Abstract
Fatty liver diseases, common metabolic diseases in chickens, can lead to a decrease in egg production and sudden death of chickens. To solve problems caused by the diseases, reliable chicken models of fatty liver disease are required. To generate chicken models of fatty liver, 7-week-old ISA female chickens were fed with a control diet (17% protein, 5.3% fat, and 1,300 mg/kg choline), a low protein and high fat diet (LPHF, 13% protein, 9.1% fat, and 1,300 mg/kg choline), a high cholesterol with low choline diet (CLC, 17% protein, 7.6% fat with additional 2% cholesterol, and 800 mg/kg choline), a low protein, high fat, high cholesterol, and low choline diet (LPHFCLC, 13% protein, 12.6% fat with additional 2% cholesterol, and 800 mg/kg choline) for 4 wk. Our data showed that the CLC and LPHFCLC diets induced hyperlipidemia. Histological examination and the content of hepatic lipids indicated that the CLC and LPHFCLC diets induced hepatic steatosis. Plasma dipeptidyl peptidase 4, a biomarker of fatty liver diseases in laying hens, increased in chickens fed with the CLC or LPHFCLC diets. Hepatic ballooning and immune infiltration were observed in these livers accompanied by elevated interleukin 1 beta and lipopolysaccharide induced tumor necrosis factor mRNAs suggesting that the CLC and LPHFCLC diets also caused steatohepatitis in these livers. These diets also induced hepatic steatosis in Plymouth Rock chickens. Thus, the CLC and LPHFCLC diets can be used to generate models for fatty liver diseases in different strains of chickens. In ISA chickens fed with the CLC diet, peroxisome proliferator-activated receptor γ, sterol regulatory element binding transcription factor 1, and fatty acid synthase mRNAs increased in the livers, suggesting that lipogenesis was enhanced by the CLC treatment. Our data show that treatment with CLC or LPHFCLC for 4 wk induces fatty liver disease in chickens. These diets can be utilized to rapidly generate chicken models for fatty liver research.
Key words: chicken model, fatty liver, cholesterol, choline
Introduction
Fatty liver commonly occurs in laying hens, for example, fatty liver syndrome and fatty liver hemorrhagic syndrome (FLHS) (Julian, 2005; Trott et al., 2014). The FLHS condition can lead to a decrease in egg production and an increase in mortality in laying hens (Julian, 2005; Trott et al., 2014). Chickens with FLHS exhibit hepatic steatosis whereas more severe cases develop blood clots and liver rupture (Trott et al., 2014; Shini et al., 2019; Zhu et al., 2021). The bleeding resulting from the rupture of the liver has been considered the cause of sudden death (Julian, 2005). Physiological status and environmental factors may positively affect the development of FLHS, for example, overweight, excess abdominal fat, high levels of plasma estrogens, or high ambient temperature (Julian, 2005; Trott et al., 2014; Rozenboim et al., 2016). Furthermore, susceptibility to fatty liver diseases in different strains varies (Thomson et al., 2003; Zhang et al., 2018). It indicates that genetic factor also contributes to the occurrence of FLHS (Trott et al., 2014). Hyperlipidemia is a feature of FLHS in chickens (Gao et al., 2019; Zhuang et al., 2019). Moreover, elevated plasma dipeptidyl peptidase 4 (DPP4), an enzyme related to nonalcoholic fatty liver disease (NAFLD) in humans, has been identified in laying hens to be associated with FLHS (Baumeier et al., 2017; Tsai et al., 2017). Fatty liver hemorrhagic syndrome is a prominent cause of chicken death in caged laying hens (Shini et al., 2019), and therefore causes a massive loss in the egg production industry.
The pathogenesis of fatty liver diseases in chickens is accompanied by an imbalance in lipid homeostasis, such as hepatic lipid accumulation, transportation, and metabolism (Zhong et al., 2019). NAFLD is a common metabolic disorder in humans caused by excessive lipid accumulation in livers (Toshikuni et al., 2014; Arab et al., 2018). As the disease progresses, inflammation may occur in livers affected by NAFLD which would be diagnosed as nonalcoholic steatohepatitis (NASH) (Estes et al., 2018). Obesity in humans is a main risk factor for NAFLD (Loomba and Sanyal, 2013; Younossi et al., 2016; Arab et al., 2018). The excess circulating free fatty acid from adipose tissue can be stored in the livers (Donnelly et al., 2005; Bril et al., 2017; Polyzos et al., 2017). Accordingly, high fat diets can be used to induce hepatic steatosis as a model of NAFLD in rodents (Van Herck et al., 2017). Insufficiency of dietary protein is associated with the occurrence of NAFLD, which may result from impaired synthesis and secretion of lipoproteins (Waterlow, 1975; Badaloo et al., 2005; Ampong et al., 2020). In chickens, high energy low protein diets induce fatty liver (Zhuang et al., 2019). Choline as a methyl donor is important for very low density lipoprotein (VLDL) secretion, and choline deficiency leads to the impairment of VLDL secretion in livers resulting in fat accumulation (Corbin and Zeisel, 2012). High cholesterol promotes the development of NAFLD in rodents (Subramanian et al., 2011; Savard et al., 2013; Ejima et al., 2016; Ho et al., 2019).
Liver is the primary organ for de novo lipogenesis in chickens and humans (Leveille et al., 1968; Griffin et al., 1992; Letexier et al., 2003), whereas both liver and adipose tissue contribute to de novo lipogenesis in rodents (Letexier et al., 2003; Laliotis et al., 2010). Models of NAFLD in chickens are limited. Currently, most of the fatty liver models induced by diet in chickens are time consuming (Ayala et al., 2009; Zhang et al., 2011; Rozenboim et al., 2016). To develop therapeutic methods for prevention of fatty liver diseases, a rapid and reliable chicken model of fatty liver disease is required. In this study, we established a dietary chicken model of fatty liver disease that occurs in 4 wk.
Materials and methods
Experimental Design
Animal experiments were approved by the Institutional Animal Care and Use Committee of National Taiwan University (NTU-109-EL-00002). A total of 40 female ISA chickens and 25 female Plymouth Rock chickens were purchased from a commercial farm in Taiwan. Chickens were individually housed in 3-tier battery cages at 25°C under a 14 h light: 10 h dark cycle. Each cage (45 cm long × 30 cm wide × 37 cm high) was utilized to cage single chickens. Water and feed were provided ad libitum except for the 12-hour duration prior to the collection of fasting blood.
Experimental Design 1
Forty 7-week-old female ISA chickens were randomly divided into 4 groups (10 chickens per group) and randomly distributed in the battery cage. The chickens were allotted to 4 dietary treatments for 4 wk: a basal diet (CON), a low protein and high fat diet (LPHF), a high cholesterol and low choline diet (CLC), and a low protein, high fat, high cholesterol, and low choline diet (LPHFCLC). The diet ingredients and calculated nutritional values are shown in Table 1. Fasting blood was collected from a brachial wing vein before treatment (week 0), and after treatment for 2 wk (week 2) and 4 wk (week 4). After treatment for 4 wk, chickens were euthanized by electrical stunning. Liver samples were collected immediately after euthanasia.
Table 1.
Diet ingredients of CON, LPHF, CLC, and LPHFCLC.
| Ingredient (%) | CON | LPHF | CLC | LPHFCLC |
|---|---|---|---|---|
| Cornmeal | 67.043 | 68.862 | 65.761 | 67.546 |
| Soybean protein | 8.046 | 1.839 | 7.892 | 1.804 |
| Soybean meal | 9.835 | 9.685 | 9.647 | 9.500 |
| Wheat bran | 4.468 | 4.587 | 4.382 | 4.500 |
| Rice bran | 4.468 | 4.587 | 4.382 | 4.500 |
| CaCO3 | 2.239 | 2.299 | 2.196 | 2.255 |
| CaHPO4 | 1.789 | 1.839 | 1.755 | 1.804 |
| Beef tallow | 1.339 | 5.507 | 1.314 | 5.402 |
| NaCl | 0.360 | 0.370 | 0.353 | 0.363 |
| DL-Methionine | 0.360 | 0.370 | 0.353 | 0.363 |
| Premix1 | 0.005 | 0.005 | 0.005 | 0.005 |
| Choline | 0.050 | 0.050 | - | - |
| Cholesterol | - | - | 1.961 | 1.961 |
| Total | 100 | 100 | 100 | 100 |
| Nutrient composition (%) | ||||
| Crude protein2 | 16.55 | 12.42 | 16.94 | 12.80 |
| Fat2 | 5.28 | 9.1 | 7.55 | 12.57 |
| Moisture2 | 11.48 | 10.72 | 10.54 | 9.98 |
| Ash2 | 5.39 | 5.09 | 5.14 | 4.76 |
| Methionine3 | 0.63 | 0.59 | 0.63 | 0.59 |
| Lysine3 | 0.94 | 0.6 | 0.94 | 0.6 |
| Choline3 | 0.13 | 0.13 | 0.08 | 0.08 |
| Cholesterol3 | 0 | 0 | 2 | 2 |
| Calculated metabolic energy (kcal/kg) | 3,043.20 | 3,209.71 | 3,043.20 | 3,209.71 |
Abbreviations: CLC, high cholesterol and low choline diet; CON, control diet; LPHF, low protein and high fat diet; LPHFCLC, low protein, high fat, high cholesterol, and low choline diet; Vit, vitamin.
Premix supplied per kg of diet: Vit A, 1.8 mg; Vit D3, 0.005 mg; Vit E, 9.09 mg; Vit K, 0.5 mg; Vit B12, 0.007 μg; pantothenic acid, 2.99 mg; riboflavin, 1.63 mg; Cu, 1.25 mg; Mn, 24.06 mg; Zn, 12.7 mg; Se, 0.06 mg; iodide, 0.35 mg.
The compositions of crude protein, fat, moisture, and ash in diets were analyzed via proximate analysis.
Calculated compositions of methionine, choline, and lysine in diets.
Experimental Design 2
Twenty-five 7-week-old female Plymouth Rock chickens were randomly divided into 4 groups (CON, n = 6; LPHF, n = 5; CLC, n = 8; LPHFCLC, n = 6) and randomly distributed in the battery cages. The chickens were fed with CON, LPHF, CLC, or LPHFCLC diets for 4 wk. Fasting blood was collected at weeks 0, 2, and 4. Chickens were euthanized by electrical stunning after feeding with various diets for 4 wk. Liver samples were collected immediately after euthanasia.
Proximate Analysis
To determine the percentage of moisture in the feed, 2 g of sample was obtained and placed in weighing bottles. After weighing the weighing bottles with samples, the samples were dried in a vacuum oven (Memmert GmbH, Schwabach, Germany) at 105°C. All samples were analyzed in triplicate. The percentage of moisture content (wet basis) was calculated using the following equation: . To determine the percentage of crude fat in the feed, 2 g of sample, which had been dried using a vacuum oven, was wrapped in a filter paper. Fat was extracted into a fat beaker with 35 mL of ethyl ether (Sigma-Aldrich Corporation, St. Louis, MO) using Goldfisch Fat Extractor (Labconco Corporation, Kansas City, MO) for 4 h. After extraction, the fat beakers were placed in a fume hood to evaporate the ethyl ether. All samples were analyzed in triplicate. The percentage of crude fat in feed was calculated using the following equation: . The percentage of crude protein in the feeds was analyzed by Kjeldahl method (Maehre et al., 2018). Briefly, 0.3 g of sample was wrapped in a nitrogen-free weighing paper (GE Healthcare Life Sciences, Chicago, IL) and a weighing paper without any sample was used for blank determination. The wrapped samples and blank papers were transferred into digestion flasks. A Kjeldahl tablet (Sigma-Aldrich Corporation) and 10 mL of concentrated sulfuric acid (Sigma-Aldrich Corporation) were added into a digestion flask to perform digestion using SpeedDigester K-439 (BÜCHI Labortechnik AG, Flawil, Switzerland) for 3 h. Next, a Distillation Unit K-355 (BÜCHI Labortechnik AG) was used to distill and capture ammonia in 25 mL of 4% boric acid (Sigma-Aldrich Corporation) containing 0.1% bromocresol green (Sigma-Aldrich Corporation) and 0.1% methyl red (Sigma-Aldrich Corporation) as pH indicators. The distillates were titrated using 0.1 N sulfuric acid (Sigma-Aldrich Corporation) until the color changed from light green to pinkish. All samples were analyzed in triplicate.
Measurement of Plasma Triglyceride (TG) and Cholesterol
Chicken blood samples were collected from brachial wing veins using 23-gauge needles (Kelly and Alworth, 2013). The blood samples were transferred into EDTA-coated blood collection tubes (Becton Dickinson, Franklin Lakes, NJ) and mixed gently for 30 min. After centrifugation at 1,000 × g for 10 min, the supernatant fraction was collected for TG and cholesterol measurement using Triglycerides and Cholesterol kits (Randox Laboratories, County Antrim, UK). The detection kits were used according to the manufacturer's instructions. The signals were detected by an Epoch Microplate Spectrophotometer (BioTek, Winooski, VT) by measuring absorbance at 500-nm wavelength.
Histological Analyses
Liver samples were fixed in 10% formaldehyde. The paraffin-embedded liver sections were sliced at 5 μm and stained with hematoxylin and eosin as described previously (Tsai et al., 2017). For Oil Red O staining, the fixed liver samples were embedded in CryomatrixTM embedding resin (Thermo Fisher Scientific, Waltham, MA) and stored at −80°C. The OCT-embedded specimens were sliced at 5 μm with a cryostat microtome. After incubation with absolute propylene glycol for 5 min, sections were stained using Oil Red O solution (Sigma-Aldrich Corporation) at 60°C for 10 min. The stained sections were incubated with 85% propylene glycol for 5 min. Then, they were washed twice with water and stained with hematoxylin. To calculate the inflammatory foci in livers, each liver section was photographed under 200× magnification (0.145 mm2 per field). Ten random fields of each section were photographed. The number of inflammatory foci was determined as the average numbers of inflammatory foci in 10 fields in each liver section.
Liver TG and Cholesterol Measurement
Twenty milligrams of chicken liver was homogenized in 1 mL of PBS. Five milliliters of chloroform/methanol (2/1, v/v) was added; samples were vortexed for 1 min followed by incubation at 4°C for 2 h. After centrifugation at 1,650 × g for 10 min the bottom phase was collected and dried using nitrogen gas. Isopropanol/Nonidet P-40 (9:1, v/v) solution was used to dissolve the lipid samples. The levels of liver TG and cholesterol were measured using Triglycerides and Cholesterol kits (Randox Laboratories). Values of liver TG and cholesterol were normalized with tissue weight.
Real-Time PCR Analysis
To collect the samples for real-time PCR analysis, liver samples were harvested in tubes and snap-frozen in liquid nitrogen. Liver samples were stored at −80°C for further RNA extraction. Total RNA of chicken liver was extracted by using GENEzol Reagent (Geneaid Biotech, Ltd., New Taipei City, Taiwan). Concentrations of RNA samples were measured by a spectrophotometer (NanoDrop One, Thermo Fisher Scientific) at 260/280 nm. Total RNA (4 μg) of each sample was treated with TURBO DNase (Thermo Fisher Scientific). Then, reverse transcription was performed using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Triplicates of each sample were used for real-time PCR analysis. The levels of gene expression in the cDNA were analyzed using a SensiFAST SYBR No-ROX Kit (Bioline, London, UK) in a CFX96 Real-Time System (Bio-Rad Laboratories, Inc., Irvine, CA). The PCR program was set as follows: 2 min at 95°C for polymerase activation, then 40 cycles at 95°C for 5 s for denaturation, followed by 60°C for 30 s for annealing/extension. The primer sets are listed in Table 2. Threshold cycle (Ct) values were obtained by using Bio-Rad CFX Manager 3.1 software (Bio-Rad Laboratories, Inc.). The fold changes of gene expressions were calculated by 2−ΔΔCT method (Livak and Schmittgen, 2001). The value of each gene was normalized to an internal control gene, peptidylprolyl isomerase A (Feroze-Merzoug et al., 2002; Tatsumi et al., 2008), in the same sample using the following equation: . Subsequently, fold changes of gene expressions were calculated using the equation: .
Table 2.
Primer sets for real-time PCR.
| Target genes | Primer sequence | Reference sequence |
|---|---|---|
| Chicken PPARG | F: CAAGGCAGCGGCAAAATAAC R: GTGCCCATAAATGATGGCCTAA |
NM_001001460.1 |
| Chicken SREBF1 | F: GCCCTCTGTGCCTTTGTCTTC R: ACTCAGCCATGATGCTTCTTC |
NM_204126.2 |
| Chicken FASN | F: CTATCGACACAGCCTGCTCCT R: CAGAATGTTGACCCCTCCTACC |
NM_205155.3 |
| Chicken ACC | F: AGTCCTGATTGAGCATGGCA R: CTCCAGATGGCGGTAGATTC |
NM_205505.1 |
| Chicken PPARA | F: ACGGAGTTCCAATCGC R: AACCCTTACAACCTTCACAA |
NM_001001464.1 |
| Chicken CPT1A | F: CTGGGTTATTGCCACGAAGC R: GCCATGGCTAAGGTTTTCGT |
NM_001012898.1 |
| Chicken DPP4 | F: AGTGTGGAATAGCGGTTGCC R: GCTCTGGCCATTACCGTTGA |
NM_001031255.3 |
| Chicken LITAF | F: ACAAGTACACCTGTTACAGTTCAGA R: AACTGGGCGGTCATAGAAC |
NM_204267.1 |
| Chicken IL1B | F: TGCCTGCAGAAGAAGCCTCG R: CTCCGCAGCAGTTTGGTCAT |
NM_204524.1 |
| Chicken PPIA | F: AGGTGCCCATAACAGCAGAG R: CACCACCCTGACACATGAAG |
NM_001166326.1 |
Abbreviations: ACC, acetyl-CoA carboxylase; CPT1A, carnitine palmitoyltransferase 1A; DPP4, dipeptidyl peptidase 4; F, forward primer; FASN, fatty acid synthase; IL1B, interleukin 1 beta; LITAF, lipopolysaccharide induced tumor necrosis factor; R, reverse primer; PPARA, peroxisome proliferator-activated receptor α; PPARG, peroxisome proliferator-activated receptor γ; PPIA, peptidylprolyl isomerase A; SREBF1, sterol regulatory element binding transcription factor 1.
ELISA Assay
Chicken plasma was used to analyze the concentration of DPP4 by an ELISA kit according to the manufacturer's instructions (MyBioSource, San Diego, CA). The signals were detected using a spectrophotometer at 570 nm.
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) and have been presented as mean ± SEM. Group comparisons of the changes of plasma TG and cholesterol were analyzed by 2-way ANOVA followed by Tukey's multiple comparison test, with the treatments and time points as the main factors. Group comparisons of the results of body weight, liver TG and cholesterol, plasma DPP4 concentration, number of inflammatory foci, and the expression levels of lipopolysaccharide induced tumor necrosis factor (LITAF) and interleukin 1 beta were analyzed by 1-way ANOVA followed by Tukey's multiple comparison test, with treatments as the main factor. Group comparisons of the gene expression levels in the livers between CON and CLC groups were analyzed by unpaired t test. Data are shown as mean ± SEM. A P-value ≤ 0.05 was considered statistically different.
Results
CLC and LPHFCLC Diets Induced Hyperlipidemia in ISA Chickens
When 7-week-old female ISA chickens were fed with either the CON, LPHF, CLC, or LPHFCLC diets for 4 wk, there was no treatment effect on body weight at weeks 0, 2, or 4 (Figure 1A). After being fed with various diets, the concentrations of TG in the CON and LPHF groups did not change at week 2 and 4 (Figure 1B). However, the plasma concentrations of TG in CLC and LPHFCLC groups increased (P < 0.05) at week 2 and 4 (Figure 1B). Moreover, the increase (P < 0.05) of plasma TG was time dependent in CLC and LPHFCLC groups (Figure 1B). At week 2 and 4, the concentrations of plasma cholesterol in the CON and LPHF groups did not change (Figure 1C). Plasma cholesterol in the CLC and LPHFCLC groups increased (P < 0.05) at week 2 and 4 (Figure 1C). These data indicated that hyperlipidemia can be induced by feeding the CLC and LPHFCLC diets.
Figure 1.
CLC and LPHFCLC induced hyperlipidemia in ISA chickens. (A) Body weights at week 0, 2, and 4 of ISA chickens fed either CON, LPHF, CLC, or LPHFCLC (1-way ANOVA) (B) Plasma TG and (C) cholesterol concentrations in ISA chickens at weeks 0, 2, and 4. Data are shown as mean ± SEM (n = 10, each group). Groups with no significant difference were labeled with a common letter (2-way ANOVA). Abbreviations: CLC, high cholesterol and low choline diet; CON, control diet; LPHF, low protein and high fat diet; LPHFCLC, low protein, high fat, high cholesterol, and low choline diet; TG, triglyceride.
CLC and LPHFCLC Diets Induced Hepatic Steatosis in ISA Chickens
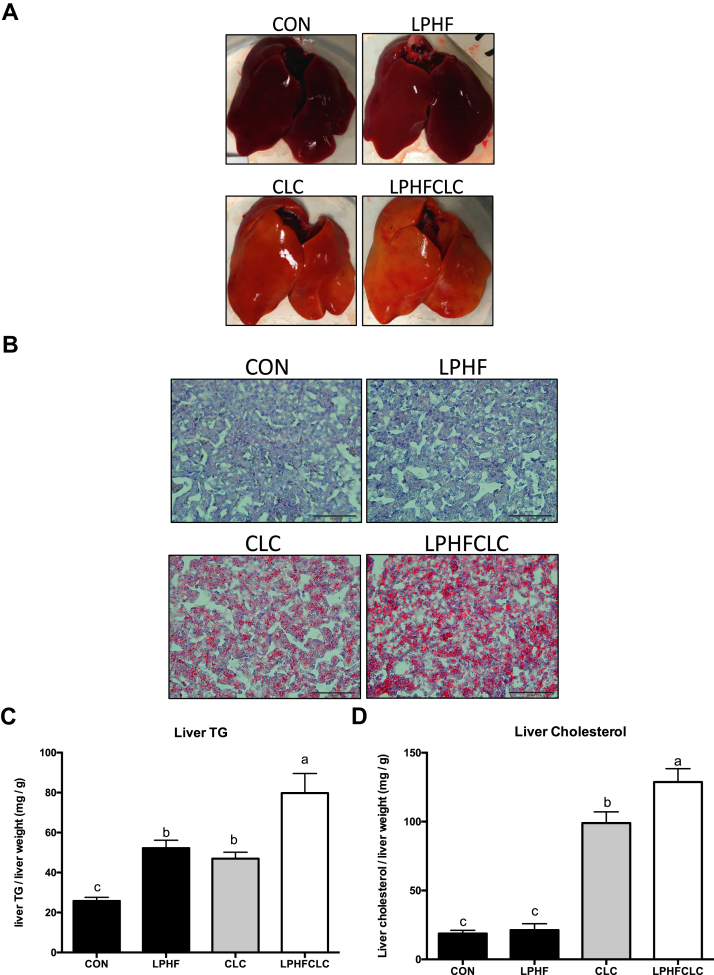
After feeding the different diets for 4 wk, the color of livers in the CON and LPHF groups was red (Figure 2A). However, in the CLC and LPHFCLC groups, the livers were yellow (Figure 2A). This observation indicated that hepatic steatosis occurred in ISA chickens fed with the CLC or LPHFCLC diets for 4 wk. To verify the occurrence of hepatic steatosis, sections of livers were stained with Oil Red O. Using this stain, livers in the CLC and LPHFCLC groups showed considerable lipid accumulation compared to the CON group (Figure 2B). Quantification of hepatic lipids indicated that the TG in LPHF, CLC, and LPHFCLC groups (4 wk feeding) was all greater (P < 0.05) than the CON group (Figure 2C). Hepatic cholesterol at 4 wk in the CLC and LPHFCLC groups increased (P < 0.05) compared to the CON group (Figure 2D). However, the hepatic cholesterol in the LPHF group was comparable to CON (Figure 2D). These data showed that the CLC and LPHFCLC diets induced hepatic steatosis in ISA chickens. Moreover, the plasma TG and cholesterol values in LPHFCLC were both higher (P < 0.05) than CLC (Figures 2C and 2D). This suggested that feeding the LPHFCLC diet enhanced the severity of hepatic steatosis caused by feeding the CLC diet.
Figure 2.
CLC and LPHFCLC induced hepatic steatosis in ISA chickens. (A) Appearance of livers in ISA chickens fed with CON, LPHF, CLC, or LPHFCLC for 4 wk. (B) Oil Red O staining was used to detect the severity of lipid accumulation in livers of the above indicated chickens. Scale bar = 50 μm. The amount of (C) TG and (D) cholesterol in the livers of the above indicated chickens. The values for liver TG and cholesterol were normalized with the weights of liver samples. Data are shown as mean ± SEM (n = 10, each group). Groups with no significant difference were labeled with a common letter (1-way ANOVA). Abbreviations: CLC, high cholesterol and low choline diet; CON, control diet; LPHF, low protein and high fat diet; LPHFCLC, low protein, high fat, high cholesterol, and low choline diet; TG, triglyceride.
Plasma DPP4 was Elevated in ISA Chickens Fed With CLC and LPHFCLC Diets
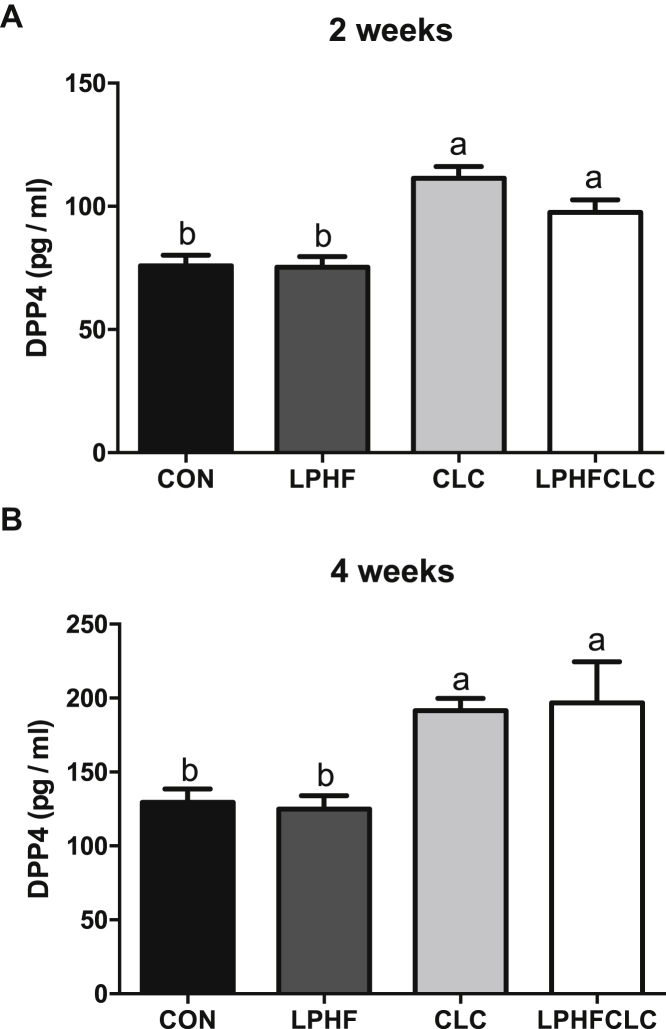
To examine the influence of these diets described previously on the levels of DPP4, ELISA assays were performed to determine the concentrations of plasma DPP4 at week 2 and 4. At week 2 and 4, the concentrations of plasma DPP4 in the LPHF group were comparable to CON (Figures 3A and 3B). However, after feeding the CLC or LPHFCLC diets, plasma DPP4 increased (P < 0.05) at both week 2 and 4 (Figures 3A and 3B). These data further confirmed that CLC and LPHFCLC diets induced fatty liver in ISA chickens.
Figure 3.
Plasma DPP4 was elevated in ISA chickens fed with CLC or LPHFCLC. The levels of plasma DPP4 in ISA chickens fed with CON, LPHF, CLC, or LPHFCLC for (A) 2 wk and (B) 4 wk. Data are shown as mean ± SEM (n = 10, each group). Groups with no significant difference were labeled with a common letter (1-way ANOVA). Abbreviations: CLC, high cholesterol and low choline diet; CON, control diet; DPP4, dipeptidyl peptidase 4; LPHF, low protein and high fat diet; LPHFCLC, low protein, high fat, high cholesterol, and low choline diet.
CLC Diet Induced NASH in Livers of ISA Chickens
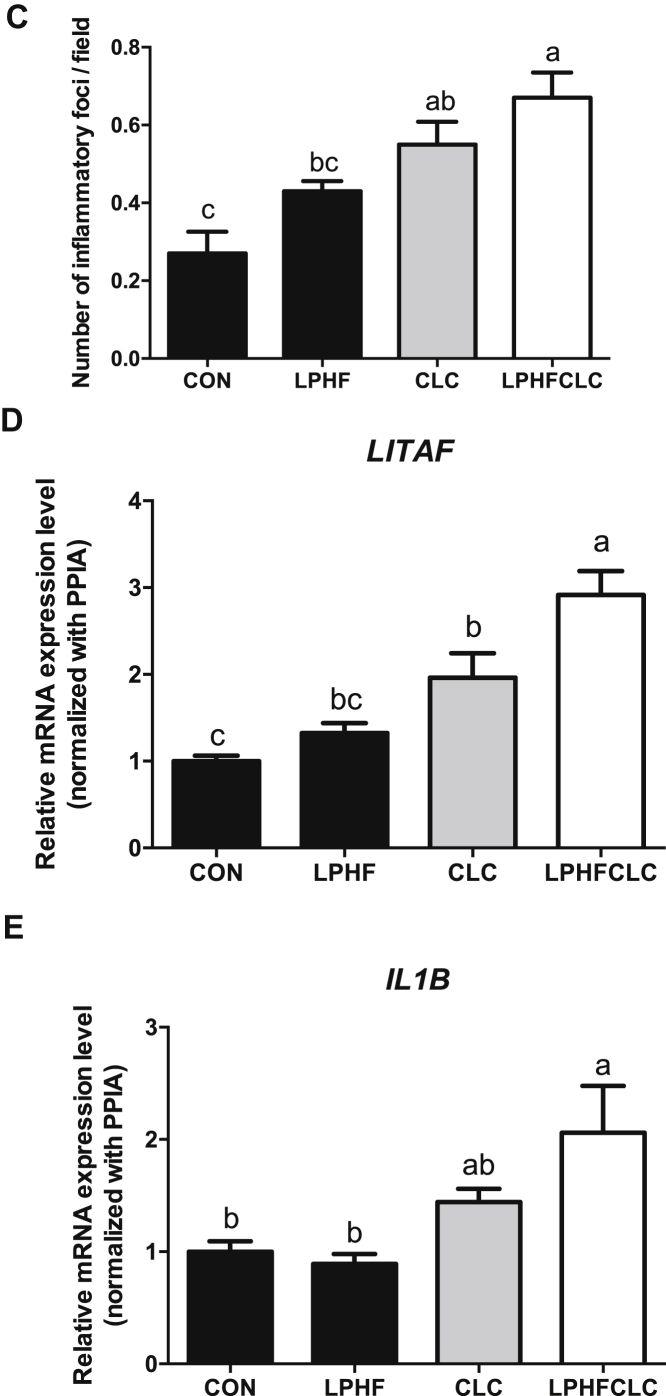
To determine whether NASH occurred in the livers, hepatic morphology and the inflammation-related genes were analyzed. Histological sections of livers showed that cells displayed the morphology of hepatic ballooning in the CLC and LPHFCLC groups (Figure 4A). The number of inflammatory foci in fields under 200× magnification in the liver section was counted to estimate the severity of immune infiltration in the livers (Figure 4B). The number of inflammatory foci increased in the CLC and LPHFCLC groups compared to CON (Figure 4C). The mRNA levels of LITAF were higher in CLC and LPHFCLC groups compared to CON (Figure 4D). Besides, the level of LITAF in the LPHFCLC group was greater than in CLC (Figure 4D). In addition, interleukin 1 beta increased in LPHFCLC-fed group compared to the CON group ( Figure 4E). These data indicated that feeding ISA chickens with the CLC and LPHFCLC diets can cause steatohepatitis in the liver.
Figure 4.
CLC and LPHFCLC induced nonalcoholic steatohepatitis in the livers of ISA chickens. (A) Livers of ISA chickens fed with CON, LPHF, CLC, or LPHFCLC stained with H&E. The white arrow indicates hepatic ballooning. Scale bar = 50 μm. (B) Livers of ISA chickens fed with CON, LPHF, CLC, or LPHFCLC stained with H&E. The green arrow indicates foci of immune infiltration. Scale bar = 100 μm. (C) Numbers of inflammatory foci in each section (H&E staining) were counted in 10 fields under 200× magnification. Field area = 0.145 mm2. The values for the 10 fields for each chicken were averaged. The relative levels of (D) hepatic LITAF and (E) IL1B mRNA in chickens fed with the indicated diets were analyzed by real-time PCR. The level of each gene was normalized with its internal control gene, PPIA. Data are shown as mean ± SEM (n = 10, each group). Groups with no significant difference were labeled with a common letter (1-way ANOVA). Abbreviations: CLC, high cholesterol and low choline diet; CON, control diet; H&E, hematoxylin and eosin; IL1B, interleukin 1 beta; LITAF, lipopolysaccharide induced tumor necrosis factor; LPHF, low protein and high fat diet; LPHFCLC, low protein, high fat, high cholesterol, and low choline diet; PPIA, peptidylprolyl isomerase A.
CLC and LPHFCLC Diets Induced Hepatic Steatosis in Plymouth Rock Chickens
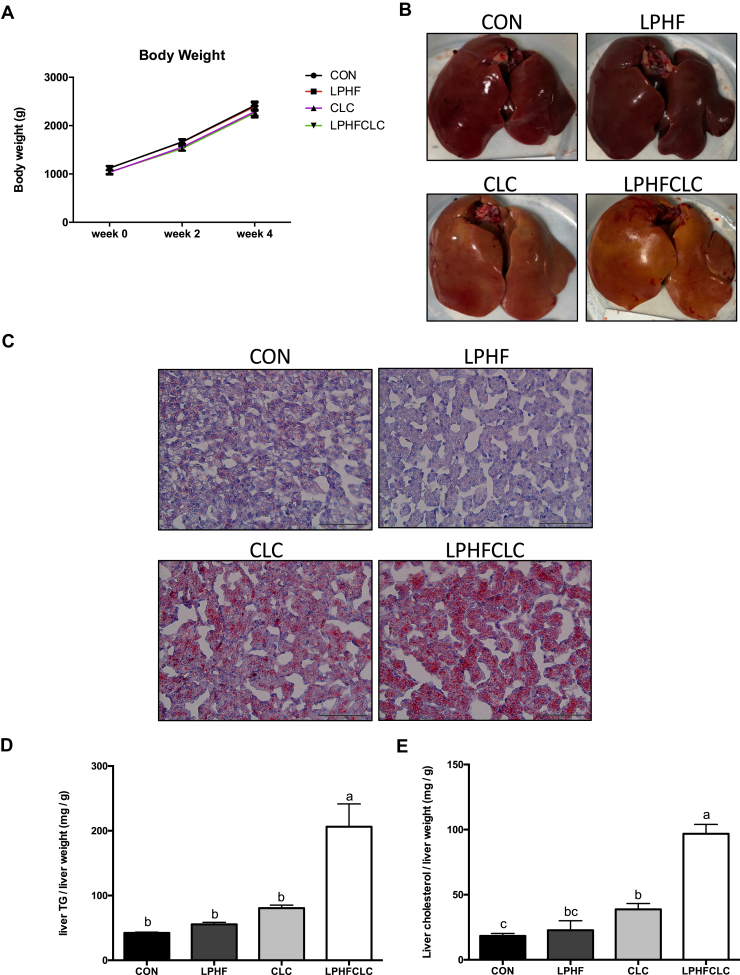
In order to examine whether the CLC and LPHFCLC diets can induce fatty liver disease in other strains of chickens, we fed 7-week-old female Plymouth Rock chickens with the CON, LPHF, CLC, or LPHFCLC diets for 4 wk. There was no treatment effect on body weight at any time (Figure 5A). Livers from chickens fed the CON and LPHF diets were normal, healthy, and red colored at week 4; however, livers of the CLC and LPHFCLC diet groups were yellow (Figure 5B). Oil Red O staining showed that the livers of the LPHF group had no obvious lipid accumulation (Figure 5C). In the CLC and LPHFCLC groups, there was lipid accumulation in the livers. The levels of hepatic TG showed no effects of LPHF and CLC diets (Figure 5D). However, hepatic TG increased (P < 0.05) in the LPHFCLC group (Figure 5D). The level of hepatic cholesterol did not change in the LPHF group compared to the CON group (Figure 5E). Feeding the CLC and LPHFCLC diets increased (P < 0.05) hepatic cholesterol compared with the CON group (Figure 5E). Thus, the CLC and LPHFCLC diets induced fatty liver disease in Plymouth Rock chickens.
Figure 5.
CLC and LPHFCLC induced hepatic steatosis in Plymouth Rock chickens. (A) Body weights of Plymouth Rock chickens fed CON, LPHF, CLC, or LPHFCLC at weeks 0, 2, and 4. (B) Appearance of livers in Plymouth Rock chickens fed the above indicated diets for 4 wk. (C) Oil Red O staining was used to detect the severity of lipid accumulation in livers of Plymouth Rock chickens fed the indicated diets for 4 wk. The amount of (D) TG and (E) cholesterol in the livers of Plymouth Rock chickens fed with the indicated diets for 4 wk. The values for hepatic TG and cholesterol were normalized with the weights of liver samples. CON, n = 6; LPHF, n = 5, CLC, n = 8; LPHFCLC, n = 6. Data are shown as mean ± SEM. Groups with no significant difference were labeled with a common letter (1-way ANOVA). Abbreviations: CLC, high cholesterol and low choline diet; CON, control diet; LPHF, low protein and high fat diet; LPHFCLC, low protein, high fat, high cholesterol, and low choline diet; TG, triglyceride.
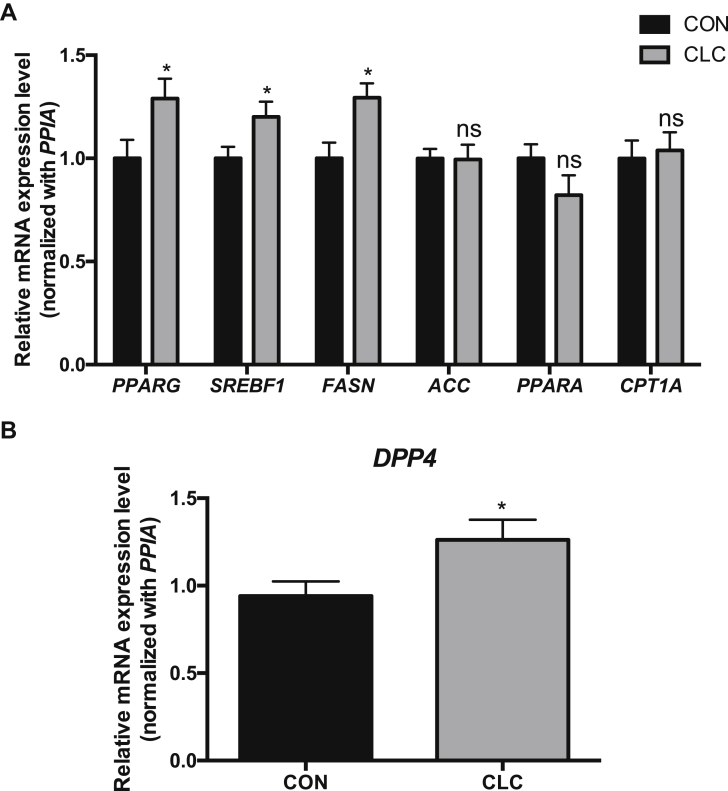
Genes Related to Lipogenesis and Fatty Liver Were Upregulated in the Livers of ISA Chickens Fed With CLC Diet
We demonstrated that diets with low choline and additional 2% cholesterol (CLC diets) were sufficient to induce fatty liver disease in chickens. Accordingly, we identified the characteristic of the fatty liver model induced by CLC. For gene expression analysis, the peroxisome proliferator-activated receptor γ, sterol regulatory element binding transcription factor 1, and fatty acid synthase mRNAs in the CLC group were elevated (P < 0.05) compared to the CON group (Figure 6A). However, the genes related to lipid catabolism, such as peroxisome proliferator-activated receptor α and carnitine palmitoyltransferase 1A, in the CLC group did not change (Figure 6A). We also examined the expression of the DPP4 gene in CLC and CON-fed ISA chickens. The CLC treatment increased (P < 0.05) the levels of DPP4 mRNA in livers (Figure 6B). Data suggested that CLC diets can upregulate lipogenic genes and DPP4 in the livers.
Figure 6.
The genes related to lipogenesis were upregulated in the livers of ISA chickens fed with CLC. (A) The mRNA levels of genes related to lipid metabolism and (B) DPP4 in the ISA chickens fed CON or CLC for 4 wk were analyzed using real-time PCR. The level of each gene was normalized with its internal control gene, PPIA. Data are shown as mean ± SEM (n = 10, each group). Unpaired t test, ∗P < 0.05, ∗∗P < 0.01. Abbreviations: ACC, acetyl-CoA carboxylase; CLC, high cholesterol and low choline diet; CON, control diet; CPT1A, carnitine palmitoyltransferase 1A; DPP4, dipeptidyl peptidase 4; FASN, fatty acid synthase; ns, not significant; PPIA, peptidylprolyl isomerase A; PPARA, peroxisome proliferator-activated receptor α; PPARG, peroxisome proliferator-activated receptor γ; SREBF1, sterol regulatory element binding transcription factor 1.
Discussion
We established chicken models of fatty liver disease using diets containing CLC or LPHFCLC. Hyperlipidemia was induced in ISA chickens after feeding with the CLC or LPHFCLC diets. The CLC and LPHFCLC diets induced fatty liver both in ISA and Plymouth Rock chickens in 4 wk. Our data suggested that the CLC diet was a rapid fatty liver model for chickens.
In laying hens, liver weight, hepatic TG concentration, and the levels of genes related with lipid synthesis and transport, such as peroxisome proliferator-activated receptor γ, ACYL, fatty acid synthase, and APOB were increased by elevation of estradiol, an estrogen steroid hormone necessary for egg production (Haghighi-Rad and Polin, 1981; Lee et al., 2010; Dong and Tong, 2019). Moreover, estradiol suppresses gga-miR-221-5p, a microRNA targeting elongation of very long chain fatty acids protein 6 as well as squalene epoxidase, to increase the amount of TG and cholesterol in the livers of chickens (Zhang et al., 2020), suggesting that estradiol contributes to lipid synthesis in the livers of laying hens. Therefore, hormones for egg production are considered to contribute to the development of FLHS in chickens (Rozenboim et al., 2016). The accumulation of lipids nutritionally supports egg production in chickens (Julian, 2005). However, excess lipids accumulated in the livers of chickens can cause a reduction of egg production and an increase in mortality (Julian, 2005; Mete et al., 2013; Shini et al., 2019). These previous studies suggested that preventing liver accumulation of excess lipid may be a strategy to prevent the decrease in egg production and sudden death of chickens. However, research on fatty liver disease of chickens is limited. Most of the studies were performed on chickens that were 6-month-old, or older (Zhang et al., 2011; Rozenboim et al., 2016; Tsai et al., 2017; Zhu et al., 2021). High energy diets also can be used to establish fatty livers in chickens. Chickens fed with a high fat, low protein or high carbohydrate diet develop symptoms of NAFLD, including hyperlipidemia, and hepatic steatosis (Ayala et al., 2009; Zhang et al., 2011; Zhuang et al., 2019). Moreover, high fat or low protein diets induce hepatic ballooning and elevation of pro-inflammatory cytokines, interleukin-6 and tumor necrosis factor α, indicating that livers have progressed to NASH (Ayala et al., 2009; Rozenboim et al., 2016). Our data showed that fatty livers could be induced in ISA and Plymouth Rock chickens after feeding CLC or LPHFCLC diets for a period of 4 wk.
The insufficiency of dietary protein also contributes to the fatty liver diseases (van Zutphen et al., 2016; Ampong et al., 2020; Chakravarthy et al., 2020). In contrast, high protein diets have been shown to be beneficial in improving hepatic steatosis in mice and humans (Bortolotti et al., 2011; Garcia Caraballo et al., 2017; Xu et al., 2020). Low protein diets can result in insufficiency of essential amino acids, such as lysine and methionine. Lysine and methionine are the precursor of carnitine (Longo et al., 2016). Carnitine is involved in the transportation of long-chain fatty acids from the cytoplasm into mitochondria in which fatty acids can be catabolized via β-oxidation (Longo et al., 2016). The low intake of essential amino acids, particularly methionine and lysine, results in decreased level of carnitine to cause NAFLD (Krajcovicova-Kudlackova et al., 2000; Savic et al., 2020). Methionine can be converted to S-adenosylmethionine, a primary methyl donor transferring its methyl group to other molecules, such as phospholipids, nucleic acids, and proteins (Noureddin et al., 2015). S-adenosylmethionine is involved in the synthesis of phosphatidylcholine which is required for the assembly and secretion of VLDL (Radziejewska et al., 2020). Afterward, homocysteine is produced in methionine metabolism (Skovierova et al., 2016). Homocysteine can obtain the methyl group from betaine to form methionine via remethylation (Radziejewska et al., 2020). In the livers, betaine can be obtained from diets or derived from choline (Radziejewska et al., 2020). Liver is the primary organ responsible for choline metabolism (Corbin and Zeisel, 2012). In the liver, choline can be converted to phosphatidylcholine (Sherriff et al., 2016). Previous study showed the impairment of synthesis and secretion of VLDL leads to NAFLD (Fujita et al., 2009). Therefore, supplementations of choline, methionine, or lysine ameliorate fatty liver diseases (Cordero et al., 2013; Lin et al., 2014; Sato et al., 2018; Zang et al., 2019). The current study showed that the LPHFCLC diet leads to higher levels of hepatic TG and cholesterol than LPHF or CLC does. It indicated that the LPHF diet exacerbated hepatic steatosis induced by the CLC diet in chickens. The effects may be attributed to the combination of high fat and low protein.
DPP4 is a membrane protein expressed in most tissues; it can be cleaved by a protease and released into the blood (Nargis and Chakrabarti, 2018). In humans, the levels of plasma DPP4 are elevated in type 2 diabetes, obesity, and NAFLD (Nargis and Chakrabarti, 2018; Niu et al., 2019). DPP4 plays a critical role in the development of insulin resistance, hepatic steatosis, pro-inflammation, metastasis of cancers, and drug resistance of cancers (Itou et al., 2013). DPP4 performs the activity of a protease to regulate the functions of its target proteins, including chemokines and incretin hormones (Nargis and Chakrabarti, 2018; Enz et al., 2019). Glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide, 2 incretins that function in insulin secretion, are inactivated by degradation via DPP4 (Nargis and Chakrabarti, 2018). Therefore, inhibiting the activity of DPP4 can suppress the degradation of glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide to improve blood sugar management in diabetes (Mulvihill, 2018). DPP4 promotes lipid accumulation in liver by suppressing insulin secretion (Xiao et al., 2014). Moreover, DPP4 inhibits PPARα and activates sterol regulatory element binding transcription factor 1 to promote lipid accumulation in the liver (Conarello et al., 2003). In diabetic mouse, systemic inhibition of DPP4 suppresses hepatic steatosis (Gorgens et al., 2019), suggesting that DPP4 is involved in lipid accumulation in the liver. Although DPP4 is expressed in most tissues, recent studies show that the source of plasma DPP4 is primarily from the liver in the mouse (Varin et al., 2019). The DPP4 secreted from the liver participates in inflammation of the liver and adipose tissue and plays an important role in the development of NAFD (Ghorpade et al., 2018; Varin et al., 2019). Our previous study showed that the levels of DPP4 were elevated in plasma and livers in laying hens with fatty livers (Tsai et al., 2017). Our current data showed that chickens fed with the CLC or LPHFCLC diet demonstrated an increase in plasma DPP4. Although the increase in DPP4 is associated with fatty liver in chickens, the detailed mechanism for the role of DPP4 in the fatty liver of chickens is still unknown.
In this study, we established a short-term chicken model of fatty liver induced by feeding a CLC or LPHFCLC diet for 4 wk. Therefore, fatty liver induced by CLC or LPHFCLC may be used to study fatty liver-associated disease, such as FLHS, or therapies for FLHS in chickens.
Acknowledgments
This research was supported by Ministry of Science and Technology, Republic of China (Taiwan) (grant number 109-2321-B-002-055).
Disclosures
The authors declare no conflict of interests.
References
- Ampong I., Watkins A., Gutierrez-Merino J., Ikwuobe J., Griffiths H.R. Dietary protein insufficiency: an important consideration in fatty liver disease? Br. J. Nutr. 2020;123:601–609. doi: 10.1017/S0007114519003064. [DOI] [PubMed] [Google Scholar]
- Arab J.P., Arrese M., Trauner M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu. Rev. Pathol. 2018;13:321–350. doi: 10.1146/annurev-pathol-020117-043617. [DOI] [PubMed] [Google Scholar]
- Ayala I., Castillo A.M., Adanez G., Fernandez-Rufete A., Perez B.G., Castells M.T. Hyperlipidemic chicken as a model of non-alcoholic steatohepatitis. Exp. Biol. Med. (Maywood) 2009;234:10–16. doi: 10.3181/0807-RM-219. [DOI] [PubMed] [Google Scholar]
- Badaloo A., Reid M., Soares D., Forrester T., Jahoor F. Relation between liver fat content and the rate of VLDL apolipoprotein B-100 synthesis in children with protein-energy malnutrition. Am. J. Clin. Nutr. 2005;81:1126–1132. doi: 10.1093/ajcn/81.5.1126. [DOI] [PubMed] [Google Scholar]
- Baumeier C., Schluter L., Saussenthaler S., Laeger T., Rodiger M., Alaze S.A., Fritsche L., Haring H.U., Stefan N., Fritsche A., Schwenk R.W., Schurmann A. Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease. Mol. Metab. 2017;6:1254–1263. doi: 10.1016/j.molmet.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotti M., Maiolo E., Corazza M., Van Dijke E., Schneiter P., Boss A., Carrel G., Giusti V., Le K.A., Quo Chong D.G., Buehler T., Kreis R., Boesch C., Tappy L. Effects of a whey protein supplementation on intrahepatocellular lipids in obese female patients. Clin. Nutr. 2011;30:494–498. doi: 10.1016/j.clnu.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Bril F., Barb D., Portillo-Sanchez P., Biernacki D., Lomonaco R., Suman A., Weber M.H., Budd J.T., Lupi M.E., Cusi K. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65:1132–1144. doi: 10.1002/hep.28985. [DOI] [PubMed] [Google Scholar]
- Chakravarthy M.V., Waddell T., Banerjee R., Guess N. Nutrition and nonalcoholic fatty liver disease: current Perspectives. Gastroenterol. Clin. North Am. 2020;49:63–94. doi: 10.1016/j.gtc.2019.09.003. [DOI] [PubMed] [Google Scholar]
- Conarello S.L., Li Z., Ronan J., Roy R.S., Zhu L., Jiang G., Liu F., Woods J., Zycband E., Moller D.E., Thornberry N.A., Zhang B.B. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6825–6830. doi: 10.1073/pnas.0631828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin K.D., Zeisel S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012;28:159–165. doi: 10.1097/MOG.0b013e32834e7b4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero P., Gomez-Uriz A.M., Campion J., Milagro F.I., Martinez J.A. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr. 2013;8:105–113. doi: 10.1007/s12263-012-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Tong J. Different susceptibility to fatty liver-haemorrhagic syndrome in young and older layers and the interaction on blood LDL-C levels between oestradiols and high energy-low protein diets. Br. Poult. Sci. 2019;60:265–271. doi: 10.1080/00071668.2019.1571164. [DOI] [PubMed] [Google Scholar]
- Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima C., Kuroda H., Ishizaki S. A novel diet-induced murine model of steatohepatitis with fibrosis for screening and evaluation of drug candidates for nonalcoholic steatohepatitis. Physiol. Rep. 2016;4:e13016. doi: 10.14814/phy2.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz N., Vliegen G., De Meester I., Jungraithmayr W. CD26/DPP4 - a potential biomarker and target for cancer therapy. Pharmacol. Ther. 2019;198:135–159. doi: 10.1016/j.pharmthera.2019.02.015. [DOI] [PubMed] [Google Scholar]
- Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feroze-Merzoug F., Berquin I.M., Dey J., Chen Y.Q. Peptidylprolyl isomerase A (PPIA) as a preferred internal control over GAPDH and beta-actin in quantitative RNA analyses. Biotechniques. 2002;32:776–778. doi: 10.2144/02324st03. 780, 782. [DOI] [PubMed] [Google Scholar]
- Fujita K., Nozaki Y., Wada K., Yoneda M., Fujimoto Y., Fujitake M., Endo H., Takahashi H., Inamori M., Kobayashi N., Kirikoshi H., Kubota K., Saito S., Nakajima A. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–780. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- Gao X., Liu P., Wu C., Wang T., Liu G., Cao H., Zhang C., Hu G., Guo X. Effects of fatty liver hemorrhagic syndrome on the AMP-activated protein kinase signaling pathway in laying hens. Poult. Sci. 2019;98:2201–2210. doi: 10.3382/ps/pey586. [DOI] [PubMed] [Google Scholar]
- Garcia Caraballo S.C., Comhair T.M., Dejong C.H.C., Lamers W.H., Koehler S.E. Dietary treatment of fatty liver: high dietary protein content has an antisteatotic and antiobesogenic effect in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1789–1804. doi: 10.1016/j.bbadis.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Ghorpade D.S., Ozcan L., Zheng Z., Nicoloro S.M., Shen Y., Chen E., Bluher M., Czech M.P., Tabas I. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature. 2018;555:673–677. doi: 10.1038/nature26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgens S.W., Jahn-Hofmann K., Bangari D., Cummings S., Metz-Weidmann C., Schwahn U., Wohlfart P., Schafer M., Bielohuby M. A siRNA mediated hepatic dpp4 knockdown affects lipid, but not glucose metabolism in diabetic mice. PLoS One. 2019;14:e0225835. doi: 10.1371/journal.pone.0225835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin H.D., Guo K., Windsor D., Butterwith S.C. Adipose tissue lipogenesis and fat deposition in leaner broiler chickens. J. Nutr. 1992;122:363–368. doi: 10.1093/jn/122.2.363. [DOI] [PubMed] [Google Scholar]
- Haghighi-Rad F., Polin D. The relationship of plasma estradiol and progesterone levels to the fatty liver hemorrhagic syndrome in laying hens. Poult. Sci. 1981;60:2278–2283. doi: 10.3382/ps.0602278. [DOI] [PubMed] [Google Scholar]
- Ho C.M., Ho S.L., Jeng Y.M., Lai Y.S., Chen Y.H., Lu S.C., Chen H.L., Chang P.Y., Hu R.H., Lee P.H. Accumulation of free cholesterol and oxidized low-density lipoprotein is associated with portal inflammation and fibrosis in nonalcoholic fatty liver disease. J. Inflamm. (Lond.) 2019;16:7. doi: 10.1186/s12950-019-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itou M., Kawaguchi T., Taniguchi E., Sata M. Dipeptidyl peptidase-4: a key player in chronic liver disease. World J. Gastroenterol. 2013;19:2298–2306. doi: 10.3748/wjg.v19.i15.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian R.J. Production and growth related disorders and other metabolic diseases of poultry--a review. Vet. J. 2005;169:350–369. doi: 10.1016/j.tvjl.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Kelly L.M., Alworth L.C. Techniques for collecting blood from the domestic chicken. Lab Anim. (Ny) 2013;42:359–361. doi: 10.1038/laban.394. [DOI] [PubMed] [Google Scholar]
- Krajcovicova-Kudlackova M., Simoncic R., Bederova A., Babinska K., Beder I. Correlation of carnitine levels to methionine and lysine intake. Physiol. Res. 2000;49:399–402. [PubMed] [Google Scholar]
- Laliotis G.P., Bizelis I., Rogdakis E. Comparative Approach of the de novo Fatty Acid Synthesis (Lipogenesis) between Ruminant and Non Ruminant Mammalian Species: from Biochemical Level to the Main Regulatory Lipogenic Genes. Curr. Genomics. 2010;11:168–183. doi: 10.2174/138920210791110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.K., Kim J.S., Ahn H.J., Hwang J.H., Kim J.M., Lee H.T., An B.K., Kang C.W. Changes in hepatic lipid parameters and hepatic messenger ribonucleic acid expression following estradiol administration in laying hens (Gallus domesticus) Poult. Sci. 2010;89:2660–2667. doi: 10.3382/ps.2010-00686. [DOI] [PubMed] [Google Scholar]
- Letexier D., Pinteur C., Large V., Frering V., Beylot M. Comparison of the expression and activity of the lipogenic pathway in human and rat adipose tissue. J. Lipid Res. 2003;44:2127–2134. doi: 10.1194/jlr.M300235-JLR200. [DOI] [PubMed] [Google Scholar]
- Leveille G.A., O'Hea E.K., Chakbabarty K. In vivo lipogenesis in the domestic chicken. Proc. Soc. Exp. Biol. Med. 1968;128:398–401. doi: 10.3181/00379727-128-33022. [DOI] [PubMed] [Google Scholar]
- Lin H.Y., Chen C.C., Chen Y.J., Lin Y.Y., Mersmann H.J., Ding S.T. Enhanced amelioration of high-fat diet-induced fatty liver by docosahexaenoic acid and lysine supplementations. Biomed. Res. Int. 2014;2014:310981. doi: 10.1155/2014/310981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Longo N., Frigeni M., Pasquali M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta. 2016;1863:2422–2435. doi: 10.1016/j.bbamcr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R., Sanyal A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- Maehre H.K., Dalheim L., Edvinsen G.K., Elvevoll E.O., Jensen I.J. Protein Determination-method Matters. Foods. 2018;7:5. doi: 10.3390/foods7010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mete A., Giannitti F., Barr B., Woods L., Anderson M. Causes of mortality in backyard chickens in northern California: 2007-2011. Avian Dis. 2013;57:311–315. doi: 10.1637/10382-092312-Case.1. [DOI] [PubMed] [Google Scholar]
- Mulvihill E.E. Dipeptidyl peptidase inhibitor therapy in type 2 diabetes: control of the incretin axis and regulation of postprandial glucose and lipid metabolism. Peptides. 2018;100:158–164. doi: 10.1016/j.peptides.2017.11.023. [DOI] [PubMed] [Google Scholar]
- Nargis T., Chakrabarti P. Significance of circulatory DPP4 activity in metabolic diseases. IUBMB Life. 2018;70:112–119. doi: 10.1002/iub.1709. [DOI] [PubMed] [Google Scholar]
- Niu L., Geyer P.E., Wewer Albrechtsen N.J., Gluud L.L., Santos A., Doll S., Treit P.V., Holst J.J., Knop F.K., Vilsboll T., Junker A., Sachs S., Stemmer K., Muller T.D., Tschop M.H., Hofmann S.M., Mann M. Plasma proteome profiling discovers novel proteins associated with non-alcoholic fatty liver disease. Mol. Syst. Biol. 2019;15:e8793. doi: 10.15252/msb.20188793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noureddin M., Mato J.M., Lu S.C. Nonalcoholic fatty liver disease: update on pathogenesis, diagnosis, treatment and the role of S-adenosylmethionine. Exp. Biol. Med. (Maywood) 2015;240:809–820. doi: 10.1177/1535370215579161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyzos S.A., Kountouras J., Mantzoros C.S. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva. Endocrinol. 2017;42:92–108. doi: 10.23736/S0391-1977.16.02563-3. [DOI] [PubMed] [Google Scholar]
- Radziejewska A., Muzsik A., Milagro F.I., Martinez J.A., Chmurzynska A. One-carbon metabolism and nonalcoholic fatty liver disease: the Crosstalk between Nutrients, Microbiota, and genetics. Lifestyle Genom. 2020;13:53–63. doi: 10.1159/000504602. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Mahato J., Cohen N.A., Tirosh O. Low protein and high-energy diet: a possible natural cause of fatty liver hemorrhagic syndrome in caged White Leghorn laying hens. Poult. Sci. 2016;95:612–621. doi: 10.3382/ps/pev367. [DOI] [PubMed] [Google Scholar]
- Sato T., Muramatsu N., Ito Y., Yamamoto Y., Nagasawa T. L-lysine Attenuates hepatic steatosis in Senescence-Accelerated mouse Prone 8 mice. J. Nutr. Sci. Vitaminol (Tokyo) 2018;64:192–199. doi: 10.3177/jnsv.64.192. [DOI] [PubMed] [Google Scholar]
- Savard C., Tartaglione E.V., Kuver R., Haigh W.G., Farrell G.C., Subramanian S., Chait A., Yeh M.M., Quinn L.S., Ioannou G.N. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57:81–92. doi: 10.1002/hep.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic D., Hodson L., Neubauer S., Pavlides M. The importance of the fatty acid transporter L-carnitine in non-alcoholic fatty liver disease (NAFLD) Nutrients. 2020;12:2178. doi: 10.3390/nu12082178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherriff J.L., O'Sullivan T.A., Properzi C., Oddo J.L., Adams L.A. Choline, its potential role in nonalcoholic fatty liver disease, and the case for human and Bacterial genes. Adv. Nutr. 2016;7:5–13. doi: 10.3945/an.114.007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shini A., Shini S., Bryden W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: impact of production system. Avian Pathol. 2019;48:25–34. doi: 10.1080/03079457.2018.1538550. [DOI] [PubMed] [Google Scholar]
- Skovierova H., Vidomanova E., Mahmood S., Sopkova J., Drgova A., Cervenova T., Halasova E., Lehotsky J. The molecular and Cellular effect of homocysteine metabolism imbalance on human Health. Int. J. Mol. Sci. 2016;17:1733. doi: 10.3390/ijms17101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S., Goodspeed L., Wang S., Kim J., Zeng L., Ioannou G.N., Haigh W.G., Yeh M.M., Kowdley K.V., O'Brien K.D., Pennathur S., Chait A. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J. Lipid Res. 2011;52:1626–1635. doi: 10.1194/jlr.M016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi K., Ohashi K., Taminishi S., Okano T., Yoshioka A., Shima M. Reference gene selection for real-time RT-PCR in regenerating mouse livers. Biochem. Biophys. Res. Commun. 2008;374:106–110. doi: 10.1016/j.bbrc.2008.06.103. [DOI] [PubMed] [Google Scholar]
- Thomson A.E., Gentry P.A., Squires E.J. Comparison of the coagulation profile of fatty liver haemorrhagic syndrome-susceptible laying hens and normal laying hens. Br. Poult. Sci. 2003;44:626–633. doi: 10.1080/00071660310001616228. [DOI] [PubMed] [Google Scholar]
- Toshikuni N., Tsutsumi M., Arisawa T. Clinical differences between alcoholic liver disease and nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20:8393–8406. doi: 10.3748/wjg.v20.i26.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott K.A., Giannitti F., Rimoldi G., Hill A., Woods L., Barr B., Anderson M., Mete A. Fatty liver hemorrhagic syndrome in the backyard chicken: a retrospective histopathologic case series. Vet. Pathol. 2014;51:787–795. doi: 10.1177/0300985813503569. [DOI] [PubMed] [Google Scholar]
- Tsai M.T., Chen Y.J., Chen C.Y., Tsai M.H., Han C.L., Chen Y.J., Mersmann H.J., Ding S.T. Identification of potential plasma biomarkers for nonalcoholic fatty liver disease by Integrating Transcriptomics and Proteomics in laying hens. J. Nutr. 2017;147:293–303. doi: 10.3945/jn.116.240358. [DOI] [PubMed] [Google Scholar]
- Van Herck M.A., Vonghia L., Francque S.M. Animal models of nonalcoholic fatty liver disease-A Starter's Guide. Nutrients. 2017;9 doi: 10.3390/nu9101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zutphen T., Ciapaite J., Bloks V.W., Ackereley C., Gerding A., Jurdzinski A., de Moraes R.A., Zhang L., Wolters J.C., Bischoff R., Wanders R.J., Houten S.M., Bronte-Tinkew D., Shatseva T., Lewis G.F., Groen A.K., Reijngoud D.J., Bakker B.M., Jonker J.W., Kim P.K., Bandsma R.H. Malnutrition-associated liver steatosis and ATP depletion is caused by peroxisomal and mitochondrial dysfunction. J. Hepatol. 2016;65:1198–1208. doi: 10.1016/j.jhep.2016.05.046. [DOI] [PubMed] [Google Scholar]
- Varin E.M., Mulvihill E.E., Beaudry J.L., Pujadas G., Fuchs S., Tanti J.F., Fazio S., Kaur K., Cao X., Baggio L.L., Matthews D., Campbell J.E., Drucker D.J. Circulating levels of Soluble dipeptidyl peptidase-4 are Dissociated from inflammation and induced by Enzymatic DPP4 inhibition. Cell Metab. 2019;29:320–334 e325. doi: 10.1016/j.cmet.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Waterlow J.C. Amount and rate of disappearance of liver fat in malnourished infants in Jamaica. Am. J. Clin. Nutr. 1975;28:1330–1336. doi: 10.1093/ajcn/28.11.1330. [DOI] [PubMed] [Google Scholar]
- Xiao C., Dash S., Morgantini C., Patterson B.W., Lewis G.F. Sitagliptin, a DPP-4 inhibitor, acutely inhibits intestinal lipoprotein particle secretion in healthy humans. Diabetes. 2014;63:2394–2401. doi: 10.2337/db13-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Markova M., Seebeck N., Loft A., Hornemann S., Gantert T., Kabisch S., Herz K., Loske J., Ost M., Coleman V., Klauschen F., Rosenthal A., Lange V., Machann J., Klaus S., Grune T., Herzig S., Pivovarova-Ramich O., Pfeiffer A.F.H. High-protein diet more effectively reduces hepatic fat than low-protein diet despite lower autophagy and FGF21 levels. Liver Int., 2020;40:2982–2997. doi: 10.1111/liv.14596. [DOI] [PubMed] [Google Scholar]
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- Zhang J.W., Chen D.W., Yu B., Wang Y.M. Effect of dietary energy source on deposition and fatty acid synthesis in the liver of the laying hen. Br. Poult. Sci. 2011;52:704–710. doi: 10.1080/00071668.2010.547457. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu Z., Liu R., Wang J., Zheng M., Li Q., Cui H., Zhao G., Wen J. Alteration of hepatic gene expression along with the Inherited Phenotype of Acquired fatty liver in chicken. Genes (Basel) 2018;9:199. doi: 10.3390/genes9040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y., Samii S.S., Myers W.A., Bailey H.R., Davis A.N., Grilli E., McFadden J.W. Methyl donor supplementation suppresses the progression of liver lipid accumulation while modifying the plasma triacylglycerol lipidome in periparturient Holstein dairy cows. J. Dairy Sci. 2019;102:1224–1236. doi: 10.3168/jds.2018-14727. [DOI] [PubMed] [Google Scholar]
- Zhang D.D., Wang D.D., Wang Z., Wang Y.B., Li G.X., Sun G.R., Tian Y.D., Han R.L., Li Z.J., Jiang R.R., Liu X.J., Kang X.T., Li H. Estrogen Abolishes the Repression role of gga-miR-221-5p targeting ELOVL6 and SQLE to promote lipid synthesis in chicken liver. Int. J. Mol. Sci. 2020;21:1624. doi: 10.3390/ijms21051624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong F., Zhou X., Xu J., Gao L. Rodent models of nonalcoholic fatty liver disease. Digestion. 2020;101:522–535. doi: 10.1159/000501851. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Mao H., Peng G., Zeng Q., Ruan J., Huang J. Effect of JAK-STAT pathway in regulation of fatty liver hemorrhagic syndrome in chickens. Anim Biosci. 2021;34:143–153. doi: 10.5713/ajas.19.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y., Xing C., Cao H., Zhang C., Luo J., Guo X., Hu G. Insulin resistance and metabonomics analysis of fatty liver haemorrhagic syndrome in laying hens induced by a high-energy low-protein diet. Sci. Rep. 2019;9:10141. doi: 10.1038/s41598-019-46183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]