Abstract
A reliable and reproducible in vivo experimental model is an essential tool to study the pathogenesis of broiler necrotic enteritis and to evaluate control methods. Most current in vivo models use Eimeria as predisposing factor. Nevertheless, most models only result in a limited number of animals with intestinal necrosis. This research describes the necrotic enteritis incidence and severity using 2 previously described experimental models varying in the time point and frequency of Eimeria administration: single late and early repeated Eimeria administration models. In an in vivo model in which Clostridium perfringens is administered at 3 consecutive days between day 18 and 20 of age, birds belonging to the single late Eimeria administration regimen received a single administration of a tenfold dose of a live attenuated Eimeria vaccine on the second day of C. perfringens challenge. Broilers belonging to the early repeated administration regimen were inoculated with the same Eimeria vaccine 4 and 2 d before the start of the C. perfringens challenge. Early repeated coccidial administration resulted in a significant increase in average necrotic lesion score (value 3.26) as compared with a single late Eimeria administration regimen (value 1.2). In addition, the number of necrotic enteritis–positive animals was significantly higher in the group that received the early repeated coccidial administration. Single Eimeria administration during C. perfringens challenge resulted in a skewed distribution of lesion scoring with hardly any birds in the high score categories. A more centered distribution was obtained with the early repeated Eimeria administration regimen, having observations in every lesion score category. These findings allow better standardization of a subclinical necrotic enteritis model and reduction of the required numbers of experimental animals.
Key words: necrotic enteritis, coccidiosis, experimental model
Introduction
Necrotic enteritis (NE) is an enteric disease caused by Clostridium perfringens toxin type G strains that are characterized by their ability to produce the NetB toxin. Restrictions in the use of antimicrobials due to legislation and increased consumer awareness can impact NE prevalence in the future, increasing the demand for research on the pathogenesis of the disease, and on alternatives for antimicrobials that prevent and control NE.
To evaluate and develop novel control strategies (vaccines, drugs, feed additives) and to study the disease pathogenesis, reliable and reproducible in vivo challenge models are an essential tool. However, research on NE is hindered by the multifactorial nature of the disease, which has led to a variety of different NE challenge models described in the scientific literature. Remarkably, a large variation in the percentage of animals developing clinical signs and lesions has been reported throughout literature in the different disease models (Lee et al., 2011; Shojadoost et al., 2012; Alnassan et al., 2014; Van Waeyenberghe et al., 2016; Bortoluzzi et al., 2019). The lack of uniformity between these performed trials has made comparison of the results difficult. Ideally, the NE challenge model should be reproducible and resemble the situation described in the field as closely as possible because implementation of certain parameters can greatly impact the outcome of results (Park et al., 2008; Van Damme et al., 2020). Preferably all challenged animals should develop the characteristic necrotic lesions without manifestation of sever clinical disease or mortality, reducing the experimental sample sizes while maintaining statistical power. Therefore, careful selection of experimental models is needed.
An important variable that differs between the different infection models is the use of predisposing factors. The list of confirmed predisposing factors is long, ranging from coinfection with Eimeria or viruses to nutritional (i.e., nonstarch polysaccharides, animal protein, poorly digested protein, antinutritional factors,…) and management factors (i.e., stress, feeding regimen, rapid growth, stock density,…). Experimental model design is based on the implementation of one or multiple of these predisposing factors, of which Eimeria coinfection, high protein diets (fishmeal), high-density housing, and mild forms of immunosuppression are most often described (Shojadoost et al., 2012). Coccidiosis is considered the most important risk factor associated with NE disease development based on the strong correlation between the prevalence of both in the field (Al-Sheikhly and Al-Saieg, 1980). Therefore, implementation of a predisposing coccidiosis challenge in the NE challenge model seems essential to link experimental studies to the field situation.
Throughout literature, a large variability in implementation of this predisposing factor in NE models has been described, differing in Eimeria species and time point, frequency, and route of administration (Gholamiandehkordi et al., 2007; Park et al., 2008; Cooper, 2016; Van Waeyenberghe et al., 2016). In the present study, a literature search was performed in which NE in vivo models were selected varying in the Eimeria administration regimen: single late Eimeria administration (on second day of C. perfringens challenge) and early repeated Eimeria administration (4 and 2 d before C. perfringens challenge). Literature data on results of trials implementing both models cannot be compared because they were not carried out simultaneously under the same conditions. Therefore, both models were compared in an in vivo trial in which all other environmental factors apart from the Eimeria administration were kept equal between both groups, so that the effect of timing and frequency of the Eimeria administration in experimental NE models could be evaluated.
Materials and methods
Model Descriptions Based on Previously Published NE Trials
A literature search was performed in which NE challenge models varying in frequency and timing of Eimeria administration were selected. Two types of NE challenge models, in which C. perfringens oral administration was performed on 3 consecutive days between day 18 and 20, were compared: single late Eimeria administration (on second day of C. perfringens challenge) and early repeated Eimeria administration (4 and 2 d before C. perfringens challenge). Among these articles published between 2010 and 2020, a further selection was made based on comparable diet composition, C. perfringens challenge strain, stocking density, inoculation schedule, type of scoring system and the availability of data on the mean lesion score and percentage of NE-positive animals. Based on these restrictions, four articles were withheld in which 5 trials were described in total. The single late Eimeria administration (during C. perfringens challenge) was described by Mot et al. (2013) (trial A and B), Van Waeyenberghe et al. (2016) (trial C), and Da Costa et al. (2013) (trial D). The early repeated Eimeria administration (before C. perfringens challenge) was described by Dierick et al. (2019) (trial E) and Van Damme et al. (2020) (trial F). A summary of experimental setup of the models and their results is given in Table 1.
Table 1.
Summary of the experimental setup parameters and results of the NE trials selected from literature.
| Parameters & results | Single laste Eimeria administration |
Early repeated Eimeria administration |
|||||
|---|---|---|---|---|---|---|---|
| Trial A | Trial B | Trial C | Trial D | Trial E | Trial F | ||
| Setup parameters | Reference | Mot et al. (2013) | Mot et al. (2013) | Van Waeyenberghe et al. (2016) | Da Costa et al. (2013) | Dierick et al. (2019) | Van Damme et al. (2020) |
| Housing density (birds/m2) | 15.3 | 19.3 | 20 | 16.6 | 18.7 | 18.7 | |
| Feed | Wheat/rye (43%/7.5%) | Wheat/rye (43%/7.5%) | Wheat/corn (48%/10%) | Wheat/rye (43%/7.5%) | Wheat/rye (43%/7.5%) | Wheat/rye (43%/7.5%) | |
| Protein source | Soybean meal | Soybean meal | Soybean meal | Soybean meal | Soybean meal | Soybean meal | |
| Day to switch to fishmeal | 17 | 17 | 17 | 17 | 17 | 17 | |
| Concentration fishmeal (%) | 30 | 30 | 40 | 30 | 30 | 30 | |
| Immunosuppression | Nobilis Gumboro D78 (in drinking water—day 16) | Nobilis Gumboro D78 (in drinking water—day 16) | / | Nobilis Gumboro D78 (in drinking water—day 16) | Nobilis Gumboro D78 (oral gavage—day 4 and 9) | Nobilis Gumboro D78 (oral gavage—day 4 and 9) | |
| Type of Eimeria | 10x Paracox-5 (oral gavage) | 10x Paracox-5 (oral gavage) | 10x Paracox-8 (oral gavage) | 10x Paracox-5 (oral gavage) | 10x Hipracox or Paracox-5 (oral gavage) | 10x Hipracox or Paracox-8 (oral gavage) | |
| Timing Eimeria challenge | Second day of CP challenge | Second day of CP challenge | Second day of CP challenge | Second day of CP challenge | Two and 4 days before CP challenge | Two and 4 days before CP challenge | |
| CP strain | CP56 | CP56 | CP56 | CP56 | CP56 | CP56 | |
| Timing CP challenge | Days 17–20 | Days 17–20 | Days 18–21 | Days 17–20 | Days 17–19 | Days 18–20 | |
| Lesion scoring system | Keyburn et al. (2006) | Keyburn et al. (2006) | Keyburn et al. (2006) | Keyburn et al. (2006) | Keyburn et al. (2006) | Keyburn et al. (2006) | |
| Timing necropsy | 4 to 6 d after first CP challenge | 4 to 6 d after first CP challenge | 1 to 5 d after first CP challenge | 1 to 3 d after first CP challenge | 3 d after first CP challenge | 3 d after first CP challenge | |
| Results | NE + animals | 48% | 52% | 32% | 48% | 62% | 85% |
| Mean lesion score (total) | 1.03 | 1.57 | 0.68 | 1.04 | 2.10 | 3.33 | |
| Mean lesion score (NE+) | 2.14 | 3 | 2.17 | 2.17 | 3.48 | 3.91 | |
Eimeria challenge was induced by oral gavage with a tenfold dose of a live attenuated vaccine: Hipracox (containing E. tenella, E. acervulina, E. maxima, E. praecox, and E. mitis), Paracox-5 (containing E. acervulina, E. maxima, E. mitis, and E. tenella), or Paracox-8 (containing E. acervulina, E.brunetti, E. maxima, E. mitis, E. necatrix, E. praecox, and E. tenella).
Abbreviations: CP, Clostridium perfringens; NE + animals, amount of animals with an NE lesion score equal to or higher than 2.
Necrotic Enteritis In Vivo Trial
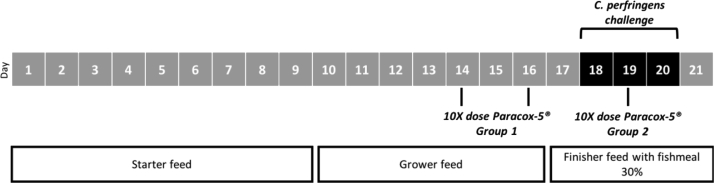
Seventy-two mixed sex Ross 308 broilers were housed in the same room and divided into 4 equal groups (duplicate per condition). Each group was housed with a density of 18 birds per square meter. Water and feed were supplied ad libitum. A schematic overview of the model is depicted in Figure 1. The feed was a wheat/rye-based (43%/7.5%) diet containing soybean meal as a protein source. Soybean meal was replaced by fishmeal (30%) from day 17 on, as a source of dietary animal protein, which is a known predisposing factor for induction of NE. A tenfold dose of Paracox-5 (MSD Animal Health) was orally administered at day 14 and 16 for group 1 or day 19 for group 2. Subclinical NE was induced by oral administration of 1 mL overnight culture (in brain heart infusion broth (Bio-Rad, Temse, Belgium)) of the pathogenic C. perfringens type G strain CP56 (netB+, alpha toxin+, pfoA+) at day 18, 19, and 20 (Timbermont et al., 2014). In contrast to most published studies, no predisposing immunosuppression was applied as this would make the model less suitable for vaccination studies. Furthermore, previous results have shown that predisposing challenge with the Nobilis Gumboro D78 vaccine had no effect on the degree and severity of birds developing NE (own unpublished results). At day 21, birds were euthanized. At necropsy, the lesions in the duodenum, jejunum, and ileum were scored using a well-established scoring system (Keyburn et al., 2006). In short, score 0: no gross lesions; score 1: thin or friable walls, score 2: focal necrosis and ulceration (1-5 foci); score 3: focal necrosis and ulceration (6-15 foci); score 4: focal necrosis and ulceration (16 or more foci); score 5: patches of necrosis 2 to 3 cm long and score 6: diffuse necrosis. Owing to its subjective nature, score 1 was not assigned. The experiment was carried out in accordance with the recommendations and following approval from the Ethical Committee of the faculty of Veterinary Medicine at Ghent University (EC2018_17). No mortality was observed.
Figure 1.
Timeline of the necrotic enteritis in vivo experiment. The feeding regimen was soybean-based and replaced with fishmeal from day 17 onward for all models. Predisposing factors are indicated below. Oral administration of a tenfold dose of Paracox-5 at d 14 and 16 for group 1 (early repeated Eimeria administration, 4 and 2 d before Clostridium perfringens challenge) and day 19 for group 2 (single late Eimeria administration, during C. perfringens challenge). All broilers were challenged with C. perfringens CP56 (black bar), resulting in the induction of subclinical NE. Here for 1 mL overnight culture of the pathogenic C. perfringens strain CP56 was orally administered. Afterward, birds were euthanized.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 8 software. Normality of the data set was checked using the Kolmogorov-Smirnov normality test. The difference in mean lesion score of both groups was assessed using the nonparametric Mann Whitney test with a significance level of 95%.
Results and discussion
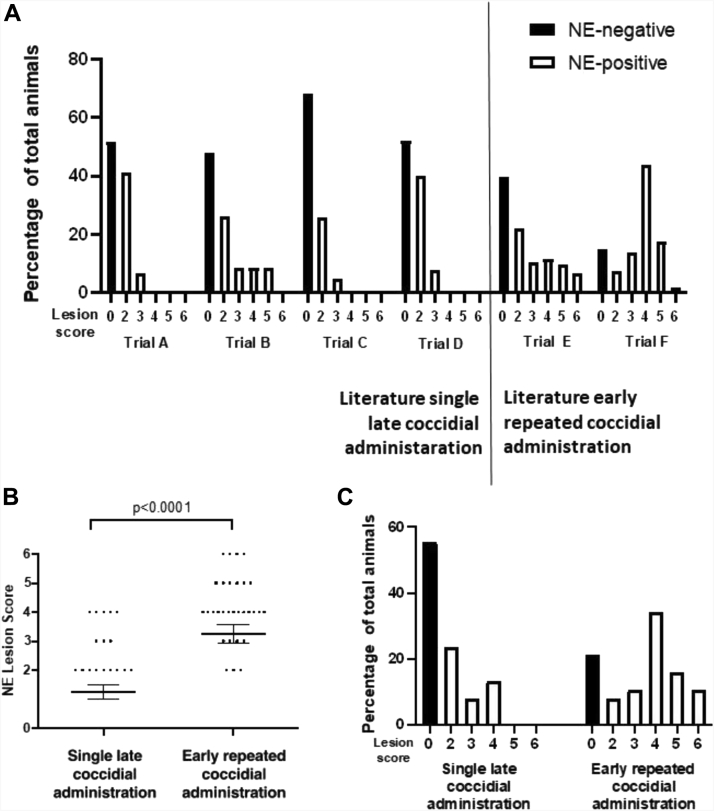
Timing of coccidiosis administration is crucial in NE lesion development. In search of the optimal NE challenge model, a literature search was performed in which NE models with variable Eimeria timing and frequency were selected. We focussed on 2 types of NE challenge models that have been described previously: single late Eimeria administration (during C. perfringens challenge) and early repeated Eimeria administration (before C. perfringens challenge). Their NE-inducing potential in previously described NE trials is summarized in Figure 2A and the results section of Table 1.
Figure 2.
Lesion scoring and distribution after single and repeated coccidial challenge in in vivo NE trials using 2 different coccidial administration models. (A) NE trials described in literature using the single late coccidial administration model (trials A and B by Mot et al. (2013), trial C by Van Waeyenberghe et al. (2016), and trial D by Da Costa et al. (2013)) and the early repeated coccidial administration model (trial E by Dierick et al. (2019) and trial F by Van Damme et al. (2020)). (B) NE lesion score obtained in present in vivo study. Birds were pretreated by administration of a tenfold dose of Paracox-5 on day 19 (single late coccidial challenge) or at day 14 and 16 (early repeated coccidial challenge). Feed and water was provided at libitum. From day 17 onward the feed was supplemented with 30% fishmeal. On day 18, 19, and 20, the birds were challenged by oral administration of 1 mL overnight culture of the pathogenic Clostridium perfringens strain CP56. Birds were euthanized and lesions were scored on day 21. In short, score 0: no gross lesions; score 2: focal necrosis and ulceration (1-5 foci); score 3: focal necrosis and ulceration (6-15 foci); score 4: focal necrosis and ulceration (16 or more foci); score 5: patches of necrosis 2 to 3 cm long and score 6: diffuse necrosis. The distribution of the lesion scores is shown in panel C. Black and open bars indicate the necrotic enteritis–negative and positive birds, respectively.
In accordance with literature data, single late Eimeria administration results in a rather limited percentage of animals developing gross necrotic lesions in the small intestine, ranging from 32 to 53%. The average NE lesion score calculated for all animals ranged from 0.68 (trial C) to 1.57 (trial B), whereas this value ranged from 2.14 (trial A) to 3 (trial B) when only taking the NE-positive animals into account. A double administration regimen in which a tenfold dose of a live attenuated Eimeria vaccine was administered twice before C. perfringens challenge results in a higher number of NE-positive animals, ranging from 62% to 85%. The average NE lesion score is also higher, ranging from 2.10 (trial E) to 3.33 (trial F) for all animals in the trial and from 3.48 (trial E) to 3.91 (trial F) for NE-positive animals.
Although both models have been used previously, a side-by-side comparison in NE-inducing potential has never been made. To unambiguously confirm that the observed difference in NE lesion development is due to the timing of Eimeria administration, an in vivo trial was performed with timing of Eimeria administration as sole variable parameter.
In the present in vivo study, single late Eimeria administration during C. perfringens challenge resulted in 45% NE-positive animals and an average lesion score of 1.2 for all animals (average lesion score of 2.77 for only the NE-positive animals), which is in agreement with previously published trials (Figure 2B). The distribution of the observed lesion scores is depicted in Figure 2C. A clear skewed distribution toward low lesions scores can be observed for the single late Eimeria administration regimen, comparable with previous NE trials. Mostly focal necrosis and ulcerations with only one to 5 foci throughout the small intestine were observed (score 2). Only sporadically more severe necrotic lesions (scores higher than 2) were observed. Compared with the single late Eimeria administration protocol, the early repeated coccidial administration regimen resulted in significantly more NE-positive animals (79%; P = 0.0059), which is comparable with previously described NE trials implementing this model (Figure 2C). The average lesion score of all animals in the trial with repeated coccidial regimen was 3.26 (average lesion score of 4.13 when only NE-positive animals were taken into account) which was significantly more severe than obtained after single coccidial administration (P < 0.0001) (Figure 2B). The distribution of lesions scores obtained after repeated administration was not skewed, having observations in all lesion score categories (Figure 2C). Throughout the trial, no mortality was observed for both models.
In the present study, we show that the timing and frequency of the Eimeria administration is crucial in NE disease development. A hypothesis explaining the underlying reason for these observed differences is based on the Eimeria life cycle. It has been suggested that the epithelial damage, induction of mucogenesis or serum leakage are the underlying reasons for the predisposing nature of a coccidiosis infection (Timbermont et al., 2011; Adhikari et al., 2020). The exact time point during the Eimeria life cycle which is responsible for this phenomenon is however unclear. The 48-h administration interval between the Eimeria administrations in the early repeated regimen was chosen based on the life cycle duration of multiple precocious Eimeria strains composing the commercial vaccine. These values range from 60 to 120 h (Shirley and Bedrník, 1997). By choosing an intermediate time point of 48 h, both asexual schizogony and the sexual gametogony stages (both resulting in epithelial cell death) of the Eimeria cycle might be represented when challenging with C. perfringens. This is in contrast to the single late coccidiosis administration protocol, where Eimeria administration coincides with C. perfringens challenge so not all stages of the life cycle of Eimeria will be represented. Alternatively, Eimeria field strains can be used in NE model development, either as a single strain or a mix (Gholamiandehkordi et al., 2007) (Van Waeyenberghe et al., 2016). However, the optimal administration interval should be reassessed, taken into account the life cycle duration of the particular strains.
Overall, our findings show that early repeated administration (before C. perfringens challenge) of a tenfold dose of a live attenuated Eimeria vaccine results in the development of NE in most of the challenged animals, whereas less animals develop disease when a single late (during C. perfringens challenge) coccidiosis administration protocol is used, all in combination with the predisposing effect of fishmeal supplementation. Furthermore, both described models have shown to be reproducible in time, with our results being similar to the results previously described in literature. The use of an NE challenge model that consistently yields high numbers of animals with lesions, without inducing mortality, reduces the number of experimental animals needed during in vivo NE trials.
Acknowledgments
The researchers Dierick Evelien and Goossens Evy were supported by Research Foundation Flanders FWO (Fonds Wetenschappelijk Onderzoek Vlaanderen) under grant numbers [1S25818N] and [12W8919N], respectively. The authors gratefully appreciated the excellent assistance of the many Ph.D. students, post-docs and scientific staff of the Department of Pathology, Bacteriology and Avian Diseases during the conduct of the necrotic enteritis in vivo trial.
Disclosures
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this article.
References
- Adhikari P., Kiess A., Adhikari R., Jha R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J. Appl. Poult. Res. 2020;29:515–534. [Google Scholar]
- Al-Sheikhly F., Al-Saieg A. Role of Coccidia in the Occurrence of necrotic enteritis of chickens. Am. Assoc. Avian Pathol. 1980;24:324–333. [PubMed] [Google Scholar]
- Alnassan A.A., Kotsch M., Shehata A.A., Krüger M., Daugschies A., Bangoura B. Necrotic enteritis in chickens: development of a straightforward disease model system. Vet. Rec. 2014;174:555. doi: 10.1136/vr.102066. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C., Vieira B.S., Hofacre C., Applegate T.J. Effect of different challenge models to induce necrotic enteritis on the growth performance and intestinal microbiota of broiler chickens. Poult. Sci. 2019;98:2800–2812. doi: 10.3382/ps/pez084. [DOI] [PubMed] [Google Scholar]
- Cooper K.K. The University of Arizona Libraries; Tucson, AZ: 2016. Pages 1–144 in Necrotic Enteritis in Broiler Chickens: Studies in Disease Reproduction and Pathogenesis in the Graduate College. [Google Scholar]
- Da Costa S.P.F., Mot D., Bokori-Brown M., Savva C.G., Basak A.K., Van Immerseel F., Titball R.W. Protection against avian necrotic enteritis after immunisation with NetB genetic or formaldehyde toxoids. Vaccine. 2013;31:4003–4008. doi: 10.1016/j.vaccine.2013.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick E., Hirvonen O.P., Haesebrouck F., Ducatelle R., Van Immerseel F., Goossens E. Rapid growth predisposes broilers to necrotic enteritis. Avian Pathol. 2019;48:416–422. doi: 10.1080/03079457.2019.1614147. [DOI] [PubMed] [Google Scholar]
- Gholamiandehkordi A., Timbermont T., Lanckriet A., Van Den Broeck W., Pedersen K., Dewulf J., Pasmans F., Haesebrouck F., Ducatelle R., Van Immerseel F. Quantification of gut lesions in subclinical necrotic enteritis model. Avian Pathol. 2007;36:375–382. doi: 10.1080/03079450701589118. [DOI] [PubMed] [Google Scholar]
- Keyburn A.L., Sheedy S.A., Ford M.E., Williamson M.M., Awad M.M., Rood J.I., Moore R.J. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Jeong W., Jeoung H.Y., An D.J. Avian necrotic enteritis: experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult. Sci. 2011;90:1381–1390. doi: 10.3382/ps.2010-01319. [DOI] [PubMed] [Google Scholar]
- Mot D., Timbermont L., Delezie E., Haesebrouck F., Ducatelle R., van Immerseel F. Day-of-hatch vaccination is not protective against necrotic enteritis in broiler chickens. Avian Pathol. 2013;42:179–184. doi: 10.1080/03079457.2013.778955. [DOI] [PubMed] [Google Scholar]
- Park S.S., Lillehoj H.S., Allen P.C., Park D.W., FitzCoy S., Bautista D.A., Lillehoj E.P. Immunopathology and Cytokine Responses in broiler chickens Coinfected with Eimeria maxima and Clostridium perfringens with the Use of an animal model of necrotic enteritis. Avian Dis. 2008;52:14–22. doi: 10.1637/7997-041707-Reg. [DOI] [PubMed] [Google Scholar]
- Shirley M.W., Bedrník P. Live attenuated vaccines against Avian coccidiosis: Success with precocious and egg-adapted lines of Eimeria. Parasitol. Today. 1997;13:481–484. doi: 10.1016/s0169-4758(97)01153-8. [DOI] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012;43:1. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., De Smet L., Van Nieuwerburgh F., Parreira V.R., Van Driessche G., Haesebrouck F., Ducatelle R., Prescott J., Deforce D., Devreese B., Van Immerseel F. Perfrin, a novel bacteriocin associated with netB positive Clostridium perfringens strains from broilers with necrotic enteritis. Vet. Res. 2014;45:1–10. doi: 10.1186/1297-9716-45-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Van Damme L., Cox N., Callens C., Haesebrouck F., Dargatz M., Ducatelle R., Van Immerseel F., Goossens E. C. perfringens challenge reduces matrix metalloproteinase activity in the jejunal mucosa of Eimeria-infected broiler chickens. Vet. Res. 2020;51:100. doi: 10.1186/s13567-020-00825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waeyenberghe L., De Gussem M., Verbeke J., Dewaele I., De Gussem J. Timing of predisposing factors is important in necrotic enteritis models. Avian Pathol. 2016;45:370–375. doi: 10.1080/03079457.2016.1156647. [DOI] [PubMed] [Google Scholar]