Abstract
Probiotics are being developed as alternatives to antibiotic growth promoters. The aim of the study was to investigate the effects of 2 novel strains of Bacillus pumilus and Bacillus subtilis on production, intestinal microbiota, gut health, and immunity of broilers raised under suboptimal conditions. Day-old chicks (Cobb 500, n = 2,073) were randomly assigned into 6 groups: Con group (group fed with basal diet), Ab group (group treated with virginiamycin), groups treated with 2 levels of B. pumilus (low dose: 3 × 108 cfu/kg of feed [BPL] and high dose: 1 × 109 cfu/kg [BPH]), and groups treated with 2 levels of B. subtilis (low dose: 3 × 108 cfu/kg [BSL] and high dose: 1 × 109 cfu/kg [BSH]). Production parameters were recorded weekly. Cecal tonsils and content as well as ileum samples were collected on day 14 and day 42. Cecal tonsils were used to sort T-regulatory cells (CD4+CD8–CD25+ and CD4+CD8+CD25+) to study expression of IL-10 and interferon gamma, whereas cecal content was used for bacterial culture. Ileum samples were used to measure gene expression of tight junction proteins, mucin, and cytokines. BW and feed intake increased in the Ab, BPL, BSL, and BSH groups compared with the Con group between day 35 and day 42. The CD4+CD8-CD25+ cells expressed high levels of IL-10 in the BSH group on day 14 and in the BPL, BSL, and BSH groups on day 42 and high levels of interferon gamma in the BPL, BSL, and BSH groups on day 14 and in the BSL and BSH groups on day 42. The expression of IL-10 and interferon gamma in CD4+CD8+CD25+ cells was higher only in the BSH group on day 14 and day 42. Cecal bacterial populations of genera, Lactobacillus (day 14 and day 42) and Clostridium (day 14), were higher in the BSH group. Expression of tight junction protein increased significantly in the ileum on day 14 in the BPL (occludin, zona occludens 1 [ZO-1]), BSL (occludin, ZO-1), and BSH (occludin, ZO-1, junctional adhesion molecule 2 [JAM-2]) groups compared with that in the Con group and declined in all groups except in the BSH group (occludin, ZO-1, JAM-2) on day 42. Expression of MUC2 and IL-17F increased in all groups on day 14 and remained high on day 42 in the BSL and BSH groups. Taken together, both Bacillus probiotics altered the intestinal and immune activities, particularly on day 14, suggesting beneficial influence of probiotics.
Key words: Bacillus probiotic, broiler, production, immunity, gut health
Introduction
To meet the growing demand for animal protein, world poultry meat production soared from 9 to 122 million tonnes between 1961 and 2017 (FAO, 2020). It is expected to continue increasing annually by 2.4% between 2015 and 2030 (FAO, 2015). To help broilers to maintain good health after the ban of subtherapeutic antibiotics as growth promoters, many different classes of alternatives are being developed, including probiotics, prebiotics, synbiotics, organic acids, phytogenics, antimicrobial peptides, and bacteriophages (Gadde et al., 2017a). Probiotics represent a nutritional approach to enhance production- and health-related parameters in broiler chickens (Grant et al., 2018). Probiotics also help in disease prevention and recovery from infections. Our laboratory has previously shown that Lactobacillus plantarum reversed Salmonella typhimurium–induced negative effects in terms of inflammation (Chen et al., 2017) and disrupted intestinal permeability (Wang et al., 2018). Bacillus-based probiotics used in recent studies showed strain-specific effects on the host, based on production and health parameters measured. Gadde et al. (2017b) and Jacquier et al. (2019) reported improvement in different growth parameters including feed conversion rate (FCR), using different Bacillus subtilis strains as probiotics. However, other authors reported no significant changes in FCR while using different Bacillus strains (Teo and Tan, 2007; Ma et al., 2018; Luan et al., 2019). Similar to growth parameters, the host immune and gut health responses to probiotics also appeared to be strain specific. Ma et al. (2018) found no significant immune response to a B. subtilis strain, whereas others reported strong activation of immune-related components by different Bacillus strains (Teo and Tan, 2007; Gadde et al., 2017b; Luan et al., 2019). In addition, while Aliakbarpour et al. (2012) and Luan et al. (2019) observed significant increases in mucin production in response to Bacillus-based probiotics, Gadde et al. (2017b) reported no difference in mucin production in response to different Bacillus strains. The strain-specific effects were also reported for gut integrity (Gadde et al., 2017b; Rhayat et al., 2019). Currently, there is no clear explanation for strain- and dose-specific effects of Bacillus probiotics. Different groups are still developing and testing novel strains of Bacillus-based probiotics that could have potential to influence hosts with broader beneficial effects.
Probiotics may induce beneficial effects through different mechanisms including modulation of intestinal microbiota, which is closely linked with maturation of the immune system (Broom and Kogut, 2018). The composition of gut microbiota in broilers is age dependent, and 2 distinct diversified sets of microbiota are present on day 14 and on day 42 during the broiler life cycle (Ocejo et al., 2019). Furthermore, commensal microbes affect various immune cells, including regulatory T cells (Treg), dendritic cells, and IgA-secreting B cells, leading to suppression of unnecessary inflammation in a mouse model (Chu and Mazmanian, 2013). Regulatory T cells are a subtype of CD4+ T cells and play an important role in keeping gut immune homeostasis as the intestinal barrier is constantly exposed to microbial antigens with a potential to induce inflammation (Sun et al., 2008). In chickens, CD4+CD25+ T cells are considered as the Treg (Lee et al., 2017), as the key Foxp3 equivalent gene, the master transcription factor for Treg, is not described in poultry yet except in peregrine falcons and saker falcons (Denyer et al., 2016). Recently, a relationship between Treg (CD4+CD25+ T cells) and gut microbiota in chicken was studied in antibiotic-treated chickens through administration of an antibiotic cocktail consisting of ampicillin, gentamycin, neomycin, metronidazole, and vancomycin in water for 7 d (Lee et al., 2018). Both CD4+CD8−CD25+ and CD4+CD8+CD25+ T cells in cecal tonsils were significantly decreased by antibiotic treatment, and gram-positive bacteria, especially Clostridia, were responsible for the changes in CD4+CD8−CD25+ or CD4+CD8+CD25+ T cells in cecal tonsils (Lee et al., 2018). These findings provided clues for potential cross talk between intestinal microbiota and Treg and influence on generation of controlled response to inflammatory signals originating from the gut environment. Probiotics, like antibiotics used as antibiotic growth promoters, remodel the diversity and richness of intestinal microbiota and may have direct or indirect influence on regulation of T cells to check the inflammatory mechanisms. However, the effects of probiotics on Treg and their anti-inflammatory response in chickens have not been investigated so far.
The present study, therefore, was designed to evaluate the effects of novel strains of Bacillus pumilus and B. subtilis on production, intestinal microbiota, gut health, and immunity (Treg) of broiler chickens raised under suboptimal conditions.
Materials and methods
Birds, Diet, and Experimental Design
A total of 2,073 one-day-old male broiler chicks (Cobb 500) were obtained from a local hatchery (Grains Natures, Tonton Falls, Quebec, Canada) and randomly divided into 36 pens (6 pens per treatment). These birds were assigned to 6 treatments and grown until 35 d (6 pens per treatment) or until 42 d (3 pens per treatment). The dietary treatments included 1) a standard basal diet (Con group), 2) a basal diet with antibiotic (virginiamycin at a dose of 16.5 mg/kg of feed) (Ab group), 3) a basal diet with a low dose of B. pumilus (3 × 108 cfu/kg of feed) (BPL group), 4) a basal diet with a high dose of B. pumilus (1 × 109 cfu/kg of feed) (BPH group), 5) a basal diet with a low dose of B. subtilis (3 × 108 cfu/kg of feed) (BSL group), and 6) a basal diet with a high dose of B. subtilis (1 × 109 cfu/kg of feed) (BSH group). The basal diet was composed of corn, soybean meal, soybean oil, amino acid supplements, vitamins, and mineral premix and mixed as per the standard of NRC (National Research Council, 1994) (Table 1). The chickens were fed with a starter feed (23% protein and 2,977 kcal of ME/kg) from day 1 to day 14 and a grower feed (20% protein and 3,056 kcal of ME/kg) from day 15 to day 42 (Table 1). The feed and water were supplied ad libitum. The probiotics (B. pumilus and B. subtilis) were provided by Lallemand SAS, Blagnac, France.
Table 1.
Composition (%) of the basal diet.
| Ingredients | Starter, % | Grower, % |
|---|---|---|
| Corn | 54.15 | 52.70 |
| Soybean meal, 48% CP | 38.55 | 30.84 |
| Soybean oil | 2.16 | 2.25 |
| Phosphorus | 1.74 | 0.93 |
| Calcium | 1.54 | 1.62 |
| Vitamin–mineral premix | 0.50 1 | 0.402 |
| Salt | 0.27 | 0.36 |
| Lysine HCL | 0.13 | 0 |
| Methionine | 0.14 | 0.12 |
| Threonine | 0.03 | 0 |
| Choline chloride | 0.10 | 0.10 |
| Sodium carbonate | 0.10 | 0.10 |
| Anticoccidial (CobanR) | 0.05 | 0.05 |
| Wheat | 0 | 10.00 |
| ME, kcal/kg | 2,977 | 3,056 |
| CP, % | 23.00 | 20 |
| Lysine total, % | 1.43 | 1.11 |
| Methionine total, % | 0.51 | 0.44 |
| Crude fat, % | 4.45 | 4.6 |
| Calcium, % | 1.05 | 0.92 |
| Phosphorus total, % | 0.75 | 0.56 |
Provided per kilogram of diet (starter): 5998.49 IU of vitamin A, 2,999.75 IU of vitamin D, 50.21 IU of vitamin E, 30 ppm of vitamin B12, 2.02 ppm of vitamin K, 1.47 mg of folic acid, 13.35 mg of pantothenic acid, 149.99 mg of biotin, 50.38 mg of niacin, 3.2 mg of pyridoxine, 6.4 mg of riboflavin, 2.23 mg of thiamine, 0.001% of magnesium, 0.02% of sulfur, 0.02% of sodium, 0.001% of potassium, 0.88 mg of iodine, 22.63 mg of iron, 12.63 mg of copper, 108.49 mg of manganese, 108.05 mg of zinc, 0.46 mg of cobalt, and 0.40 mg of selenium.
Provided per kilogram of diet (grower): 5999 IU of vitamin A, 3000 IU of vitamin D, 29.80 IU of vitamin E, 30 ppm of vitamin B12, 2.02 ppm of vitamin K, 1.47 mg of folic acid, 13.35 mg of pantothenic acid, 150 mg of biotin, 50.38 mg of niacin, 3.2 mg of pyridoxine, 6.4 mg of riboflavin, 2.22 mg of thiamine, 0.001% of magnesium, 0.02% of sulfur, 0.02% of sodium, 0.001% of potassium, 0.88 mg of iodine, 22.56 mg of iron, 12.62 mg of copper, 108.50 mg of manganese, 108.03 mg of zinc, 0.46 mg of cobalt, and 0.40 mg of selenium.
The experiment was conducted under suboptimal conditions to simulate industrial conditions and evaluate treatment responses, as previously described by Pourabedin et al., 2014. In brief, birds were reared at a higher density (16 birds/m2), colder temperature starting at day 8 (4°C lower than stipulated code of practice), and higher intestinal viscosity by adding 0.5% of guar gum in the feed (Silbergeld et al., 2008). The lightning program was 23-hour light and 1-hour darkness by day 5 of placement, and darkness was gradually increased to 4 h for the rest of the study. The feed intake (FI), body mass, and FCR were calculated on a weekly basis, and mortality was checked daily for each pen. The study protocol was approved by the Animal Care Committee of McGill University (ref # 2018-8002/150930269).
Flow Sorting of Immune Cells for RNA Extraction
Cecal tonsils (longitudinally cut), from 3 sacrificed birds per group at day 14 and day 42, were obtained, washed, and crushed using the flat end of a 3-mL syringe plunger in 1 mmol EDTA solution. The solution was passed through a 40-μm cell strainer (BD Biosciences) into a 50-mL conical tube. The cells were centrifuged for 8 min at 400 relative centrifugal force (∼1200 RPM) and washed with cold PBS twice. The cells were resuspended to a concentration of 1 × 106 cells/mL in flow staining buffer. The viability dye (eBioscience FVD eFluor 780, San Diego, CA) was added to cells at a concentration of 1 μL/mL, and the cells were incubated for 30 min on ice and in a dark place. For examination of T-cell subsets, the cells were stained with anti-chicken CD3-Dylight 405 (clone PC3/188A) (from Santa Cruz Biotechnology, Inc., Dallas, TX), TCR gamma/delta-PerCP (clone TCR1) (from Novus Biologicals, Centennial, CO), CD4-FITC (clone CT-4) and CD8a-PE (clone CT-8) (from Southern Biotech, Birmingham, AL), and CD25-Alexa Fluor 647 (clone-AbD13504) (from Bio-Rad, Philadelphia, PA). The cells were fixed using fixative solution (eBioscience 1-step Fix/Lyse Solution-10X). Different controls such as unstained, single stained for each antibody, fluorescence minus 1 for each fluorophore, and viability dye were included in the experiment. T-cell subpopulations (CD4+CD8−CD25+ and CD4+CD8+CD25+) were sorted using a BD FACSAriaTM Fusion cell sorter (BD, Franklin Lakes, NJ) and stored in Trizol solution (Invitrogen, Waltham, MA) at −20°C for RNA extraction to determine expression levels of IL-10 and interferon gamma.
RNA Isolation and Measurement of mRNA Levels of Immunity and Gut Integrity–Related Genes
Ileum tissue samples (3 cm) were collected from 3 sacrificed birds per group at day 14 and day 42. These samples were stored at −20°C in the TRIzol solution (Invitrogen) before RNA extraction. The ileum tissues and immune cells in the Trizol solution were homogenized and centrifuged at 12,000 × g for 10 min. The supernatant was mixed with chloroform (257 μL/mL) following the manufacturer's recommendations and centrifuged at 12,000 × g for 15 min at 4°C to achieve phase separation. The RNA in the supernatant was mixed with an equal quantity of 70% ethanol and passed through the membrane cartridges. The samples were treated with DNAase enzyme (Invitrogen), and after washings, the RNA was eluted in RNase-free water. RNA quantity was assessed using a spectrophotometer (DeNovix, Wilmington, DE) by measuring absorbance at 260 nm, and RNA purity was determined using the optical density ratios at 260/280 and 260/230. The eluted RNA was stored at −80°C. Total RNA (1 μg) was reverse transcribed to complementary DNA, following the manufacturer's instructions (Applied Biosystems, Beverly, MA). The cDNA samples were stored at −20°C. Expression levels of genes related to immunity (IL-10 and interferon gamma), tight junctions (junctional adhesion molecule 2 [JAM-2], occludin, and zona occludens 1 [ZO-1]), mucin (MUC2), and proinflammatory cytokine (IL-17F) was determined using specific primers (Table 2) by real-time PCR (Bio-Rad). The SYBR Green PCR master mix (Bio-Rad) was used as per the manufacturer's instructions for real-time PCR. Expression levels of target genes were normalized by β-actin and GAPDH, and relative quantification was determined via the 2−ΔΔCt method (Livak and Schmittgen, 2001). Each sample was analyzed in triplicate, and no template controls were used to assess the nonspecific primer amplification.
Table 2.
Primers used for quantitative real-time PCR.
| Gene1 | Primer sequence | |
|---|---|---|
| IL-10 | 3′-AGCTGACGGTGGACCTATTATT-5′ 3′-GGCTTTGCGCTGGATTC-5′ |
Forward Reverse |
| Interferon gamma | 3′-CGGGAGCTGAGGGTGAA-5′ 3′-GTGAAGAAGCGGTGACAGC-5′ |
Forward Reverse |
| IL-17F | 5-TGAAGACTGCCTGAACCA-3 5-AGAGACCGATTCCTGATGT-3 |
Forward Reverse |
| Occludin | 5-GAGCCCAGACTACCAAAGCAA-3 5-GCTTGATGTGGAAGAGCTTGTTG-3 |
Forward Reverse |
| ZO-1 | 5-CCGCAGTCGTTCACGATCT-3 5-GGAGAATGTCTGGAATGGTCTGA-3 |
Forward Reverse |
| JAM-2 | 5-AGCCTCAAATGGGATTGGATT-3 5-CATCAACTTGCATTCGCTTCA-3 |
Forward Reverse |
| MUC2 | 5-GCCTGCCCAGGAAATCAAG-3 5-CGACAAGTTTGCTGGCACAT-3 |
Forward Reverse |
| B-actin | 3′-CAACACAGTGCTGTCTGGTGGTA-5′ 3′-ATCGTACTCCTGCTTGCTGATCC-5′ |
Forward Reverse |
| GAPDH | 5-GGTGGTGCTAAGCGTGTTAT-3 5-ACCTCTGCCATCTCTCCACA-3 |
Forward Reverse |
IL-10: interleukin 10; IL-17F: interleukin 17F; ZO-1: zona occludens 1; JAM-2: junctional adhesion molecule 2; MUC2: mucin 2; B-actin: beta-actin; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Bacterial Culture Analyses of Cecal Content Samples
Birds were randomly selected and euthanized by cervical dislocation on day 14 (6 per group) and day 42 (3 per group). The fresh cecal contents of birds were collected and transferred to the laboratory in sterile tubes having peptone water (1 g per 9 mL). The contents were serially diluted 10-fold in 0.85% sterile saline solution. Diluted contents were plated in duplicate on sterile petri dishes having different selective agar, and mean values of cfu were recorded for the statistical analysis. Lactobacillus was detected using the de Man–Rogosa–Sharpe agar (BD, Mississauga, Ontario, Canada) after 48 h of anaerobic incubation at 37°C, whereas Clostridium was detected using the reinforced clostridial agar (Sigma-Aldrich, Cleveland, OH) after 48 h of incubation under anaerobic conditions at 35°C. Escherichia coli was detected after 24 h of aerobic incubation using the RAPID E. coli 2 selective medium (Bio-Rad, Mississauga, Ontario, Canada) at 37°C. The colonies counted after the incubation periods, and the values were expressed as cfu per gram of cecal contents.
Statistical Analysis
A completely randomized design was used for different parameters in the study. The data were analyzed via one-way ANOVA using the SPSS software (version 24; IBM, Armonk, NY). The data were presented as least squares means ± SEM for each treatment. The differences were considered significant at a P value ≤ 0.05. When the main effect was significant, differences between means were analyzed using Duncan's multiple range test.
The statistical model for completely randomized design was as follows:
where Yij represents the observation for the dependent variables at the jth replicate in the ith treatment (i = 1 to 6), μ is the overall mean, TRTi is the fixed effect of treatments (i = 1 to 6), and eij is the random residual error. The mortality was estimated using the Kaplan–Meier estimation method.
Results
Effects of B. pumilus and B. subtilis Probiotics on Production Parameters of Broilers
To evaluate the effects of Bacillus probiotics on production parameters of broiler chickens, BW, ADG, FI, FCR, and mortality were monitored (Table 3, Table 4, Table 5, Table 6, Table 7). The effects of B. pumilus and B. subtilis on BW, ADG, FI, and FCR between day 1 and day 35 were not statistically different from the Con group, but the BPL and BSH groups had the highest and lowest ADG, respectively, during the period of day 14 to day 21 (Table 6). Mortality in the BSH group was higher than in other groups (Table 3). At the end of the day 35 to day 42 period, the BW in the Ab, BPL, BSL, and BSH groups were higher than in the Con group (Table 4), whereas the FI was higher in the Ab and BSH groups and lowest in the BPH group than in the Con group (Table 5). Feed conversion ratio was not affected by dietary treatments.
Table 3.
Effects of dietary treatments on percentage of mortality of broilers.
| Treatments1 | Percentage of mortality |
|---|---|
| Con | 2.03b |
| Ab | 3.47a,b |
| BPL | 2.56b |
| BPH | 2.32b |
| BSL | 2.03b |
| BSH | 5.56a |
| P-value | 0.050 |
a,bMeans with different superscripts in the same column differ (P < 0.05).
There are a total of 342 birds for the Con, Ab, and BPL groups and a total of 348 birds for the BPH, BSL, and BSH groups.
Con: control; Ab: antibiotic (virginiamycin); BPH: high dose of B. pumilus; BPL: low dose of Bacillus pumilus; BSH: high dose of B. subtilis; BSL: low dose of B. subtilis.
Table 4.
Effects of dietary treatments on BW (g) of broilers.
| Treatment1 | Day 1 | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 |
|---|---|---|---|---|---|---|---|
| Con | 38.3 | 128 | 376 | 801 | 1,376 | 2,055 | 2,780b |
| Ab | 38.4 | 128 | 375 | 818 | 1,380 | 2,084 | 3,010a |
| BPL | 38.0 | 126 | 373 | 831 | 1,413 | 2,130 | 3,033a |
| BPH | 38.5 | 125 | 363 | 801 | 1,369 | 2,087 | 2,862a,b |
| BSL | 38.1 | 131 | 364 | 790 | 1,366 | 2,045 | 2,973a |
| BSH | 38.5 | 129 | 368 | 777 | 1,376 | 2,105 | 3,052a |
| SEM | 0.47 | 2.0 | 6.8 | 13.0 | 19 | 27 | 55 |
| P-value | 0.959 | 0.436 | 0.635 | 0.086 | 0.597 | 0.285 | 0.034 |
a,bMeans with different superscripts in the same column differ (P < 0.05).
n = 6 pens per group, 57 or 58 birds per pen.
Con: control; Ab: antibiotic (virginiamycin); BPH: high dose of B. pumilus; BPL: low dose of Bacillus pumilus; BSH: high dose of B. subtilis; BSL: low dose of B. subtilis.
Table 5.
Effects of dietary treatments on ADG (g) of broilers.
| Treatment1 | Day 1–7 | Day 7–14 | Day 14–21 | Day 21–28 | Day 28–35 | Day 35–42 |
|---|---|---|---|---|---|---|
| Con | 12.8 | 35.4 | 60.7b,c | 82.1 | 97.0 | 97 |
| Ab | 12.8 | 35.3 | 63.2a,b | 80.4 | 100.5 | 125 |
| BPL | 12.6 | 35.3 | 65.4a | 83.1 | 102.4 | 129 |
| BPH | 12.3 | 34.0 | 62.5a,b,c | 81.2 | 102.6 | 114 |
| BSL | 13.2 | 33.4 | 60.9b,c | 82.3 | 97.0 | 126 |
| BSH | 12.9 | 34.1 | 58.6c | 85.5 | 104.1 | 132 |
| SEM | 0.28 | 0.83 | 1.4 | 2.6 | 2.7 | 8.8 |
| P-value | 0.366 | 0.392 | 0.027 | 0.785 | 0.324 | 0.152 |
a,bMeans with different superscripts in the same column differ (P < 0.05).
n = 6 pens per group, 57 or 58 birds per pen.
Con: control; Ab: antibiotic (virginiamycin); BPH: high dose of B. pumilus; BPL: low dose of Bacillus pumilus; BSH: high dose of B. subtilis; BSL: low dose of B. subtilis.
Table 6.
Effects of dietary treatments on weekly feed intake (g) of broilers.
| Treatment1 | Day 1–7 | Day 7–14 | Day 14–21 | Day 21–28 | Day 28–35 | Day 35–42 |
|---|---|---|---|---|---|---|
| Con | 22.5 | 58.2 | 113 | 149 | 170 | 206d |
| Ab | 22.2 | 56.5 | 117 | 149 | 185 | 248a |
| BPL | 22.9 | 55.0 | 118 | 143 | 162 | 214c |
| BPH | 21.2 | 53.6 | 111 | 170 | 170 | 201e |
| BSL | 22.1 | 57.5 | 114 | 155 | 161 | 239b |
| BSH | 22.3 | 56.9 | 114 | 162 | 187 | 250a |
| SEM | 1.2 | 2.2 | 8.9 | 10.4 | 12.9 | 0.86 |
| P-value | 0.943 | 0.697 | 0.994 | 0.509 | 0.591 | <0.001 |
a-dMeans with different superscripts in the same column differ (P < 0.05).
n = 6 pens per group, 57 or 58 birds per pen.
Con: control; Ab: antibiotic (virginiamycin); BPH: high dose of B. pumilus; BPL: low dose of Bacillus pumilus; BSH: high dose of B. subtilis; BSL: low dose of B. subtilis.
Table 7.
Effects of dietary treatments on weekly feed conversion ratio of broilers.
| Treatment1 | Day 1–7 | Day 7–14 | Day 14–21 | Day 21–28 | Day 28–35 | Day 35–42 |
|---|---|---|---|---|---|---|
| Con | 1.77 | 1.66 | 1.94 | 1.83 | 1.83 | 2.16 |
| Ab | 1.79 | 1.61 | 1.93 | 1.87 | 1.94 | 1.99 |
| BPL | 1.84 | 1.59 | 1.91 | 1.75 | 1.63 | 1.67 |
| BPH | 1.78 | 1.61 | 1.82 | 2.13 | 1.77 | 1.79 |
| BSL | 1.71 | 1.73 | 1.94 | 1.91 | 1.72 | 1.95 |
| BSH | 1.77 | 1.75 | 1.98 | 1.95 | 1.86 | 1.91 |
| SEM | 0.11 | 0.08 | 0.17 | 0.14 | 0.15 | 0.14 |
| P-value | 0.980 | 0.665 | 0.989 | 0.488 | 0.759 | 0.318 |
n = 6 pens per group, 57 or 58 birds per pen.
Con: control; Ab: antibiotic (virginiamycin); BPH: high dose of B. pumilus; BPL: low dose of Bacillus pumilus; BSH: high dose of B. subtilis; BSL: low dose of B. subtilis.
Effects of B. pumilus and B. subtilis Probiotics on Intestinal Tight Junction and Mucin Protein Expression in the Ileum
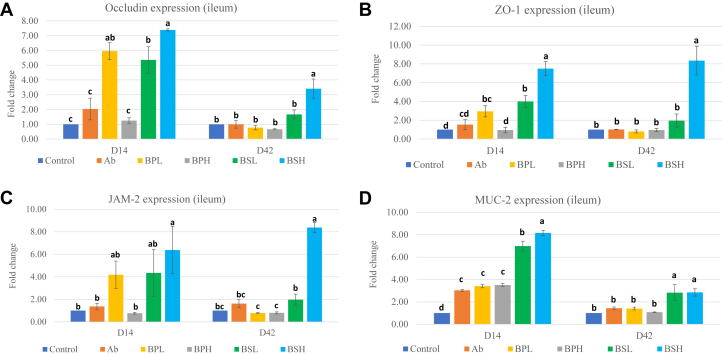
To determine whether B. pumilus and B. subtilis affect intestinal integrity of broiler chickens, expression of the selected tight junction genes (occludin, ZO-1, and JAM-2) and mucin gene (MUC2) was determined through real time- polymerase chain reaction (RT-PCR). The expression of occludin (Figure 1A), ZO-1 (Figure 1B), and JAM-2 (Figure 1C) was increased in the ileum at 14 d of age in the BPL (occludin and ZO-1), BSL (occludin and ZO-1), and BSH (occludin, ZO-1, and JAM-2) groups. However, expression of these genes in all groups except the BSH (occludin, ZO-1, and JAM-2) group became nonsignificant at day 42 in comparison with that in the Con group. Expression of occludin was different between the 2 levels of each probiotic on day 14 and between the BSL and BSH groups at day 42 (P < 0.05). Expression of ZO-1 at day 14 was significantly different between the 2 levels of each probiotic (Figure 1B). There were also significant differences in expression of ZO-1 and JAM-2 between the BSL and BSH groups at day 42. Expression of the mucin (MUC2) gene was significantly higher (Figure 1D) in all groups than in the Con group at day 14 and remained significantly higher than in the Con group at day 42 for the BSL and BSH groups. The expression of the mucin gene was significantly different between the BSL and BSH groups on day 14 (P < 0.05), but not on day 42. There were no differences in mucin expression between the BPL and BPH groups on both day 14 and day 42. These results showed that both Bacillus probiotics had potential to improve intestinal integrity and functions.
Figure 1.
(A) Expression of occludin mRNA in broiler ileum samples at 14 d and 42 d of age. Chickens were fed with basal diets (Con), diets supplemented with antibiotic (Ab), or various strains of Bacillus pumilus at low dose (BPL), B. pumilus at high dose (BPH), B. subtilis at low dose (BSL), and B. subtilis at high dose (BSH). abcDifferent letters mean significant differences between the groups (P < 0.05). Values are presented as least squares means ± SEM (n = 3). (B) Expression of ZO-1 mRNA in broiler ileum samples at 14 d and 42 d of age. Chickens were fed with basal diets (Con), diets supplemented with antibiotic (Ab), or various strains of Bacillus pumilus at low dose (BPL), B. pumilus at high dose (BPH), B. subtilis at low dose (BSL), and B. subtilis at high dose (BSH). abcdDifferent letters mean significant differences between the groups (P < 0.05). Values are presented as least squares means ± SEM (n = 3). (C) Expression of JAM-2 mRNA in broiler ileum samples at 14 d and 42 d of age. Chickens were fed with basal diets (Con), diets supplemented with antibiotic (Ab), or various strains of Bacillus pumilus at low dose (BPL), B. pumilus at high dose (BPH), B. subtilis at low dose (BSL), and B. subtilis at high dose (BSH). abcDifferent letters mean significant differences between the groups (P < 0.05). Values are presented as least squares means ± SEM (n = 3). (D) Expression of MUC-2 mRNA in broiler ileum samples at 14 d and 42 d of age. Chickens were fed with basal diets (Con), diets supplemented with antibiotic (Ab), or various strains of Bacillus pumilus at low dose (BPL), B. pumilus at high dose (BPH), B. subtilis at low dose (BSL), and B. subtilis at high dose (BSH). abcdDifferent letters mean significant differences between the groups (P < 0.05). Values are presented as least squares means ± SEM (n = 3). Abbreviations: JAM-2, junctional adhesion molecule 2; ZO-1, zona occludens 1.
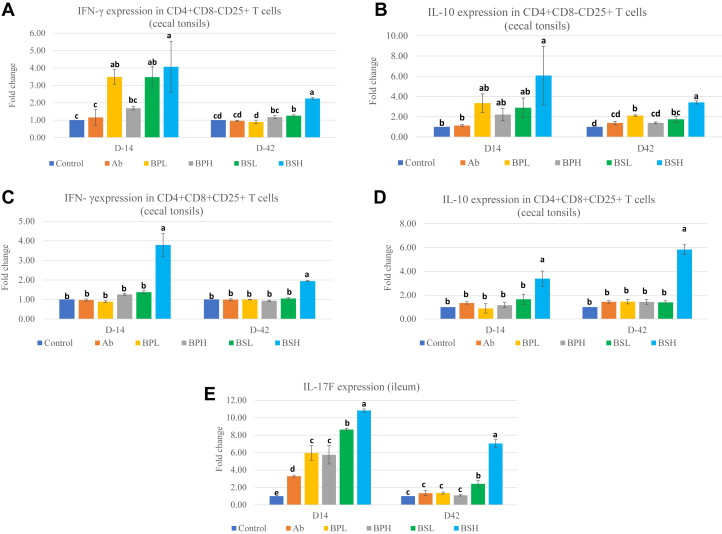
Effects of B. pumilus and B. subtilis Probiotics on Cytokines (IL-10 and Interferon Gamma) Secreted by CD4+CD8−CD25+ and CD4+CD8+CD25+ T Cells in Cecal Tonsils
To investigate how the Bacillus probiotics affect immune regulation, CD4+CD8−CD25+ T cells and CD4+CD8+CD25+ T cells were sorted from cecal tonsils, and expression of IL-10 and interferon gamma genes in these cells was evaluated by RT-PCR. Expression of interferon gamma (Figure 2A) and IL-10 (Figure 2B) in CD4+CD8−CD25+ T cells was increased in response to treatement with BPL (interferon gamma), BSL (interferon gamma), and BSH (IL-10 and interferon gamma) diets at day 14 in comparison with the Con group. Expression of IL-10 in the BPL, BSL, and BSH groups and interferon gamma in the BSL and BSH groups remained high by day 42, and expression in other groups became nonsignificant compared with that in the Con group. Expression levels of interferon gamma and IL-10 between the 2 levels of each probiotic on day 42 were significantly different. Expression of interferon gamma (Figure 2C) and IL-10 (Figure 2D) in CD4+CD8+CD25+ T cells was significantly higher only in the BSH group on day 14 and day 42. The significant differences in expression of interferon gamma and IL-10 were seen at both day 14 and 42 between the BSL and BSH groups (P < 0.050). The results of the study demonstrated that 3 probiotic groups (BPL, BSL, and BSH) potentially stimulated CD4+CD8+CD25+ and CD4+CD8-CD25+ T cells and influenced the expression of IL-10 and interferon gamma.
Figure 2.
(A) Expression of interferon gamma mRNA in CD4+CD8-CD25+ T cells in broiler cecal tonsil samples at 14 d and 42 d of age. Chickens were fed with basal diets (Con), diets supplemented with antibiotic (Ab), or various strains of Bacillus pumilus at low dose (BPL), B. pumilus at high dose (BPH), B. subtilis at low dose (BSL), and B. subtilis at high dose (BSH). abcdDifferent letters mean significant differences between the groups (P < 0.05). Values are presented as least squares means ± SEM (n = 3). (B) Expression of IL-10 mRNA in CD4+CD8–CD25+ T cells in broiler cecal tonsil samples at 14 d and 42 d of age. Chickens were fed with basal diets (Con), diets supplemented with antibiotic (Ab), or various strains of Bacillus pumilus at low dose (BPL), B. pumilus at high dose (BPH), B. subtilis at low dose (BSL), and B. subtilis at high dose (BSH). abcdDifferent letters mean significant differences between the groups (P < 0.05). Values are presented as least squares means ± SEM (n = 3). (C) Expression of interferon gamma mRNA in CD4+CD8+CD25+ T cells in broiler cecal tonsil samples at 14 d and 42 d of age. Chickens were fed with basal diets (Con), diets supplemented with antibiotic (Ab), or various strains of Bacillus pumilus at low dose (BPL), B. pumilus at high dose (BPH), B. subtilis at low dose (BSL), and B. subtilis at high dose (BSH). abDifferent letters mean significant differences between the groups (P < 0.05). Values are presented as least squares means ± SEM (n = 3). (D) Expression of IL-10 mRNA in CD4+CD8+CD25+ T cells in broiler cecal tonsil samples at 14 d and 42 d of age. Chickens were fed with basal diets (Con), diets supplemented with antibiotic (Ab), or various strains of Bacillus pumilus at low dose (BPL), B. pumilus at high dose (BPH), B. subtilis at low dose (BSL), and B. subtilis at high dose (BSH). abDifferent letters mean significant differences between the groups (P < 0.05). Values are presented as least squares means ± SEM (n = 3). (E) Expression of IL-17F mRNA in broiler ileum samples at 14 d and 42 d of age. Chickens were fed with basal diets (Con), diets supplemented with antibiotic (Ab), or various strains of Bacillus pumilus at low dose (BPL), B. pumilus at high dose (BPH), B. subtilis at low dose (BSL), and B. subtilis at high dose (BSH). abcdeDifferent letters mean significant differences between the groups (P < 0.05). Values are presented as least squares means ± SEM (n = 3).
Effects of B. pumilus and B. subtilis Probiotics on IL-17F Cytokine Expression in the Ileum
IL-17F is a proinflammatory cytokine, secreted by T cells including Th17 cells, and plays a role in immune homeostasis and regulation of gut integrity and function. To investigate whether the Bacillus probiotics affected the expression of the IL-17F gene, its expression in the ileum was measured by RT-PCR. Expression levels of IL-17F (Figure 2E) were significantly elevated in all groups compared with the Con group by day 14 and remained higher at day 42 in the BSL and BSH groups only. Expression of IL-17F in the BSH group was seen significantly higher than that in the BSL group (P < 0.050) on both day 14 and day 42. The results of the study suggested that Bacillus probiotics contributed to the activation of the IL-17F cytokine in the ileum.
Effects of B. pumilus and B. subtilis Probiotics on Cecal Bacterial Populations
To determine whether B. pumilus and B. subtilis have effects on the intestinal bacterial populations of broiler chickens, the selected genera (Lactobacillus and Clostridium) and species (E. coli) were determined via bacterial culturing. As shown in Table 8, the bacterial counts of Lactobacillus were significantly higher on day 14 in the BSH group and remained higher on day 42 than in the Con group. In contrast, Lactobacillus counts in the BPL and BSL groups were significantly lower at day 14 than in the Con group. The Lactobacillus population was higher in the BSH group than in the BSL group both at day 14 and day 42 (P < 0.05) and higher in the BPH group than in the BPL group at day 14 only (P < 0.050). The Clostridium count among different groups was not statistically different both at day 14 and 42, but in the BSH group, it was higher than that in the Con, Ab, and BSL groups at day 14 and the Ab group at day 42. There was a decrease in the cecal E. coli population in broilers fed with the BPH diet in comparison with those fed with the Con diet both at day 14 and 42 and those fed with the BSL diet at day 14. On the other hand, the E. coli population in other diet groups was not statistically different from the Con groups both at day 14 and 42. The E. coli population between the BPL and BPH groups at day 14 and 42 and between the BSL and BSH groups at day 14 (P < 0.050) was significantly different from each other.
Table 8.
Effects of dietary treatments on cecal bacterial populations (log10 cfu/g).
| Treatments1 |
Lactobacillus sp. |
Clostridium sp. |
Escherichia coli |
|||
|---|---|---|---|---|---|---|
| Day 14 | Day 42 | Day 14 | Day 42 | Day 14 | Day 42 | |
| Con | 9.12b | 8.53c,d | 9.04b,c | 8.81a,b | 8.51a | 8.39a |
| Ab | 8.97c | 8.37c,d | 8.84c | 8.60b | 8.42a,b | 8.12a |
| BPL | 8.82d | 9.09a,b | 9.10a,b,c | 8.77a,b | 8.37a,b | 8.16a |
| BPH | 9.01b,c | 8.73b,c | 9.28a,b | 8.73a,b | 7.75c | 7.49b |
| BSL | 8.79d | 8.23d | 8.94b,c | 8.73a,b | 8.34b | 8.37a |
| BSH | 9.55a | 9.36a | 9.45a | 9.01a | 8.53a | 8.51a |
| SEM | 0.04 | 0.13 | 0.05 | 0.04 | 0.05 | 0.08 |
| P-value | <0.001 | <0.001 | 0.010 | 0.131 | <0.001 | <0.001 |
a-dMeans with different superscripts in the same column differ (P < 0.05).
n = 6 per group at day 14 and n = 3 per group at day 42.
Con: control; Ab: antibiotic (virginiamycin); BPH: high dose of B. pumilus; BPL: low dose of Bacillus pumilus; BSH: high dose of B. subtilis; BSL: low dose of B. subtilis.
Discussion
In this study, we investigated the effects of B. pumilus and B. subtilis on performance of broilers under suboptimal conditions. Although effects of B. subtilis strains in broilers have been widely investigated (Grant et al., 2018), the effect of B. pumilus on broilers is rarely reported. During the first 5 wk of age (day 1–35), there was no significant effect of B. pumilus and B. subtilis strains on growth performance. However, looking at the period of day 35 to day 42, the BW and FI were significantly higher in the Ab, BPL, BSL, and BSH groups at day 42 than in the Con group, whereas FI in the BSH group was significantly lower at day 42 than in the Con group. Other authors also reported this delayed response of probiotics on growth performance. Jacquier et al. (2019) reported no change in broiler performance up to 21 d of age in response to B. subtilis strains, but later, FCR and BW improved by 35 and 42 d of age, respectively. In contrary, Gadde et al. (2017b) saw significant changes at day 14 in BW and FCR of broilers in response to B. subtilis strain 1781. B. pumilus was also reported to have beneficial effects on the BW of giant freshwater prawns (Zhao et al., 2019) and the striped catfish (Thy et al., 2017). These dissimilarities in results could be attributed to the differences in strains used, probiotic dose, diet composition, and rearing conditions.
Effects of B. pumilus and B. subtilis probiotics on expression of various intestinal tight junction (TJ) proteins were also investigated. These junctional proteins maintain the integrity of the epithelial barrier and regulate paracellular permeability. The junction complexes are composed of tight junctions, gap junctions, adherens junctions, and desmosomes. Tight junctions include 4 integral transmembrane proteins (occludin, claudin, JAM, and tricellulin) that interact with cytosolic scaffold proteins (ZO), which in turn bind to the actin cytoskeleton (Ulluwishewa et al., 2011). Therefore, to better understand how B. pumilus and B. subtilis affected tight junctions, changes in the gene expression of occludin, JAM-2, and ZO-1 at the mRNA level were determined in the ileum. Significant upregulation of expression of TJ proteins was seen in response to Bacillus treatment groups, except in the BPH group, on day 14, which became nonsignificant in all groups except in the BSH group on day 42. This increase in the first 2 wk of life and then decline in TJ protein expression in later weeks of life, especially in the B. pumilus probiotic groups, may be attributed to the bacterial species specificity and interactions of these probiotic strains with changing populations of indigenous intestinal microbiota. The intestinal microbiota or their components activate different submucosal immune cells including Th-17 cells that secrete different cytokines such IL-17A, IL-17F, and IL-22. These cytokines activate epithelial cells to increase expression of TJ proteins (Weaver et al., 2013). TJ proteins are dynamic in nature and are subject to change and remodel in response to external stimuli in the gut lumen such as food, commensals, and pathogenic bacteria (Ulluwishewa et al., 2011). Thus, when antigenic signals from the intestinal lumen decline, their expression also decreases as per conditions. Our results are in agreement with those by Gadde et al. (2017b), who used B. subtilis strain 1781 (PB1), a combination of B. subtilis strain 1,104 and strain 747 (PB2), or B. subtilis strain 1781 + strain 747 (PB3) and found that these Bacillus strains significantly increased expression of TJ proteins JAM-2, ZO-1 (PB2, PB3), and occludin (PB1, PB2) on day 14 in broilers. Rhayat et al. (2019) reported that B. subtilis strain Bs 29,784 improved expression of TJ proteins (occludin, claudin-1, and ZO-1) and transepithelial electrical resistance in CACO-2 cells in vitro. Jacquier et al. (2019) also reported significant increase in intestinal microvilli length (+18% in the ileum and +17% in the cecum) in the broiler group fed with a Bacillus strain. Improvement in TJ protein expression at day 14 would be beneficial for young chickens as higher expression of TJ proteins will reduce intestinal permeability and leakage of feed-originated toxins and contaminants across the epithelial lining. It will reduce inflammation, and more energy will be available for host production. Peng et al. (2019) used B. subtilis CW14 strain as the probiotic to mitigate tight junction injury by improving TJ protein expression and reduce apoptosis that was induced by ochratoxin A. Similarly, Emami et al. (2019) used a cocktail of probiotics to alleviate losses induced by Clostridium perfringens to production and TJ proteins. Thus, higher expression of TJ proteins in response to B. pumilus and B. subtilis at day 14 can be interpreted as an improvement of intestinal integrity.
Mucins, a major component of the mucus, are large glycoproteins with a highly polymeric protein backbone structure and can be either gel forming (secretory) or membrane bound. MUC2, the major secretory mucin, plays a vital role in keeping the architecture of the mucus layer on the intestinal surface and in preventing microorganisms from approaching the innermost mucus layer (Jiang et al., 2013). In this study, MUC2 expression increased in all treatment groups on day 14 and remained high in the B. subtilis groups at day 42. Aliakbarpour et al. (2012) reported similar increase in mucin mRNA expression in the intestine upon supplementation with Bacillus probiotics. Similarly, Luan et al. (2019) reported increases in total goblet cells and expression of mucin-2 in broiler tracheal samples in response to the Bacillus amyloliquefaciens probiotic. In contrast, Gadde et al. (2017b) observed no difference in the expression of MUC2 in any of the probiotic-treated or antibiotic-fed broilers at day 14 despite significant increases in BW and FCR. Probiotics can bring changes in intestinal microbiota, which lead to changes in bacterial fermentation products such as alterations in the short-chain fatty acid (SCFA) profile (Pan and Yu, 2014). These SCFA, especially butyrate, was considered to regulate mucin production locally (Tellez et al., 2006). The SCFA-producing bacterial populations such as genera of Lactobacillus and Clostridium were higher in our study (BPL and BSH groups), which may contribute to higher expression of mucin. Other reports describe the role of IL-22 from Th-17 and other cells in inducing goblet cells to secrete mucin in response to antigenic challenges (Sugimoto et al., 2008). A high production of mucin is a beneficial protective measure to cope with emerging intestinal challenges by invading pathogens. The high expression levels of mucin after supplementation with Bacillus probiotics during the 42 d of the life cycle could be helpful to chickens.
One subtype of CD4-positive T cells in humans, mice, and poultry expresses an added receptor, CD25. CD4+CD25+ T cells in chickens have been reported as Treg (Shanmugasundaram and Selvaraj, 2011). CD4+CD25+ can be divided into CD4+CD8−CD25+ and CD4+CD8+CD25+, although their functional differences are unknown. It has been reported that reduction of gut microbiota reduced mRNA expression of both IL-10 and interferon gamma in CD4+CD8−CD25+ T cells, but not in CD4+CD8+CD25+ T cells, from cecal tonsils in chickens, suggesting existence of potential functional differences between these 2 populations of cells (Lee et al., 2018). These CD4+CD25+ cells can regulate immune homeostasis with a key anti-inflammatory cytokine, IL-10. Lee et al. (2018) reported that the percentages of CD4+CD8-CD25+ and CD4+CD8+CD25+ cells were decreased when chickens were treated with an antibiotic cocktail and that the normal percentage was regained when cohoused with untreated birds, indicating a link between Treg and intestinal microbiota. In our study, we investigated the impact of probiotics (B. pumilus and B. subtilis) on cytokines (IL-10 and interferon gamma) of CD4+CD8-CD25+ and CD4+CD8+CD25+ T cells in cecal tonsils of chickens. We saw high expression of IL-10 and interferon gamma (coexpression) in CD4+CD8−CD25+ and CD4+CD8+CD25+ T cells in cecal tonsils in response to probiotics, particularly in the case of the BSH group. The bacterial species that belong to Clostridia and Lactobacillus in the intestine can produce SCFA and activate the Treg via GPR43 receptors and elicit their regulatory functions to maintain intestinal homeostasis (Honda and Littman, 2016; Lee et al., 2018). Our study observed increase in the cecal population of Clostridium and Lactobacillus species in the BSH group, which may provide an explanation for higher expression of IL-10 and interferon gamma in CD4+CD8−CD25+ and CD4+CD8+CD25+ T cells in this group. The coproduction of IL-10 and interferon gamma by CD4+CD8−CD25+ and CD4+CD8+CD25+ T cells may work like Tr1 cells in chickens, as suggested by Lee et al. (2018), using IL-10 to suppress and tolerate immune responses.
We also observed that expression of IL-17F in the ileum of chickens was increased in response to all 4 probiotics groups (BPL, BPH, BSL, and BSH) on day 14 and remained significantly high in the BSL and BSH groups, but not in the BPL and BPH groups, on day 42. The Th17 cells, with the help of their key cytokines, IL-17A, IL-17F, and IL-22, can stimulate the production of antimicrobial proteins by intestinal epithelial cells, formation of tight junctions between these cells, recruitment of granulocytes, and mediation in transportation of IgA across the mucosa (Weaver et al., 2013; Honda and Littman, 2016). These cells are concentrated more in barrier sites such as the intestine than in systemic sites (Weaver et al., 2013). Certain bacteria such as segmented filamentous bacteria from the family Clostridiaceae were directly linked with stimulation of Th17 cells (Ohnmacht et al., 2011). A recent study on broilers reported elevated expression of IL-17 in response to a mix of Lactobacillus, Bifidobacterium, and Enterococcus–based probiotic product (Emami et al., 2019), suggesting a potential role of IL-17 in alleviation of damage to TJ proteins and intestinal epithelial cells due to pathogenic infection. Despite the protective role of Th17 cells, they may play a role in pathological consequences if overwhelmed by large microbial intestinal breaches (Ohnmacht et al., 2011). Whether there is a link between the high mortality rate in early weeks of age and sustained higher expression of IL-17F in the BSH group is intriguing. In addition, why expression of IL-17F was reduced on day 42 in the B. pumilus groups needs further investigation.
The groups treated with Bacillus probiotics performed better than the Ab group in several aspects. Expression of many genes in the Ab group was significantly lower than in the BPL, BSL, and BSH groups for occludin and ZO-1, the BSH group for JAM-2, and the BSL and BSH groups for MUC-2 on day 14 and the BSH group for occludin, ZO-1, and JAM-2 and the BSL and BSH groups for MUC-2 on day 42. Similarly, the effect of BSH diet on CD4+CD8-CD25+ and CD4+CD8+CD25+ T cells was significantly higher than in the Ab group in terms of interferon gamma and IL-10 secretions on day 14 and 42. The BPL and BSL groups at day 14 and BSL group at day 42 showed significantly better result than the Ab group in terms of their effects on interferon gamma secretion from CD4+CD8-CD25+ T cells. Similarly, IL-10 secreted by CD4+CD8-CD25+ cells at day 42 was higher in the BPL group than in the Ab group. The expression of interferon gamma and IL-10 from CD4+CD8+CD25+ cells was not significantly different among the Ab, BPL, BPH, and BSL groups at day 14 and 42. These results are in agreement with those reported by Gadde et al. (2017b), who found that the groups treated with Bacillus probiotics generated better results than the antibiotic (bacitracin methylene disalicylate) group in terms of immunity and tight junction protein expression.
Conclusion
Taken together, this study documented the effects of B. pumilus and B. subtilis strains on growth performance, intestinal microbiota, immunity, and gut health. We observed that B. pumilus and B. subtilis supplementation conferred intestinal health benefits to broilers by promoting gut integrity and function coupled with activation of Treg of the immune system. These effects were strain, dose, and age sensitive and were different for B. pumilus and B. subtilis.
Acknowledgments
The authors are thankful to Bushansingh Baurhoo (Shyam) for provision of feed to experimental birds and to the Mitacs-Accelerate program for financial support of the study. The PhD student (Bilal) was also partially funded by the Centre de recherche en infectiologie porcine et avicole–Fonds de recherche du Québec–Nature et technologies (CRIPA-FRQNT).
Disclosures
The authors have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aliakbarpour H.R., Chamani M., Rahimi G., Sadeghi A.A., Qujeq D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Australasian J. Anim. Sci. 2012;25:1285–1293. doi: 10.5713/ajas.2012.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom J.L., Kogut M.H. Gut immunity: its development and reasons and opportunities for modulation in monogastric production animals. Anim. Health Res. Rev. 2018;19:46–52. doi: 10.1017/S1466252318000026. [DOI] [PubMed] [Google Scholar]
- Chen Q., Tong C., Ma S., Zhou L., Zhao X. Involvement of MicroRNAs in probiotics-induced reduction of the cecal inflammation by Salmonella Typhimurium. Front. Immunol. 2017;8:704. doi: 10.3389/fimmu.2017.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H., Mazmanian S.K. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat. Immunol. 2013;14:668–675. doi: 10.1038/ni.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer M.P., Pinheiro D.Y., Garden O.A., Shepherd A.J. Missed, not Missing: Phylogenomic Evidence for the existence of avian FoxP3. PLoS One. 2016;11:e0150988. doi: 10.1371/journal.pone.0150988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Calik A., White M.B., Young M., Dalloul R.A. Necrotic enteritis in broiler chickens: the role of tight junctions and mucosal immune responses in alleviating the effect of the disease. Microorganisms. 2019;7:231. doi: 10.3390/microorganisms7080231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization Livestock production. 2015. http://www.fao.org/3/y4252e/y4252e07.htm Accessed Oct. 2020.
- Food and Agriculture Organization of the United Nations Gateway to Poultry Production and Products. 2020. http://www.fao.org/poultry-production-products/production/en/ Accessed Oct. 2020.
- Gadde U., Oh S.T., Lee Y.S., Davis E., Zimmerman N., Rehberger T., Hyun S.T. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob. Proteins. 2017;9:397–405. doi: 10.1007/s12602-017-9275-9. [DOI] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Grant A., Gay C.G., Lillehoj H.S. Bacillus spp. as directed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- Honda K., Littman D.R. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- Jacquier V., Nelson A., Jlali M., Rhayat L., Brinch K.S., Devillard E. Bacillus subtilis 29784 induces a shift in broiler gut microbiome toward butyrate producing bacteria and improves intestinal histomorphology and animal performance. Poult. Sci. 2019;98:2548–2554. doi: 10.3382/ps/pey602. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Applegate T.J., Lossie A.C. Cloning, annotation and developmental expression of the chicken intestinal MUC2 gene. PLoS One. 2013;8:e53781. doi: 10.1371/journal.pone.0053781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.K., Bae S., Gu M.J., You S.J., Kim G., Park S., Jeung W., Ko K.H., Cho J.K., J. S, Kang, C. Yun H9N2-specific IgG and CD4+CD25+ T cells in broilers fed a diet supplemented with organic acids. Poult. Sci. 2017;96:1063–1070. doi: 10.3382/ps/pew382. [DOI] [PubMed] [Google Scholar]
- Lee I.K., Gu M.J., Ko K.H., Bae S., Kim G., Jin G., Kim E.B., Kong Y., Park T.S., Park B., Jung H.J., Han S.H., Yun C. Regulation of CD4+CD8−CD25+and CD4+CD8+CD25+ T cells by gut microbiota in chicken. Sci. Rep. 2018;8:8627. doi: 10.1038/s41598-018-26763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., T. D. Schmittgen Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luan S.J., Sun Y.B., Wang Y., Sa R.N., Zhang H.F. Bacillus amyloliquefaciens spray improves the growth performance, immune status, and respiratory mucosal barrier in broiler chickens. Poult. Sci. 2019;98:1403–1409. doi: 10.3382/ps/pey478. [DOI] [PubMed] [Google Scholar]
- Ma Y., Wang W., Zhang H., Wang J., Zhang W., Gao J., Wu S., Qi G. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018;8:15358. doi: 10.1038/s41598-018-33762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 9th ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ocejo M., Oporto B., Hurtado A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow growing chickens throughout their productive lifespan. Sci. Rep. 2019;9:2506. doi: 10.1038/s41598-019-39323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C., Marques R., Presley L., Sawa S., Lochner M., Eberl G. Intestinal microbiota, evolution of the immune system and the bad reputation of pro-inflammatory immunity. Cell. Microbiol. 2011;13:653–659. doi: 10.1111/j.1462-5822.2011.01577.x. [DOI] [PubMed] [Google Scholar]
- Pan D., Z. Yu Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Liu J., Liang Z. Probiotic Bacillus subtilis CW14 reduces disruption of the epithelial barrier and toxicity of ochratoxin A to Caco-2 cells. Food Chem. Toxicol. 2019;126:25–33. doi: 10.1016/j.fct.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Pourabedin M., Xu Z., Baurhoo B., Chevaux E., Zhao X. Effects of mannan oligosaccharide and virginiamycin on the cecal microbial community and intestinal morphology of chickens raised under suboptimal conditions. Can. J. Microbiol. 2014;60:255–266. doi: 10.1139/cjm-2013-0899. [DOI] [PubMed] [Google Scholar]
- Rhayat L., Maresca M., Nicoletti C., Perrier J., Brinch K.S., Christian S., Devillard E., Eckhardt E. Effect of Bacillus subtilis strains on intestinal barrier function and inflammatory response. Front. Immunol. 2019;10:564. doi: 10.3389/fimmu.2019.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugasundaram R., Selvaraj R.K. Regulatory T cell properties of chicken CD4+CD25+ cells. J. Immunol. 2011;186:1997–2002. doi: 10.4049/jimmunol.1002040. [DOI] [PubMed] [Google Scholar]
- Silbergeld E.K., Graham J., Price L.B. Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Ogawa A., Mizoguchi E., Shimomura Y., Andoh A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Yang H., Nose K. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:139–147. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- Tellez G., Higgins S.E., Donoghue A.M., Hargis B.M. Digestive physiology and the role of microorganisms. J. Appl. Poult. Res. 2006;15:136–144. [Google Scholar]
- Teo a.Y., Tan H.M. Evaluation of the performance and intestinal gut microflora of broilers fed on corn-soy diets supplemented with Bacillus subtilis PB6 (CloSTAT) J. Appl. Poult. Res. 2007;16:296–303. [Google Scholar]
- Thy H.T.T., Tri N.N., Quy O.M., Fotedar R., Kannika K., Unajak S., Areechon N. Effects of the dietary supplementation of mixed probiotic spores of Bacillus amyloliquefaciens 54A, and Bacillus pumilus 47B on growth, innate immunity and stress responses of striped catfish (Pangasianodon hypothalamus) Fish Shellfish Immunol. 2017;60:391–399. doi: 10.1016/j.fsi.2016.11.016. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson A.C., McNabb W.C., Moughan P.J., Well J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Wang L., Li L., Lv Y., Chen Q., Feng J., Zhao X. Lactobacillus plantarum restores intestinal permeability disrupted by Salmonella infection in newly hatched chicks. Sci. Rep. 2018;8:2229. doi: 10.1038/s41598-018-20752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C.T., Elson C.O., Fouser L.A., Kolls J.K. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Zhu J., Hu J., Dong X., Sun L., Zhang X., Miao M. Effects of dietary Bacillus pumilus on growth performance, innate immunity and digestive enzymes of giant freshwater prawns (Macrobrachium rosenbergii) Aquac. Nutr. 2019;25:712–720. [Google Scholar]