Abstract
Blood biochemistry and bone metabolism were evaluated to investigate the etiology and mechanism of spontaneous femoral head necrosis (FHN) in broilers. According to the femoral head score of the fourth, fifth, and sixth week old FHN-affected broilers, they were divided into 3 groups, namely Normal group, femoral head separation group, and femoral head separation with growth plate lacerations group, and then carried out a comparative study. The results showed that the liver function (alanine aminotransferase and aspartate aminotransferase) and lipid metabolism (high-density lipoprotein and triglyceride) levels of broilers with spontaneous FHN were significant changed compared with the normal group. At the same time, accumulation of lipid droplets appeared in the liver, which illustrated that the occurrence of FHN may be related to lipid metabolism disorders. Tibia and femur parameters showed significant changes in bone mineral density and bone strength. The distribution of chondrocytes in the articular cartilage of broilers with FHN was irregular and vacuoles appeared, which indicated that cartilage homeostasis was destroyed. TUNEL staining showed that the apoptosis rate of articular chondrocytes in broilers with FHN in 6-week-old was significantly higher than that of normal broilers. Meanwhile, the bone markers (bone glaprotein and bone-specific alkaline phosphatase) changed significantly, indicating that the articular chondrocyte apoptosis and bone metabolism disorder may occur in FHN-affected birds. Therefore, FHN in broilers may be caused by dyslipidemia and abnormal bone metabolism.
Key words: femoral head necrosis, lipid metabolism, bone metabolism, broiler
Introduction
Improvement of the breeding technology increases the feed utilization rate and decreases the occurrence of diseases; as a result, the growth rate of broilers has promoted by 300% compared with the 1960s (Dinev et al., 2019). However, the rapid growth not only improves the economic benefits but also causes the certain negative metabolic disorders, and the bone growth and development cannot meet the weight support of overgrown broilers (Williams et al., 2004), increasing of the incidence of bone diseases and welfare concerns (Cook, 2000; Julian, 2005; Sanchez-Rodriguez et al., 2019). The leg problems in broiler are attributed to various musculoskeletal disorders leading to lameness, infection, pain, and discomfort (Bessei, 2006; Packialakshmi et al., 2015b). As broilers gain weight, body posture significantly changes the center of gravity, making the proximal femur vulnerable to severe exercise (Paxton et al., 2014). Femoral head necrosis (FHN) is one of the most common bone diseases. It is characterized by separation of the epiphysis and articular cartilage, necrosis of trabecular bone, and finally subchondral fracture. According to the severity, it can be divided into femoral head separation (FHS) or femoral head separation with growth plate lacerations (Durairaj et al., 2009; Li et al., 2015).
Femoral head necrosis is a disorder with a variety of traumatic and nontraumatic etiologies. Traumatic factors may include physical damage to the epiphysis caused by habitat conditions, capture, handling, and other environmental factors (Packialakshmi et al., 2015b), whereas nontraumatic factors may include vascular occlusion, dyslipidemia, and chondrocyte abnormal apoptosis (Cui et al., 1997; Seamon et al., 2012; Wideman and Prisby, 2012; Li et al., 2015; Zhang et al., 2017; Yu et al., 2020). In fast growing broilers, the body weight (BW) increased linearly from 0 to 6 wk, whereas FHN occurs at 4~6 wk of age (Applegate and Lilburn, 2002). Kerachian et al. found that prednisolone can cause thromboembolism and affect the occurrence of FHN through hyperlipidemia (Kerachian et al., 2009). Excessive glucocorticoids administration induces hyperlipidemia and fat deposition within the femoral head intramedullary tissue and fat embolism, and these factors may lead to FHN and its development (Yin et al., 2006; Kitajima et al., 2007; Chen et al., 2008; Song et al., 2017; Zhang et al., 2018). Glucocorticoids treatment affects the proliferation, differentiation, endoplasmic reticulum stress, and apoptosis of articular chondrocytes (Li et al., 2015; Zhang et al., 2017, 2019; Yu et al., 2020). At present, most of the models are used to study the FHN, however, this experiment is to study the spontaneous FHN in broilers to determine the etiology and the related pathways that may be involved.
Materials and methods
Experimental Materials
Chicken IL-1β, IL-6, and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were procured from ML Biotech (Shanghai, China). Chicken bone–specific alkaline phosphatase (BALP), bone glaprotein (BGP), type I collagen carboxy-terminal peptide (CTX), and tartrate resistant acid phosphatas-5b (TRACP-5b) ELISA kits were purchased from Nanjing Angle Gene Biotechnology Co., Ltd. (Nanjing, China). Total protein, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), calcium (Ca), phosphorus (P), triglyceride (TG), total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) assay kits were purchased from Mindray Biomedical Electronics Co., Ltd. (Shenzhen, China). TUNEL assay kit was obtained from Servicebio biological technology Co., Ltd. (Wuhan, China).
Sample Collection
The broilers (Ross 308 birds) of both sexes were from a farm in Jiangsu Province. The experiment was conducted in full accordance with the “Guidelines for Laboratory Animals” issued by the Ministry of Science and Technology and approved by the Animal Protection and Utilization Committee of Nanjing Agricultural University (#NJAU-Poult-2019102205, approved on October 22, 2019). Birds were fed a 3-phase commercial diet ad libitum: a starter ration (21.00% crude protein, 1.00% Ca, 0.52% total P, 0.45% methionine) from 0 to 21 d, a grower ration (19.00% crude protein, 0.95% Ca, 0.47% total P, 0.38% methionine) from 22 to 35 d and a ration (17.00% crude protein, 0.90% Ca, 0.42% total P, 0.34% methionine) from 36 to 42 d, the end of the experimental period. At the fourth, fifth, and sixth week of age, the birds with lameness were selected for serum, plasma, and tissue samples collection. After the BW was recorded, blood samples were collected from inferior pterygoid vein and subjected to centrifugation at 4,000 × g for 10 min. The serum and plasma were collected and stored at −20°C until analysis. Then, the broilers were euthanized, and the liver was collected, and the weight was recorded. Liver index was computed as the ratio of liver weight to BW. Bone samples, including femur and tibia, were collected and cleaned of all adherent tissue, and bone weight and length were measured. In addition, bone index was calculated as the ratio of bone weight to BW. Then, the liver and femoral head tissues in broilers with FHN were collected immediately, rinsed with saline solution, and stored in 4% paraformaldehyde (PFA) and −20°C for ELISA analysis and histological analysis. According to FHN score standard (Durairaj et al., 2009), the broilers were divided into 3 groups. Eight broilers each group were used for analysis.
Biochemical Analysis
The level of biochemical was measured by BS-300 automatic biochemical detector (Mindray Biomedical Electronics Co., Ltd.). The contents of TG, total cholesterol, HDL, and LDL in plasma were used to represent the level of blood lipid components.
ELISA Analysis
The levels of BALP, BGP, CTX, and TRACP-5b in serum were used to represent the bone metabolism markers of broilers. And, the levels of IL-1β, IL-6, and TNF-α in liver homogenate were measured to reflect the level of inflammation.
Histopathological Analysis
The femoral head was fixed with 4% PFA, washed overnight with flowing water, and decalcified in 10% EDTA for 2 wk (Yu et al., 2020). The decalcified cartilage tissue and PFA fixed liver tissue were dehydrated in ethanol, transparent in xylene, and embedded in paraffin. The cartilage and liver tissues were stained with hematoxylin and eosin for histological observation.
Bone Mineral Density Analysis
The bone mineral density (BMD) of tibia and femur were tested by a dual energy X-ray bone densitometer (MEDIKORS, Gyeonggi, Korea) with a fast scan, high-energy parameters of 80 kVp/1.0 mA, and low-energy parameters of 55 kVp/1.25 mA. The acquired image was analyzed by analyzer 1.0 image processing system.
Bone Strength Analysis
Bone strength was measured by 3-point bending test. The span of bone strength measurement was determined according to the average bone length of each group. The bone was placed on the working platform of universal material testing machine (LR10K Plus, Lloyd Instruments Ltd., England, UK). The parameters were set as follows: preload 5 N, preload speed 15 mm/min, and the test was stopped when the bone was fractured. The bone strength curve was obtained by NEXYGEN Plus software. The highest point of the curve was the bone strength value (N).
TUNEL Staining
The cartilage tissue was sliced according to the manual of TUNEL assay kit and observed with Upright fluorescence microscope (NIKON ECLIPSE C1; NIKON, Tokyo, Japan) after staining.
Statistical Analysis
SPSS Statistic 25.0 Software (SPSS Software Inc., Chicago, IL) was used for statistical analysis. Differences in the samples of normal broilers and FHN broilers were determined with one-way analysis (ANOVA, LSD). The histogram was drawn by GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA). All values were expressed as mean ± SEM. All measurements were repeated 3 times. Significant differences were defined at 2 levels as follows: P < 0.05 (significant) and P < 0.01 (extremely significant).
Results
Clinical Realization of FHN Broilers
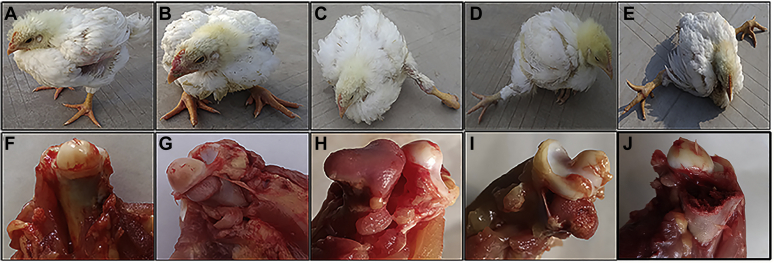
The clinical symptoms of FHN were collected and recorded in this experiment. The limbs of normal broilers were healthy (Figure 1A), whereas the broilers with FHN were in squatting state (Figure 1B) and later developed into unilateral hemiplegia or even bilateral paralysis (Figures 1C–E). Lame broilers were difficult to get food and water, resulting in artificial elimination or dehydration death. The collected femoral heads were scored according to the FHN scoring standard (Durairaj et al., 2009). As shown in Figures 1G, 1H, the cartilage of the femoral head was separated from the growth plate of the lower layer, but there was no visible lesion in the growth plate, which named FHS, if the growth plate was damaged or even the epiphysis is broken, it is named femoral head separation with growth plate laceration (Figures 1I, 1J).
Figure 1.
Clinical and anatomical manifestations of FHN broilers at 4 wk of age. (A) Normal broiler. (B) Crouching broiler. (C) Left hemiplegic broiler. (D) Right hemiplegic broiler. (E) Bilateral paralyzed broiler. (F) Normal femoral head. (G) Femoral head Incomplete separation of articular cartilage and growth plate. (H) Complete separation of cartilage and growth plate. (I) Damage to the growth plate. (J) Fracture of the epiphysis. Abbreviation: FHN, femoral head necrosis.
Biochemical Examination in Broilers With Spontaneous FHN
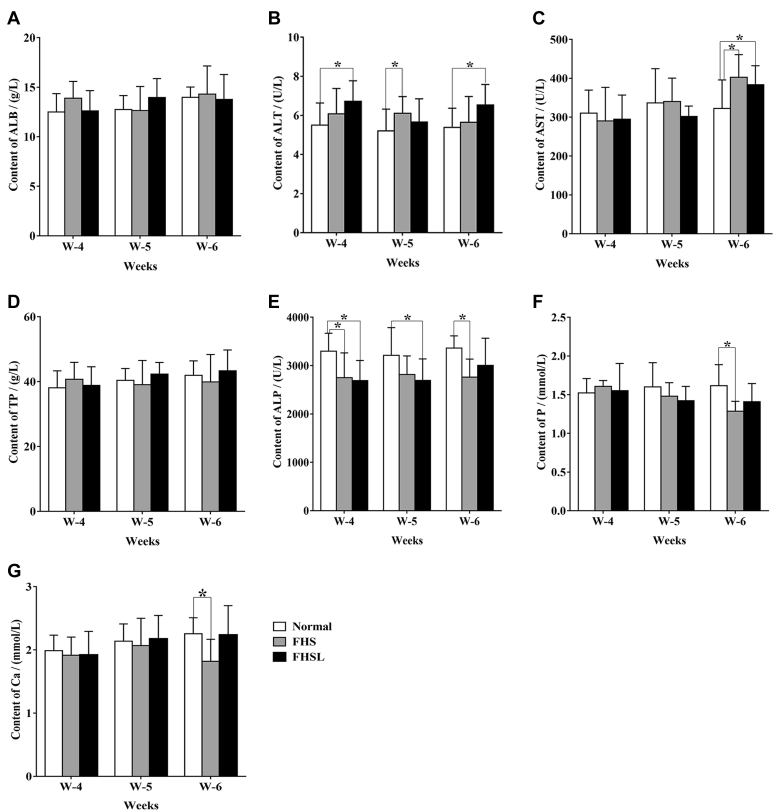
The levels of ALP, P, and Ca (Figures 2E–G) in broilers with FHN were significantly lower compared with the normal group (P < 0.05), whereas the contents of ALT (Figure 2B) and AST (Figure 2C) were significantly higher than that of normal broilers (P < 0.05), indicating that liver parenchyma was damaged.
Figure 2.
Changes of biochemical level between spontaneous FHN broilers and normal broilers. (A) The level of ALB. (B) The level of ALT. (C) The level of AST. (D) The level of TP. (E) The level of ALP. (F) The level of P. (G) The level of Ca. Date were presented as mean ± SEM (n = 3). ∗P < 0.05. Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FHN, femoral head necrosis; TP, total protein.
Changes of Liver Morphology and Lipid Metabolism in Broilers With FHN
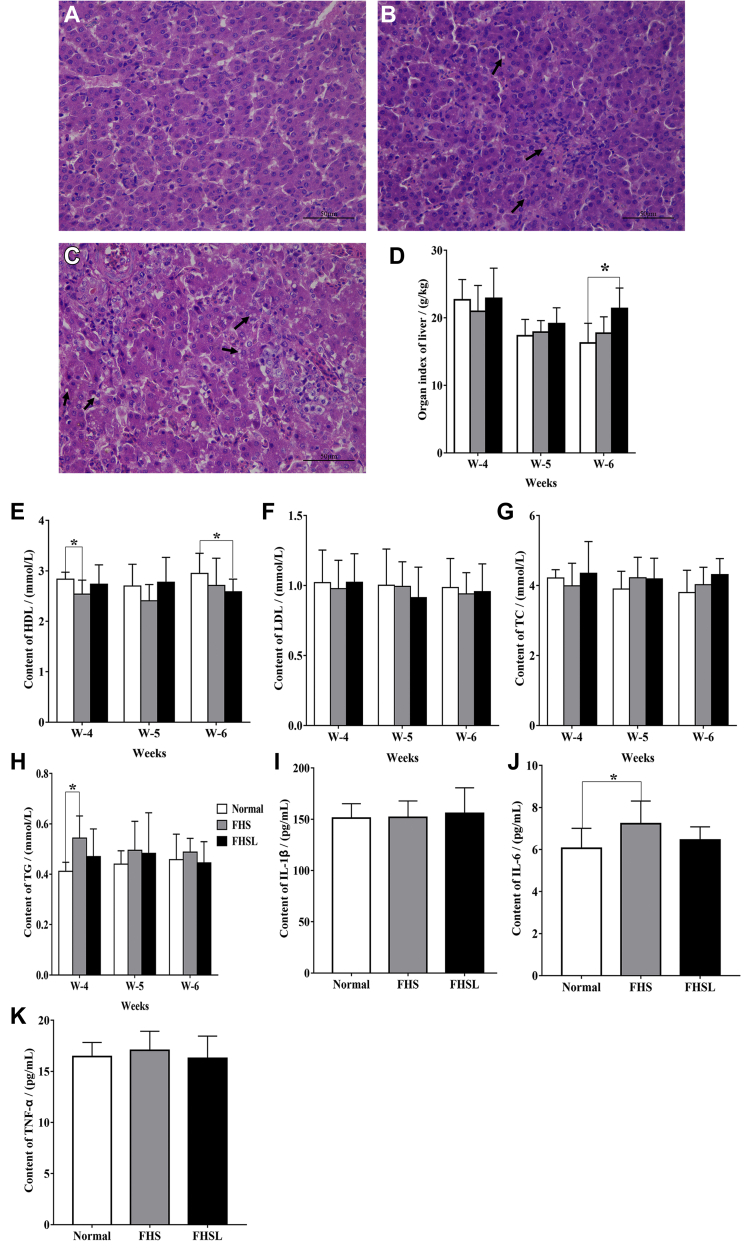
Liver tissue morphology and plasma lipid metabolism were detected to determine whether the liver and lipid metabolism were changed. Results as shown in Figures 3B, 3C, the hepatic cord structure was disordered and fat vacuoles appear in liver tissue of broilers with FHN. Compared with the normal group, the level of liver index (Figure 3D), HDL (Figure 3E), and TG (Figure 3H) in FHN birds were significantly changed (P < 0.05), whereas other lipid metabolism indexes did not change significantly (P > 0.05) and showed an upward trend, demonstrating that the occurrence of FHN may be related to liver lipid metabolism disorder.
Figure 3.
The changes of liver histomorphology, lipid metabolism factors, and proinflammatory factors. H&E staining of liver in broilers with normal (A), FHS (B), and FHSL (C) at 6 wk of age. (D) Organ index of liver. (E) The content of HDL. (F) The content of LDL. (G) The content of TC. (H) The content of TG. Levels of IL-1β (I), IL-6 (J), and TNF-α (K) in liver of 6-week-old broilers. Date were presented as mean ± SEM (n = 3). ∗P < 0.05. Scale bar: 50 μm. The area where the arrow points is fat vacuoles. Abbreviations: FHS, femoral head separation; FHSL, femoral head separation with growth plate laceration; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglyceride.
Changes of Bone Parameters Related to Tibia and Femur in Broilers With FHN
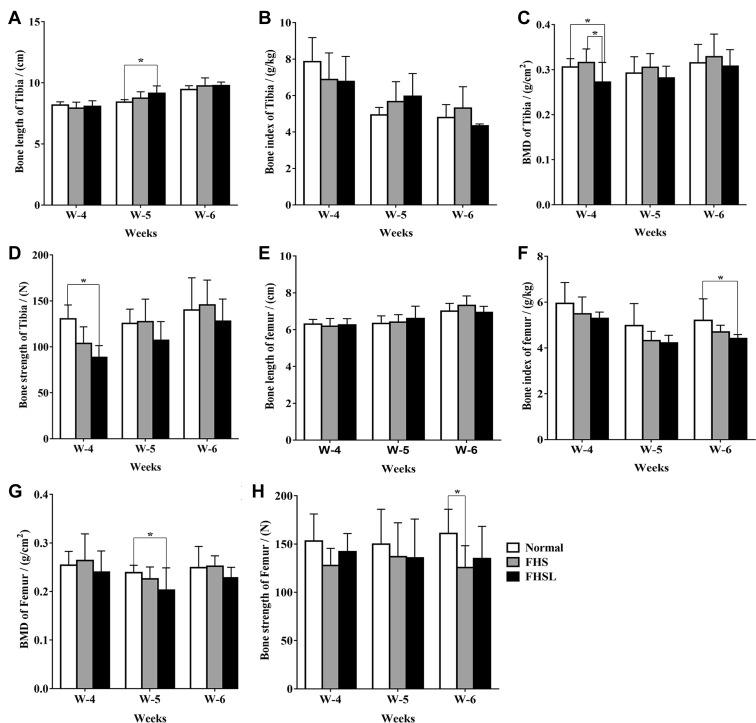
Then, the bone parameters of the long bones in broilers, namely tibia and femur, including bone length (Figures 4A, 4E), bone index (Figures 4B, 4F), BMD (Figures 4C, 4G), and bone strength (Figures 4D, 4H) were measured (Zhang et al., 2019). The results showed that the BMD and bone strength of tibia (Figures 4C, 4D) and femur (Figutres 4G, 4H) in FHN broilers were significantly changed compared with the normal group (P < 0.05), revealing that the bone density and strength of long bones may be affect in FHN-affected birds.
Figure 4.
Changes of bone parameters related to tibia and femur in broilers with FHN. (A) Bone length of tibia. (B) Bone index of tibia. (C) BMD of tibia. (D) Bone strength of tibia. (E) Bone length of femur. (F) Bone index of femur. (G) BMD of femur. (H) Bone strength of femur. Date were presented as mean ± SEM (n = 3). ∗P < 0.05. Abbreviations: BMD, bone mineral density; FHN, femoral head necrosis.
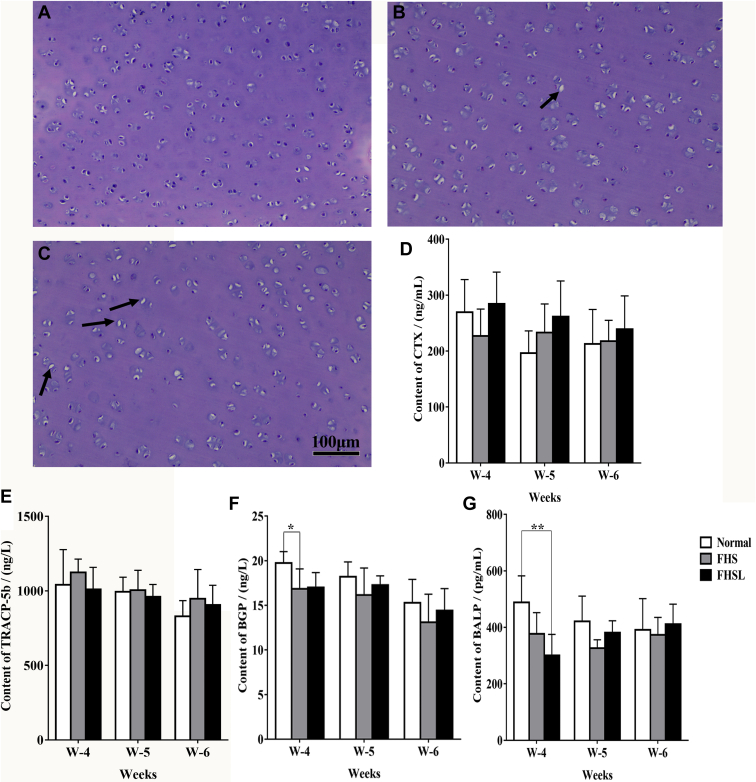
Changes of Cartilage Morphology and Bone Metabolism in Broilers With FHN
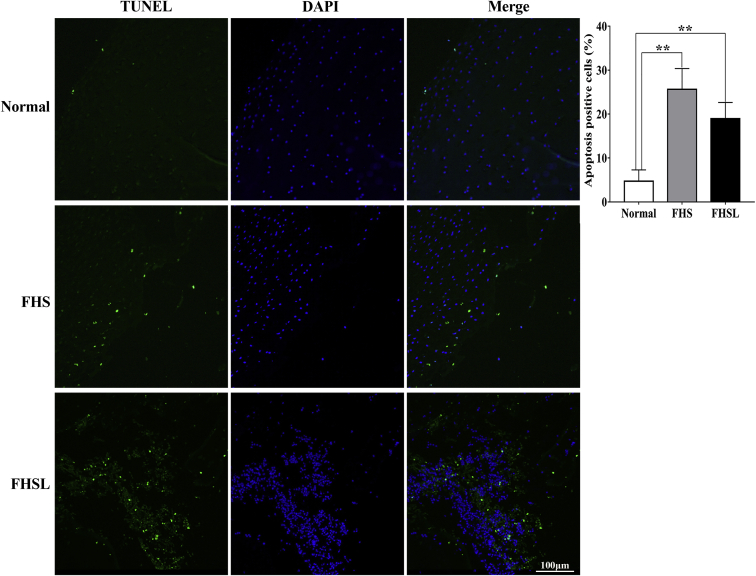
As the related bone parameters of long bone changed significantly (Figure 4), the effect of articular cartilage of femoral head on FHN was detected. The results showed that the articular chondrocytes of FHN broilers were irregularly distributed and vacuoles increased (Figures 5B, 5C), illustrating that cartilage homeostasis was damaged. At the same time, the results of TUNEL staining displayed that the apoptotic rate of articular chondrocytes in 6-week-old broilers with FHN (Figure 6) was significantly increased (P < 0.01), and the bone metabolism-related targets of BGP (Figure 5F) and BALP (Figure 5G) were significantly decreased. It was suggested that apoptosis of articular chondrocytes and bone metabolism disorder may lead to FHN in broilers.
Figure 5.
Detection of H&E staining and bone metabolism in the articular chondrocytes of femoral head. H&E staining of articular chondrocytes of broiler chickens with normal (A), FHS (B), and FHSL (C) at 6 wk of age. (D) Content of CTX. (E) Content of TRACP-5b. (F) Content of BGP. (G) Content of BALP. Date were presented as mean ± SEM (n = 3). ∗P < 0.05, ∗∗P < 0.01. Scale bar: 100 μm. The area where the arrow points was empty lacunae. Abbreviations: BALP, bone–specific alkaline phosphatase; BGP, bone glaprotein; CTX, type I collagen carboxy-terminal peptide; FHS, femoral head separation; FHSL, femoral head separation with growth plate laceration; TRACP-5b, tartrate resistant acid phosphatas-5b.
Figure 6.
TUNEL staining and positive rate of apoptotic chondrocytes in femoral head of 6-week-old broilers. Date were presented as mean ± SEM (n = 3). ∗∗P < 0.01. Scale bar: 100 μm. Abbreviations: FHS, femoral head separation; FHSL, femoral head separation with growth plate laceration.
Discussion
Many studies have shown that FHN is one of the main factors of lameness in broilers (Kincaid, 1993; McNamee et al., 1998; Dinev et al., 2019). However, most of the current research on FHN focuses on glucocorticoid modeling (Zhang et al., 2017, 2019; Yu et al., 2020), and few studies have been conducted on naturally occurring broilers to explore the causes and possible involvement of FHN-related mechanisms. In this experiment, the relevant quotas of 4~6 wk-old-broilers were detected to determine the body changes of broilers with spontaneous FHN. First, the overall level of body was judged by detecting the routine biochemical indexes of serum. The levels of ALT (Figure 2B) and AST (Figure 2C) in FHN group were significantly higher than those in normal group (P < 0.05), illustrating that liver parenchyma was damaged in FHN group. Therefore, the inflammatory factors in the liver were detected. The IL-1β is a typical inflammatory factor, which can promote inflammatory reaction. High expression of IL-1β can induce inflammatory mechanism, which can reflect the degree of cell injury and inflammation (Libby, 2017; Szekely and Arbel, 2018; He et al., 2019). The IL-6 and TNF-α are commonly used clinical markers of inflammation (Liu and Meng, 2018; Lu et al., 2019; Wang et al., 2020). Wang et al. have reported that IL-6 can be used as an early inflammatory factor to promote the secretion of other inflammatory factors and to amplify the inflammatory response (Wang et al., 2015). In this experiment, the level of IL-6 (Figure 3J) in broilers with FHN was significantly higher than that in the normal group (P < 0.05), which showed that the birds had an inflammatory reaction.
The relationship between lipid metabolism and leg diseases has been reported by many studies (Packialakshmi et al., 2015a, 2016; Wang et al., 2018). Guo et al. considered that leg diseases are associated with impaired growth, bone mass, bone structure and lipid metabolism (Guo et al., 2019). Packialakshmi et al. reported that the changes of body weight and blood lipid could be used as noninvasive biomarkers of FHN (Packialakshmi et al., 2015a, 2016). In this experiment, it showed that the content of HDL (Figure 3E) was significantly decreased (P < 0.05), the level of TG (Figure 3H) was significantly increased (P < 0.05), and the accumulation of lipid droplets in liver tissue became larger, which prompted that lipid metabolism in FHN broilers was in disorder, which was consistent with previous studies.
The serological markers of bone metabolism mainly consist of bone formation and resorption markers. The BGP and BALP are typical markers of bone formation. The BGP is involved in the regulation of bone resorption, but more importantly, it is involved in the mineralization process of the matrix and the differentiation of osteoblasts and related to bone turnover (Chopin et al., 2012; Niimi et al., 2014). The BALP participate in the process of bone formation and is stable in serum. It is considered as one of the most accurate markers of bone formation (Galliera et al., 2012; Sarvari et al., 2015). The concentration of BALP usually reflects the rate of bone formation in bone tissue (Sarvari et al., 2015). The BALP has been used in many studies to assess the extent of bone growth and bone metastasis (Schindler et al., 2008; Heidenreich et al., 2019). In this study, the level of BGP (Figure 5F) and BALP (Figure 5G) in broilers with FHN were significantly decreased than that of normal broilers, demonstrating that the bone resorption level of FHN broilers has changed. The TRACP-5b, mainly derived from osteoclasts, is a specific and highly sensitive bone resorption index (Halleen, 2003; Morisawa et al., 2017; Wu et al., 2017). The level of CTX reflects the bone resorption activity of osteoclasts and is an important bone metabolism index to reflect bone resorption (Jung et al., 2004; Zhao et al., 2011). Although TRACP-5b and CTX (Figures 5D, 5E) did not change significantly, they still showed an upward trend compared with normal broilers, and the level of P (Figure 2F) and Ca (Figure 2G) in broilers with FHN were significantly decreased than those in normal broilers (P < 0.05), which demonstrated that the bone formation level of FHN broilers was lower than bone resorption level, which led to the decrease of BMD. Relevant studies have reported that the imbalance of intrachondral homeostasis may play an important role in the occurrence and development of FHN in broilers (Zhang et al., 2017, 2019; Yu et al., 2020). In recent years, studies have shown that chondrocyte apoptosis is closely related to FHN and osteoarthritis (Matsuo et al., 2001; Calder et al., 2004; Hembree et al., 2007; Takács-Buia et al., 2008; Youm et al., 2010; Chen et al., 2014; Fan et al., 2014; Mutijima et al., 2014). The results (Figures 5, 6) obtained in this experiment are consistent with the previous results (Zhang et al., 2019; Yu et al., 2020); that is, the irregular distribution of articular chondrocytes in broilers with FHN and the appearance of vacuoles revealed that the steady state of cartilage was destroyed, and the level of apoptosis was significantly increased (P < 0.01).
In conclusion, inflammation, liver parenchyma damage, lipid metabolism disorder and bone metabolism disorder, and chondrocyte apoptosis occurred in spontaneous FHN broilers. The FHN may be related to the disorder of lipid metabolism and apoptosis of articular chondrocytes.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 32072936).
Disclosures
The authors declare no conflicts of interest.
References
- Applegate T.J., Lilburn M.S. Growth of the femur and tibia of a commercial broiler line. Poult. Sci. 2002;81:1289–1294. doi: 10.1093/ps/81.9.1289. [DOI] [PubMed] [Google Scholar]
- Bessei W. Welfare of broilers: a review. Worlds Poult. Sci. J. 2006;62:455–466. [Google Scholar]
- Calder J.D., Buttery L., Revell P.A., Pearse M., Polak J.M. Apoptosis--a significant cause of bone cell death in osteonecrosis of the femoral head. J. Bone Joint Surg. Br. 2004;86:1209–1213. doi: 10.1302/0301-620x.86b8.14834. [DOI] [PubMed] [Google Scholar]
- Chen S., Li J., Peng H., Zhou J., Fang H. Administration of erythropoietin exerts protective effects against glucocorticoid-induced osteonecrosis of the femoral head in rats. Int. J. Mol. Med. 2014;33:840–848. doi: 10.3892/ijmm.2014.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.C., Weng J., Chen X.Q., Du J.Z., Zhu M.P., Pan Y.Q., Liu M. Relationships among magnetic resonance imaging, histological findings, and IGF-I in steroid-induced osteonecrosis of the femoral head in rabbits. J. Zhejiang Univ. Sci. B. 2008;9:739–746. doi: 10.1631/jzus.B0820127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin F., Biver E., Funck-Brentano T., Bouvard B., Coiffier G., Garnero P., Thomas T. Prognostic interest of bone turnover markers in the management of postmenopausal osteoporosis. Joint Bone Spine. 2012;79:26–31. doi: 10.1016/j.jbspin.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Cook M.E. Skeletal deformities and their causes: introduction. Poult. Sci. 2000;79:982–984. doi: 10.1093/ps/79.7.982. [DOI] [PubMed] [Google Scholar]
- Cui Q., Wang G.J., Su C.C., Balian G. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin. Orthop. Relat. Res. 1997:8–19. [PubMed] [Google Scholar]
- Dinev I., Kanakov D., Kalkanov I., Nikolov S., Denev S. Comparative pathomorphologic studies on the incidence of fractures associated with leg skeletal pathology in commercial broiler chickens. Avian Dis. 2019;63:641–650. doi: 10.1637/aviandiseases-D-19-00108. [DOI] [PubMed] [Google Scholar]
- Durairaj V., Okimoto R., Rasaputra K., Clark F.D., Rath N.C. Histopathology and serum clinical chemistry evaluation of broilers with femoral head separation disorder. Avian Dis. 2009;53:21–25. doi: 10.1637/8367-051908-Reg.1. [DOI] [PubMed] [Google Scholar]
- Fan L., Li J., Yu Z., Dang X., Wang K. Hypoxia-inducible factor prolyl hydroxylase inhibitor prevents steroid-associated osteonecrosis of the femoral head in rabbits by promoting angiogenesis and inhibiting apoptosis. PLoS One. 2014;9:e107774. doi: 10.1371/journal.pone.0107774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliera E., Luzzati A., Perrucchini G., Gagliano F., Colloredo Mels L., Banfi G., Corsi Romanelli M.M., Drago L. Bone formation and resorption markers as diagnostic tools for bone metastases evaluation. Int. J. Biol. Markers. 2012;27:e395–e399. doi: 10.5301/JBM.2012.9579. [DOI] [PubMed] [Google Scholar]
- Guo Y., Tang H., Wang X., Li W., Wang Y., Yan F., Kang X., Li Z., Han R. Clinical assessment of growth performance, bone morphometry, bone quality, and serum indicators in broilers affected by valgus-varus deformity. Poult. Sci. 2019;98:4433–4440. doi: 10.3382/ps/pez269. [DOI] [PubMed] [Google Scholar]
- Halleen J.M. Tartrate-resistant acid phosphatase 5B is a specific and sensitive marker of bone resorption. Anticancer Res. 2003;23:1027–1029. [PubMed] [Google Scholar]
- He Y.K., Cen X.T., Liu S.S., Lu H.D., He C.N. Protective effects of ten oligostilbenes from Paeonia suffruticosa seeds on interleukin-1β-induced rabbit osteoarthritis chondrocytes. BMC Chem. 2019;13:72. doi: 10.1186/s13065-019-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich A., Gillessen S., Heinrich D., Keizman D., O'Sullivan J.M., Carles J., Wirth M., Miller K., Reeves J., Seger M., Nilsson S., Saad F. Radium-223 in asymptomatic patients with castration-resistant prostate cancer and bone metastases treated in an international early access program. BMC Cancer. 2019;19:12. doi: 10.1186/s12885-018-5203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree W.C., Ward B.D., Furman B.D., Zura R.D., Nichols L.A., Guilak F., Olson S.A. Viability and apoptosis of human chondrocytes in osteochondral fragments following joint trauma. J. Bone Joint Surg. Br. 2007;89:1388–1395. doi: 10.1302/0301-620X.89B10.18907. [DOI] [PubMed] [Google Scholar]
- Julian R.J. Production and growth related disorders and other metabolic diseases of poultry - a review. Vet. J. 2005;169:350–369. doi: 10.1016/j.tvjl.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Jung K., Lein M., Stephan C., Von Hösslin K., Semjonow A., Sinha P., Loening S.A., Schnorr D. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int. J. Cancer. 2004;111:783–791. doi: 10.1002/ijc.20314. [DOI] [PubMed] [Google Scholar]
- Kerachian M.A., Séguin C., Harvey E.J. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J. Steroid Biochem. Mol. Biol. 2009;114:121–128. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid S.A. Bone biology and skeletal disorders in poultry, Poultry Science Symposium 23. Poult. Sci. 1993;72 [Google Scholar]
- Kitajima M., Shigematsu M., Ogawa K., Sugihara H., Hotokebuchi T. Effects of glucocorticoid on adipocyte size in human bone marrow. Med. Mol. Morphol. 2007;40:150–156. doi: 10.1007/s00795-007-0367-6. [DOI] [PubMed] [Google Scholar]
- Li P.F., Zhou Z.L., Shi C.Y., Hou J.F. Downregulation of basic fibroblast growth factor is associated with femoral head necrosis in broilers. Poult. Sci. 2015;94:1052–1059. doi: 10.3382/ps/pev071. [DOI] [PubMed] [Google Scholar]
- Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J. Am. Coll. Cardiol. 2017;70:2278–2289. doi: 10.1016/j.jacc.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Meng J. Tanshinone IIA ameliorates lipopolysaccharide-induced inflammatory response in bronchial epithelium cell line BEAS-2B by down-regulating miR-27a. Biomed. Pharmacother. 2018;104:158–164. doi: 10.1016/j.biopha.2018.05.021. [DOI] [PubMed] [Google Scholar]
- Lu J., Liu K., Qi M., Geng H., Hao J., Wang R., Zhao X., Liu Y., Liu J. Effects of Cr(VI) exposure on electrocardiogram, myocardial enzyme parameters, inflammatory factors, oxidative kinase, and ATPase of the heart in Chinese rural dogs. Environ. Sci. Pollut. Res. Int. 2019;26:30444–30451. doi: 10.1007/s11356-019-06253-0. [DOI] [PubMed] [Google Scholar]
- Matsuo M., Nishida K., Yoshida A., Murakami T., Inoue H. Expression of caspase-3 and -9 relevant to cartilage destruction and chondrocyte apoptosis in human osteoarthritic cartilage. Acta Med. Okayama. 2001;55:333–340. doi: 10.18926/AMO/32000. [DOI] [PubMed] [Google Scholar]
- McNamee P.T., McCullagh J.J., Thorp B.H., Ball H.J., Graham D., McCullough S.J., McConaghy D., Smyth J.A. Study of leg weakness in two commercial broiler flocks. Vet. Rec. 1998;143:131–135. doi: 10.1136/vr.143.5.131. [DOI] [PubMed] [Google Scholar]
- Morisawa T., Nakagomi A., Kohashi K., Kusama Y., Shimizu W. Serum tartrate-resistant acid phosphatase-5b levels are associated with the severity and extent of coronary atherosclerosis in patients with coronary artery disease. J. Atheroscler. Thromb. 2017;24:1058–1068. doi: 10.5551/jat.39339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutijima E., De Maertelaer V., Deprez M., Malaise M., Hauzeur J.P. The apoptosis of osteoblasts and osteocytes in femoral head osteonecrosis: its specificity and its distribution. Clin. Rheumatol. 2014;33:1791–1795. doi: 10.1007/s10067-014-2607-1. [DOI] [PubMed] [Google Scholar]
- Niimi R., Kono T., Nishihara A., Hasegawa M., Matsumine A., Kono T., Sudo A. Determinants associated with bone mineral density increase in response to daily teriparatide treatment in patients with osteoporosis. Bone. 2014;66:26–30. doi: 10.1016/j.bone.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Packialakshmi B., Liyanage R., Lay J., Jr., Okimoto R., Rath N. Prednisolone-induced predisposition to femoral head separation and the accompanying plasma protein changes in chickens. Biomark. Insights. 2015;10:1–8. doi: 10.4137/BMI.S20268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packialakshmi B., Liyanage R., Lay J.O., Jr., Okimoto R., Rath N.C. Proteomic changes in the plasma of broiler chickens with femoral head necrosis. Biomark. Insights. 2016;11:55–62. doi: 10.4137/BMI.S38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packialakshmi B., Rath N.C., Huff W.E., Huff G.R. Poultry femoral head separation and necrosis: a review. Avian Dis. 2015;59:349–354. doi: 10.1637/11082-040715-Review.1. [DOI] [PubMed] [Google Scholar]
- Paxton H., Tickle P.G., Rankin J.W., Codd J.R., Hutchinson J.R. Anatomical and biomechanical traits of broiler chickens across ontogeny. Part II. Body segment inertial properties and muscle architecture of the pelvic limb. PeerJ. 2014;2:e473. doi: 10.7717/peerj.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Rodriguez E., Benavides-Reyes C., Torres C., Dominguez-Gasca N., Garcia-Ruiz A.I., Gonzalez-Lopez S., Rodriguez-Navarro A.B. Changes with age (from 0 to 37 D) in tibiae bone mineralization, chemical composition and structural organization in broiler chickens. Poult. Sci. 2019;98:5215–5225. doi: 10.3382/ps/pez363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvari B.K., Sankara Mahadev D., Rupa S., Mastan S.A. Detection of bone metastases in breast cancer (BC) patients by serum tartrate-resistant acid phosphatase 5b (TRACP 5b), a bone resorption marker and serum alkaline phosphatase (ALP), a bone formation marker, in lieu of whole body skeletal Scintigraphy with Technetium99m MDP. Indian J. Clin. Biochem. 2015;30:66–71. doi: 10.1007/s12291-013-0399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler F., Lajolo P.P., Pinczowski H., Fonseca F.L., Barbieri A., Massonetto L.H., Katto F.T., Del Giglio A. Bone and total alkaline phosphatase for screening skeletal metastasis in patients with solid tumours. Eur. J. Cancer Care (Engl.) 2008;17:152–156. doi: 10.1111/j.1365-2354.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- Seamon J., Keller T., Saleh J., Cui Q. The pathogenesis of nontraumatic osteonecrosis. Arthritis. 2012;2012:601763. doi: 10.1155/2012/601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Du Z., Chen B., Ren M., Yang Q., Sui Y., Wang Q., Wang A., Zhao H., Qin Y., Zhang G. Association of SREBP2 gene polymorphisms with the risk of osteonecrosis of the femoral head relates to gene expression and lipid metabolism disorders. Mol. Med. Rep. 2017;16:7145–7153. doi: 10.3892/mmr.2017.7473. [DOI] [PubMed] [Google Scholar]
- Szekely Y., Arbel Y. A review of interleukin-1 in heart disease: where do we stand today? Cardiol. Ther. 2018;7:25–44. doi: 10.1007/s40119-018-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács-Buia L., Iordachel C., Efimov N., Caloianu M., Montreuil J., Bratosin D. Pathogenesis of osteoarthritis: chondrocyte replicative senescence or apoptosis? Cytometry B Clin. Cytom. 2008;74:356–362. doi: 10.1002/cyto.b.20428. [DOI] [PubMed] [Google Scholar]
- Wang H.T., Fang Y.Q., Bao X.C., Yuan H.R., Ma J., Wang F.F., Zhang S., Li K.C. Expression changes of TNF-α, IL-1β and IL-6 in the rat lung of decompression sickness induced by fast buoyancy ascent escape. Undersea Hyperb. Med. 2015;42:23–31. [PubMed] [Google Scholar]
- Wang Y., Hao J., Zhang S., Li L., Wang R., Zhu Y., Liu Y., Liu J. Inflammatory injury and mitophagy induced by Cr(VI) in chicken liver. Environ. Sci. Pollut. Res. Int. 2020;27:22980–22988. doi: 10.1007/s11356-020-08544-3. [DOI] [PubMed] [Google Scholar]
- Wang A., Ren M., Wang J. The pathogenesis of steroid-induced osteonecrosis of the femoral head: a systematic review of the literature. Gene. 2018;671:103–109. doi: 10.1016/j.gene.2018.05.091. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Prisby R.D. Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis. Front. Endocrinol. (Lausanne) 2012;3:183. doi: 10.3389/fendo.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B., Waddington D., Murray D.H., Farquharson C. Bone strength during growth: influence of growth rate on cortical porosity and mineralization. Calcif. Tissue Int. 2004;74:236–245. doi: 10.1007/s00223-002-2124-0. [DOI] [PubMed] [Google Scholar]
- Wu Z.Q., Chen X.T., Xu Y.Y., Tian M.J., Chen H.Y., Zhou G.P., Xu H.G. High uric acid (UA) downregulates bone alkaline phosphatase (BALP) expression through inhibition of its promoter activity. Oncotarget. 2017;8:85670–85679. doi: 10.18632/oncotarget.21110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Li Y.B., Wang Y.S. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: mechanism of steroid-induced osteonecrosis. Chin. Med. J. (Engl.) 2006;119:581–588. [PubMed] [Google Scholar]
- Youm Y.S., Lee S.Y., Lee S.H. Apoptosis in the osteonecrosis of the femoral head. Clin. Orthop. Surg. 2010;2:250–255. doi: 10.4055/cios.2010.2.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Wang S., Zhou Z. Cartilage homeostasis affects femoral head necrosis induced by methylprednisolone in broilers. Int. J. Mol. Sci. 2020;21:4841. doi: 10.3390/ijms21144841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Jin V.L., Jin L. Role of coagulopathy in glucocorticoid-induced osteonecrosis of the femoral head. J. Int. Med. Res. 2018;46:2141–2148. doi: 10.1177/0300060517700299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Li S., Pang K., Zhou Z. Endoplasmic reticulum stress affected chondrocyte apoptosis in femoral head necrosis induced by glucocorticoid in broilers. Poult. Sci. 2019;98:1111–1120. doi: 10.3382/ps/pey474. [DOI] [PubMed] [Google Scholar]
- Zhang M., Shi C.Y., Zhou Z.L., Hou J.F. Bone characteristics, histopathology, and chondrocyte apoptosis in femoral head necrosis induced by glucocorticoid in broilers. Poult. Sci. 2017;96:1609–1614. doi: 10.3382/ps/pew466. [DOI] [PubMed] [Google Scholar]
- Zhao J., Xia W., Nie M., Zheng X., Wang Q., Wang X., Wang W., Ning Z., Huang W., Jiang Y., Li M., Wang O., Xing X., Sun Y., Luo L., He S., Yu W., Lin Q., Pei Y., Zhang F., Han Y., Tong Y., Che Y., Shen R., Hu Y., Zhou X., Xu L. The levels of bone turnover markers in Chinese postmenopausal women: Peking vertebral fracture study. Menopause. 2011;18:1237–1243. doi: 10.1097/gme.0b013e31821d7ff7. [DOI] [PubMed] [Google Scholar]