Poultry Industry Co. Ltd. This work was supported by grants from the National Key R&D Program of China (2018YFD0501301) and National Natural Science Foundation of China (31772585).
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this study.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.12.005.
Supplementary data
References
- Abrahams V.M. Thirty years of reproductive immunology: an introduction. Am. J. Reprod. Immunol. 2010;63:411–412. doi: 10.1111/j.1600-0897.2010.00849.x. [DOI] [PubMed] [Google Scholar]
- Andersson L., Bostrom P., Ericson J., Rutberg M., Magnusson B., Marchesan D., Ruiz M., Asp L., Huang P., Frohman M.A., Boren J., Olofsson S.O. PLD1 and ERK2 regulate cytosolic lipid droplet formation. J. Cell Sci. 2006;119:2246–2257. doi: 10.1242/jcs.02941. [DOI] [PubMed] [Google Scholar]
- Atikuzzaman M., Mehta Bhai R., Fogelholm J., Wright D., Rodriguez-Martinez H. Mating induces the expression of immune- and pH-regulatory genes in the utero-vaginal junction containing mucosal sperm-storage tubuli of hens. Reproduction. 2015;150:473–483. doi: 10.1530/REP-15-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizoglu D.B., Braitsch C., Marciano D.K., Cleaver O. Afadin and RhoA control pancreatic endocrine mass via lumen morphogenesis. Genes Dev. 2017;31:2376–2390. doi: 10.1101/gad.307637.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakst M., Wishart G., Brillard j. p. Oviducal sperm selection, transport, and storage in poultry. Poult. Sci. Rev. 1994;5:117–143. [Google Scholar]
- Bakst M.R., Bauchan G. Apical blebs on sperm storage tubule epithelial cell microvilli: their release and interaction with resident sperm in the Turkey hen oviduct. Theriogenology. 2015;83:1438–1444. doi: 10.1016/j.theriogenology.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Baylis D., Bartlett D.B., Patel H.P., Roberts H.C. Understanding how we age: insights into inflammaging. Longev. Healthspan. 2013;2:8. doi: 10.1186/2046-2395-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont C., Brillard J.P., Millet N., De Reviers M. Comparison of various characteristics of duration of fertility in hens. Br. Poult. Sci. 1992;33:649–661. doi: 10.1080/00071669208417503. [DOI] [PubMed] [Google Scholar]
- Birkhead T.R., Møller A.P. Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Biol. J. Linn. Soc. 2008;50:295–311. [Google Scholar]
- Brillard J.P. Sperm storage and transport following natural mating and artificial insemination. Poult. Sci. 1993;72:923–928. doi: 10.3382/ps.0720923. [DOI] [PubMed] [Google Scholar]
- Campo J.L. Comparative yolk cholesterol content in four Spanish breeds of hens, an F2 cross, and a White Leghorn population. Poult. Sci. 1995;74:1061–1066. doi: 10.3382/ps.0741061. [DOI] [PubMed] [Google Scholar]
- Chiocchetti A., Zhou J., Zhu H., Karl T., Haubenreisser O., Rinnerthaler M., Heeren G., Oender K., Bauer J., Hintner H., Breitenbach M., Breitenbach-Koller L. Ribosomal proteins Rpl10 and Rps6 are potent regulators of yeast replicative life span. Exp. Gerontol. 2007;42:275–286. doi: 10.1016/j.exger.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Chung E.Y., Liu J., Homma Y., Zhang Y., Brendolan A., Saggese M., Han J., Silverstein R., Selleri L., Ma X. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27:952–964. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran S.P., Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007;3:e56. doi: 10.1371/journal.pgen.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza T., Sherman-Baust C.A., Poosala S., Mullin J.M., Morin P.J. Age-related changes of claudin expression in mouse liver, kidney, and pancreas. J. Gerontol. Ser. A-biol. Sci. Med. Sci. 2009;64:1146–1153. doi: 10.1093/gerona/glp118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.C., Isobe N., Nishibori M., Yoshimura Y. Expression of transforming growth factor-beta isoforms and their receptors in utero-vaginal junction of hen oviduct in presence or absence of resident sperm with reference to sperm storage. Reproduction. 2006;132:781–790. doi: 10.1530/rep.1.01177. [DOI] [PubMed] [Google Scholar]
- Das S.C., Isobe N., Yoshimura Y. Mechanism of prolonged sperm storage and sperm survivability in hen oviduct: a review. Am. J. Reprod. Immunol. 2008;60:477–481. doi: 10.1111/j.1600-0897.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- Das S.C., Nagasaka N., Yoshimura Y. Changes in the localization of antigen presenting cells and T cells in the utero-vaginal junction after repeated artificial insemination in laying hens. J. Reprod. Dev. 2005;51:683–687. doi: 10.1262/jrd.17027. [DOI] [PubMed] [Google Scholar]
- Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasenko G.M., Hardin R.T., Robinson F.E., Wilson J.L. Relationship of hen age and egg sequence position with fertility, hatchability, viability, and preincubation embryonic development in broiler breeders. Poult. Sci. 1992;71:1374–1383. doi: 10.3382/ps.0711374. [DOI] [PubMed] [Google Scholar]
- Forster R., Davalos-Misslitz A.C., Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- Gangemi S., Basile G., Monti D., Merendino R.A., Di Pasquale G., Bisignano U., Nicita-Mauro V., Franceschi C. Age-related modifications in circulating IL-15 levels in humans. Mediat. Inflamm. 2005;2005:245–247. doi: 10.1155/MI.2005.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A.B., Reynolds M.E., Lorenz F.W. Distribution of spermatozoa in the oviduct and fertility in domestic birds. VII. Innervation and vascular supply of the uterovaginal sperm-host glands of the domestic hen. J. Reprod. Fertil. 1968;17:305–310. doi: 10.1530/jrf.0.0170305. [DOI] [PubMed] [Google Scholar]
- Goldberg A.A., Bourque S.D., Kyryakov P., Boukh-Viner T., Gregg C., Beach A., Burstein M.T., Machkalyan G., Richard V., Rampersad S., Titorenko V.I. A novel function of lipid droplets in regulating longevity. Biochem. Soc. Trans. 2009;37:1050–1055. doi: 10.1042/BST0371050. [DOI] [PubMed] [Google Scholar]
- Gorelik L., Flavell R.A. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- Goto T., Ishikawa A., Onitsuka S., Goto N., Fujikawa Y., Umino T., Nishibori M., Tsudzuki M. Mapping quantitative trait loci for egg production traits in an F2 intercross of Oh-Shamo and White Leghorn chickens. Anim. Genet. 2011;42:634–641. doi: 10.1111/j.1365-2052.2011.02190.x. [DOI] [PubMed] [Google Scholar]
- Gumulka M., Kapkowska E. Age effect of broiler breeders on fertility and sperm penetration of the perivitelline layer of the ovum. Anim. Reprod. Sci. 2005;90:135–148. doi: 10.1016/j.anireprosci.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Hansen M., Taubert S., Crawford D., Libina N., Lee S.J., Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Herrera J., Saldana B., Camara L., Berrocoso J.D., Mateos G.G. Influence of grinding size of the main cereal of the diet on egg production and eggs quality of brown egg laying hens from 33 to 65 weeks of age. Poult. Sci. 2018;97:2506–2515. doi: 10.3382/ps/pey098. [DOI] [PubMed] [Google Scholar]
- Hodges B.D., Wu C.C. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J. Lipid Res. 2010;51:262–273. doi: 10.1194/jlr.R003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D.J., Thomson S.L., Wu J., Ottinger M.A. Reproductive aging in female birds. Exp. Gerontol. 2003;38:751–756. doi: 10.1016/s0531-5565(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Holt W.V., Fazeli A. Sperm storage in the female reproductive tract. Annu. Rev. Anim. Biosci. 2016;4:291–310. doi: 10.1146/annurev-animal-021815-111350. [DOI] [PubMed] [Google Scholar]
- Holt W.V., Lloyd R.E. Sperm storage in the vertebrate female reproductive tract: how does it work so well? Theriogenology. 2010;73:713–722. doi: 10.1016/j.theriogenology.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Huang A., Isobe N., Obitsu T., Yoshimura Y. Expression of lipases and lipid receptors in sperm storage tubules and possible role of fatty acids in sperm survival in the hen oviduct. Theriogenology. 2016;85:1334–1342. doi: 10.1016/j.theriogenology.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Huang A., Isobe N., Yoshimura Y. Changes in localization and density of CD63-positive exosome-like substances in the hen oviduct with artificial insemination and their effect on sperm viability. Theriogenology. 2017;101:135–143. doi: 10.1016/j.theriogenology.2017.06.028. [DOI] [PubMed] [Google Scholar]
- Ito T., Yoshizaki N., Tokumoto T., Ono H., Yoshimura T., Tsukada A., Kansaku N., Sasanami T. Progesterone is a sperm-releasing factor from the sperm-storage tubules in birds. Endocrinology. 2011;152:3952–3962. doi: 10.1210/en.2011-0237. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M., Powers R.W., 3rd, Steffen K.K., Westman E.A., Hu D., Dang N., Kerr E.O., Kirkland K.T., Fields S., Kennedy B.K. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Korovila I., Hugo M., Castro J.P., Weber D., Hohn A., Grune T., Jung T. Proteostasis, oxidative stress and aging. Redox Biol. 2017;13:550–567. doi: 10.1016/j.redox.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R., Caffarini M., Tang H., Cerqueni G., Pellegrino P., Monsurro V., Di Primio R., Orciani M. The senescent status of endothelial cells affects proliferation, inflammatory profile and SOX2 expression in bone marrow-derived mesenchymal stem cells. Exp. Gerontol. 2019;120:21–27. doi: 10.1016/j.exger.2019.02.014. [DOI] [PubMed] [Google Scholar]
- Ledur M.C., Fairfull R.W., McMillan I., Gowe R.S., Asselstine L. Genetic effects of ageing on fertility and hatchability in the first laying cycle of three white leghorn strains and their two-way crosses. Br. Poult. Sci. 2000;41:552–561. doi: 10.1080/713654980. [DOI] [PubMed] [Google Scholar]
- Marshall L.L., Stimpson S.E., Hyland R., Coorssen J.R., Myers S.J. Increased lipid droplet accumulation associated with a peripheral sensory neuropathy. J. Chem. Biol. 2014;7:67–76. doi: 10.1007/s12154-014-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M., Sasanami T. Sperm storage in the female reproductive tract: a conserved reproductive Strategy for better fertilization Success. Adv. Exp. Med. Biol. 2017;1001:173–186. doi: 10.1007/978-981-10-3975-1_11. [DOI] [PubMed] [Google Scholar]
- May-Panloup P., Boucret L., Chao de la Barca J.M., Desquiret-Dumas V., Ferre-L'Hotellier V., Moriniere C., Descamps P., Procaccio V., Reynier P. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum. Reprod. Update. 2016;22:725–743. doi: 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- Mouasni S., Tourneur L. FADD at the Crossroads between cancer and inflammation. Trends Immunol. 2018;39:1036–1053. doi: 10.1016/j.it.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Murase R., Sato H., Yamamoto K., Ushida A., Nishito Y., Ikeda K., Kobayashi T., Yamamoto T., Taketomi Y., Murakami M. Group X secreted Phospholipase A2 releases omega3 polyunsaturated fatty acids, Suppresses colitis, and Promotes sperm fertility. J. Biol. Chem. 2016;291:6895–6911. doi: 10.1074/jbc.M116.715672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T. Roles of lipid-modulating enzymes diacylglycerol kinase and cyclooxygenase under pathophysiological conditions. Anat. Sci. Int. 2015;90:22–32. doi: 10.1007/s12565-014-0265-7. [DOI] [PubMed] [Google Scholar]
- Pantsulaia I., Iobadze M., Kikodze N., Pantsulaia N., Chikovani T. Lipid profile and cytokines interactions during successful aging. Georgian Med. News. 2015:46–51. (243):46–51. [PubMed] [Google Scholar]
- Park H., Staehling K., Tsang M., Appleby M.W., Brunkow M.E., Margineantu D., Hockenbery D.M., Habib T., Liggitt H.D., Carlson G., Iritani B.M. Disruption of Fnip1 reveals a metabolic checkpoint controlling B lymphocyte development. Immunity. 2012;36:769–781. doi: 10.1016/j.immuni.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Tsang M., Iritani B.M., Bevan M.J. Metabolic regulator Fnip1 is crucial for iNKT lymphocyte development. Proc. Natl. Acad. Sci. U. S. A. 2014;111:7066–7071. doi: 10.1073/pnas.1406473111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish A.R. The impact of aging on epithelial barriers. Tissue Barriers. 2017;5:e1343172. doi: 10.1080/21688370.2017.1343172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perheentupa A., Huhtaniemi I. Aging of the human ovary and testis. Mol. Cell. Endocrinol. 2009;299:2–13. doi: 10.1016/j.mce.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Primiani C.T., Ryan V.H., Rao J.S., Cam M.C., Ahn K., Modi H.R., Rapoport S.I. Coordinated gene expression of neuroinflammatory and cell signaling markers in dorsolateral prefrontal cortex during human brain development and aging. PLoS One. 2014;9:e110972. doi: 10.1371/journal.pone.0110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos T.C., Murakami A.E., Oliveira C.A.L., Giraldelli N. Sperm-egg interaction and fertility of Japanese breeder quails from 10 to 61 weeks. Poult. Sci. 2013;92:205–210. doi: 10.3382/ps.2012-02536. [DOI] [PubMed] [Google Scholar]
- Scott C.C., Vossio S., Rougemont J., Gruenberg J. TFAP2 transcription factors are regulators of lipid droplet biogenesis. eLife. 2018;7:e36330. doi: 10.7554/eLife.36330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehadeh L.A., Webster K.A., Hare J.M., Vazquez-Padron R.I. Dynamic regulation of vascular myosin light chain (MYL9) with injury and aging. PLoS One. 2011;6:e25855. doi: 10.1371/journal.pone.0025855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasuna K., Iwata H. Effect of aging on the female reproductive function. Contracept. Reprod. Med. 2017;2:23. doi: 10.1186/s40834-017-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel J.C., Waldmann T.A., Morris J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Ohtsu A., Shiratsuki S., Kawahara-Miki R., Iwata H., Kuwayama T., Shirasuna K. Age-dependent changes in inflammation and extracellular matrix in bovine oviduct epithelial cells during the post-ovulatory phase. Mol. Reprod. Dev. 2016;83:815–826. doi: 10.1002/mrd.22693. [DOI] [PubMed] [Google Scholar]
- Velarde M.C., Menon R. Positive and negative effects of cellular senescence during female reproductive aging and pregnancy. J. Endocrinol. 2016;230:R59–R76. doi: 10.1530/JOE-16-0018. [DOI] [PubMed] [Google Scholar]
- Vollenhoven B., Hunt S. Ovarian ageing and the impact on female fertility. F1000Res. 2018;7:e1835. doi: 10.12688/f1000research.16509.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther T.C., Farese R.V., Jr. The life of lipid droplets. Biochim. Biophys. Acta. 2009;1791:459–466. doi: 10.1016/j.bbalip.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Idrissi F.Z., Hermansson M., Grippa A., Ejsing C.S., Carvalho P. Seipin and the membrane-shaping protein Pex30 cooperate in organelle budding from the endoplasmic reticulum. Nat. Commun. 2018;9:2939. doi: 10.1038/s41467-018-05278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D., Weinberger B., Grubeck-Loebenstein B. The aging of the immune system. Transpl. Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- Welte M.A., Gould A.P. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:1260–1272. doi: 10.1016/j.bbalip.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistedt A., Ridderstrale Y., Wall H., Holm L. Age-related changes in the shell gland and duodenum in relation to shell quality and bone strength in commercial laying hen hybrids. Acta Vet. Scand. 2019;61:14. doi: 10.1186/s13028-019-0449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Akutsu H., Satoh Y. The morphological and functional observation of the gap junction proteins in the oviduct epithelia in young and adult hamsters. Okajimas Folia Anat. Jpn. 2011;88:57–64. doi: 10.2535/ofaj.88.57. [DOI] [PubMed] [Google Scholar]
- Yang L., Zheng X., Mo C., Li S., Liu Z., Yang G., Zhao Q., Li S., Mou C. Transcriptome analysis and identification of genes associated with chicken sperm storage duration. Poult. Sci. 2020;99:1199–1208. doi: 10.1016/j.psj.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y., Barua A. Female reproductive system and immunology. Adv. Exp. Med. Biol. 2017;1001:33–57. doi: 10.1007/978-981-10-3975-1_3. [DOI] [PubMed] [Google Scholar]
- Yu H., Zhao Z., Yu X., Li J., Lu C., Yang R. Bovine lipid metabolism related gene GPAM: molecular characterization, function identification, and association analysis with fat deposition traits. Gene. 2017;609:9–18. doi: 10.1016/j.gene.2017.01.031. [DOI] [PubMed] [Google Scholar]
- Zheng W.M., Yoshimura Y., Tamura T. Effects of age and gonadal steroids on the localization of antigen-presenting cells, and T and B cells in the chicken oviduct. J. Reprod. Fertil. 1998;114:45–54. doi: 10.1530/jrf.0.1140045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.