Abstract
With global warming and ban on antibiotics, it occurs occasionally that deoxynivalenol (DON) together with Clostridium perfringens impairs the gut health of broiler chickens. However, the interactive effect of DON and C. perfringens on intestinal health is still unknown. A total of 120 one-day-old Arbor Acres broilers were randomly distributed to 4 groups. Birds were gavaged with C. perfringens (8 × 108 CFU/d per bird) or sterile medium and fed a DON diet (0 or 5 mg of DON per kg diet) to investigate the interactive effects. The main effect analysis showed that DON diet significantly downregulated (P < 0.05) the mRNA expression of mucin-2, B-cell lymphoma-2–associated X, and cysteinyl aspartate–specific proteinase-3 of jejunal mucosa; decreased (P < 0.05) the indexes of ACE, Chao1, Shannon, and Simpson; and also decreased the relative abundance of the phylum Bacteroidete and the genera Lactococcus in jejunal contents of broilers chickens. Meanwhile, C. perfringens significantly increased (P < 0.05) crypt depth; decreased (P < 0.05) the ratio of villi height to crypt depth, the activity of jejunal diamine oxidase, and the relative abundance of Lactococcus; and upregulated (P < 0.05) the relative expression of B-cell lymphoma-2 and cysteinyl aspartate–specific proteinase-8. Furthermore, the interactions between DON and C. perfringens were most significant (P < 0.05) in the mRNA expression of lipopolysaccharide-induced TNF factor (LITAF) and TLR-4, the abundance of the genera Lactococcus in jejunal contents, and butyric acid concentrations in cecal contents of birds. Finally, Spearman correlation analysis suggested that the most negative correlations (P < 0.05) with the abundance of the genera except Lactobacillus were observed within the mRNA expression of LITAF. The abundance of Lactococcus had a positive correlation (P < 0.05) with the expression of Caspase-3. Most genera except Lactobacillus negatively correlated (P < 0.05) with acetic acid, butyric acid, and total short-chain fatty acids. In conclusion, dietary deoxynivalenol and C. perfringens challenge had a harmful effect on the jejunal health and should be carefully monitored in broiler production.
Key words: broiler chicken, Clostridium perfringens, deoxynivalenol, jejunal health, microbiota
Introduction
Deoxynivalenol (DON), a mycotoxin belongs to group B of trichothecene, is the secondary metabolites of Fusarium, especially Fusarium graminearum and Fusarium culmorum (Khaneghah et al., 2018). The detection rate of DON could be up to 70%, which is often found in wheat, barley, oat, corn, and other crops (Streit et al., 2013). It could exist from agriculture crops to human food and animal feed, hence it brings great risk to the human food safety and the animal feeding industry because of its resistance to grind, process, and heat. In addition, DON has been shown to involve in acute and chronic illnesses in both humans and farm animals (Girardet et al., 2011). DON could bind to ribosome, causing dysfunction of protein and nucleic acid biosynthesis so as to influence cell proliferation, differentiation, and apoptosis (Ueno, 1983). Therefore, the high-protein turnover tissues such as the intestine and liver are major targets of the DON. DON is reported to damage the barrier function of the intestine, leading to mucosal necrosis, diarrhea, vomiting, reduced feed conversion, and immune dysfunction of human and poultry (Chen et al., 2017; Mishra et al., 2020). As a biotic barrier that separate pathogenic agent from visceral organs, “leaky gut” indicates a serious health challenge to the animal. What's more, DON could affect the absorption of amino acid, carbohydrate, and other nutrients, and the fermentation of mass nutriment in the large intestine could disrupt the balance of intestinal microflora, which could be a potential mechanism of its harmful effects (Awad and Zentek, 2015). The toxicity of the DON is dependent on exposal dose and duration (Payros et al., 2016). Low-dose DON promotes the secretion of cytokines, induces inflammatory responses, and increases the risk of chronic immune disease infection, whereas acute feeding leads to immune cell apoptosis, immunosuppression, and impaired intestinal function of the animal.
Clostridium perfringens, a resident gram-positive bacteria of poultry intestinal contents, is the major etiologic agent for necrotic enteritis (NE). C. perfringens is classed into type-A to type-E by the 4 major secreted toxins (α, β, ε, and ι). The intestine of healthy birds typically contains approximately 1 × 104 CFU C perfringens/g of digesta, but it would overgrow reaching a critical concentration of about 1 × 107 to 1 × 109 CFU/g of digesta with various unfavorable factors such as intestinal injury, excessive animal protein content in the diet, impaired immune function, a plethora of coccidiosis, and intestinal microflora disorder (Uzal et al., 2014). Some exotoxin and harmful active substances such as hyaluronidase, acid phosphatase, protease, collagenase, sulfatase, and so on would be secreted excessively to injure the intestinal mucosa of poultry (Wu et al., 2018b). Then the bacteria would pass the mucosa layer and attach to intestinal epithelial cells, active pathogen-associated molecular patterns, and result in an inflammation response, inducing cell death. It is reported that the pathogenicity of C. perfringens is associated with the secreted exotoxin such as α-toxin, NE B–like toxin, and so on. α-Toxin, a toxin secreted by all type (type A, B, C, D, and E) of C. perfringens, is a zinc-containing metalloenzyme, which has the function same as phospholipase and sphingomyelinase, could induce cell death and destroy the epithelial cell membrane (Immerseel et al., 2009). NE B–like toxin, a novel exotoxin discovered, is produced by type-A C. perfringens, which could induce avian intestinal cells hemolytic and pore-forming (Zhou et al., 2017). But the pathogenesis of NE is still contradictory, and the mechanisms need to be further explored.
Owing to the change of climate with higher temperature and humidity and global ban on the use of antibiotics, mycotoxin and pathogens take great challenges to the poultry industry (Antonissen et al., 2015). And the cooccurrence of mycotoxin and pathogens happened occasionally (Antonissen et al., 2014). Studies have revealed that mycotoxin could exacerbate the pathogens' toxicity on the intestine through injuring the intestine, but few studies have concentrated on the interaction between mycotoxin and pathogens. Thus, we choose broiler chicken as an animal model to investigate the interactive effects of dietary DON and C. perfringens challenge on gut health, trying to provide another insight into the interaction of mycotoxin and pathogens.
Materials and methods
Ethics Statement
The protocol for the animal experimental procedures was approved by the Institutional Animal Care and Use Committee of Northwest A&F University (Permit Number: NWAFAC 1008).
Birds and Treatment
In a 2 × 2 factorial arrangement, a total of 120 hatched 1-day-old healthy (Arbor Acres) broilers with similar body weight were randomly assigned into 4 treatment groups: 1) Birds of group CON were fed a basal diet; 2) Birds of group DON were fed a basal diet contaminated with DON at 5 mg per kg diet; 3) Birds of group CP were challenged with C. perfringens CVCC2030 culture medium at 8 × 108 CFU/mL by instilling using 1-mL injector, and the dose was l mL 1 d per bird from day 17 to 20; 4) Birds of group DC were fed basal diet contaminated with 5 mg of DON per kg diet and challenged with C. perfringens CVCC2030 medium at 8 × 108 CFU/mL by instilling using 1-mL injector, and the dose was l mL 1 d per bird from day 17 to 20. To avoid cross-contamination, the unchallenged birds and challenged birds were reared in 2 separate rooms with 2 layers of metal cage, and the brooding temperature was maintained at 35°C for the first week and gradually decreased to 27°C in the 3rd wk. All birds were allowed ad libitum access to feed and water. The experiment lasted for 21 d.
Diets, C. perfringens Challenge, and Sampling
The ingredients and nutrient levels of the basic diet are shown in Table 1. The DON was produced by inoculating Fusarium graminea PH-1 into the rice at 25°C. The final DON concentration in fermentative rice analyzed by LC-MS/MS method was 453.9 ppm. And the DON feed was mixed with fermentative rice and basic diet by a ratio of 1:90 to reach 5 mg of DON/kg of diet approximately referred to European Commission (European Commission, 2006), which was a maximum observed level in animal feeds (Rodrigues and Naehrer, 2012). The C. perfringens challenged method was based on the study by Antonissen et al. (2014) with slight modifications. Briefly, C. perfringens (CVCC2030) was obtained from the China Veterinary Culture Collection Center (Beijing, China) which isolated them from NE-infective broilers. C. perfringens was cultured anaerobically on tryptose-sulfite-cycloserine for 18 h at 37°C and then aseptically transferred into a cooked meat medium (HB0259; Qingdao Hope Bio-Technology Co., Ltd.) and incubated anaerobically for 18 h at 37°C.
Table 1.
Ingredients and composition of the control experimental diets.
| Ingredients | Content (%) | Nutrient levels2 | Content (%) |
|---|---|---|---|
| Corn | 60.86 | Crude protein | 21.00 |
| Soybean meal | 23.52 | Calcium | 0.90 |
| Corn gluten meal | 4.00 | Total phosphorus | 0.70 |
| Cottonseed meal | 3.00 | Available phosphorus | 0.46 |
| Distiller's dried grain | 3.00 | ME/(MJ/kg) | 12.13 |
| CaHPO4 | 1.83 | Lysine | 1.40 |
| Soybean oil | 1.10 | Methionine | 0.55 |
| Limestone | 1.00 | Available lysine | 1.20 |
| L-Lys•H2SO4 | 0.80 | Available methionine | 0.50 |
| Salt | 0.30 | ||
| DL-Met | 0.23 | ||
| L-Threonine | 0.12 | ||
| Choline chloride | 0.10 | ||
| Premix1 | 0.40 | ||
| Total | 100.00 |
The premix provided the following per kg of diets: VA 8,000 IU, VB1 4 mg, VB2 3.6 mg, VB5 40 mg, VB6 4 mg, VB12 0.02 mg, VD3 3,000 IU, VE 20 IU, VK3 2 mg, biotin 0.15 mg, folic acid 1.0 mg, D-pantothenic acid 11 mg, nicotinic acid 10 mg, antioxidant 100 mg, Cu (as copper sulfate) 10 mg, Fe (as ferrous sulfate) 80 mg, Mn (as manganese sulfate) 80 mg, Zn (as zinc sulfate) 75 mg, I (as potassium iodide) 0.40 mg, Se (as sodium selenite) 0.30 mg.
All the nutrient levels were calculated values.
At day 21, 6 birds per group were randomly selected, weighed, and euthanized by cervical dislocation. The middle complete jejunal segments which were fixed in 10% buffered formalin were used for the analysis of intestinal morphology (Yang et al., 2017). The jejunal mucosa scraped from the jejunum was snap-frozen in liquid nitrogen and stored at −80°C until further mRNA analysis. The jejunal and cecal content samples were collected into 2-mL Eppendorf tubes and frozen immediately in liquid nitrogen, and the rest were stored at −80°C immediately for 16s rDNA and short-chain fatty acid (SCFA) contents analysis.
Quantitative RT-PCR Analysis of Gene Expression
The total RNA of mucosa samples was extracted using the Trizol Reagent kit (Invitrogen Co., Carlsbad, CA). The quantity and quality of extracted total RNA were determined using a spectrophotometer (NanoDrop-2000; Thermo Fisher Scientific, Waltham, MA) at 260 and 280 nm. First-strand cDNA was synthesized from 2 μg of total RNA using an EasyScript First-Strand cDNA Synthesis SuperMix kit (TransGen Biotech, Beijing, China) according to the manufacturer's instructions. Primer sequences of the β-actin, zona occludens-1 (ZO-1), occludin, claudin-1, mucin-2 (Muc-2), toll-like receptor-2 (TLR-2), lipopolysaccharide-induced TNF factor (LITAF), interleukin-1β (IL-1β), interferon-γ (IFN-γ), toll-like receptor-4 (TLR-4), B-cell lymphoma-2 (Bcl-2), C-myc, cysteinyl aspartate specific proteinase-3 (Caspase-3), cysteinyl aspartate–specific proteinase-8 (Caspase-8), and Bcl2-associated X (Bax) are listed in Table 2, and all the genes' sequences were quoted from NCBI. A total volume of 20 μL of reaction system included 10 μL of SYBR Promerx Ex Taq, 0.4 μL upstream primers (10 μM/L), 0.4 μL downstream primers (10 μM/L), 2 μL cDNA, and 7.2 μL DEPC H2O. The reactions of real-time PCR were carried out at 95°C for 15 s, followed by 40 cycles at 95°C for 15 s, and at 60°C for 60 s. The mRNA expression was calculated as being equal to 2 −ΔΔCT and normalized for GAPDH gene expression.
Table 2.
Primers used in real-time quantitative PCR.
| Gene | Gene bank ID | Primer sequence (5′-3′) |
|---|---|---|
| GAPDH | L08165 | F: TGGGCATCAAGGGCTACA R: TCGGGTTGGTTGGTGATG |
| Bax | XM_025145467.1 | F: ATCGTCGCCTTCTTCGAGTT R: ATCCCATCCTCCGTTGTCCT |
| Bcl-2 | NM_205339.2 | F: TCGCGCCGCTACCAGAGGGACTTC R: CCGGTTGACGCTCTCGACGCACAT |
| Caspase-3 | NM_204725.1 | F: GGCTCCTGGTTTATTCAGTCTC R: ATTCTGCCACTCTGCGATTT |
| Caspase-8 | NM_204592.3 | F: TCACTTTGCCAGAGCCTGAG R: CCCTTGTTCCTCCTGTCGTC |
| Claudin-1 | NM_001013611.2 | F: ACCCACAGCCTAAGTGCTTC R: AGGTCTCATAAGGCCCCACT |
| C-myc | NM_001030952.1 | F: ACAGTGGCAGGGTCCTCAAACAGA R: GTCACGCAGGGCAAAGAAACTCAG |
| IFN-γ | NM_205149.1 | F: AAGCTCCCGATGAACGACT R: CCTCTGAGACTGGCTCCTTTT |
| IL-1β | NM_204524.1 | F: TGGGCATCAAGGGCTACA R: TCGGGTTGGTTGGTGATG |
| LITAF | NM_204267.1 | F: TGTGTATGTGCAGCAACCCGTAGT R: GGCATTGCAATTTGGACAGAAGT |
| Muc-2 | NM_001318434.1 | F: CTACTTCACCTTCAACCATTACAAC R: TCATAGTCACCACCATCTTCTTCAG |
| Occludin | NM_205128.1 | F: TCATCGCCTCCATCGTCTAC R: TCTTACTGCGCGTCTTCTGG |
| TLR-2 | NM_204278.1 | F: GATTGTGGACAACATCATTGACTC R: AGAGCTGCTTTCAAGTTTTCCC |
| TLR-4 | NM_001030693.1 | F: AGTCTGAAATTGCTGAGCTCAAAT R: GCGACGTTAAGCCATGGAAG |
| ZO-1 | XM_015278981.2 | F: GGGATGTTTATTTGGGCGGC R: TCACCGTGTGTTGTTCCCAT |
Abbreviations: Bax, Bcl2-associated X; Bcl-2, B-cell lymphoma-2; Caspase-3, cysteinyl aspartate specific proteinase-3; Caspase-8, cysteinyl aspartate specific proteinase-8; F, forward; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IFN-γ, interferon-γ; IL-1β, interleukin-1β; LITAF, lipopolysaccharide induced TNF factor; Muc-2, mucin-2; R, reverse; TLR-2, toll-like receptor-2; TLR-4, toll-like receptor-2; ZO-1, zona occludens-1.
Determination of Jejunal Diamine Oxidase Activity
The frozen jejunal tissue samples were thawed on ice, and 0.1 g of each tissue was homogenized in 0.9 mL of cold phosphate buffered saline. The resulting homogenate was then centrifuged at 2,500 rpm under 4°C for 10 min, and the supernatant was collected for estimation of diamine oxidase (DAO) activity (A088-2-1, DAO assay kit; Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The DAO activity was calculated by the decline rate of OD 340 nm.
Microbial DNA Extraction, 16S rRNA Gene Amplification of the V3 + V4 Region, Sequencing, and Bioinformatics Analysis
A total of 24 collected jejunal content samples (1 g) from CON, DON, CP, and DC groups of 21-day-old broilers were used for DNA extraction using DNeasy PowerSoil Kit (QIAGEN, Inc., Netherlands). The quantity and quality of extracted DNA were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and agarose gel electrophoresis, respectively.
PCR amplification of the bacterial 16S rRNA genes V3–V4 region was performed and purified with Agencourt AMPure Beads (Beckman Coulter, Indianapolis, IN) and quantified using the PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA). After the individual quantification step, amplicons were pooled in equal amounts, and paired-end 2 × 300-bp sequencing was performed using the Illumina MiSeq platform with MiSeq Reagent Kit v3 at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China). The Quantitative Insights into Microbial Ecology (QIIME, v1.8.0) pipeline was used to process the sequencing data. Briefly, raw sequencing reads with exact matches to the barcodes were assigned to respective samples and identified as valid sequences. The low-quality sequences were filtered through the following criteria: sequences that had a length of less than 150 bp, sequences that had average Phred scores less than 20, sequences that contained ambiguous bases, and sequences that contained mononucleotide repeats more than 8 bp. Paired-end reads were assembled using FLASH (v1.2.7, http://ccb.jhu.edu/software/FLASH/). After chimera detection, the remaining high-quality sequences were clustered into operational taxonomic units (OTUs) at 97% sequence identity by UCLUST. A representative sequence was selected from each OTU using default parameters. OTU taxonomic classification was conducted by BLAST searching the representative sequences set against the Green genes Database (http://greengenes.lbl.gov/cgi-bin/nph-index.cgi) using the best hit. The taxon abundance of each sample was separated into the phylum, class, order, family, and genera levels. The MetaStat (http://metastats.cbcb.umd.edu/) was used to identify the effect of DON and C. perfringens on gut microbiota, and the R software 2.15.3 was used to calculate Spearman's rank correlation coefficients between the relative abundance of the gut microbiome and the concentrations of SCFAs, as well as the mRNA expression of immunity-related and apoptosis-regulatory genes, and exhibited the result by the heatmap.
Determination of Bacterial Metabolites
The cecal contents were collected and immediately placed into sterile plastic tubes, sealed, and frozen at −80°C for subsequent SCFAs analysis. The concentrations of SCFAs (acetic acid, propionic acid, isobutyric acid, n-butyric acid, isopentanoic acid, n-pentanoic acid) were measured using gas chromatography (Agilent 7820A GC). SP-2560 capillary column and hydrogen flame ion detector was used. The chromatographic conditions are as follows: The carrier gas was N2, the split ratio was 40:1, the flow rate was 2.0 mL/min, the average linear velocity was 38 cm/s, the column pressure was 11.3 psi (1psi≈6.89kpa). The initial temperature of the capillary column was 120°C (3 min), and the temperature is increased to 180°C at 10°C/min (1 min). The temperature of the hydrogen flame detector was 250°C, the flow rate of H2 was 40 mL/min, and the airflow rate was 450 mL/min. Column flow + makeup gas flow was 45 mL/min, the temperature of the inlet was 210°C, and the injection volume was 0.6 μL.
Statistical Analysis
The SPSS 13.0 software for Windows was used for all statistical analyses in this study. Univariate analysis was used to analyze the main effect and interactive effect. All results were represented in terms of mean ± SEM, and P < 0.05 was implied a significant difference.
Results
Effect of DON and C. perfringens on the Jejunal Morphology
The gut is the first target of DON and C. perfringens, so we explored the effect of dietary DON and C. perfringens challenge on the morphology of jejunum. As shown in Table 3, C. perfringens challenge increased (P < 0.05) the jejunal crypt depth (CD) and decreased (P < 0.05) the ratio of villi height to crypt depth (VH/CD) showing that C. perfringens could damage the construction of jejunum. Dietary DON had no effect on the morphology of jejunum. And no interaction was seen between dietary DON and C. perfringens challenge.
Table 3.
The effect of DON and C. perfringens on jejunal morphology.
| Parameter | CON | DON | CP | DC | SEM |
P values |
||
|---|---|---|---|---|---|---|---|---|
| DON | C. perfringens | DON × C. perfringens | ||||||
| VH (μm) | 734.28 | 675.33 | 700.53 | 678.11 | 18.33 | 0.311 | 0.689 | 0.669 |
| CD (μm) | 69.22 | 63.82 | 73.15 | 86.95 | 2.72 | 0.691 | 0.015 | 0.103 |
| VH/CD | 10.59 | 10.61 | 9.62 | 8.13 | 0.32 | 0.325 | 0.009 | 0.315 |
Abbreviations: CD, crypt depth; CON, basal diet; CP, basal diet + C. perfringens challenge; DC, basal diet extra deoxynivalenol + C. perfringens challenge; DON, basal diet extra deoxynivalenol; VH, villi height; VH/CD, the ratio of villi height to crypt depth.
Effect of DON and C. perfringens on the Gut Barrier Permeability
The intestinal mucosa acts as a selectively permeable barrier, absorbing the nutrients and defending against pathogens and toxins. We tested the mRNA expression of tight junctions, Muc-2, and activity of the related enzyme (Table 4). It was shown that dietary DON and C. perfringens challenge had no significant effect on the gene expression of occludin, claudin-1, and ZO-1, while dietary DON significantly downregulated (P < 0.05) the mRNA expression of Muc-2 and C. perfringens reduced (P < 0.05) the activity of DAO of jejunum. These results indicated impaired barrier function. There was no interaction between dietary DON and C. perfringens challenge.
Table 4.
The effect of DON and C. perfringens on the relative expression of tight junction, Muc-2 and DAO activity of jejunum.
| Parameter | CON | DON | CP | DC | SEM |
P values |
||
|---|---|---|---|---|---|---|---|---|
| DON | C. perfringens | DON × C. perfringens | ||||||
| Claudin | 1.14 | 0.49 | 0.57 | 0.52 | 0.12 | 0.121 | 0.224 | 0.178 |
| Muc-2 | 1.13 | 0.21 | 1.20 | 0.69 | 0.14 | 0.013 | 0.299 | 0.434 |
| Occludin | 1.02 | 0.89 | 1.36 | 1.36 | 0.11 | 0.768 | 0.104 | 0.768 |
| ZO-1 | 0.79 | 0.54 | 0.62 | 0.95 | 0.11 | 0.872 | 0.608 | 0.209 |
| DAO (U/L) | 1.97 | 2.29 | 1.17 | 1.29 | 0.15 | 0.286 | <0.001 | 0.618 |
Abbreviations: CON, basal diet; CP, basal diet + C. perfringens challenge; DAO, diamine oxidase; DC, basal diet extra deoxynivalenol + C. perfringens challenge; DON, basal diet extra deoxynivalenol; Muc-2, mucin-2; ZO-1, zona occludens-1.
Effect of DON and C. perfringens on the Inflammation and Apoptosis Response
In the present study, we tried to investigate the effects of dietary DON and C. perfringens challenge and their interactive effects on the inflammation response in a broiler jejunum model. For this purpose, we determined the relative expression of cytokines using RT-qPCR (Table 5). As shown, dietary DON and C. perfringens challenge had no effect on the relative expression of cytokines. However, the significant interactions between DON and C. perfringens were clear (P < 0.05) in the expression of TLR-4 and LITAF.
Table 5.
The effect of DON and C. perfringens on the relative expression of cytokines.
| Parameter | CON | DON | CP | DC | SEM |
P-values |
||
|---|---|---|---|---|---|---|---|---|
| DON | C. perfringens | DON × C. perfringens | ||||||
| IFN-γ | 1.42 | 0.80 | 0.61 | 0.75 | 0.19 | 0.508 | 0.244 | 0.309 |
| IL-1β | 1.04 | 1.81 | 1.44 | 2.14 | 0.18 | 0.231 | 0.543 | 0.950 |
| LITAF | 1.02c | 2.17b | 2.50a | 1.81b | 0.17 | 0.565 | 0.162 | 0.028 |
| TLR-2 | 1.14 | 1.13 | 1.72 | 0.71 | 0.3 | 0.425 | 0.896 | 0.437 |
| TLR-4 | 0.81b | 1.60a | 0.99b | 0.63c | 0.11 | 0.438 | 0.154 | 0.046 |
Numbers within a row with different superscripts differ statistically at P ≤ 0.05.
Abbreviations: CON, basal diet; CP, basal diet + C. perfringens challenge; DC, basal diet extra deoxynivalenol + C. perfringens challenge; DON, basal diet extra deoxynivalenol; IFN-γ, interferon-γ; IL-1β, interleukin-1β; LITAF, lipopolysaccharide-induced TNF factor; TLR-2, toll-like receptor-2; TLR-4, toll-like receptor-2.
Inflammation is accompanied by cell death, so we test the expression of apoptosis-related genes (Table 6). Results showed dietary DON significantly downregulated (P < 0.05) the relative expression of Bax and Caspase-3, inversely C. perfringens significantly upregulated (P < 0.05) the relative expression of Bcl-2 and Caspase-8. The trend of interactions (P = 0.086) between DON and C. perfringens was in the expression of Caspase-8.
Table 6.
The effect of DON and C. perfringens on the relative expression of apoptotic genes.
| Parameter | CON | DON | CP | DC | SEM |
P values |
||
|---|---|---|---|---|---|---|---|---|
| DON | C. perfringens | DON × C. perfringens | ||||||
| Bax | 0.80 | 0.42 | 1.00 | 0.34 | 0.12 | 0.038 | 0.802 | 0.551 |
| Bcl-2 | 0.79 | 0.30 | 1.67 | 1.27 | 0.18 | 0.192 | 0.012 | 0.883 |
| Bcl-2/Bax | 0.92 | 0.79 | 0.74 | 0.86 | 0.04 | 0.964 | 0.605 | 0.220 |
| Caspase-3 | 1.03 | 0.42 | 1.67 | 0.45 | 0.20 | 0.018 | 0.355 | 0.402 |
| Caspase-8 | 0.84 | 0.85 | 3.59 | 1.10 | 0.17 | 0.090 | 0.046 | 0.086 |
| C-myc | 0.92 | 0.72 | 1.34 | 0.45 | 0.20 | 0.213 | 0.879 | 0.407 |
Abbreviations: Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X; Caspase-3, cysteinyl aspartate specific proteinase-3; Caspase-8, cysteinyl aspartate specific proteinase-8; CON, basal diet; CP, basal diet + C. perfringens challenge; DC, basal diet + 5 mg of deoxynivalenol per kg diet + C. perfringens challenge; DON, basal diet + 5 mg of deoxynivalenol per kg diet.
Effect of DON and C. perfringens on the Jejunal Microflora and Bacterial Metabolites
To get the diversity and abundance of microflora in jejunal digesta, we performed 16s rDNA gene sequencing. It can be seen from Table 7 that dietary DON decreased (P < 0.05) the ACE, Chao1, Shannon, and Simpson index significantly. We analyzed the effect of dietary DON and C. perfringens challenge on the relative abundance of top 5 phylum and genera in jejunal digesta (Table 8). Results showed that dietary DON tended to increase the relative abundance of Firmicutes (P = 0.079) and decrease Proteobacteria (P = 0.085), Actinobacteria (P = 0.070), and Bacteroidetes (P < 0.05) significantly. In the genus level, similarly, dietary DON increased (P < 0.05) the relative abundance of Lactobacillus, decreased (P < 0.05) the relative abundance of Lactococcus, and tended to decrease (P = 0.074) the relative abundance of Sediminibacterium. In addition, C. perfringens challenge decreased (P < 0.05) the relative abundance of Lactococcus. There was significant interaction (P < 0.05) between DON and C. perfringens for the abundance of Lactococcus.
Table 7.
The effect of DON and C. perfringens on the abundance and diversity of microflora in jejunal digesta.
| Parameter | CON | DON | CP | DC | SEM |
P values |
||
|---|---|---|---|---|---|---|---|---|
| DON | C. perfringens | DON × C. perfringens | ||||||
| ACE | 1,152.69 | 600.59 | 1,022.24 | 639.85 | 86.71 | 0.007 | 0.772 | 0.591 |
| Chao1 | 1,078.02 | 580.43 | 971.15 | 620.66 | 81.97 | 0.011 | 0.826 | 0.628 |
| Shannon | 5.88 | 3.30 | 4.53 | 3.61 | 0.33 | 0.007 | 0.380 | 0.170 |
| Simpson | 0.90 | 0.65 | 0.75 | 0.72 | 0.03 | 0.037 | 0.514 | 0.107 |
Abbreviations: CON, basal diet; CP, basal diet + C. perfringens challenge; DC, basal diet + 5 mg of deoxynivalenol per kg diet + C. perfringens challenge; DON, basal diet + 5 mg of deoxynivalenol per kg diet.
Table 8.
The effect of DON and C. perfringens on the relative abundance of jejunal microbial flora.
| Parameter | CON | DON | CP | DC | SEM |
P values |
||
|---|---|---|---|---|---|---|---|---|
| DON | C. perfringens | DON × C. perfringens | ||||||
| Phylum level | ||||||||
| Firmicutes | 49.35 | 75.86 | 54.50 | 73.53 | 6.18 | 0.079 | 0.909 | 0.763 |
| Proteobacteria | 30.22 | 13.84 | 27.83 | 16.62 | 3.81 | 0.085 | 0.980 | 0.737 |
| Actinobacteria | 13.13 | 6.76 | 11.63 | 6.78 | 1.48 | 0.070 | 0.803 | 0.797 |
| Bacteroidetes | 2.83 | 1.23 | 2.72 | 1.18 | 0.39 | 0.050 | 0.913 | 0.969 |
| Cyanobacteria | 2.18 | 1.07 | 1.62 | 0.99 | 0.25 | 0.102 | 0.527 | 0.642 |
| Genera level | ||||||||
| Lactobacillus | 34.95 | 71.72 | 50.02 | 71.24 | 6.62 | 0.032 | 0.567 | 0.542 |
| Ochrobactrum | 14.55 | 6.63 | 14.68 | 9.79 | 1.9 | 0.109 | 0.670 | 0.696 |
| Amycolatopsis | 9.26 | 4.87 | 7.71 | 5.05 | 1.05 | 0.113 | 0.751 | 0.689 |
| Lactococcus | 0.52a | 0.11b | 0.21b | 0.13b | 0.04 | 0.026 | 0.001 | 0.013 |
| Sediminibacterium | 1.97 | 0.82 | 1.79 | 0.91 | 0.27 | 0.074 | 0.934 | 0.797 |
Numbers within a row with different superscripts differ statistically at P ≤ 0.05.
Abbreviations: CON, basal diet; CP, basal diet + C. perfringens challenge; DC, basal diet + 5 mg of deoxynivalenol per kg diet + C. perfringens challenge; DON, basal diet + 5 mg of deoxynivalenol per kg diet.
SCFAs are produced by the microflora, and it is not only the energy source of bacteria and intestinal cells but it also plays potent roles in maintaining intestinal barrier function, regulation on immune response, and cell proliferation (Dalile et al., 2019). We examined the content of SCFAs in the cecal digesta (Table 9). Results showed that dietary DON and C. perfringens challenge had no main effect (P > 0.05) on the content of SCFAs. The interaction between DON and C. perfringens was most significant (P < 0.05) in the content of butyric acid.
Table 9.
The effect of DON and C. perfringens on the contents of short chain fatty acids of cecal digesta.
| Parameter | CON | DON | CP | DC | SEM |
P values |
||
|---|---|---|---|---|---|---|---|---|
| DON | C. perfringens | DON × C. perfringens | ||||||
| Acetic acid | 63.16 | 76.11 | 72.13 | 56.97 | 4.01 | 0.891 | 0.532 | 0.095 |
| Propionic acid | 3.83 | 3.88 | 4.26 | 3.23 | 0.29 | 0.430 | 0.866 | 0.384 |
| Butyric acid | 9.91a,b | 13.41a | 12.56a | 8.46b | 0.89 | 0.862 | 0.508 | 0.039 |
| Isobutyric acid | 0.44 | 0.44 | 0.51 | 0.46 | 0.04 | 0.781 | 0.538 | 0.768 |
| Valeric acid | 1.05 | 1.07 | 1.22 | 0.98 | 0.04 | 0.342 | 0.728 | 0.237 |
| Isovaleric acid | 0.72 | 0.62 | 0.63 | 0.51 | 0.05 | 0.146 | 0.171 | 0.927 |
| Total SCFAs | 79.11 | 95.53 | 91.31 | 70.61 | 4.91 | 0.827 | 0.518 | 0.070 |
Numbers within a row with different superscripts differ statistically at P ≤ 0.05.
Abbreviations: CON, basal diet; CP, basal diet + C. perfringens challenge; DC, basal diet + 5 mg of deoxynivalenol per kg diet + C. perfringens challenge; DON, basal diet + 5 mg of deoxynivalenol per kg diet.
The Analysis of KEGG Pathway PICRUSt
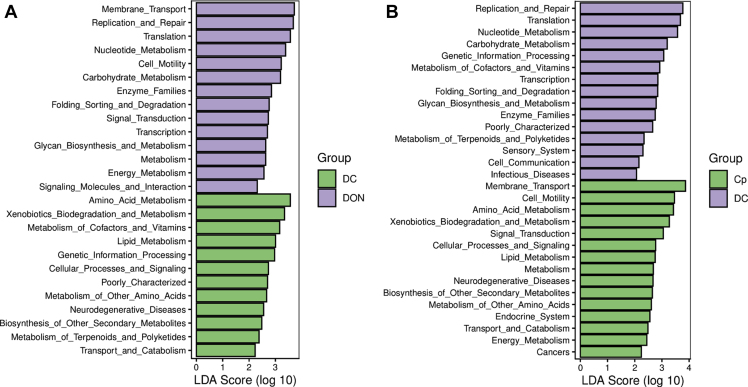
To best understand the function of microflora, we used LDA analysis on the Kyoto Encyclopedia of Genes and Genomes pathway PICRUSt in level 2 of microflora in the jejunal digesta (Figure 1). As found, compared with group DC, the most abundant KEGG pathways of group DC were amino acid metabolism, xenobiotic biodegradation and metabolism, and metabolism of cofactors, vitamins. However, the pathways predicted of group DON were membrane transport, replication, and repair, translation et al. When compared between groups CP and DC, the pathways enriched in group CP were membrane transport, cell motility, and amino acid metabolism, and those in group DC were replication and repair, translation, and nucleotide metabolism.
Figure 1.
The linear discriminant analysis (LDA) of KEGG pathway PICRUSt of microflora in jejunal digesta of 21-day-old broilers at level 2. (A) between group DON and DC and (B) between group CP and DC, of which the LDA scores ≥2. The treatments were as follows: DON = basal diet + 5 mg of deoxynivalenol per kg diet; Abbreviations: CP, basal diet + C. perfringens challenge; DC, DON diet + C. perfringens challenge.
Correlation Analysis
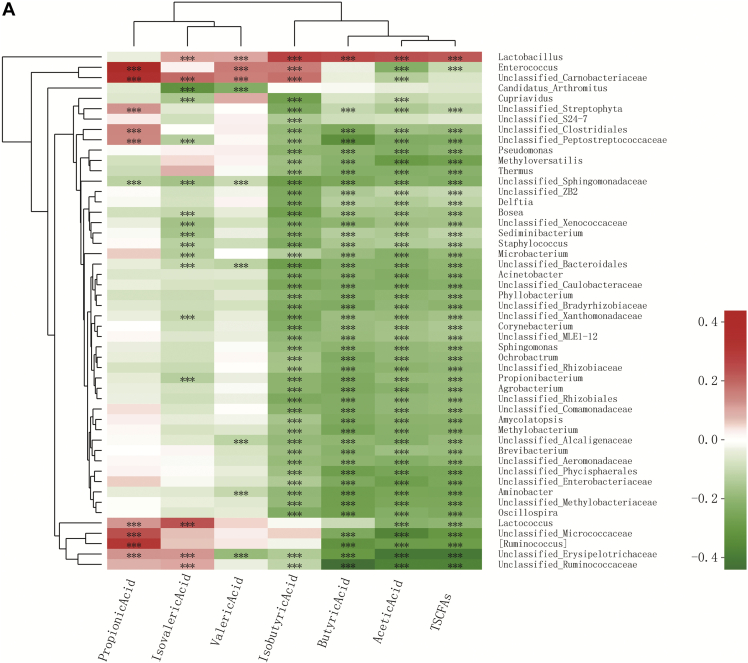
Spearman correlation analysis was conducted to characterize the associations of top 50 genera bacterial abundances with SCFAs' concentrations and the relative expression of apoptosis and inflammation genes. In total, the most abundance of the genera was significantly negatively correlated with acetic acid, butyric acid, isobutyric acid, and total SCFAs' concentrations (Figure 2A). However, the abundance of Lactobacillus had a positive (P < 0.05) correlation with all the SCFA indicator except for propionic acid. The abundance of Lactococcus was significantly negatively (P < 0.05) correlated with acetic acid and total SCFAs and positively correlated (P < 0.05) with isovaleric acid and propionic acid contents.
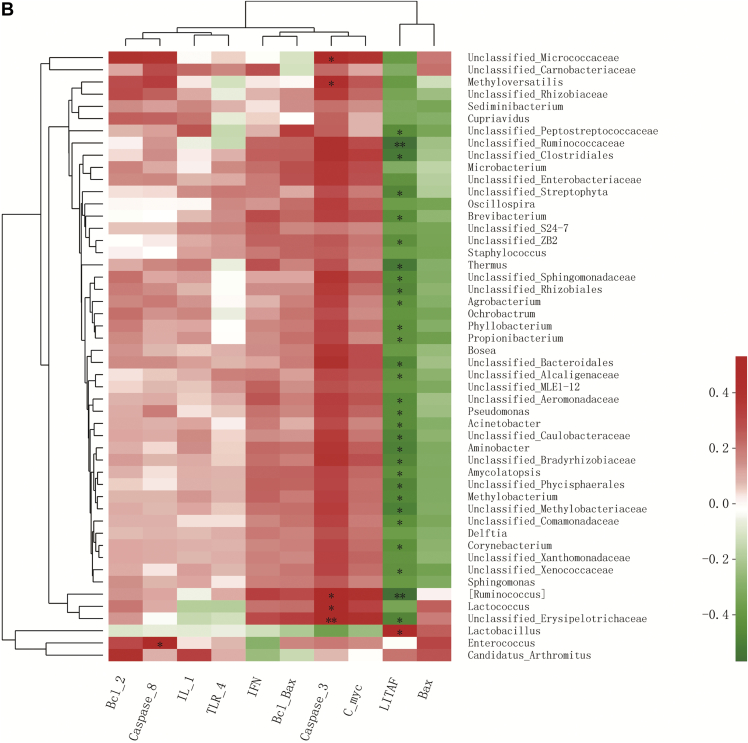
Figure 2.
Correlation analysis (A) between SCFA concentrations in cecal digesta and relative abundance of the top 50 genera in jejunal digesta of 21-day-old broilers and (B) between mRNA expression of immunity-related and apoptosis-regulatory genes with the relative abundance of the top 50 genera in jejunal digesta of 21-day-old broilers. Colors refer to the degree of correlation. ∗0.01 < P ≤ 0.05, ∗∗0.001 < P ≤ 0.01, ∗∗∗P ≤ 0.001.
To study the role of gut microbiota in DON-induced and C. perfringens–induced inflammation and apoptosis response of broiler chickens, we examined the relationship between the relative abundance of gut microbiota and the mRNA expression of immunity-related and apoptosis-regulatory genes (Figure 2B). In total, most negative correlations (P < 0.05) were observed between genera abundances and LITAF, a proinflammation cytokine. And surprisingly, Lactobacillus was positively correlated (P < 0.05) with LITAF. Lactococcus was not correlated (P > 0.05) with LITAF, but had a positive correlation (P < 0.05) with Caspase-3. Furthermore, the abundance of Enterococcus was positively correlatied (P < 0.05) with Caspase-8.
Discussion
The digestion and absorption capacity are tight correlative with intestinal morphology, and VH, CD, and VH/CD are sensitive indicators reflecting the development and function of the gut. As the main target of the DON, the development of the gut would be impaired when DON was consumed by animals. But the toxicity of DON for chicken is still controversial. Osselaere et al. (2013) reported that jejunal VH was decreased and CD was increased when 7.54 mg of DON/kg of feed was consumed. On the contrary, dietary DON would not influence the morphology of jejunum when the dose was 10 mg/kg (Ghareeb et al., 2015; Wu et al., 2018a). In this study, it is found that dietary DON had no influence on the morphology of jejunum. The result may be related to the dose we choose according to the European Commission standards (European Commission, 2006). And the tolerant capacity of broilers for DON is strengthened with the breeding and genetic modification. C. perfringens is an intestinal pathogenic agent that could induce impairment on the mucosa for its secretion of exotoxin and bioactive matter. Xue et al. (2018) reported that C. perfringens infection could result in obstacle to the development of villi and crypt and decrease the VH/CD ratio. Partially consistent with the aforementioned report, the results of this study noted that C. perfringens could increase the CD and decrease the VH/CD ratio. Variation of results among different studies may be due to the different effects from different C. perfringens species and infection methods. Crypt, the villus factory, palyes potent role in the renewal of villus as needed in response to inflammation from pathogens or toxins (Willing and Van, 2007). In accordance with the results of morphology, C. perfringens significantly reduced the activity of jejunal DAO. As a sensitive indicator of intestinal barrier function, DAO is an intracellular enzyme in the intestinal cells that would be released into blood from mucosal cells when the intestine was damaged (Liu et al., 2020). In this experiment, the decreased DAO indicated impaired intestine.
Intestinal mucosa layer act as a selective barrier that prevents intestinal microbial from translocation and maintains intestinal homeostasis, among which Muc-2 is an important part of the mucosa layer secreted by global cells. Results showed that dietary DON downregulated the mRNA expression of Muc-2, and this may be the inhibition effect of dietary DON on resistin-like molecule beta through the activation of protein kinase R and mitogen-activated protein kinase p38 (Pinton et al., 2015). Intestinal immune function plays a potent role in defending against gut pathogenic agents. DON could bind to the ribosome to induce “ribotoxic stress response,” activating downstream mitogen-activated protein kinase, which plays a critical role in modulating cell growth, differentiation, apoptosis, and immune response, of which p38 and ERK affect cytokine production and lead to the upregulation of LITAF gene expression (Ueno, 1983). Especially p38 appears to be involved in the genotoxicity of DON. Our laboratory reported that 10 mg/kg of DON in the diet had no influence on the relative expression of IL-10, IL-12, LITAF, and IFN-γ (Wu et al., 2018a). According to the present result, the extra addition of DON has no influence on the mRNA expression of TLRs and cytokines. To conclude, the low content of DON cannot induce inflammation response of broilers. C. perfringens could attach to epithelial cells, and the pathogen-associated molecular patterns, which are unique “microbial signatures” found in different pathogens, are recognized by pathogen-recognition receptors, triggering the final activation of nuclear factor kappa-light-chain-enhancer of activated B cells and inducing inflammation response (Oh and Lillehoj, 2016). Previous studies revealed that the relative expression of IFN-γ, LITAF, and IL-1β was upregulated in C. perfringens–induced NE broilers (Li et al., 2018; Wang et al., 2019). However, another study demonstrated C. perfringens challenge failed to influence the expression of IFN-γ, LITAF, and IL-10 in the NE model (Li et al., 2018). In this study, we found that C. perfringens did not change the expression of TLRs and cytokines. The difference in results may be attributed to the difference in infection patterns. However, interactive analysis results showed that dietary DON and C. perfringens challenge had an antagonistic effect on the expression of TLR-4 and LITAF. TLRs are sensitive sites that participate in the host immune response by recognizing molecular patterns associated with pathogens. They can active downstream TIR domain-containing adaptor inducing interferon-β or myeloid differentiation factor 88 to regulate the chemoresistance, cytokine, and chemokine production inducing cell apoptosis or proliferation (Lu et al., 2019). Among those, TLR4 is the key factor mediating LPS-induced inflammation. LITAF, a proinflammation cytokine that could promote the activation and chemotaxis of inflammatory cells, could induce acute inflammatory response (Sattler, 2017). Above all, dietary DON at 5 mg/kg and C. perfringens challenge (duration of day 17–day 21) had no effect on the jejunal inflammation response, but they had an interactive effect on the inflammation response.
Inflammation responce can eliminate antigens, but too acute inflammation response could result in cell death and injure the intestine. Apoptosis and proliferation are important mechanisms to update epithelial cells to maintain the normal construction and function of the intestine (Der Flier and Clevers, 2009). Meanwhile, abnormal apoptosis would lead to various diseases and dysfunction of the intestine. Apoptosis, an ATP-dependent cell death method, is a procedural death controlled by cells (Benjelloun et al., 2010). Under basal conditions, Bax is inactive and localized to cytoplasm. When various unfavorable conditions happened, Bax accumulates at the mitochondria and can be directly activated by BH3-only proteins (Letai et al., 2002). Afterward, BH3-only proteins inhibit antiapoptotic Bcl-2 proteins and activate the effector proapoptotic Bcl-2 proteins Bax and Bcl-2-antagonist/killer1, leading to mitochondrial outer membrane permeabilization which activated Caspase-3 and 7 to facilitate apoptosis (Cogliati et al., 2013). In addition, Caspase-8–mediated cleavage and activation of BH3-only protein BID also regulated the apoptosis pathway (Bock and Tait, 2020). Results showed that dietary DON significantly downregulated the expression of proapoptosis genes Bax and Caspase-3 indicating an antiapoptosis state, and this may be due to the dose- and time-dependent toxicity of DON (Payros et al., 2016). Bcl-2 can inhibit apoptosis by limiting the release of cytochrome c from mitochondria, which has the ability as Caspases to activate apoptosis (Yang et al., 1997). Furthermore, Bcl-2 impedes the activation of Bax to prevent apoptosis (Scaffidi et al., 1998). Caspase-8, as a cell death regulator, could induce cell apoptosis via death receptors such as tumor necrosis factor receptor 1 (TNFR1). Moreover, it could depress cell necroptosis via regulating the receptor-interacting serine/threonine kinase 3 and mixed lineage kinase domain-like protein (Alvarez-Diaz et al., 2016). Effects of C. perfringens on the upregulated relative expression of Bcl-2 and Caspase-8 indicated that the broilers were in a proapoptosis status. In effect, C. perfringens promoted the apoptosis of jejunal epithelial cells. The death receptors pathway is accomplished by the combination of a signal molecule and death receptors located in the cell surface, such as LITAF and TNFR. When secreted, signal molecule LITAF combined with TNFR1, TNFR1-associated death domain, receptor-interacting protein kinase 1, TNF receptor–associated factor 2, and cellular inhibitor of apoptosis proteins 1/2 were recruited, and Caspase-8 was activated (Haas et al., 2009). Activated Caspase-8 cleaves and activates Caspases-3 and Caspases-7, leading to wide-scale cleavage of cellular components and rapid cell death (Bock and Tait, 2020). The interactive analysis noted that the combined effect of DON and C. perfringens trended to antagonize the expression of Caspase-8. Combined with their antagonistic effect on the expression of TLR-4 and LITAF, it could be conjectured that DON and C. perfringens may have interaction in the apoptotic death receptor pathway of epithelial cells by antagonizing the expression of LITAF in the TLR4 pathway.
There is abundant microbiota harbored in the intestine, which plays an important role in the regulation of digestion, absorption, and immunity. As known the toxicity of DON on intestine is through the impact on the microflora (Robert et al., 2017). To verify whether the interactive effect of DON and C. perfringens is through their impact on the microflora, the construction of microflora in the jejunum was determined using the 16s rDNA gene-sequencing approach. Alpha diversity indices including Chao1, ACE, Shannon, and Simpson indices, are commonly used to measure the abundance and diversity of the community. Chao1 and ACE indices suggest the abundance of the community, and the Shannon and Simpson indexes suggest diversity (Shannon, 1948; Chao, 1984; Chao and Yang, 1993). In this experiment, dietary DON significantly reduced Simpson, Chao1, ACE, and Shannon indices of the flora, exhibiting that dietary DON reduced the abundance and diversity of microflora, according to previous studies (Vignal et al., 2018; Awad et al., 2019). In agreement with the aforementioned results, dietary DON reduced the abundance of Bacteroidetes and tended to reduce the abundance of Firmicutes, Proteobacteria, and Actinobacteria. C. perfringens challenge had no effect on the abundance of microbiota on the phylum level. On the genus level, results revealed that dietary DON affected the abundance of Lactobacillus and Lactococcus. Similarly, the C. perfringens challenge had the same effect on Lactococcus, but interactive analysis revealed that they antagonized the relative abundance of Lactococcus among the top 5 genera. Variation of bacteria mainly related to the shift of SCFAs' contents. Dietary DON and C. perfringens have no effect on the content of SCFAs in the cecal contents of broilers. However, dietary DON and C. perfringens challenge had an interaction effect on butyric acid content. The changes of apoptosis and intestinal inflammation status are driven by the modified microbiota (Yang et al., 2020). Spearman correlation analysis was used to characterize associations of bacterial relative abundances with SCFAs' concentrations in cecal digesta and mRNA expression of inflammation and apoptosis-relative genes. In total, most negative correlations were observed within the isobutyric acid, butyric acid, acetic acid, and total acid contents. In contrast, most positive correlations were observed within the genus Lactobacillus. These results indicated that the composition differences of bacterial metabolites reflected the changes of microbiota. To study the role of gut microbiota in dietary DON and C. perfringens challenge–induced apoptosis and intestinal inflammation in broilers, the relationship between the relative abundance of the gut microbiome and the mRNA expression of immunity-related and apoptosis-regulatory genes was also examined. Results revealed most of the top 50 genera were negatively correlative with the mRNA expression of LITAF. Interestingly, the relative abundance of Lactobacillus was positively correlated with LITAF. We speculate that it may be the regulative role of the Lactobacillus under infection conditions (Sanders et al., 2019). The relative abundance of Lactococcus had no significant correlation with LITAF gene expression, but a significant positive correlation was seen with Caspase-3 gene expression. Lactococcus are among the most important lactic acid bacteria involved in the dairy industry (Barbieri et al., 2019), and some species are the pathogenic agent in fish such as Lactococcus garvieae (Raissy et al., 2018). Above all, we speculated that the interactive effect of dietary DON and C. perfringens challenge on the relative abundance of Lactococcus to influence the death receptor apoptosis pathway may be a potential mechanism of their interactive effect on the apoptosis response of jejunal epithelial cells. The results of the KEGG pathway PICRUSt agreed with the aforementioned results, showing the metabolism was altered by the shifty microflora. Different stimuli such as DNA damage and mitotic processes accompany with apoptosis. DON is potentially genotoxic, and exposure to DON enhances DNA damage and apoptosis (Mishra et al., 2016). Pierron et al. (2016) reported that the de-epoxidation or epimerization of DON led to an absence of MAPK activation and a reduced toxicity, and it is reported that there are intestinal bacteria that belong to the Clostridium sp. category which had the ability to de-epoxidize or epimerize DON (Li et al., 2017). Furthermore, it is reported that the downregulated expression of genes related to the energy metabolism of Salmonella may be the reason of the toxicity of DON to Salmonella (Vandenbroucke et al., 2011). The toxicity between DON and pathogenic bacteria may be another explanation for the interactive effect of dietary DON and C. perfringens challenge. Although the underlying mechanism still needs further investigation, our results confirm that there are antagonistic effects between DON and C. perfringens on the enterotoxication of broilers, implying that the complex mode of mycotoxin and pathogenic bacteria and microflora may play a crucial role in their interaction.
Conclusions
Both DON and C. perfringens had a harmful effect on the jejunal health of broiler chickens. The interaction effect between the 2 factors on the mRNA expression of LITAF and TLR-4 may have evolved by manipulating the bacterial community composition and bacterial metabolites, especially Lactococcus and butyric acid.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (grant number 31402095), the National Key Research and Development Program of China (grant number 2018YFD0500600), the Program for Shaanxi Science and Technology (grant number 2017ZDXMNY-087), and the Fundamental Research Funds for the Central Universities (grant number 2452019203). The authors are grateful to Pro. Xu Jinrong for providing the strain of F. graminea PH-1 and Pro. Zhong Wang for providing the strain of C. perfringens CVCC2030.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.10.061.
Supplementary Data
References
- Alvarez-Diaz S., Dillon C.P., Lalaoui N., Tanzer M.C., Rodriguez D.A., Lin A., Lebois M., Hakem R., Josefsson E.C., O’Reilly L.A. The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity. 2016;45:513–526. doi: 10.1016/j.immuni.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonissen G., Van Immerseel F., Pasmans F., Ducatelle R., Haesebrouck F., Timbermont L., Verlinden M., Janssens G.P.J., Eeckhaut V., Eeckhout M., De Saeger S., Hessenberger S., Martel A., Croubels S. The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS One. 2014;9:e108775. doi: 10.1371/journal.pone.0108775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonissen G., Croubels S., Pasmans F., Ducatelle R., Eeckhaut V., Devreese M., Verlinden M., Haesebrouck F., Eeckhout M., De Saeger S., Antlinger B., Novak B., Martel A., Van Immerseel F. Fumonisins affect the intestinal microbial homeostasis in broiler chickens, predisposing to necrotic enteritis. Vet. Res. 2015;46:98. doi: 10.1186/s13567-015-0234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.A., Zentek J. The feed contaminant deoxynivalenol affects the intestinal barrier permeability through inhibition of protein synthesis. Arch. Toxicol. 2015;89:961–965. doi: 10.1007/s00204-014-1284-9. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Ruhnau D., Hess C., Doupovec B., Schatzmayr D., Hess M. Feeding of deoxynivalenol increases the intestinal paracellular permeability of broiler chickens. Arch. Toxicol. 2019;93:2057–2064. doi: 10.1007/s00204-019-02460-3. [DOI] [PubMed] [Google Scholar]
- Barbieri F., Montanari C., Gardini F., Tabanelli G. Biogenic amine production by lactic acid bacteria: a review. Foods Basel Switz. 2019;8:17. doi: 10.3390/foods8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjelloun N., Joly L.M., Palmier B., Plotkine M., Charriautmarlangue C. Apoptotic mitochondrial pathway in neurones and astrocytes after neonatal hypoxia-ischaemia in the rat brain. Neuropathol. Appl. Neurobiol. 2010;29:350–360. doi: 10.1046/j.1365-2990.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Bock F.J., Tait S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020;21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- Chao A. Nonparametric estimation of the number of classes in a population. Scand. J. Statist. 1984;11:265–270. [Google Scholar]
- Chao A., Yang M.C.K. Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika. 1993;80:193–201. [Google Scholar]
- Chen S.S., Li Y.H., Lin M.F. Chronic exposure to the Fusarium mycotoxin deoxynivalenol: impact on performance, immune organ, and intestinal integrity of slow-growing chickens. Toxins (Basel) 2017;9:334. doi: 10.3390/toxins9100334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliati S., Frezza C., Soriano M.E., Varanita T., Quintanacabrera R., Corrado M., Cipolat S., Costa V., Casarin A., Gomes L.C. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155:160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- Der Flier L.G.V., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- European Commission Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC) Official J. Eur. Union. 2006 L229, 7–9. [Google Scholar]
- Girardet C., Bonnet M.S., Jdir R., Sadoud M., Thirion S., Tardivel C., Roux J., Lebrun B., Wanaverbecq N., Mounien L., Trouslard J., Jean A., Dallaporta M., Troadec J.D. The food-contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PLoS One. 2011;6:e26134. doi: 10.1371/journal.pone.0026134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghareeb K., Awad W.A., Bohm J., Zebeli Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: poultry and swine. J. Appl. Toxicol. 2015;35:327–337. doi: 10.1002/jat.3083. [DOI] [PubMed] [Google Scholar]
- Haas T.L., Emmerich C.H., Gerlach B.R., Schmukle A.C., Cordier S.M., Rieser E., Feltham R., Vince J.E., Warnken U., Wenger T. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell. 2009;36:831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Immerseel F.V., Rood J.I., Moore R.J., Titball R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Khaneghah A.M., Martins L.M., von Hertwig A.M., Bertoldo R., Sant’Ana A.S. Deoxynivalenol and its masked forms: Characteristics, incidence, control and fate during wheat and wheat based products processing - a review. Trends Food Sci. Technol. 2018;71:13–24. [Google Scholar]
- Letai A., Bassik M.C., Walensky L.D., Sorcinelli M.D., Weiler S., Korsmeyer S.J. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Li F., Wang J., Huang L., Chen H., Wang C. Effects of adding Clostridium sp. wj06 on intestinal morphology and microbial diversity of growing pigs fed with natural deoxynivalenol contaminated wheat. Toxins. 2017;9:383. doi: 10.3390/toxins9120383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang W., Liu D., Guo Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2018;9:25. doi: 10.1186/s40104-018-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhang D., Liu Y., Zhou D., Yang H., Zhang K., Zhang D. Circular RNA circ_0001105 protects the intestinal barrier of septic rats by inhibiting inflammation and oxidative damage and YAP1 expression. Gene. 2020;755:144897. doi: 10.1016/j.gene.2020.144897. [DOI] [PubMed] [Google Scholar]
- Lu Y., Xu X., Jiang T., Jin L., Zhao X.D., Cheng J.H., Jin X.J., Ma J., Piao H.N., Piao L.X. Sertraline ameliorates inflammation in CUMS mice and inhibits TNF-α-induced inflammation in microglia cells. Int. Immunopharmacol. 2019;67:119–128. doi: 10.1016/j.intimp.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Mishra S., Srivastava S., Dewangan J., Divakar A., Kumar Rath S. Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: a survey. Crit. Rev. Food Sci. Nutr. 2020;60(8):1346–1374. doi: 10.1080/10408398.2019.1571479. [DOI] [PubMed] [Google Scholar]
- Mishra S., Tewari P., Chaudhari B.P., Dwivedi P.D., Pandey H.P., Das M. Deoxynivalenol induced mouse skin tumor initiation: Elucidation of molecular mechanisms in human HaCaT keratinocytes. Int. J. Cancer. 2016;139:2033–2046. doi: 10.1002/ijc.30260. [DOI] [PubMed] [Google Scholar]
- Oh S., Lillehoj H.S. The role of host genetic factors and host immunity in necrotic enteritis. Avian Pathol. 2016;45:313–316. doi: 10.1080/03079457.2016.1154503. [DOI] [PubMed] [Google Scholar]
- Osselaere A., Santos R., Hautekiet V., Backer P.D., Chiers K., Ducatelle R., Croubels S. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS One. 2013;8:e69014. doi: 10.1371/journal.pone.0069014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payros D., Alassanekpembi I., Pierron A., Loiseau N., Pinton P., Oswald I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016;90:2931–2957. doi: 10.1007/s00204-016-1826-4. [DOI] [PubMed] [Google Scholar]
- Pierron A., Mimoun S., Murate L.S., Loiseau N., Lippi Y., Bracarense A.P.F.R.L., Schatzmayr G., He J.W., Zhou T., Moll W. Microbial biotransformation of DON: molecular basis for reduced toxicity. Sci. Rep. 2016;6 doi: 10.1038/srep29105. 29105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P., Graziani F., Pujol A., Nicoletti C., Paris O., Ernouf P., Di Pasquale E., Perrier J., Oswald I.P., Maresca M. Deoxynivalenol inhibits the expression by goblet cells of intestinal mucins through a PKR and MAP kinase dependent repression of the resistin-like molecule β. Mol. Nutr. Food Res. 2015;59:1076–1087. doi: 10.1002/mnfr.201500005. [DOI] [PubMed] [Google Scholar]
- Raissy M., Hashemi S., Roushan M., Jaafarian M., Momtaz H., Soltani M., Kheirabadi E.P. Effects of essential oils of Satureja bachtiarica and Nigella sativa on the efficacy of lactococcosis vaccine in rainbow trout (Oncorhynchus mykiss) Iran. J. Fish. Sci. 2018;17:95–106. [Google Scholar]
- Robert H., Payros D., Pinton P., Theodorou V., Mercier-Bonin M., Oswald I.P. Impact of mycotoxins on the intestine: are mucus and microbiota new targets? J. Toxicol. Environ. Health B Crit. Rev. 2017;20:249–275. doi: 10.1080/10937404.2017.1326071. [DOI] [PubMed] [Google Scholar]
- Rodrigues I., Naehrer K. A Three-Year survey on the worldwide occurrence of mycotoxins in Feedstuffs and feed. Toxins. 2012;4:663. doi: 10.3390/toxins4090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- Sattler S. The role of the immune system beyond the Fight against infection. Adv. Exp. Med. Biol. 2017;1003:3–14. doi: 10.1007/978-3-319-57613-8_1. [DOI] [PubMed] [Google Scholar]
- Scaffidi C., Fulda S., Srinivasan A., Friesen C., Li F., Tomaselli K.J., Debatin K., Krammer P.H., Peter M.E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948;27:379–423. [Google Scholar]
- Streit E., Naehrer K., Rodrigues I., Schatzmayr G. Mycotoxin occurrence in feed and feed raw materials worldwide: long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013;93:2892–2899. doi: 10.1002/jsfa.6225. [DOI] [PubMed] [Google Scholar]
- Ueno Y. General toxicology. In: Ueno Y., editor. Trichothecenes: Chemical, Biological, and Toxicological Aspects. Elsevier; New York, NY: 1983. pp. 135–146. [Google Scholar]
- Uzal F.A., Freedman J.C., Shrestha A., Theoret J.R., Garcia J., Awad M.M., Adams V., Moore R.J., Rood J.I., McClane B.A. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. 2014;9:361–377. doi: 10.2217/fmb.13.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke V., Croubels S., Martel A., Verbrugghe E., Goossens J., Van Deun K., Boyen F., Thompson A.R., Shearer N., De Backer P. The mycotoxin deoxynivalenol potentiates intestinal inflammation by Salmonella typhimurium in porcine ileal loops. PLoS One. 2011;6:e23871. doi: 10.1371/journal.pone.0023871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignal C., Djouina M., Pichavant M., Caboche S., Waxin C., Beury D., Hot D., Gower-Rousseau C., Body-Malapel M. Chronic ingestion of deoxynivalenol at human dietary levels impairs intestinal homeostasis and gut microbiota in mice. Arch. Toxicol. 2018;92:2327–2338. doi: 10.1007/s00204-018-2228-6. [DOI] [PubMed] [Google Scholar]
- Wang H., Latorre J.D., Bansal M., Abraha M., Al-Rubaye B., Tellez-Isaias G., Hargis B., Sun X. Microbial metabolite deoxycholic acid controls Clostridium perfringens-induced chicken necrotic enteritis through attenuating inflammatory cyclooxygenase signaling. Sci. Rep. 2019;9:14541. doi: 10.1038/s41598-019-51104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing B.P., Van K.A.G. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J. Anim. Sci. 2007;12:3256–3266. doi: 10.2527/jas.2007-0320. [DOI] [PubMed] [Google Scholar]
- Wu S.R., Liu Y.L., Duan Y.L., Wang F.Y., Guo F.S., Yan F., Yang X.J., Yang X. Intestinal toxicity of deoxynivalenol is limited by supplementation with Lactobacillus plantarum JM113 and consequentially altered gut microbiota in broiler chickens. J. Anim. Sci. Biotechnol. 2018;9:74. doi: 10.1186/s40104-018-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Shao Y., Song B., Zhen W., Wang Z., Guo Y., Shahid M., Nie W. Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens -induced necrotic enteritis. J. Anim. Sci. Biotechnol. 2018;9:9. doi: 10.1186/s40104-017-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G.D., Barekatain R., Wu S.B., Choct M., Swick R.A. Dietary L-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poult. Sci. 2018;97:1334–1341. doi: 10.3382/ps/pex444. [DOI] [PubMed] [Google Scholar]
- Yang J., Liu X., Bhalla K., Kim C.N., Ibrado A.M., Cai J., Peng T.I., Jones D.P., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yang X., Li L., Duan Y.L., Yang X.J. Antioxidant activity of Lactobacillus plantarum JM113 in vitro and its protective effect on broiler chickens challenged with deoxynivalenol. J. Anim. Sci. 2017;95:837–846. doi: 10.2527/jas.2016.0789. [DOI] [PubMed] [Google Scholar]
- Yang X., Liang S., Guo F., Ren Z., Long F. Gut microbiota mediates the protective role of Lactobacillus plantarum in ameliorating deoxynivalenol-induced apoptosis and intestinal inflammation of broiler chickens. Poult. Sci. 2020;99:2395–2406. doi: 10.1016/j.psj.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Lepp D., Pei Y., Liu M., Yin X., Ma R., Prescott J.F., Gong J. Influence of pCP1NetB ancillary genes on the virulence of Clostridium perfringens poultry necrotic enteritis strain CP1. Gut Pathog. 2017;9:6. doi: 10.1186/s13099-016-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.